Abstract
Anticipatory nausea (AN) is a conditioned nausea reaction experienced by chemotherapy patients upon returning to the clinic. Currently, there are no specific treatments for this phenomenon, with the classic anti-emetic treatments (e.g., ondansetron) providing no relief. The rat model of AN, contextually elicited conditioned gaping reactions in rats, provides a tool for assessing potential treatments for this difficult to treat disorder. Systemically administered drugs which elevate the endocannabinoids, anandamide (AEA) and 2-arachodonyl glycerol (2-AG), by interfering with their respective degrading enzymes, fatty acid amide hydrolase (FAAH) and monoacyl glycerol lipase (MAGL) interfere with AN in the rat model. We have shown that MAGL inhibition within the visceral insular cortex (VIC) interferes with acute nausea in the gaping model (Sticht et al, 2015b). Here we report that bilateral infusion of the MAGL inhibitor, MJN110 (but neither the FAAH inhibitor, PF3845, nor ondansetron) into the VIC suppressed contextually – elicited conditioned gaping and this effect was reversed by co-administration of the CB1 antagonist, AM251. These findings suggest that 2-AG within the VIC plays a critical role in the regulation of both acute nausea and AN. As there are currently no specific therapeutics for chemotherapy patients that develop anticipatory nausea, MAGL inhibition by MJN110 may be a candidate treatment.
Keywords: Monoacylglycerol lipase, Fatty acid amide hydrolase, visceral insular cortex, cannabinoid, anticipatory nausea, serotonin, CB1 antagonist
Introduction
Approximately 25%–50% of cancer patients receiving highly emetic chemotherapy treatment develop anticipatory nausea (AN) as a result of the association between the contextual cues of the chemotherapy clinic with the subsequent nausea experienced by their treatment (Akechi et al., 2010; Bovberg et al., 1992; Hickok, Roscoe & Morrow, 2001; Nesse et al., 1980; Tyc, Mulhern & Bieberich, 1997; Watson McCarron & Law, 1992; Zachariaie et al., 2007). The risk of developing AN increases with the number of chemotherapy cycles during which nausea is not properly managed (Aapro, Molassiotis & Oliver 2005; Hickok et al., 2003; Janelsins et al., 2013; Morrow et al., 1998; Roscoe et al., 2011). Once AN develops it is not well controlled by currently available antiemetics used to treat acute nausea such as the classic 5-HT3 receptor antagonist, ondansetron (OND; Aapro et al., 2005; Foubert & Vaessen, 2005; Morrow et al., 1998), and is currently treated with non-specific anti-anxiety drugs (benzodiazepines, such as lorazepam), which have sedating side effects (Malik et al., 1995; Razavi et al., 1993). Since AN is not well managed in the clinic there is a need for more selective and effective treatments for this distressing side effect of chemotherapy treatment in cancer patients. The development of new treatments, however, requires preclinical evaluation in animal models. Such an animal model has been recently reported, contextually elicited conditioned gaping in rats (Limebeer et al., 2006; Limebeer et al., 2008; Rock et al., 2008).
Although rats do not vomit when injected with a toxin, they display a distinctive conditioned gaping reaction with re-exposed to a toxin-induced flavor cue (Grill & Norgren, 1978) or contextual cue (Limebeer et al., 2008). Only emetic treatments produce conditioned gaping in rats and anti-emetic treatments suppress these conditioned gaping reactions (see Parker 2014). Interestingly, once contextually elicited conditioned gaping reactions are established, they cannot be attenuated by the classical anti-emetic treatment, OND, as is also evident in human chemotherapy patients expressing AN elicited by the treatment paired context (Limebeer et al 2006; Rock et al., 2014). On the other hand, cannabinoid treatments are effective in suppressing the expression of contextually-elicited conditioned gaping in rats (see Rock et al., 2014 for review). As well, evidence indicates that systemic elevation of endogenous cannabinoids anandamide (AEA) and 2-arachidonyl glycerol (2-AG) by suppression of their selective degrading enzymes fatty acid amide hydrolase (FAAH; Cravatt et al., 1996) and monoacylglycerol lipase (MAGL; Dinh, et al 2002) respectively, attenuates the expression of contextually elicited conditioned gaping in rats (Rock et al., 2008; Parker et al 2015).
Recent evidence implicates the visceral insular cortex (VIC) as the site responsible for the generation of acute nausea-induced conditioned gaping in rats (Kiefer & Orr, 1992; Tuerke et al., 2012; Limebeer et al., 2010; Sticht et al., 2015a; Sticht et al., 2015b). Sticht et al (2015a) demonstrated that exogenous administration of 2-AG, but not AEA, into the VIC suppressed acute nausea produced by LiCl, thereby preventing the establishment of LiCl-induced conditioned gaping. Furthermore, intra-VIC infusion of the MAGL inhibitor MJN110, but not the FAAH inhibitors URB597 or PF3845, suppressed LiCl-induced acute nausea by selective elevation of 2-AG in this region (Sticht et al, 2015b). Although evidence clearly implicates the VIC in the generation of acute nausea, its role in AN remains to be elucidated. Here we evaluate the potential of bilateral intra-VIC infusions of the MAGL inhibitor, MJN110, and FAAH inhibitor, PF3845, and OND to interfere with the expression of LiCl-induced contextually elicited conditioned gaping in rats during a test for AN. Given that attenuation of acute nausea by manipulations of endocannabinoids in the VIC is mediated by 2-AG, not AEA (Sticht et al, 2015a; Sticht et al, 2015b), we predicted that intra-VIC administration of MJN110, but not PF3845 would also interfere with the expression of AN. As well, given that systemic administration of OND is ineffective as a treatment of AN in the rodent model and in humans, we predicted that OND treatment to this region would not interfere with AN, even though it effectively interferes with acute nausea when delivered to this region (Tuerke et al, 2012).
Method
Subjects
A total of 48 näive male Sprague-Dawley rats, obtained from Charles River Laboratories (St Constant, Quebec), were used for assessment of AN, The number reflects only those rats with correctly placed cannaule. They were individually housed in shoebox cages in a colony room kept at an ambient temperature of 21°C with a 12/12 hour light-dark schedule (lights off at 7 am), and maintained on food (Highland Rat Chow[8640]) and water ad-libitum. Their body weights ranged from 300 to 440 g on the day of testing for AN. Animal procedures complied with the Canadian Council on Animal Care guidelines and the protocols were approved by the Institutional Animal Care Committee at University of Guelph.
Drugs
Lithium Chloride (Sigma Aldrich, Canada) was prepared in a 0.15 M solution with sterile water and was administered at a volume of 20 ml kg−1 (127.2 mg kg−1 dose) intraperitoneally (ip). OND (Sigma Aldrich, Canada) was prepared at a concentration of 1 mg ml−1 in saline. The drugs MJN110 and PF3845 (generously donated by BF Cravatt), AM251 (generously donated by A Makriyiannis) were prepared in a 1:9 solution of Tween 80: physiological saline at concentrations of 2 and 4 mg ml−1 (MJN110), PF 1 and 2 mg ml−1 (AM251), 1 mg ml−1 (OND). The drugs were first dissolved in ethanol (and sonicated if necessary) then Tween 80 was added to the solution and the ethanol was evaporated off with a nitrogen stream after which the saline was added. The final VEH consisted of 1:9 Tween:saline. At test, all drugs were microinfused bilaterally to the VIC at a rate of 0.5 ul/min. The VEH (1 ul), MJN (2 ug; Sticht, et al., 2015b), PF (2 ug; Sticht et al., 2015b) and OND (1 ug; Tuerke et al. 2012) groups were microinfused for two minutes and the injector was left in place for one minute following the infusion.. The MJN was microinfused 60 min and the OND was microinfused 30 min before the rat was put in the conditioning chamber for assessing AN. The VEH-AM251 (1 μg/.5 μl; Limbeer et al, 2012) and MJN (2 μg/.5μl)-AM251 (1 μg/.5μl) groups were microinfused one minute for each drug (VEH-MJN 60 min and the AM251 15 minutes before being placed in the conditioning chamber). Following the microinfusion the injector was left in place for one minute before returning the obdurator.
Surgery
All rats were surgically implanted with bi-lateral guide cannulas directed to the VIC as described in detail in Limebeer et al. (2012). Briefly, once animals were prepared for surgery and fully anesthetized (using isoflurane) they were placed in a stereotaxic frame and stainless steel guide cannulas (6 mm below pedestal; Plastics One, Roanoke, Virginia) placed at a 10° divergent angle were lowered using coordinates relative to Bregma: AP −0.5 mm; ML ± 5.0 mm; DV – 4.5 mm from the skull surface. Once the guide cannulas were set in place using dental cement adhered to six screws placed in the skull and an obdurator was put into the cannulas. The rats were then removed from the frame and returned to the colony room when they were fully ambulatory. They were monitored daily and allowed to recover for five days before the experimental procedures began.
Histology
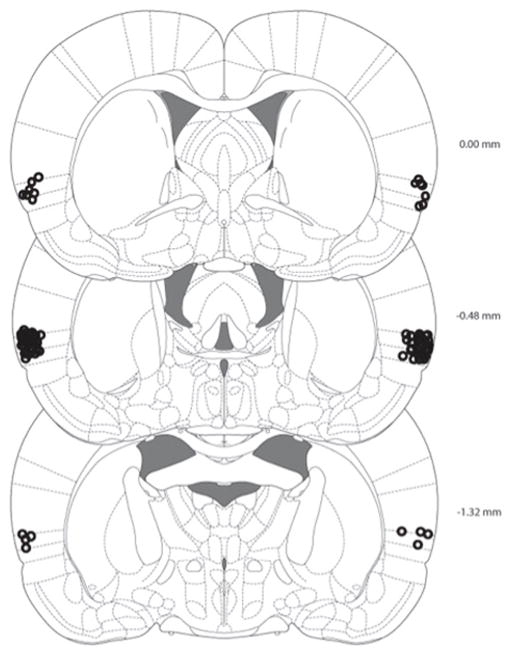
Guide cannula placements were verified by histological evaluation of tissue. Rats were deeply anaesthetized using Sodium Pentobarbitol (Intervet Canada Corp, Kirkland, QC, Canada) and were then transcardially perfused using PBS (0.1 M) and 4% formalin. The brains were removed and placed on a shaker overnight at room temperature in a 20% Sucrose/Formalin solution. The relevant brain regions were then sectioned (50 um sections) using a Leica 1930 cryostat (Leica Microsystems, Concord, ON, Canada). Twenty four hr later the sections were thionin stained, coverslipped and afterwards placements were evaluated using a MZ6 Leica Stereomicroscope, Leica DFC420 Digital Camera and Leica Application Suite software (Leica Microsystems, Concord, ON, Canada). Any rats with improper placements (n=15) were removed from statistical analysis. Group N’s reported represent rats with proper placements. Figure 1 presents a schematic representation of the microinfusion cannula tip placements in the VIC for rats with correct placements. All cannaula tips were located between 0.00 and −1.32 mm posterior to bregma according to Paxinos and Watson (2007).
Figure 1.
A schematic representation of the microinfusion cannula tip placements in the VIC for all rats. All cannula tips were located between 0.00 and −1.32mm posterior to bregma. From The Rat Brain in Stereotaxic Coordinates (6th ed.), Figures 33 – 44 inclusive by G. Paxinos and C. Watson, 2007, New York, NY: Academic Press. Copyright 2007 by Elsevier Academic Press. Adapted with permission.
Apparatus
The conditioning chamber used to assess AN was constructed of black Plexiglas sides (22.5 × 26 × 20 cm) with an opaque lid.. The testing room was dark with two 40-Watt lights on either side of the conditioning chamber. The chamber was placed on a table with a clear Plexiglas top. A mirror was located beneath the chamber at a 45° angle which facilitated viewing of the ventral surface of the rat. A Sony videocamera (Handycam, Henry’s Camera, Waterloo, ON, Canada) was used to videotape the rats from the mirror beneath the chamber. The videotapes were later scored using “The Observer” Event recording software (Noldus, Inc, Netherlands).
The activity chamber was constructed of white Plexiglas with the dimensions of 60 cm × 25 cm × 25 cm., located in a different room than the conditioning chamber for assessing AN and was illuminated with a red-light. A video camera mounted on an extension pole captured the activity of the rat and was sent to a computer for analysis of distance (cm) traveled using the Ethovision software program (Noldus, Inc, NL).
Behavioral Procedures
Effect of intra-VIC MAGL inhibition, FAAH inhibition and 5-HT3 antagonism on AN
The rats received four conditioning trials, during which the conditioning chamber was paired with LiCl (127 mg kg−1). On each conditioning trial, each rat was injected with LiCl and immediately placed in the conditioning chamber for a 30-min period. This procedure occurred on a total of four conditioning trials, with 48 hr between each trial.
The AN test trial occurred 72 hr after the final conditioning trial. The groups were administered a bilateral microinfusion into the VIC of VEH (n=12) MJN110 (n=10), PF3845 (n=12) or OND (n=9) prior to placement in the conditioning chamber. Groups MJN110 and PF3845 received the microinfusion 60 min prior to the AN test to ensure sufficient elevation of 2-AG and AEA respectively (Niphakis et al., 2013; Ahn et al., 2009) whereas group VEH and OND were infused 30 min (Tuerke et al., 2012) prior to placement in the chamber.
Immediately following the 5 min AN test trial rats were removed from the chamber and taken to a separate room where their locomotor activity was recorded for 15 min. When the activity test was completed rats were returned to their home cages and the colony room.
The videos from the AN test trial were scored for the response of gaping (retraction of the corners of the mouth with the lower incisors exposed) by an observer blind to the experimental conditions.
Role of CB1 in reversing suppressive effect of MAGL inhibition on AN
Since only intra-VIC administration of MJN110 suppressed AN, the potential of the CB1 antagonist/inverse agonist, AM251, to reverse this effect was evaluated and compared with the VEH and MJN alone group. The groups included: VEH (n=12), MJN 110 (n=10), VEH-AM251 (n=8), MJN-AM251 (n=9). Following the 4 conditioning trials, on the AN test trial, rats were bilaterally microinfused with VEH (1 μl) or MJN110 (2μg) into the VIC 1 hr prior to the AN test (flow rate of 0.5 ul/min for two min leaving the injector in for 1 min post infusion) and rats in group VEH-AM251 or MJN110 – AM251 were also infused bilaterally with 1 μg AM251 45 min later (flow rate of 0.5 ul/min for 0.5 min leaving the injector in place for 1 min post infusion).
Statistical Analyses
Conditioned gaping reactions for the AN test and distance travelled for the activity test were analyzed using a one-way ANOVA. Post-hoc pairwise comparisons were examined with Fisher’s LSD test. Statistical significance was defined as p<0.05.
Results
Effect of intra-VIC MAGL inhibition, FAAH inhibition and 5-HT3 antagonism on AN
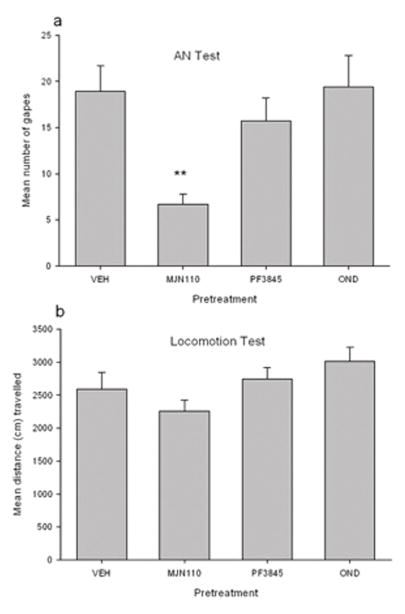
Group MJN110, but neither PF3845 nor OND groups, displayed suppressed contextually elicited conditioned gaping reactions during the test for AN. The upper section of Figure 2 presents the mean (±sem) number of gapes displayed by the various pretreatment groups. A single factor ANOVA of gaping reactions revealed a significant effect of pretreatment condition, F (3, 39) = 4.8; p = 0.006. Subsequent Fisher LSD post-hoc comparison tests revealed that only Group MJN110 differed significantly from Group VEH (p’s < 0.01); neither Group PF3845 nor Group OND differed significantly from Group VEH. The lower section of Figure 2 presents the mean (±sem) distance (cm) travelled during the 15 min locomotor test that immediately followed the AN test. As is apparent, the single factor ANOVA was not statistically significant; none of the VIC pretreatments modified general activity level.
Figure 2.
a) Mean (+sem) number of gapes following intra-VIC administration of VEH (n=12), MJN110 (2μg/μl; n=10), PF3845 (2 μg/μl; n=12) or OND (1μg/μl; n=9) during a 5-min test of contextually-elicited conditioned gaping (AN test) in rats. **=p < 0.01 different than all other groups. b) Mean distance (cm) traveled during a 15-min test of activity immediately following the AN test.
Experiment 2: Role of CB1 receptor in suppression of AN by intra-VIC MJN110
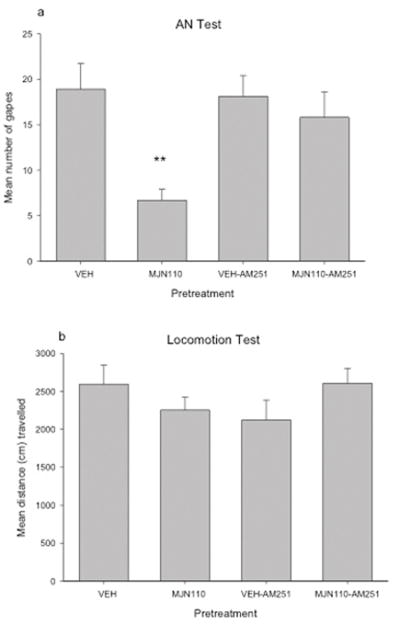
Intra-VIC administration of AM251 reversed the suppression of contextually elicited conditioned gaping produced by the MAGL inhibitor, MJN110, as seen in the upper section of Figure 3. A single factor ANOVA of gaping reactions revealed a significant effect of pretreatment condition, F (3, 35) = 5.5; p = 0.003. Subsequent LSD pairwise comparisons revealed that Group MJN110 displayed fewer gapes than did group MJN110-AM251 (p < 0.01). The reversal was complete because group MJN110-AM251 did not differ from Group VEH or Group VEH-AM251. The lower section of Figure 3 presents the mean (±sem) distance (cm) travelled during the 15 min locomotor test that immediately followed the AN test. There were no significant differences in activity among the various pretreatment conditions.
Figure 3.
a) Mean number of gapes during a 5-min test of contextually-elicited conditioned gaping (AN Test) following intra-VIC administration of VEH (n=12), MJN110(2μg/μl; n=10), VEH-AM251 (1μg/.5 μl; n=8) or MJN110-AM251 (2μg/.5μl-1μg/.5μl; n=9). b) Mean distance (cm) travelled during a 15-min test of activity immediately following the AN test
Discussion
Intra-VIC administration of the MAGL inhibitor, MJN110, interfered with the expression of contextually-elicited conditioned gaping reactions in a test of AN in rats. Neither the FAAH inhibitor, PF 3845, nor the 5-HT3 antagonist, OND, interfered with AN when bilaterally delivered to the VIC. Since MAGL inhibition selectively elevates 2-AG, but not AEA, these results suggest that elevation of 2-AG within the VIC regulates AN as we have previously reported it regulates acute nausea (Sticht et al., 2015a; Sticht et al, 2015b). Indeed, we have shown that either systemically administered MJN110 (20 mg/kg, ip) or intra-VIC administered MJN110 (2 μg/μl) selectively elevated 2-AG within the VIC. On the other hand, consistent with our report, neither systemic nor intra-VIC administered PF3845 (at the same dose as used here) elevated AEA in the VIC but both did elevate other fatty acids (N-oleoylethanolamide [OEA] and N-palymitolylethanolamide [PEA]) in this region indicating that this dose did suppress FAAH, but anandamide was not available in the VIC (Sticht et al, 2015b). As well, systemically administered LiCl also selectively elevated 2-AG, but not AEA, within the VIC when assessed 20 min following LiCl administration. Finally, LiCl produced c-fos activation in the VIC, an effect that was suppressed by systemic pretreatment with MJN110 (20 mg/kg, ip). The current findings suggest that 2-AG release in the VIC is critical for the regulation of not only acute nausea, but also anticipatory nausea.
The failure of OND within the VIC to modulate AN is consistent with the systemic effect of OND on contextually elicited conditioned gaping in rats (Limebeer & Parker, 2006; Rock et al., 2014) and on anticipatory nausea in human patients (eg. Morrow et al., 1998). However, this finding contrasts with the effect of systemic (Limebeer et al., 2000) or intra-VIC (Tuerke et al., 2012) administration of OND on acute nausea. These results suggest that the role of 5-HT3 in the generation of acute and anticipatory nausea differs in both rats and humans.
Finally, FAAH inhibition by systemic administration of URB597 has previously been reported to suppress contextually-elicited conditioned gaping in rats (Rock et al., 2008). However, here we report that intra VIC administration of the more selective FAAH inhibitor, PF3845 (Ahn et al., 2009), was without effect on AN. We have recently reported that systemic PF 3845 and URB597 are equally effective in suppressing contextually-elicited conditioned gaping and both effects are reversed by CB1 antagonism (Rock et al., 2015). Therefore, these effects are presumably mediated by the action of AEA either peripherally or at some other region than the VIC. Future research will be aimed at determining the site of action of AEA in attenuating AN. We are currently investigating the potential of the ventral pallidum in this role, given its role in the generation of disgust (Calder et al, 2007) and aversive responding in rats (Smith and Berridge, 2007).
Acknowledgments
This work was supported by NSERC (92057) and CIHR (137122) grants to LAP and by DAO32933 and DA33760 to BFC
References
- Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2005;13:117–121. doi: 10.1007/s00520-004-0745-8. [DOI] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chemistry & Biology. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akechi T, Okuyama T, Endo C, Sagawa R, Uchida M, Nakaguchi T, Furukawa TA. Anticipatory nausea among ambulatory cancer patients undergoing chemotherapy: prevalence, associated factors, and impact on quality of life. Cancer Science. 2010;101:2596–2600. doi: 10.1111/j.1349-7006.2010.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berr
- Bovbjerg DH, Redd WH, Jacobsen PB, Manne SL, Taylor KL, Surbone A, Hakes MD. An experimental analysis of classically conditioned nausea during cancer chemotherapy. Psychosomatic Medicine. 1992;54:623–637. doi: 10.1097/00006842-199211000-00001. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrade neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensis SL, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert J, Vaessen G. Nausea: the neglected symptom? European Journal of Oncology Nursing. 2005;9:21–32. doi: 10.1016/j.ejon.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Hickok JT, Roscoe JA, Morrow GR. The role of patients’ expectations in the development of anticipatory nausea related to chemotherapy for cancer. Journal of Pain and Symptom Management. 2001;22:843–850. doi: 10.1016/S0885-3924(01)00317-7. [DOI] [PubMed] [Google Scholar]
- Hickok JT, Roscoe JA, Morrow GR, Kink DK, Atkins JN, Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opinion on Pharmacotherapy. 2013;14:757–766. doi: 10.1517/14656566.2013.776541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behavioral Neuroscience. 1992;106:140–146. doi: 10.1037/0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. Journal of Experimental Psychology Animal Behavior Processes. 2000;26:371–384. doi: 10.1037/0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Hall G, Parker LA. Exposure to a lithium-paired context elicits gaping in rats: A model of anticipatory nausea. Physiology & Behavior. 2006;88:398–403. doi: 10.1016/j.physbeh.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Krohn JP, Cross-Mellor S, Litt DE, Ossenkopp KP, Parker LA. Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behavioral Brain Research. 2008;187:33–40. doi: 10.1016/j.bbr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, Parker LA. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. British Journal of Pharmacology. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limebeer CL, Rock EM, Mechoulam R, Parker LA. The anti-nausea effects of CB1 agonists are mediated by an action at the visceral insular cortex. British Journal of Pharmacology. 2012;167:1126–1136. doi: 10.1111/j.1476-5381.2012.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik IA, Khan WA, Qazibash M, Ata E, Butt A, Khan MA. Clinical efficacy of lorazepam in prophylaxis of anticipatory, acute, and delayed nausea and vomiting induced by high doses of cisplatin. A prospective randomized trial. American Journal of Clinical Oncology. 1995;18:170–175. doi: 10.1097/00000421-199504000-00017. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ. Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer. 1998;6:244–247. doi: 10.1007/s005200050161. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Carli T, Curtis GC, Kleinman PD. Pretreatment nausea in cancer chemotherapy: a conditioned response? Psychosomatic Medicine. 1980;42:33–36. doi: 10.1097/00006842-198001000-00004. [DOI] [PubMed] [Google Scholar]
- Roscoe JA, Morrow GR, Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2011;19:1533–1538. doi: 10.1007/s00520-010-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB, Chang JW, Buczynski MW, Parsons LH, Byrne F, Cravatt BF. Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chemical Neuroscience. 2013;4:1322–1332. doi: 10.1021/cn400116z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. European Journal of Pharmacology. 2014;722:122–133. doi: 10.1016/j.ejphar.2013.09.070. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Sticht MA, Limebeer CL. Cannabinoids suppress acute and anticipatory nausea in preclinical rat models of conditioned gaping. Clinical Pharmacology and Therapeutics. 2015;97:559–561. doi: 10.1002/cpt.98. doi:10.1002.cpt.98. [DOI] [PubMed] [Google Scholar]
- Razavi D, Delvaux N, Farvacques C, DeBrier F, Van Heer C, Kaufmans L, Piccart M. Prevention of adjustment disorders and anticipatory nausea secondary to adjuvant chemotherapy: a double-blind, placebo-controlled study assessing the usefulness of alprazolam. Journal of Clinical Oncology. 1993;11:1384–1390. doi: 10.1200/JCO.1993.11.7.1384. [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology. 2008;196:389–395. doi: 10.1007/s00213-007-0970-1. [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Navaratnam R, Sticht MA, Bonner N, Engeland K, Parker LA. A comparison of cannabidiolic acid with other treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology. 2014;231:3207–3215. doi: 10.1007/s00213-014-3498-1. [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Ward JM, Cohen A, Grove K, Niphakis MJ, Cravatt BF, Parker LA. Interference with acute nausea and anticipatory nausea in rats by fatty acid amide hydrolase (FAAH) inhibition through a PPARα and CB1 receptor mechanism, respectively: a double dissociation. Psychopharmacology. 2015;232:3841–3848. doi: 10.1007/s00213-015-4050-7. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose ‘liking’ and food intake. J Neurosi, 2005. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticht MA, Limebeer CL, Rafla BR, Parker LA. Intra-visceral insular cortex 2-arachidonoylglycerol, but not N-arachidonoylethanolamide, suppresses acute nausea-induced conditioned gaping in rats. Neuroscience. 2015a;286:338–344. doi: 10.1016/j.neuroscience.2014.11.058. [DOI] [PubMed] [Google Scholar]
- Sticht MA, Limebeer CL, Rafla BR, Abdullah RA, Poklis JL, Ho W, Parker LA. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology. 2015b;102:92–102. doi: 10.1016/j.neuropharm.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerke KJ, Limebeer CL, Fletcher PJ, Parker LA. Double dissociation between regulation of conditioned disgust and taste avoidance by serotonin availability at the 5-HT(3) receptor in the posterior and anterior insular cortex. Journal of Neuroscience. 2012;32:13709–13717. doi: 10.1523/JNEUROSCI.2042-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc VL, Mulhern RK, Bieberich AA. Anticipatory nausea and vomiting in pediatric cancer patients: an analysis of conditioning and coping variables. Journal of Developmental and Behavioral Pediatrics. 1997;18:27–33. doi: 10.1097/00004703-199702000-00006. [DOI] [PubMed] [Google Scholar]
- Watson M, McCarron J, Law M. Anticipatory nausea and emesis, and psychological morbidity: assessment of prevalence among out-patients on mild to moderate chemotherapy regimens. British Journal of Cancer. 1992;66:862–866. doi: 10.1038/bjc.1992.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariae R, Paulsen K, Mehlsen M, Jensen AB, Johansson A, von der Maase H. Anticipatory nausea: the role of individual differences related to sensory perception and autonomic reactivity. Annals of Behavioral Medicine. 2007;33:69–79. doi: 10.1207/s15324796abm3301_8. [DOI] [PubMed] [Google Scholar]