Abstract
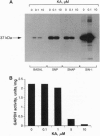
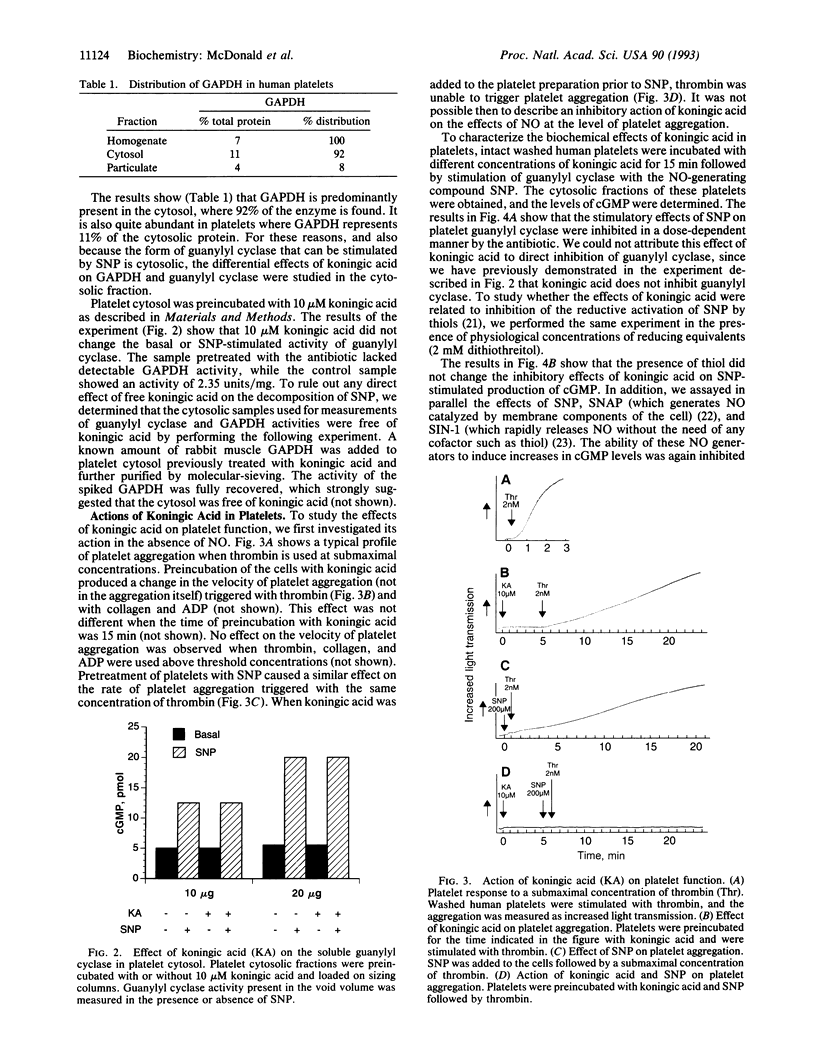
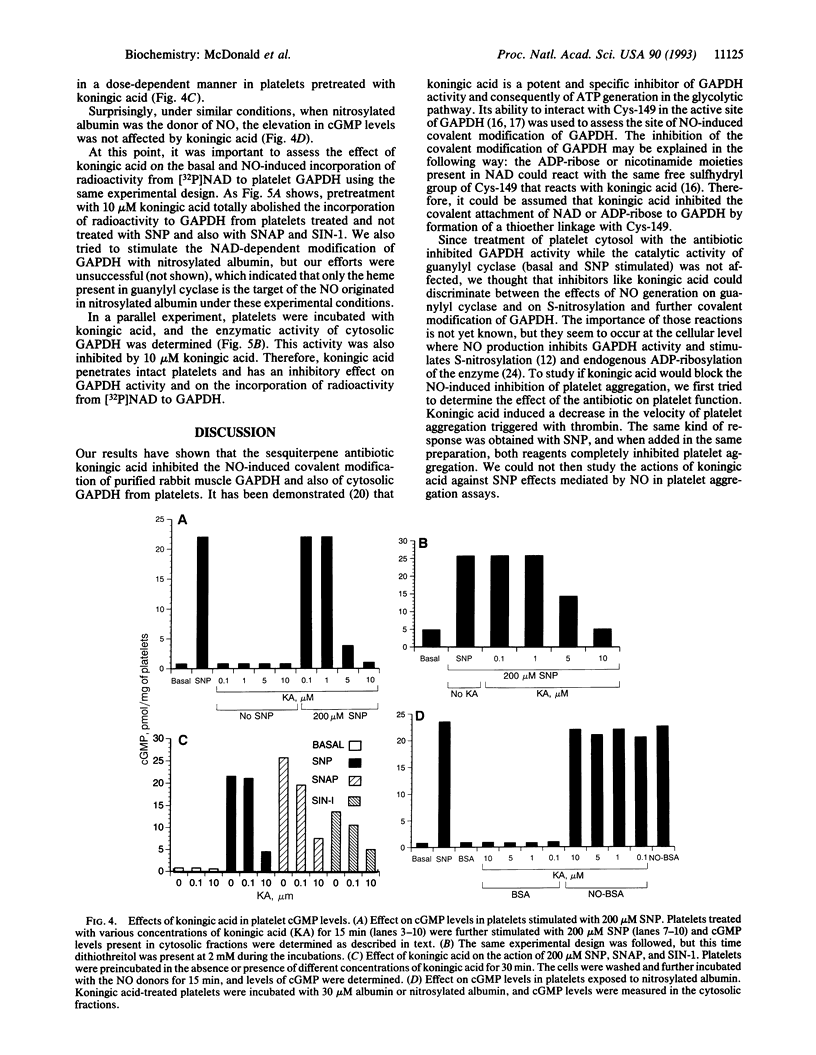
Nitric oxide (NO) or NO-generating compounds like sodium nitroprusside (SNP) increase cellular levels of cGMP and produce S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase [GAPDH; D-glyceraldehyde-3-phosphate:NAD+ oxidoreductase (phosphorylating), EC 1.2.1.12]. In search of a reagent that could discriminate between these two effects, we used the sesquiterpene antibiotic koningic acid, which binds to GAPDH at the Cys-149 of the active site. Koningic acid inhibited basal and sodium nitroprusside-stimulated NAD-dependent covalent modification of purified rabbit muscle GAPDH in a dose-dependent manner. Furthermore, we tested the effect of koningic acid on human platelets. Approximately 90% of GAPDH is present in the cytosol of human platelets, and the exposure of platelet cytosol to koningic acid inhibited GAPDH activity, while the soluble guanylyl cyclase (basal and sodium nitroprusside-stimulated) activity remained unaltered. Pretreatment of intact platelets with koningic acid slowed the rate of aggregation induced by a submaximal concentration of thrombin. In addition, the antibiotic also inhibited the cGMP increases triggered by SNP, S-nitroso-N-acetylpenicillamine (SNAP), and 3-morpholinosyndomidine (SIN-1) but failed to prevent an increase in cGMP caused by nitrosylated albumin. Under the same conditions, koningic acid also inhibited basal and SNP- SNAP-, and SIN-1-stimulated NAD-dependent modification of GAPDH and its enzymatic activity. These results suggest that the mechanism of delivery of NO from SNP, SNAP, and SIN-1 to platelets may require the active form of GAPDH. When NO is delivered by nitrosylated albumin, active GAPDH was not necessary.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Bloch W., MacQuarrie R. A., Bernhard S. A. The nucleotide and acyl group content of native rabbit muscle glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1971 Feb 10;246(3):780–790. [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Requirement for heme in the activation of purified guanylate cyclase by nitric oxide. Biochim Biophys Acta. 1983 Jun 29;745(3):310–321. doi: 10.1016/0167-4838(83)90063-8. [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Ankarcrona M., Nicotera P., Brüne B. Exogenous nitric oxide (NO) generation or IL-1 beta-induced intracellular NO production stimulates inhibitory auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase in RINm5F cells. J Immunol. 1993 Apr 1;150(7):2964–2971. [PubMed] [Google Scholar]
- Dimmeler S., Lottspeich F., Brüne B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992 Aug 25;267(24):16771–16774. [PubMed] [Google Scholar]
- Duman R. S., Terwilliger R. Z., Nestler E. J. Endogenous ADP-ribosylation in brain: initial characterization of substrate proteins. J Neurochem. 1991 Dec;57(6):2124–2132. doi: 10.1111/j.1471-4159.1991.tb06431.x. [DOI] [PubMed] [Google Scholar]
- Endo A., Hasumi K., Sakai K., Kanbe T. Specific inhibition of glyceraldehyde-3-phosphate dehydrogenase by koningic acid (heptelidic acid). J Antibiot (Tokyo) 1985 Jul;38(7):920–925. doi: 10.7164/antibiotics.38.920. [DOI] [PubMed] [Google Scholar]
- Goretski J., Hollocher T. C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988 Feb 15;263(5):2316–2323. [PubMed] [Google Scholar]
- Ignarro L. J., Adams J. B., Horwitz P. M., Wood K. S. Activation of soluble guanylate cyclase by NO-hemoproteins involves NO-heme exchange. Comparison of heme-containing and heme-deficient enzyme forms. J Biol Chem. 1986 Apr 15;261(11):4997–5002. [PubMed] [Google Scholar]
- Ignarro L. J., Degnan J. N., Baricos W. H., Kadowitz P. J., Wolin M. S. Activation of purified guanylate cyclase by nitric oxide requires heme. Comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. Biochim Biophys Acta. 1982 Sep 17;718(1):49–59. doi: 10.1016/0304-4165(82)90008-3. [DOI] [PubMed] [Google Scholar]
- Kots AYa, Skurat A. V., Sergienko E. A., Bulargina T. V., Severin E. S. Nitroprusside stimulates the cysteine-specific mono(ADP-ribosylation) of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. FEBS Lett. 1992 Mar 23;300(1):9–12. doi: 10.1016/0014-5793(92)80153-8. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- McDonald L. J., Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6238–6241. doi: 10.1073/pnas.90.13.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina y Vedia L., McDonald B., Reep B., Brüne B., Di Silvio M., Billiar T. R., Lapetina E. G. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992 Dec 15;267(35):24929–24932. [PubMed] [Google Scholar]
- Moss J., Vaughan M. NAD: arginine mono-ADP-ribosyltransferases from animal cells. Methods Enzymol. 1984;106:430–437. doi: 10.1016/0076-6879(84)06046-8. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D. N., Elguindi S., O'Brien P. J. Reductive metabolism of nitroprusside in rat hepatocytes and human erythrocytes. Arch Biochem Biophys. 1991 Apr;286(1):30–37. doi: 10.1016/0003-9861(91)90005-4. [DOI] [PubMed] [Google Scholar]
- Sakai K., Hasumi K., Endo A. Identification of koningic acid (heptelidic acid)-modified site in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1991 Apr 8;1077(2):192–196. doi: 10.1016/0167-4838(91)90058-8. [DOI] [PubMed] [Google Scholar]
- Sakai K., Hasumi K., Endo A. Inactivation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase by koningic acid. Biochim Biophys Acta. 1988 Feb 10;952(3):297–303. doi: 10.1016/0167-4838(88)90130-6. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Snyder S. H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]