Abstract
Noroviruses (NV) are the most common cause of acute gastrointestinal illness in the United States and worldwide. The development of specific antiviral countermeasures has lagged behind that of other viral pathogens, primarily because norovirus disease has been perceived as brief and self-limiting and robust assays suitable for drug discovery have been lacking. The increasing recognition that NV illness can be life-threatening, especially in immunocompromised patients who often require prolonged hospitalization and intensive supportive care, has stimulated new research to develop an effective antiviral therapy. Here, we propose a path forward for evaluating drug therapy in norovirus-infected immunocompromised individuals, a population at high risk for serious and prolonged illness. The clinical and laboratory features of norovirus illness in immunocompromised patients are reviewed, and potential markers of drug efficacy are defined. We discuss the potential design of clinical trials in these patients and how an anti-viral therapy that proves effective in immunocompromised patients might also be used in the setting of acute outbreaks, especially in confined settings such as nursing homes, to block the spread of infection and reduce the severity of illness. We conclude by reviewing the current status of approved and experimental compounds that might be evaluated in a hospital setting.
Keywords: Norovirus, Transplant, Antiviral, Immunocompromised, Drug treatment, Diarrhea
1. Introduction
Noroviruses (NV) are notorious for causing epidemics of acute gastrointestinal illness in settings such as schools, cruise ships, nursing homes, and communities. Less widely recognized, however, is the severe burden of chronic NV infection in immunocompromised patients, particularly solid organ and stem-cell transplant recipients, who may suffer prolonged, debilitating diarrheal disease that requires careful fluid replacement and intensive supportive care (Kaufman et al., 2005; Bok and Green, 2012). Detailed understanding of the pathobiology of NV infection and discovery of human anti-NV drugs have been complicated by the absence of a permissive cell culture system for NV or an authentic animal disease model for these important positive-strand RNA viruses, handicaps that have been overcome for other viral infections such as hepatitis C virus. Thus, cellular targets of NV in the intestinal mucosa remain incompletely defined (Bok et al., 2011; Taube et al., 2013), and no antiviral therapies are currently licensed, either to slow the spread of NV outbreaks in healthy populations or to prevent or treat infections in immunodeficient persons. Furthermore, no formal clinical anti-NV drug trials are currently in progress.
Despite the numerous impediments to NV research, important advances have been achieved. Susceptibility to NV infection has been linked to host expression of histo-blood group antigens (HBGA) on the intestinal epithelium that serve as factors involved with NV attachment. Individuals expressing HGBA are designated secretor-positive and susceptible to a wide range of strains; those not expressing HGBA, i.e. who are secretor-negative, may be markedly less susceptible to infection (Tan and Jiang, 2007; Jin et al., 2013). Various components of the adaptive immune system including antibodies, CD-4 lymphocytes, and CD-8 lymphocytes contribute to disease recovery and virus elimination (Fang et al., 2013; Tomov et al., 2013). Resistance to NV re-infection is apparently variable and strain-dependent (Zhu et al., 2013). The contribution of specific antibody to protection appears to be based in part on binding to the NV capsid at sites of attachment to HBGA (Higo-Moriguchi et al., in press; Chen et al., 2013). Recent discoveries such as these justify optimism that specific therapeutic countermeasures to NV can be developed in the near future (Rohayem et al., 2010).
Here, we describe acute and chronic NV infection in immuno-compromised patients, focusing specifically on organ transplant recipients who have an urgent need for antiviral therapy. We propose options for the potential design of clinical trials in this cohort and outline the clinical and laboratory features of NV illness that might be employed as criteria to evaluate the efficacy of therapy. We follow this discussion by considering how drugs that prove beneficial against chronic infection in immunodeficient patients might also be used to limit the impact of naturally occurring NV epidemics, especially among vulnerable populations such as nursing home or other long-term care facility residents. We conclude by discussing the current status of a number of experimental compounds and drugs that are FDA-approved for other indications or that have shown evidence of anti-NV activity in the laboratory, preclinical investigations, and pilot clinical studies and that might provide promising candidates for testing in a hospital setting.
2. The clinical challenge of norovirus infection
2.1. Impact of the disease
The RNA virus family Caliciviridae, of which the genus Norovirus is the most consequential member in clinical medicine, was first recognized approximately 40 years ago as a cause of intense, albeit usually self-limited vomiting and/or watery diarrhea (Kapikian et al., 1997; Green, 2013). The recent, marked reduction in the prevalence of rotavirus infection following successful vaccine development, together with the increased availability of sensitive and practical methods for NV detection have established NV as the most common cause of both epidemic and endemic viral enteritis in the US and worldwide (Hall et al., 2011, 2013a). In the US alone, NV is estimated to be responsible for 19–21 million episodes of gastroenteritis and 56,000–71,000 hospitalizations annually, about 570–800 of which are fatal (lifetime risk equal to 1 in 5000–7000) (Gastañaduy et al., 2013; Hall et al., 2011, 2013a; Koo et al., 2013). NV infections are responsible for 1.1 million hospitalizations and 218,000 deaths annually in children in the developing world (Hall et al., 2011, 2013a). In the US, 58% of an estimated annual 9.4 million episodes of food borne illness are caused by NV, making these infections the leading identified causative agent in all age groups of this significant public health problem (Hall et al., 2011, 2013a; Scallan et al., 2011). In a recent survey of 921 hospitals in the US, NV was the most frequent hospital-acquired infection, accounting for 18% of all cases, but more importantly, 65% of all hospital unit closures (Rhinehart et al., 2012).
Nearly two-thirds of all NV outbreaks reported in the US occur in long-term care facilities (Greig and Lee, 2009; Hall et al., 2011, 2013b; Rhinehart et al., 2012). Factors that promote widespread endemic NV infection and epidemic disease, particularly in confined institutional settings, include:
short incubation time (median 1.2 days) (Lee et al., 2013);
high virulence and infectivity (Greig and Lee, 2009; Hall et al., 2011; Kroneman et al., 2008; Seitz et al., 2009; Teunis et al., 2008);
strong resistance to common disinfectants (Park et al., 2010);
persistence on surfaces and in water (Seitz et al., 2009); and
fecal shedding of virus, which may last up to 1–2 months in infected persons who have resolved symptoms and are otherwise healthy (Hall et al., 2011, 2013a; Glass et al., 2009; Koo et al., 2013; Milbrath et al., 2013).
Furthermore, the inconsistent and incomplete immune protection that is obtained after a single NV infection, in some circumstances lasting for only up to 30 weeks, maintains susceptibility of all age groups to recurring, acute disease (Hall et al., 2011, 2013a,b; Glass et al., 2009; Koo et al., 2013).
Emphasizing the relationship between immune-competence and control of NV infection, roughly one-third of fatal NV cases occur in the setting of chemotherapy for malignancy or organ transplantation (Trivedi et al., 2013). This number may underestimate the true risk of fatal disease in immunocompromised populations, because until very recently, NV testing for gastrointestinal symptoms was rarely performed in clinical practice (Bok and Green, 2012). Despite the increased mortality risk, most immunocompromised individuals infected with NV survive. However, cumulative disease morbidity experienced by survivors is typically much greater than that experienced by infected individuals who are immunocompetent.
Differences in the clinical presentation of NV enteritis between immunocompetent and immunocompromised hosts are summarized in Table 1. Healthy individuals who acquire NV enteritis typically experience brief but severe vomiting that is often followed by diarrhea that lasts no more than 4 days, often less. Intravenous (IV) fluid resuscitation in hospital emergency departments is not invariable, and hospitalization is infrequent (Atmar and Estes, 2006; Bresee et al., 2012). In contrast, NV infection in immunocompromised patients often causes relentless watery, secretory diarrhea, i.e. not requiring stimulation by food or fluid intake, that may last for 3–4 months or longer; stool volume may initially amount to several liters per day, comparable to volumes observed with cholera (Kaufman et al., 2005; Alam et al., 2008). Severe, protracted diarrhea due to NV has been reported in numerous immune-compromised populations, including recipients of a hematopoietic stem cell transplant (Roddie et al., 2009), intestinal transplant (Kaufman et al., 2005), and kidney transplant (Schorn et al., 2010; Roos-Weil et al., 2011), as well as patients with malignancy (Simon et al., 2006).
Table 1.
Clinical differences in norovirus infection in immunocompetent and immunocompromised populations. Based on: Atmar and Estes (2006), Glass et al. (2009), Kaufman et al. (2005), Ludwig et al. (2008), Milbrath et al. (2013), Roddie et al. (2009), Roos-Weil et al. (2011), Saif et al. (2011), Schorn et al. (2010), Schwartz et al. (2011), and Siebenga et al. (2008).
| Immunocompetent | Immunocompromised | |
|---|---|---|
| Acuity Vomiting |
Usually sudden
|
Usually sudden
|
| Diarrhea |
|
|
| Fecal Shedding Mortality |
|
|
Effective management of acute NV infection in immunocompromised individuals mandates emergent, large-volume IV fluid replacement, since rapid oral rehydration is difficult if not impossible. These medically fragile patients typically have serious co-morbidities associated with malignant disease or an organ transplant, and solid-organ allografts can be easily and irreparably injured by fluid losses resulting from severe diarrhea. The common inability to tolerate food beyond one week after the start of infection requires IV nutrition therapy, also known as total parenteral nutrition (TPN). Moreover, the only well-established means of suppressing NV replication in the setting of therapeutic immunosuppression or chemotherapy is curtailment of treatment that risks loss of tumor control or rejection of the transplant. Because these patients are typically placed on dedicated transplant or oncology wards in proximity to other patients who are similarly immunocompromised, and because fecal excretion of NV exceeding 6 months in immunocompromised patients is not unusual (Ludwig et al., 2008), endemic perpetuation of highly morbid and protracted disease in hospital settings can occur (Kaufman et al., 2005; Roddie et al., 2009; Roos-Weil et al., 2011).
2.2. The focus on drug development to combat NV
Whereas development and universal application of vaccines have profoundly reduced the incidence and severity of rotavirus enteritis, another cause of acute infectious enteritis in infants and children, NV vaccines remain unavailable. Properties of NV that serve as major impediments to extended vaccine efficacy include marked antigenic diversity that presumably requires incorporation of antigens derived from an unusually large number of NV strains, the marked propensity of replicating NV for antigenic drift, and a lack of naturally-occurring long-term immunity following NV infection (Esposito et al., 2014). Thus, a pattern of repeated vaccinations over time, every subsequent dose incorporating newly evolved NV antigens, may be necessary to achieve temporary protection from contemporaneous strains in the manner reminiscent of influenza vaccination practice. Preliminary testing of one human NV vaccine has revealed efficacy superior to placebo, but 37% of vaccine recipients developed symptoms (Atmar and Estes, 2006), and neither widespread cross-protection against dissimilar NV strains nor duration of protection have been established (Dicaprio et al., 2013).
In the absence of an early and/or foreseeable breakthrough in NV vaccine availability, there will remain great interest in development of anti-NV drugs based on the successful precedents established in the treatment of infections with hepatitis C virus and other positive-strand RNA viruses. Persons who have serious and prolonged reductions in immune function would be likely to obtain the greatest overall benefit from effective drug therapy for NV (Kaufman et al., 2005; Roos-Weil et al., 2011; Saif et al., 2011). Effective therapy for NV could reduce the general human disease burden by the interruption of virus spread in potentially epidemic settings including health care workers, sailors on naval vessels, soldiers in military barracks, passengers on cruise ships, residents of chronic care facilities, nursing homes and other long-term residences, students in school dormitories, food handlers, guests at large hotels, and individuals at risk in potentially severe community outbreaks (Gaulin et al., 2011; Huynen et al., 2013).
3. Therapeutic drug trials in immunocompromised subjects with norovirus infection
3.1. Study of immunocompromised populations with acute norovirus enteritis
Enhanced prioritization of early therapeutic drug investigation to immunocompromised patients, relative to groups without underlying complex medical problems, may be contrary to usual practice. However, the extraordinary morbidity from NV gastroenteritis that is experienced by immunocompromised populations and the probability that disease severity in this group will facilitate recognition of anti-NV drug efficacy are relevant when regulatory authorities consider populations for inclusion in clinical trials during the drug development and approval process. Similarly, the pharmaceutical community may recognize that performing anti-NV trials in immunocompromised persons in chronic medical care could prove advantageous in comparison with immunocompetent populations that experience acute illness, because drug trials in immunocompromised groups are unlikely to be undermined by subject drop-out due to loss to follow-up, and drug utilization may ultimately prove high if prophylactic efficacy can be confirmed.
3.1.1. Considerations for subject enrollment
The key enrollment criterion is sudden gastrointestinal illness that is severe enough to require immediate IV fluid resuscitation and hospitalization. Symptoms that suggest onset of NV enteritis include vomiting that is followed within minutes to a few hours by near-continuous, explosive watery diarrhea or sudden explosive diarrhea alone, either with or without fever. Diagnoses to be excluded include other bacterial, viral, and protozoan intestinal infections and non-infectious processes such as graft versus host disease (Schwartz et al., 2011). Testing of whole stool specimens is more sensitive than rectal swab (Bresee et al., 2012).
Because candidate anti-NV agents should be judged on their ability to lessen frequency, volume, and duration of vomiting and diarrhea and duration of virus transmission, anti-NV agents are likely to demonstrate the greatest clinical benefit when initiated immediately after symptom onset. Consequently, it should be acceptable, if not essential, to recruit subjects into trials before confirmation of NV infection, which because of the limited number of commercial reference laboratories currently offering NV detection by qualitative PCR, may require up to one working week (Rossignol and El-Gohary, 2006). Although enrollees who demonstrate pathogens other than or in addition to NV must be dropped from anti-NV protocols, these subjects might continue treatment under alternative protocols, particularly if there are early indications of benefit.
Establishing fecal density and molecular properties of NV isolates at enrollment establishes a baseline for subsequent determinations of drug impact on NV replication, for recognition of genome mutations over the duration of infection, and for correlation of shedding with symptoms (Schorn et al., 2010). Similarly, establishing general immune responsiveness, evidence of previous NV infection, and susceptibility to NV infection based on HGBA secretor status form the basis for stratifying efficacy of candidate drugs. Proposed subject evaluation at recruitment is summarized in Table 2.
Table 2.
Patient testing at enrollment. There are two important principles underlying enrollment of immunocompromised subjects into anti-NV drug investigations. Mixed and potentially confounding infections must be excluded as properties of each NV isolate that may affect drug responses are characterized. Also, susceptibility of each subject to opportunistic infections generally and to NV specifically should be determined. Based on: Higo-Moriguchi et al. (2014), So et al. (2013), Zhu et al. (2013), Tomov et al. (2013).
| Establishing NV as the sole enteric pathogen |
|
| Characterization of NV isolates |
|
| Assessment of previous host exposure to NV and susceptibility |
|
| Evaluation of general immune competence |
|
3.1.2. Considerations for trial design
Use of randomized, placebo-controlled, and double blinded (RPCDB) study design for clinical trials of anti-NV drugs in immunocompromised subjects is justified by the expectation of multiple-site testing, the paucity of information concerning impact of the type of immunocompromise on outcome, the typically variable clinical course of infection, and the overall complexity of subject populations including their increased potential for actual and apparent adverse drug effects and interactions. As conceived for therapies against other viruses, dosing regimens of orally-delivered anti-NV agents established in Phase 1 trials can be initially based on achieving adequate tissue (i.e. intestinal mucosal) concentrations using trough plasma concentration as the surrogate measure for achieving the 50% effective concentration (EC50) at a minimum (Reddy et al., 2012). Repetition of pharmacokinetic assessment with symptomatic immunocompromised subjects in Phase 2 and possibly Phase 3 trials should be essential, as drug absorption and clearance are likely to be affected by NV-induced diarrhea per se as well as by concomitant gastrointestinal disorders such as graft-versus-host disease and chemotherapy to name a few examples. For these reasons, trials that use a range of doses may be needed to define both efficacy and toxicity in immunosuppressed patients (Yamazaki et al., 2009; van der Vries et al., 2013). Development of IV-delivered drugs may circumvent some of these problems.
When considering trial duration, single-patient case studies have suggested that drug responses can be obvious and rapid, e.g. in 1 day (Siddiq et al., 2011). In prospective drug trials, length of treatment can be based on time to suppress virus replication in cell culture models employing murine NV (MNV) (Rocha-Pereira et al., 2012b) or human NV replicons (Chang and George, 2007; Rocha-Pereira et al., 2013), or animal models of infection and disease such as MNV in mice (Rocha-Pereira et al., 2013) or human NV in gnotobiotic pigs (Jung et al., 2012). However, continuation of trials until actual or anticipated cessation of fecal shedding can be justified by a prime therapeutic objective of interrupting disease transmission that may occur in the setting of protracted excretion (Sukhrie et al., 2012). Subjects initially randomized to placebo should cross-over to active drug if symptomatic at trial conclusion, both in furtherance of establishing drug efficacy and to serve as an inducement to subject enrollment. Isolated reports of successful, uncontrolled use of drugs against NV off-label imply that Independent Data Monitoring Committees should be integral components of anti-NV drug trials in order to facilitate earlier crossover from placebo to test drug if efficacy is suggested before trial completion (Deng et al., 2012). Similarly, in the event of symptomatic relapse after trial completion, extended treatment should be an option.
3.1.3. Considerations for patient management during trials
The key clinical indicator of drug efficacy in immunocompromised populations is time of recovery, as indicated by extended cessation of IV fluid therapy. Although optimal clinical management of acute infectious enteritis has been of great interest for several decades, particularly in children (Pieścik-Lech et al., 2013), management of protracted infectious enteritis associated with states of immunocompromise remains largely empiric. In clinical drug trials, standardization of patient management among test sites to the greatest degree possible is essential, since IV fluid therapy and related clinical measures of therapeutic outcome are directly influenced by management approach. Basic management principles are summarized in Table 3.
Table 3.
Patient management during drug trials. Accurate assessment of responses to trial medications will most probably be benefited by consistently similar treatment of hospitalized patients with NV enteritis. In particular, evolving symptoms should anticipate programmed reductions in IV fluid therapy and restorations of diet to the greatest extent possible.
| Phases of disease evolution | Clinical presentation | Treatment |
|---|---|---|
| Day 0: acute dehydration phase |
|
|
| Up to 1 week: sub-acute, potential early recovery phase |
|
|
| 1 week – 6 months or beyond: chronic phase |
|
|
| Recovery phase |
|
|
3.1.4. Assessment of drug efficacy
The assessment of drug efficacy for treatment of NV gastroenteritis in the immunocompromised patient has three components: The clinical response, virological response, and immunological response to the drug, in comparison with placebo (summarized in Table 4). Clinical response focuses on quantifiable measures of symptom resolution and is thereby a primary trial endpoint (Rossignol and El-Gohary, 2006). Clinical measures enumerated in Table 4 are all variants on the common theme of symptom resolution that differ mainly in applicability to various subject populations, e.g. whether an intestinal stoma or fecal continence is likely to be present. The virological response focuses on suppression of viral replication and shedding, suppression of virus mutation, and, possibly, reduction in infectivity; new animal models of human NV infection may facilitate these analyses (Cheetham et al., 2006; Taube et al., 2013). Because of the epidemic potential of NV, these outcome measures should also be of primary interest. Finally, integrity of the adaptive immune response affects NV disease expression, rate of mutation that may lead to immune evasion, and potentially, efficacy of candidate anti-NV drugs (Siebenga et al., 2008; Roddie et al., 2009; Robles et al., 2012). Because changes in patient management during the course of NV infection such as alteration of immunosuppression or chemotherapy regimens may affect disease outcomes independent of anti-NV therapy (Kaufman et al., 2005; Roos-Weil et al., 2011; Schorn et al.,2010;), general and NV-specific immunity should be retested at various time points during trials in the same fashion as at enrollment (see Section 3.1.1).
Table 4.
Criteria to assess drug efficacy. In immunocompromised subjects, therapeutic efficacy of anti-NV drugs can be defined in several ways, reflecting the multiple goals of treatment in this population. Those goals include, first and foremost, the alleviation of symptoms of NV enteritis and also interruption of disease transmission without undermining NV-specific immune responses. Optimally, all of these criteria would be used comprehensively for drug evaluations.
| Clinical criteriaa |
|
| Virological criteria |
|
| Immunological criteria |
Ancillary data that are likely to be important in evaluation of anti-NV drug efficacy include trends in concurrent administration of chemotherapy and immunosuppressive medications, including trends in blood levels of these drugs where applicable. As noted above, such trends focus on identifying clinician-initiated interruptions in treatment of underlying conditions, specifically chemotherapy for malignancy and immunosuppression after transplant that risk tumor regrowth and allograft rejection or graft versus host disease, respectively (Kaufman et al., 2005; Roddie et al., 2009; Roos-Weil et al., 2011). Routine blood chemistry and hematological monitoring of patient status are essential for identification of side effects of anti-NV drugs that may not have been apparent when given to healthy subjects. In addition, when endoscopic small intestinal biopsy is performed as a component of clinical care, histopathology can be correlated with clinical symptoms and fecal RNA shedding (Kaufman et al., 2005; Schwartz et al., 2011).
3.2. Study of immunocompromised populations with chronic norovirus enteritis
A truly effective anti-NV agent should prevent chronic diarrheal disease and/or infection by arresting acute infection. However, by analogy with chronic hepatitis C, it does not automatically follow that agents with demonstrated value for acute disease would retain comparable efficacy against more rapidly evolving, genetically diverse chronic NV infections (Vermehren and Sarrazin, 2012); rather, assessment of anti-NV drugs after chronic infection may require dedicated evaluation. Drug trials in immunocompromised patients with chronic NV disease differ somewhat from acute infection trials. Insuring similarity of subject cohorts requires definition of chronicity based on length of symptoms, viral shedding, or both that may be somewhat arbitrary. Although use of placebos in chronically infected patients remains optimal, recognition of clinical response to candidate drugs should be facilitated by the established character of symptoms at enrollment. Virological monitoring remains important to test the goal of virus clearance.
3.3. Study of uninfected immunocompromised populations in a high-risk setting
The standard practice of clustering hospitalized immunocompromised patients in specialty care units creates opportunities for NV outbreaks given the potential for virus spread from patients with protracted NV disease or asymptomatic fecal shedding (Sukhrie et al., 2012; Doshi et al., 2013). As with immunocompromised patients acquiring NV in the community, patients confined to the hospital are especially likely to benefit from effective drug prophylaxis.
3.3.1. Considerations in subject enrollment
In contrast with the initiation of treatment protocols for immunocompromised subjects with acute gastroenteritis, prophylactic anti-NV drug trials are initiated when an index case has confirmed NV disease. Suitable candidates for enrollment include those sharing living spaces and health care providers of the index case up to 48 h before onset of symptoms, the maximum duration of NV latency (Harris et al., 2013; Lee et al., 2013). Assessment at subject enrollment can be the same as in treatment protocols (see Section 3.1.1) excepting that fecal testing need only include NV RNA by qualitative PCR to establish or exclude existing asymptomatic infection and documentation of pre-morbid stool patterns. Because successful prophylaxis may be restricted to sub-populations with relative immune preservation, testing of NV-specific and general immune competence is especially important in these studies (Boudreault et al., 2011).
3.3.2. Considerations for trial design
Because of the sporadic and often unpredictable nature of NV outbreaks, drug prophylaxis trials that are RDBPC and conducted at multiple sites are preferred, because risks of NV transmission may vary between institutions based on differences in subject population susceptibility, infection control practices, and pathogenicity of responsible NV strains (Hassine-Zaafrane et al., 2013).
Current concepts of NV pathobiology suggest two prophylactic strategies. The first strategy would use drugs effective for treatment of established NV disease in a prophylactic role. Although effective prophylactic antiviral drug doses can often be lower than those needed to treat active infection (Kotton, 2013), the very short incubation time of NV infection in combination with very high virulence and potential for reduced drug bio-availability suggests that initial prophylaxis trials should employ therapeutic doses to establish benefit. The second prophylactic strategy would employ drugs that interfere with virus attachment to (and assimilation by) the small bowel, for example, via epithelial histo-blood group antigens (HBGA) in those individuals expressing these binding ligands (Tan and Jiang, 2010). Dosing of virus-attachment inhibitors would be based on considerations of small bowel lumen volume, cross-sectional surface area, and estimates of initial infecting NV load.
3.3.3. Clinical monitoring and assessment of drug efficacy
Clinical monitoring includes bi-weekly to monthly subject surveillance with fecal NV RNA qualitative PCR for detection of sub-clinical infection in addition to ad hoc NV testing for symptoms of acute gastroenteritis. When prophylaxis (drug or placebo) has failed, treatment protocols as delineated in Section 3.1 may be initiated, including continuation of the study drug for evaluation of potential disease attenuation and crossover to trial drugs of subjects who have received placebo.
3.4. Extending anti-NV therapeutic trials to other populations at risk
Acute NV enteritis can be extremely unpleasant, a major source of economic loss, and a significant burden to healthcare services even in situations when it is unlikely to be life-threatening. In the absence of a vaccine that delivers long-lasting immunity against a broad spectrum of NV genotypes, adults and children who are susceptible to NV and who would benefit from effective anti-NV therapy for acute disease and prophylaxis in high-risk settings will remain high. As with drug trials in immunocompromised populations, trials in other groups are most effectively performed in confined settings (Zelner et al., 2013). In contrast, conducting robust studies of subjects coming to urgent care clinics and emergency departments may be difficult when there is no medical need for follow-up after clinical disease resolution (Bresee et al., 2012. Furthermore, any institution wishing to participate in anti-NV trials must be prepared to include testing for NV by PCR in all patients presenting with compatible symptoms, which has historically not been standard practice in many settings (Bresee et al., 2012).
Subject populations who fulfill these criteria and should be considered priorities for testing include:
Young adults in school dormitories, military barracks, and navy ships (Arness et al., 2000; MMWR, 2009; Morillo et al., 2012).
Persons of all ages with various physical infirmities other than primary immune deficiencies, particularly the elderly in nursing homes and chronic care facilities, in recognition that mortality risk associated with NV infection is 10-fold greater in the elderly in comparison with all age-groups combined (Bernard et al., 2014).
4. Current state of anti-norovirus drug discovery
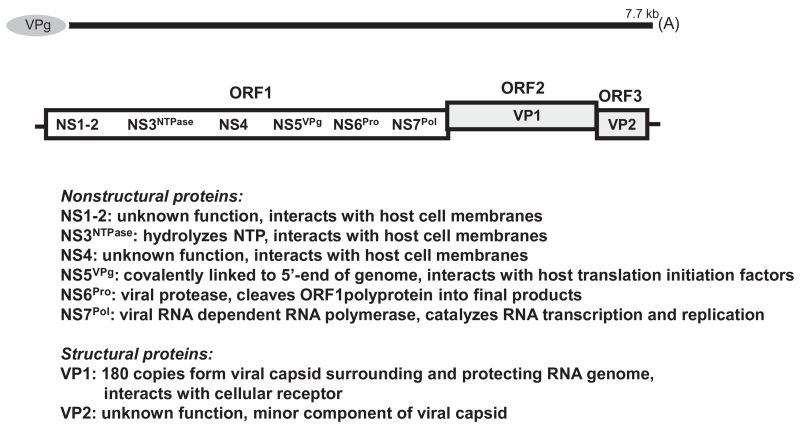
Knowledge of the NV replication strategy and pathogenesis in the host are critical in the search for antiviral drugs. Like other positive-strand RNA viruses, the NV undergo a cycle of entry into cells, RNA genome replication, maturation of progeny virus, and spread of this virus to new cells (Green, 2013). Any of these steps in the virus life cycle, as well as several of the viral proteins (Fig. 1), might serve as potential targets for the development of inhibitors. There has been little, if any systematic effort to date by the pharmaceutical community to develop drugs that inhibit human NV infection. Although only limited antiviral discovery attempts from a handful of investigative groups have been discussed in the published literature, these studies have revealed important insights into potential approaches and demonstrated the potential accessibility of viral targets. Over the past few years, there has been a slow, but steady increase in anti-NV discovery investigations.
Fig. 1.
See (Green, 2013) for a review. The norovirus positive-sense RNA genome is approximately 7700 nucleotides in length, polyadenylated, and organized into three open reading frames (ORFs). ORF1 encodes three obligatory enzymes (NTPase, pro, pol) involved in virus replication that might serve as targets for antiviral drugs. The other three ORF1-encoded non-structural proteins are also essential for replication. VPg, which is covalently attached to the 5′ end of the viral genome, interact with host cell proteins, especially translation initiation factors, and the remaining two proteins interact with host factors and membranes. Interruption of such virus-host interactions may offer additional potential drug targets. The major structural protein of the virus, VP1, is encoded by ORF2 and the minor structural protein, VP2 is encoded by ORF3. Compounds that block viral entry into cells or inhibit virus assembly might prove effective. Both structural proteins are translated separately from a single sub-genomic messenger RNA. ORF1 is translated as a single polyprotein directly from the poly-adenylated genomic RNA, and is cleaved into individual non-structural proteins by the viral protease. ORF2 is depicted as offset to denote short overlaps with both ORF1 and ORF3.
Until recently, discovery efforts have been hampered by technical challenges such as the absence of a human NV cell culture system, as noted above. The availability of permissive cell culture systems for MNV and feline calicivirus (FCV) (reviewed in Green, 2013), and especially the development of a robust human NV replicon cell line (Chang et al., 2006; Chang and George, 2007), have helped in the search for broad-spectrum calicivirus inhibitors. Although in vitro assays for the key enzymes involved in human NV RNA replication, the viral cysteine protease (Pro) and RNA dependent-RNA polymerase (Pol) have existed for a decade or more, and stable human NV replicon cell lines were developed several years ago, until most recently these reagents have primarily been utilized for investigations of fundamental aspects of viral processes or host–virus interactions. The enzymatic assays have generally not been sufficiently robust or appropriately formatted to support large-scale drug discovery efforts. While the necessary developments for formatting robust NV polymerase antiviral assays have lacked progress, there have recently been significant improvements in NV protease assays that support utility in high-throughput formats (Chang et al., 2012; Viswanathan et al., 2013).
Animal models for human NV infection have recently come into focus as potentially practical tools for antiviral development. A mouse model supporting human NV has been recently developed but, while representing a substantial breakthrough for the field, exhibits low virus production and little, if any pathology (Taube et al., 2013). A gnotobiotic pig model of human NV infection has been established for several years but not widely exploited, presumably due to larger animal size and expense (Cheetham et al., 2006). However, the pig model does support high yields of virus shedding, significant pathology, and disease comparable to humans, which are amenable to quantification. A mouse model of MNV infection utilizing interferon receptor deficient mice has been shown to exhibit modest, but quantifiable, GI symptoms, as well as high yields of virus (Rocha-Pereira et al., 2013). Both of the latter models have been recently used in antiviral studies (see below).
Table 5 presents a list of compounds in the published literature that have been shown to exhibit antiviral activity against human NV utilizing a variety of experimental assays. A discussion of the status of these compounds is presented below.
Table 5.
Compounds exhibiting antiviral activity against human NV and other caliciviruses. Abbreviations: pol, polymerase; pro, protease; nucl., nucleoside/nucleotide; NNI, non-nucleoside inhibitor; RdRp, RNA-dependent RNA polymerase: MNV, murine norovirus; NV, human norovirus.
| Target | Compound | Assays/stage | Virus | Reference |
|---|---|---|---|---|
| Pol (nucl.) | 2′C-methylcytidine | Cell culture (infection) | MNV | Costantini et al. (2012), Rocha-Pereira et al. (2012b) |
| Cell culture (replicon) | NV | Costantini et al. (2012), Rocha-Pereira et al. (2013) | ||
| Mouse infection | MNV | Rocha-Pereira et al. (2013) | ||
| Pol (nucl.) | Ribavirin | Cell culture (replicon) | NV | Chang and George (2007) |
| Cell culture (infection) | FCV | Belliot et al. (2005) | ||
| Co-crystallization w/pol | NV | Alam et al. (2012) | ||
| Pol (nucl.) | 2′-F-2′-methylcytidine | Cell culture (infection) | MNV | Costantini et al. (2012) |
| Cell culture (replicon) | NV | |||
| Pol (nucl.) | B-D-N(4) hydroxycytidine | Cell culture (infection) | MNV | Costantini et al. (2012) |
| Cell culture (replicon) | NV | |||
| Pol (nucl.) | 2-Thio-uridine | Cell culture (infection), RdRp in vitro assay | FCV | Belliot et al. (2005) |
| Pol (nucl.) | 6-Aza-uridine | Cell culture (infection), RdRp in vitro assay |
FCV | Belliot et al. (2005) |
| Pol (nucl.) | Favipiravir (T-705) | Cell culture (infection) | MNV | Rocha-Pereira et al. (2012a) |
| Pol (nucl.) | 2-thiouridine | Co-crystallization w/pol | NV | Alam et al. (2012) |
| Pol (nucl.) | 5-nitrocytidine | Co-crystallization w/pol | NV | Zamyatkin et al. (2008) |
| Pol (nucl.) | 2′-amino-2′-deoxycytidine | Co-crystallization w/pol | NV | Zamyatkin et al. (2008) |
| Pol (NNI) | Suramin | RdRp in vitro assay, co-crystallization w/pol, in silico docking |
NV, MNV |
Mastrangelo et al. (2012) |
| Pol (NNI) | NF203 | RdRp in vitro assay, co-crystallization w/pol, in silico docking |
NV, MNV |
Mastrangelo et al. (2012) |
| Pol (NNI) | PPNDS | RdRp in vitro assay, co-crystallization w/pol | NV | Tarantino et al. (2014) |
| Pro | Acyclic sulfamide-based compounds |
In vitro protease assay, cell culture (replicon) | NV | Dou et al. (2012c) |
| Pro | Piperazine derivatives | In vitro protease assay, cell culture (replicon) | NV | Dou et al. (2012a) |
| Pro | Pyranobenopyrone compounds | In vitro protease assay, cell culture (replicon) | NV | Pokhrel et al. (2012) |
| Pro | Cyclosulfamide-based derivatives |
In vitro protease assay, cell culture (replicon) | NV | Dou et al. (2011,2012b) |
| Pro | Dipeptidyl or tripeptidyl transition state inhibitors |
In vitro protease assay, cell culture (replicon), co-crystallization w/pro |
NV, MNV |
Kim et al. (2012), Mandadapu et al. (2013), Prior et al. (2013), Takahashi et al. (2013) |
| Pro | Chymostatin | in vitro protease assay | NV | Chang et al. (2012) |
| Entry (HBGA) | Multiple compound classes | in vitro binding assay | NV | Feng and Jiang (2007), Zhang et al. (2013) |
| Entry (virus) | Monoclonal antibodies | in vitro binding assay, chimpanzee infection | NV | Lindesmith et al. (2012), Chen et al. (2013) |
| Human interferon alpha | cell culture (replicon) genobiotic pig infection |
NV |
Chang and George, 2007
Jung et al., 2012 |
|
| Nitazoxanide (tizoxanide, Alinia®) |
Phase II trial transplant patient |
NV |
Rossignol and El-Gohary (2006)
Siddiq et al. (2011) |
|
| (E)-2-styrylchromones | Cell culture (infection) | MNV | Rocha-Pereira et al. (2010) | |
| Unfolded protein response |
Deubiquitinase inhibitors | Cell culture (infection), cell culture (replicon) | MNV NV |
Perry et al. (2012) |
4.1. Drugs and investigational compounds previously administered to humans
Several antiviral agents previously given therapeutically to humans have been utilized against NV infections, with limited success. The investigational antiviral, favipiravir (T-705), currently under clinical development for the treatment of influenza virus infections, also exhibits activity against a variety of other, unrelated RNA viruses in preclinical culture and animal models (Furuta et al., 2013). In a cell culture model of MNV infection, favipiravir displayed a modest potency against viral replication (EC50, 124 μM) and CPE (EC50, 50 μM) (Rocha-Pereira et al., 2012a). Two other clinically available antiviral drugs, human alpha-interferon and ribavirin, have been reported to reduce replication of a human NV replicon in cell culture (EC50, ca. 2.0 IU/ml and 50 μM, respectively) (Chang and George, 2007). Administration of human interferon-alpha (3000 IU/kg/day) was reported to significantly delay the onset (3.0 vs. 1.3 days), but not the duration, of virus shedding in gnotobiotic pigs infected with human NV (Jung et al., 2012). Interestingly, in this same model, administration of high doses of the anti-cholesterol drug, simvastatin, enhanced viral titers during shedding, presumably due to an observed increase in low-density lipoprotein that compensated for an overall, drug-induced reduction of total cholesterol (Jung et al., 2012). Similar effects of an enhancement of human NV replication have been observed in replicon-containing cell cultures treated with simvastatin (Chang, 2009).
Currently, the only drug successfully utilized against NV infections in preliminary clinical studies, and that which has shown the most promise thus far, is the broad-spectrum anti-infective, nitazoxanide (NTZ) (Alinia®, Romark Laboratories, LC), which is licensed for use against infections with Cryptosporidium and Giardia. Thirteen individuals with NV infections were identified in an out-patient clinical trial for treatment of viral gastroenteritis (Rossignol and El-Gohary, 2006). Treatment with NTZ significantly reduced the median time to resolution of symptoms in 6 patients relative to that observed in the 7 receiving placebo (1.5 d (0.5–2.5 d) treated vs. 2.5 d (1.5–6.5 d) placebo). Of direct relevance to immunocompromised persons, the primary subject of this article, a case report of a hematopoietic stem cell transplant recipient with a 10-day history of NV infection described an 80% reduction in diarrhea episodes within 24 h of initiating NTZ treatment, and a complete resolution of symptoms after 4 days of treatment, importantly, without reduction of immunosuppressive therapy (Siddiq et al., 2011).
The nucleoside analogue, 2′-C-methylcytidine (2′-C-MeC) (Pierra et al., 2006; Gardelli et al., 2009), previously investigated in clinical trials for utility against hepatitis C virus infection has demonstrated early promise as a direct-acting anti-NV drug. This nucleoside has been reported to be a potent inhibitor of the replication of human NV in a replicon cell culture model (EC50, 1.3 μM), along with two related analogues, 2′-F-2′-C-MeC, and B-D-N(4)-hydroxycytidine (NHC) (EC50, 1.5 and 3.2 μM, respectively) (Costantini et al., 2012). Both 2′-C-MeC and 2′-F-2′-C-MeC were also active at higher concentrations against MNV replication in cell culture infection model (EC50, 6.9 and 12.7 μM, respectively) (Costantini et al., 2012). In a separate report, 2′-C-MeC was also effective at comparable concentrations (EC50, 1.4–2.0 μM) against virus replication, plaque formation, and CPE in MNV-infected cells (Rocha-Pereira et al., 2012b). In another study, 2′-C-MeC was found to inhibit replication of a human NV replicon in cell culture, albeit at a higher concentration than earlier reports (EC50, 18 μM), but more importantly, was able to clear cells of the NV replicon after only 4 serial passages under a high treatment concentration (5× EC50), with no apparent cytotoxic effects (Rocha-Pereira et al., 2013). In a mouse model of MNV infection, 2′-C-MeC (50 mg/kg, twice daily, sub-cutaneous injection) prevented death, lessened diarrhea, and reduced intestinal virus loads (Rocha-Pereira et al., 2013).
4.2. Other nucleosides
Recent co-crystallography studies of the human NV polymerase with nucleotides have provided some initial insight into features that are likely to be important for the design of effective NV nucleoside inhibitors. Co-crystals of CTP or 5-nitrocytidine triphosphate with the NV polymerase indicated that the positioning of the nitro group of 5-nitrocytidine triphosphate blocked the interaction of the 3′-hydroxyl group from interactions with the RNA primer, suggesting a general approach for the design of anti-NV nucleosides (Zamyatkin et al., 2008). In another co-crystallization study utilizing CTP and 2′-amino-2′-deoxycytidine-5′-triphosphate with the human NV polymerase suggested that replacement of the 2′-hydroxyl group with an amino group would be important for nucleoside design as this induced a rearrangement of the polymerase active site that disrupted interactions with active-site metal ions (Zamyatkin et al., 2009). Crystal structures of MNV-1 RdRp in complex with 2-thiouridine or ribavirin have revealed additional important information on nucleotide interactions in the active site (Alam et al., 2012).
A series of uridine analogues was examined utilizing the feline Calicivirus (FCV) polymerase and FCV infection in cell culture (Belliot et al., 2005). While the triphosphates of these analogues exhibited only modest inhibition (less than 10-fold) of RdRp activity in enzymatic assays at high concentrations (200 μM), two analogues, 2-thio-uridine and 6-aza-uridine, reduced FCV titers in cell culture 100- to 1000-fold after exposing cells to high concentrations of compounds (2 mM) for only one hour immediately prior to infection. In these studies, ribavirin was modestly effective, reducing viral production less than 10-fold.
4.3. Non-nucleoside RdRp inhibitors
The non-nucleoside, suramin, and a related molecule, NF203, have been shown to be potent inhibitors of human NV (IC50, 25 and 7 nM, respectively) and MNV (IC50, 70 and 200 nM, respectively) RdRp activity in enzymatic assays (Mastrangelo et al., 2012). Crystallographic and in silico docking studies demonstrated that these molecules specifically complexed with both viral polymerases and predicted that mutation of Tyr41 of the human NV polymerase and Trp42 of the MNV polymerase to alanine would hinder interactions of these inhibitors. Subsequent assays with the mutated enzymes demonstrated that these mutations reduced potencies of the two inhibitors by 4- to 6-fold. The same group of investigators has recently discovered that pyridoxal-50-phosphate-6-(20-naphthylazo-60-nitro-40,80-disulfonate) tetrasodium salt (PPNDS) is also an effective inhibitor of NV RdRp activity in enzymatic assays (IC50, 45 nM) (Tarantino et al., 2014). Interestingly, crystal structures of the RdRp/PPNDS complex indicate that this compound binds to a second site in the polymerase, distinct from that occupied by suramin and NF203.
4.4. Protease inhibitors
The human NV antiviral target studied in greatest detail thus far is the viral 3C-like, chymotrypsin-like, cysteine protease. Not surprisingly, the chymotrypsin inhibitor, chymostatin, has been shown to be a relatively potent inhibitor (IC50, ca. 1.0 μM) in an enzymatic assay, although due to its peptide-based structure, this molecule has limited cellular uptake potential (Chang et al., 2012). During the past two years, a group of collaborative investigators has described a diverse spectrum of molecules displaying a wide range of antiviral potencies in both enzymatic and culture-based assays, including compounds based on acyclic sulfamide scaffolds, piperazine derivatives, pyranobenopyrone compounds, and cyclo-sulfamide-based derivatives (Dou et al., 2011, 2012a, 2012b, 2012c; Chang et al., 2012; Pokhrel et al., 2012). Although antiviral potencies for the vast majority of these compounds is modest (cell culture EC50 ca. 5–10 μM), cytotoxicity in cell culture (CC50 > 100 −μM) was predominantly favorable for most of these molecules.
Most recently, the same group of investigators has described a class of molecules, generally termed dipeptidyl or tripeptidyl transition state inhibitors, which display potent, broad-spectrum activity in enzymatic and cell culture-based assays against several 3C-like proteases of viruses from the families Picornaviridae, Coronaviridae, and Caliciviridae (including NV), which share conserved features in the protease catalytic site (Kim et al., 2012; Mandadapu et al., 2013; Prior et al., 2013; Takahashi et al., 2013). Mechanistically, these molecules have been shown to be covalently bound to the nucleophilic cysteine residue in the catalytic sites of the proteases of NV and poliovirus in co-crystallography studies (Kim et al., 2012). Several of these molecules demonstrate IC50 as low as 150 nM in NV protease enzymatic as-says and EC50 as low as 40nM against NV replication in replicon-based cell culture assays (Prior et al., 2013). However, cytotoxicity information on this potentially promising class of compounds is presented for only two compounds in the on-line version of one publication (Prior et al., 2013), making evaluation of the potential utility of these molecules for antiviral development difficult.
4.5. Entry inhibitors
Finally, there has been work in the identification of compounds and therapeutic antibodies that might inhibit virus entry and spread. As noted in Section 3.3.2 above, human NV strains may use HBGA molecules as binding ligands to intestinal epithelial cells. Inhibitors that block this interaction may therefore prove effective in treatment (Feng and Jiang, 2007; Zhang et al., 2013). However, these studies indicate that not all strains of NV are universally blocked by individual compounds. These screening studies utilized both in silico modeling and in vitro binding assays to identify a wide range of compound structures with the potential to block NV infection. No additional work to optimize these compounds has yet been published.
Monoclonal antibodies with therapeutic potential have been described (Lindesmith et al., 2012; Chen et al., 2013), but additional work on their mechanisms of virus neutralization is needed. One antibody, when incubated with virus prior to inoculation, was able to prevent human NV infection in a chimpanzee (Chen et al., 2013).
4.6. Other compounds
A group of (E)-2-styrylchromones has been shown to have modest antiviral activity (7-35 μM) in MNV-infected cultures, preventing both replication and plaque formation (Rocha-Pereira et al., 2010). The mechanism of action of this class of flavonoid-type compounds is unknown, and a related group of 2-styrylchromones has been shown to have activity against picornaviruses (Conti et al., 2005). Time of addition studies indicated that these compounds appear to inhibit steps following entry (Rocha-Pereira et al., 2010). Limited structure–activity analysis noted that the (E)-2-styryl group in the chromone moiety was critical for anti-NV activity. A small-molecule deubiquitinase inhibitor has been shown to inhibit the replication of MNV in cultured murine macrophages (EC90 c.a. 5 μM) and the human NV replicon (EC50 c.a. 5–10 μM), apparently though induction of the unfolded protein response (Perry et al., 2012).
Despite recent advances in anti-NV drug discovery, it will take a considerable amount of time, most likely years, before definitive clinical trials or treatments are available to immunocompromised patients infected with NV. The only current prospect for immediate clinical trials is nitazoxanide, since it is already FDA-approved and has shown efficacy against NV disease, albeit in only a handful of patients. The only other current prospect for clinical trials in the near future is 2′-C-methylcytidine, which has been used safely in humans, although the original studies were terminated due to GI side effects following several weeks to months of administration. Additional aspects of intervention and control strategies against NV infections not addressed in this paper can be found in recent reviews (Rohayem et al., 2010; Li et al., 2012; De Clercq, in press).
5. Summary
Experimental researchers have laid the groundwork for rapid progress in the discovery of agents to inhibit NV replication. Although prospects for clinical development of the therapies discussed here are not yet clear, synthesis and testing of these molecules in the pre-clinical setting have collectively established important principles for the development of NV antivirals. These studies have demonstrated that it should be possible for small molecules to interrupt NV infection successfully, for example, by inhibiting critical viral enzymes such as the proteases and polymerases. As a result, inhibition of viral replication, infection, and virus-induced cytopathology can be demonstrated in cell culture. More encouraging evidence indicates that new animal models of NV infection may be used to assess drug efficacy.
The limited clinical reports reviewed above offer hope that human NV disease can be significantly shortened using currently available agents. Immunocompromised patients with NV disease are in greatest need of an effective treatment. Furthermore, as emphasized in this review, immunocompromised patients also collectively represent a highly appropriate clinical model for the evaluation of test molecules, both by virtue of the chronicity and severity of their disease and their close connection to healthcare services, ensuring long-term, in-depth assessment of drug effects and safety. It should be noted that the use of any agent shown to have only limited toxicity in the general population must be approached with caution in immunocompromised individuals, due to their special clinical condition.
Development of safe and promising antiviral therapy in immunocompromised populations offers the opportunity to decrease the NV disease burden not only in these individuals in the near future, but in others at high risk of infection by this ubiquitous enteric pathogen. Ultimately, the greatest public health impact of an effective antiviral therapy will perhaps be seen in prophylactic use during NV outbreaks to prevent epidemic spread to exposed, uninfected individuals, especially persons in relatively closed environments. The ultimate goal of a consistently safe and effective treatment of human NV disease will be realized sooner if the pharmaceutical industry assumes a leadership role in these efforts.
References
- Alam I, Lee JH, Cho KJ, Han KR, Yang JM, Chung MS, Kim KH. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012;426:143–151. doi: 10.1016/j.virol.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Alam NH, Ashraf H, Sarker SA, Olesen M, Troup J, Salam MA, Gyr N, Meier R. Efficacy of partially hydrolyzed guar gum-added oral rehydration solution in the treatment of severe cholera in adults. Digestion. 2008;78:24–29. doi: 10.1159/000152844. [DOI] [PubMed] [Google Scholar]
- Arness MK, Feighner BH, Canham ML, Taylor DN, Monroe SS, Cieslak TJ, Hoedebecke EL, Polyak CS, Cuthie JC, Fankhauser RL, Humphrey CD, Barker TL, Jenkins CD, Skillman DR. Norwalk-like viral gastroenteritis outbreak in U.S. Army trainees. Emerg. Infect Dis. 2000;6:204–207. doi: 10.3201/eid0602.009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmar RL, Estes MK. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. North Am. 2006;35:275–290. doi: 10.1016/j.gtc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Belliot G, Sosnovtsev SV, Chang K-O, Babu V, Uche U, Arnold JJ, Cameron CE, Green KY. Norovirus proteinase-polymerase and polymerase are both active forms of RNA-dependent RNA polymerase. J. Virol. 2005;79:2393–2403. doi: 10.1128/JVI.79.4.2393-2403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H, Höhne M, Niendorf S, Altmann D, Stark K. Epidemiology of norovirus gastroenteritis in Germany 2001-2009: eight seasons of routine surveillance. Epidemiol. Infect. 2014;142:63–74. doi: 10.1017/S0950268813000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N. Engl. J. Med. 2012;367:2126–2132. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok K, Parra GI, Mitra T, Abente E, Shaver CK, Boon D, Engle R, Yu C, Kapikian AZ, Sosnovtsev SV, Purcell RH, Green KY. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc. Natl. Acad. Sci. U.S.A. 2011;108:325–330. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault AA, Xie H, Leisenring W, Englund J, Corey L, Boeckh M. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol. Blood Marrow Transplant. 2011;17:979–986. doi: 10.1016/j.bbmt.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresee JS, Marcus R, Venezia RA, Keene WE, Morse D, Thanassi M, Brunett P, Bulens S, Beard RS, Dauphin LA, Slutsker L, Bopp C, Eberhard M, Hall A, Vinje J, Monroe SS, Glass RI. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J. Infect. Dis. 2012;205:1374–1381. doi: 10.1093/infdis/jis206. [DOI] [PubMed] [Google Scholar]
- Chang K-O. Role of cholesterol pathways in norovirus replication. J. Virol. 2009;83:8587–8595. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, George DW. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. J. Virol. 2007;81:12111–12118. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology. 2006;353:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Chang K-O, Takahashi D, Prakash O, Kim Y. Characterization and inhibition of Norovirus proteases of genogroups I and II using a florescence resonance energy transfer assay. Virology. 2012;423:125–133. doi: 10.1016/j.virol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham S, Souza M, Meulia T, Grimes S, Han MG, Saif LJ. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 2006;80:10372–10381. doi: 10.1128/JVI.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Sosnovtsev SV, Bok K, Parra GI, Makiya M, Agulto L, Green KY, Purcell RH. Development of Norwalk virus-specific monoclonal antibodies with therapeutic potential for the treatment of Norwalk virus gastroenteritis. J. Virol. 2013;87:9547–9557. doi: 10.1128/JVI.01376-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti C, Mastromarino P, Goldoni P, Portalone G, Desideri N. Synthesis and anti-rhinovirus properties of fluoro-substituted flavonoids. Antivir. Chem. Chemother. 2005;16:267–276. doi: 10.1177/095632020501600406. [DOI] [PubMed] [Google Scholar]
- Choung RS, Locke GR, 3rd, Zinsmeister AR, Schleck CD, Talley NJ. Epidemiology of slow and fast colonic transit using a scale of stool form in a community. Aliment. Pharmacol. Ther. 2007;26:1043–1050. doi: 10.1111/j.1365-2036.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- Costantini VP, Whitaker T, Barclay L, Lee D, McBrayer TR, Schinazi RF, Vinje J. Antiviral activity of nucleoside analogues against Norovirus. Antivir. Ther. 2012;17:981–991. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Dancing with chemical formulae of antivirals: a panoramic view (part 2) Biochem. Pharm. doi: 10.1016/j.bcp.2013.09.010. in press. http://dx.doi.org/10.1016/j.bcp.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Deng C, Hanna K, Bril V, Dalakas MC, Donofrio P, van Doorn PA, Hartung HP, Merkies IS. Challenges of clinical trial design when there is lack of clinical equipoise: use of a response-conditional crossover design. J. Neurol. 2012;259:348–352. doi: 10.1007/s00415-011-6200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicaprio E, Ma Y, Hughes J, Li J. Epidemiology, prevention, and control of the number one foodborne illness: human norovirus. Infect. Dis. Clin. North Am. 2013;27:651–674. doi: 10.1016/j.idc.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi M, Woodwell S, Kelleher K, Mangan K, Axelrod P. An outbreak of norovirus infection in a bone marrow transplant unit. Am. J. Infect. Control. 2013;41:820–823. doi: 10.1016/j.ajic.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Design and synthesis of inhibitors of noroviruses by scaffold hopping. Bioorg. Med. Chem. 2011;19:5749–5755. doi: 10.1016/j.bmc.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou D, He G, Mandadapu SR, Aravapalli S, Kim Y, Chang K-O, Groutas WC. Inhibition of noroviruses by piperazine derivatives. Bioorg. Med. Chem. Lett. 2012a;22:377–379. doi: 10.1016/j.bmcl.2011.10.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou D, Mandadapu SR, Alliston KR, Kim Y, Chang K-O, Groutas WC. Cyclosulfamide-based derivatives as inhibitors of noroviruses. Eur. J. Med. Chem. 2012b;47:59–64. doi: 10.1016/j.ejmech.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou D, Tiew K-C, Mandadapu SR, Gunnam MR, Alliston KR, Kim Y, Chang KO, Groutas WC. Potent norovirus inhibitors based on the acyclic sulfamide scaffold. Bioorg. Med. Chem. 2012c;20:2111–2118. doi: 10.1016/j.bmc.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, Ascolese B, Senatore L, Codecà C. Pediatric norovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:285–290. doi: 10.1007/s10096-013-1967-9. [DOI] [PubMed] [Google Scholar]
- Fang H, Tan M, Xia M, Wang L, Jiang X. Norovirus P particle efficiently elicits innate, humoral and cellular immunity. PLoS One. 2013;8:e63269. doi: 10.1371/journal.pone.0063269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Jiang X. Library screen for inhibitors targeting norovirus binding to histo-blood group antigen receptors. Antimicrob. Agents Chemother. 2007;51:324–331. doi: 10.1128/AAC.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardelli C, Attenni B, Donghi M, Meppen M, Pacini B, Harper S, Di Marco A, Fiore F, Giuliano C, Pucci V, Laufer R, Gennari N, Marcucci I, Leone JF, Olsen DB, MacCoss M, Rowley M, Narjes F. Phosphoramidate prodrugs of 2′-C-methylcytidine for therapy of hepatitis C virus infection. J. Med. Chem. 2009;52:5394–5407. doi: 10.1021/jm900447q. [DOI] [PubMed] [Google Scholar]
- Gastañaduy PA, Hall AJ, Curns AT, Parashar UD, Lopman BA. Burden of norovirus gastroenteritis in the ambulatory setting - United States, 2001-2009. J. Infect. Dis. 2013;207:1058–1065. doi: 10.1093/infdis/jis942. [DOI] [PubMed] [Google Scholar]
- Gaulin C, Nguon S, Leblanc MA, Ramsay D, Roy S. Multiple outbreaks of gastroenteritis that were associated with 16 funerals and a unique caterer and spanned 6 days. J. Food Prot. 2011;76:1582–1589. doi: 10.4315/0362-028X.JFP-13-079. [DOI] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N. Engl. J. Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KY. Caliciviridae: the noroviruses. In: Knipe DM, Howley P, editors. Fields Virology. Sixth ed. Lippincott Williams& Wilkins; Philadelphia: 2013. pp. 582–608. [Google Scholar]
- Greig JD, Lee MB. Enteric outbreaks in long-term care facilities and recommendations for prevention: a review. Epidemiol. Infect. 2009;137:145–155. doi: 10.1017/S0950268808000757. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Vinjé J, Lopman B, Park GW, Yen C, Gregoricus N, Parashar U. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm. Rep. 2011;60:1–18. [PubMed] [Google Scholar]
- Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, Parashar UD. Norovirus disease in the United States. Emerg. Infect. Dis. 2013a;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013b;19:1305–1309. doi: 10.3201/eid1908.130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JP, Lopman BA, Cooper BS, O’Brien SJ. Does spatial proximity drive norovirus transmission during outbreaks in hospitals? BMJ Open. 2013;13(3):e003060. doi: 10.1136/bmjopen-2013-003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassine-Zaafrane M, Sdiri-Loulizi K, Kaplon J, Salem IB, Pothier P, Aouni M, Ambert-Balay K. Prevalence and genetic diversity of norovirus infection in Tunisian children (2007-2010) J. Med. Virol. 2013;85:1100–1110. doi: 10.1002/jmv.23552. [DOI] [PubMed] [Google Scholar]
- Higo-Moriguchi K, Shirato H, Someya Y, Kurosawa Y, Takeda N, Taniguchi K. Isolation of cross-reactive human monoclonal antibodies that prevent binding of human noroviruses to histo-blood group antigens. J. Med. Virol. 2014;86:558–567. doi: 10.1002/jmv.23734. [DOI] [PubMed] [Google Scholar]
- Huynen P, Mauroy A, Martin C, Savadogo LG, Boreux R, Thiry E, Melin P, De Mol P. Molecular epidemiology of norovirus infections in symptomatic and asymptomatic children from Bobo Dioulasso, Burkina Faso. J. Clin. Virol. 2013;58:515–521. doi: 10.1016/j.jcv.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Jin M, He Y, Li H, Huang P, Zhong W, Yang H, Zhang H, Tan M, Duan ZJ. Two gastroenteritis outbreaks caused by GII Noroviruses: host susceptibility and HBGA phenotypes. PLoS One. 2013;8:e58605. doi: 10.1371/journal.pone.0058605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Wang Q, Kim Y, Scheuer K, Zhang Z, Shen Q, Chang K-O, Saif LJ. The effect of simvastatin or interferon-a on infectivity of human Norovirus using a gnotobiotic pig model for the study of antivirals. PLoS One. 2012;7:e4619, 1–11. doi: 10.1371/journal.pone.0041619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian AZ, Estes MK, Channok RM. Norwalk group of viruses. In: Fields BN, Knipe DM, Howley P, editors. Fields Virology. Lippincott-Raven, Phil.; PA, USA: 1997. pp. 783–810. [Google Scholar]
- Kaufman SS, Chatterjee NK, Fuschino ME, Morse DL, Morotti RA, Magid MS, Gondolesi GE, Florman SS, Fishbein TM. Characteristics of human Calicivirus enteritis in intestinal transplant recipients. J. Pediatr. Gastroenterol. Nutr. 2005;40:328–333. doi: 10.1097/01.mpg.0000155182.54001.48. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lovell S, Tiew K-C, Mandadapu SR, Alliston KR, Battaile KP, Groutas WC, Chang K-O. Broad-spectrum antivirals against 3C or 3C-like proteases of Picornaviruses, Noroviruses, and Coronaviruses. J. Virol. 2012;86:11754–11762. doi: 10.1128/JVI.01348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HL, Neill FH, Estes MK, Munoz FM, Cameron A, Dupont HL, Atmar RL. Noroviruses: the most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J. Pediatr. Infect. Dis. Soc. 2013;2:57–60. doi: 10.1093/jpids/pis070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton CN. CMV: prevention, diagnosis and therapy. Am. J. Transplant. 2013;13(Suppl. 3):24–40. doi: 10.1111/ajt.12006. [DOI] [PubMed] [Google Scholar]
- Kroneman A, Verhoef L, Harris J, Vennema H, Duizer E, van Duynhoven Y, Gray J, Iturriza M, Böttiger B, Falkenhorst G, Johnsen C, von Bonsdorff CH, Maunula L, Kuusi M, Pothier P, Gallay A, Schreier E, Höhne M, Koch J, Szücs G, Reuter G, Krisztalovics K, Lynch M, McKeown P, Foley B, Coughlan S, Ruggeri FM, Di Bartolo I, Vainio K, Isakbaeva E, Poljsak-Prijatelj M, Grom AH, Mijovski JZ, Bosch A, Buesa J, Fauquier AS, Hernandéz-Pezzi G, Hedlund KO, Koopmans M. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J. Clin. Microbiol. 2008;46:2959–2965. doi: 10.1128/JCM.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RM, Lessler J, Lee RA, Rudolph KE, Reich NG, Perl TM, Cummings DA. Incubation periods of viral gastroenteritis: a systematic review. BMC Infect. Dis. 2013;13:446. doi: 10.1186/1471-2334-13-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Predmore A, Divers E, Lou F. New interventions against human Norovirus; progress, opportunities, and challenges. Annu. Rev. Food Sci. Technol. 2012;3:331–352. doi: 10.1146/annurev-food-022811-101234. [DOI] [PubMed] [Google Scholar]
- Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, Lanzavecchia A, Baric RS. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog. 2012;8(5):e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Adams O, Laws HJ, Schroten H, Tenenbaum T. Quantitative detection of norovirus excretion in pediatric patients with cancer and prolonged gastroenteritis and shedding of norovirus. J. Med. Virol. 2008;80:1461–1467. doi: 10.1002/jmv.21217. [DOI] [PubMed] [Google Scholar]
- Mandadapu SR, Gunnam MR, Tiew K-C, Uy RAZ, Prior AM, Alliston KR, Hua DH, Kim Y, Chang K-O, Groutas WC. Inhibition of norovirus 3CL protease by bisulfite adducts of transition state inhibitors. Bioorg. Med. Chem. Lett. 2013;23:62–65. doi: 10.1016/j.bmcl.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo E, Pezzullo M, Tarantino D, Petazzi R, Germani F, Kramer D, Robel I, Rohayem J, Bolognesi M, Milani M. Structure-based inhibition of Norovirus RNA-dependent RNA polymerases. J. Mol. Biol. 2012;419:198–210. doi: 10.1016/j.jmb.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Milbrath MO, Spicknall IH, Zelner JL, Moe CL, Eisenberg JN. Heterogeneity in norovirus shedding duration affects community risk. Epidemiol. Infect. 2013;141:1572–1584. doi: 10.1017/S0950268813000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo SG, Luchs A, Cilli A, do Carmo Sampaio Tavares Timenetsky M. Rapid detection of norovirus in naturally contaminated food: foodborne gastroenteritis outbreak on a cruise ship in Brazil, 2010. Food Environ. Virol. 2012;4:124–129. doi: 10.1007/s12560-012-9085-x. [DOI] [PubMed] [Google Scholar]
- MMWR Norovirus outbreaks on three college campuses - California, Michigan, and Wisconsin, 2008. Morb. Mortal. Wkly Rep. 2009;58:1095–1100. [PubMed] [Google Scholar]
- Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew C, Vinjé J. Comparative efficacy of seven hand sanitizers against murine Norovirus, feline Calicivirus, and GII.4 Norovirus. J. Food Prot. 2010;12:2232–2238. doi: 10.4315/0362-028x-73.12.2232. [DOI] [PubMed] [Google Scholar]
- Perry JW, Ahmed M, Chang KO, Donato NJ, Showalter HD, Wobus CE. Antiviral activity of a small molecule deubiquitinase inhibitor occurs via induction of the unfolded protein response. PLoS Pathog. 2012;8(7):e1002783. doi: 10.1371/journal.ppat.1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierra C, Amador A, Benzaria S, Cretton-Scott E, D’Amours M, Mao J, Mathieu S, Moussa A, Bridges EG, Standring DN, Sommadossi JP, Storer R, Gosselin G. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2′-C-methylcytidine. J. Med. Chem. 2006;49:6614–6620. doi: 10.1021/jm0603623. [DOI] [PubMed] [Google Scholar]
- Pieścik-Lech M, Shamir R, Guarino A, Szajewska H. Review article: the management of acute gastroenteritis in children. Aliment. Pharmacol. Ther. 2013;37:289–303. doi: 10.1111/apt.12163. [DOI] [PubMed] [Google Scholar]
- Pokhrel L, Kim Y, Nguyen TDT, Prior AM, Lu J, Chang K-O, Hua DH. Synthesis and anti-norovirus activity of pyranobenzopyrone compounds. Bioorg. Med. Chem. Lett. 2012;22:3480–3484. doi: 10.1016/j.bmcl.2012.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior AM, Kim Y, Weerasekara S, Moroze M, Alliston KR, Uy RAZ, Groutas WC, Chang K-O, Hua DA. Design, synthesis, and bioevaluation of viral 3C and 3C-like protease inhibitors. Bioorg. Med. Chem. Lett. 2013;23:6317–6320. doi: 10.1016/j.bmcl.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MB, Morcos PN, Le Pogam S, Ou Y, Frank K, Lave T, Smith P. Pharmacokinetic/pharmacodynamic predictors of clinical potency for hepatitis C virus nonnucleoside polymerase and protease inhibitors. Antimicrob. Agents Chemother. 2012;56:3144–3156. doi: 10.1128/AAC.06283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart E, Walker S, Murphy D, O’Reilly K, Leeman P. Frequency of outbreak investigations in US hospitals: results of a national survey of infection preventionists. Am. J. Infect. Control. 2012;40:2–8. doi: 10.1016/j.ajic.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Norovirus infection in pediatric hematopoietic stem cell transplantation recipients: incidence, risk factors, and outcome. Biol. Blood Marrow Transplant. 2012;18:1883–1889. doi: 10.1016/j.bbmt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Cunha R, Pinto DC, Silva AM, Nascimento MS. (E)-2-styrylchromones as potential anti-norovirus agents. Bioorg. Med. Chem. 2010;18:4195–4201. doi: 10.1016/j.bmc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Nascimento MSJ, Neyts J. Favipiravir (T-705) inhibits in vitro Norovirus replication. Biochem. Biophys. Res. Commun. 2012a;424:777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Cunha R, Costa I, Nascimento MSJ, Neyts J. Inhibition of Norovirus replication by the nucleoside analogue 2′-C-methylcytidine. Biochem. Biophys. Res. Commun. 2012b;427:796–800. doi: 10.1016/j.bbrc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MSJ, Neyts J. The viral polymerase inhibitor 2′-C-methylcytidine inhibits Norwalk virus replication and protects against Norovirus-induced diarrhea and mortality in a mouse model. J. Virol. 2013;87:11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddie C, Paul JP, Benjamin R, Gallimore CI, Xerry J, Gray JJ, Peggs KS, Morris EC, Thomson KJ, Ward KN. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin. Infect. Dis. 2009;49:1061–1068. doi: 10.1086/605557. [DOI] [PubMed] [Google Scholar]
- Rohayem J, Bergmann M, Gebhardt J, Gould E, Tucker P, Mattevi A, Unge T, Hilgenfeld R, Neyts J. Antiviral strategies to control calicivirus infections. Antivir. Res. 2010;87:162–178. doi: 10.1016/j.antiviral.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos-Weil D, Ambert-Balay K, Lanternier F, Mamzer-Bruneel MF, Nochy D, Pothier P, Avettand-Fenoel V, Anglicheau D, Snanoudj R, Bererhi L, Thervet E, Lecuit M, Legendre C, Lortholary O, Zuber J. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92:61–69. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- Rossignol JF, El-Gohary YM. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment. Pharmacol. Ther. 2006;24:1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368:124–129. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- Saif MA, Bonney DK, Bigger B, Forsythe L, Williams N, Page J, Babiker ZO, Guiver M, Turner AJ, Hughes S, Wynn RF. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr. Transplant. 2011;15:505–509. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States - major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorn R, Höhne M, Meerbach A, Bossart W, Wüthrich RP, Schreier E, Müller NJ, Fehr T. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin. Infect. Dis. 2010;51:307–314. doi: 10.1086/653939. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Vergoulidou M, Schreier E, Loddenkemper C, Reinwald M, Schmidt-Hieber M, Flegel WA, Thiel E, Schneider T. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;2(117):5850–5856. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz SR, Leon JS, Schwab KJ, Lyon GM, Dowd M, McDaniels M, Abdulhafid G, Fernandez ML, Lindesmith LC, Baric RS, Moe CL. Norovirus infectivity in humans and persistence in water. Appl. Environ. Microbiol. 2009;77:6884–6888. doi: 10.1128/AEM.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq DM, Koo HL, Adachi JA, Viola GM. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 2011;63:394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenga JJ, Beersma MF, Vennema H, van Biezen P, Hartwig NJ, Koopmans M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J. Infect. Dis. 2008;198:994–1001. doi: 10.1086/591627. [DOI] [PubMed] [Google Scholar]
- Simon A, Schildgen O, Maria Eis-Hübinger A, Hasan C, Bode U, Buderus S, Engelhart S, Fleischhack G. Norovirus outbreak in a pediatric oncology unit. Scand. J. Gastroenterol. 2006;41:693–699. doi: 10.1080/00365520500421694. [DOI] [PubMed] [Google Scholar]
- So CW, Kim DS, Yu ST, Cho JH, Kim JD. Acute viral gastroenteritis in children hospitalized in Iksan, Korea during December 2010-June 2011. Korean J. Pediatr. 2013;56:383–388. doi: 10.3345/kjp.2013.56.9.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhrie FH, Teunis P, Vennema H, Copra C, Thijs Beersma MF, Bogerman J, Koopmans M. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin. Infect. Dis. 2012;54:931–937. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- Tan M, Jiang X. Norovirus-host interaction: implications for disease control and prevention. Expert Rev. Mol. Med. 2007;9:1–22. doi: 10.1017/S1462399407000348. [DOI] [PubMed] [Google Scholar]
- Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Kima Y, Lovell S, Prakash O, Groutas WC, Chang K-O. Structural and inhibitor studies of norovirus 3C-like proteases. Virus Res. 2013;178:437–444. doi: 10.1016/j.virusres.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino D, Pezzullo M, Mastrangelo E, Croci R, Rohayem J, Robel I, Bolognesi M, Milani M. Naphthalene-sulfonate inhibitors of human norovirus RNA-dependent RNA-polymerase. Antiviral Res. 2014;102:23–28. doi: 10.1016/j.antiviral.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Taube S, Kolawole AO, Höhne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE. A mouse model for human norovirus. MBio. 2013;4(4):i:e00450–13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomov VT, Osborne LC, Dolfi DV, Sonnenberg GF, Monticelli LA, Mansfield K, Virgin HW, Artis D, Wherry EJ. Persistent enteric murine norovirusinfection is associated with functionally suboptimal virus-specific CD8 T cell responses. J. Virol. 2013;87:7015–7031. doi: 10.1128/JVI.03389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Le Pendu J, Calderon RL. Norwalk virus: how infectious is it? J. Med. Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- Trivedi TK, Desai R, Hall AJ, Patel M, Parashar UD, Lopman BA. Clinical characteristics of norovirus-associated deaths: a systematic literature review. Am. J. Infect. Control. 2013;41:654–657. doi: 10.1016/j.ajic.2012.08.002. [DOI] [PubMed] [Google Scholar]
- van der Vries E, Stittelaar KJ, van Amerongen G, Veldhuis Kroeze EJ, de Waal L, Fraaij PL, Meesters RJ, Luider TM, van der Nagel B, Koch B, Vulto AG, Schutten M, Osterhaus AD. Prolonged influenza virus shedding and emergence of antiviral resistance in immunocompromised patients and ferrets. PLoS Pathog. 2013;9:e1003343. doi: 10.1371/journal.ppat.1003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermehren J, Sarrazin C. The role of resistance in HCV treatment. Best Pract. Res. Clin. Gastroenterol. 2012;26:487–503. doi: 10.1016/j.bpg.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Viswanathan P, May J, Uhm S, Yon C, Korba B. RNA binding by human Norovirus 3C-like proteases inhibits protease activity. Virology. 2013;438:20–27. doi: 10.1016/j.virol.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Nakamura H, Yamagata S, Miura G, Hattori N, Shinozaki K, Sadahiro T, Toyoda A, Nakasa H, Ariyoshi N, Oda S, Harigaya K, Kitada M. Unexpected serum level of vancomycin after oral administration in a patient with severe colitis and renal insufficiency. Int. J. Clin. Pharmacol. Ther. 2009;47:701–706. doi: 10.5414/cpp47701. [DOI] [PubMed] [Google Scholar]
- Zamyatkin DF, Parra F, Alonso JMM, Harki DA, Peterson BR, Grochulski P, Ng KK-S. Structural insights into mechanisms of catalysis and inhibition in Norwalk virus polymerase. JBC. 2008;283:7705–7712. doi: 10.1074/jbc.M709563200. [DOI] [PubMed] [Google Scholar]
- Zamyatkin DF, Parra F, Machin A, Grochulski P, Ng KK-S. Binding of 2′ amino-2′-deoxycytidine-5′-triphosphate to Norovirus polymerase induces rearrangement of the active site. J. Mol. Biol. 2009;390:10–16. doi: 10.1016/j.jmb.2009.04.069. [DOI] [PubMed] [Google Scholar]
- Zelner JL, Lopman BA, Hall AJ, Ballesteros S, Grenfell BT. Linking time-varying symptomatology and intensity of infectiousness to patterns of Norovirus transmission. PLoS One. 2013;8(7):e68413. doi: 10.1371/journal.pone.0068413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Tan M, Chhabra M, Dai YC, Meller J, Jiang X. Inhibition of histo-blood group antigen binding as a novel strategy to block norovirus infections. PLoS One. 2013;8(7):e69379. doi: 10.1371/journal.pone.0069379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Regev D, Watanabe M, Hickman D, Moussatche N, Jesus DM, Kahan SM, Napthine S, Brierley I, Hunter RN, 3rd, Devabhaktuni D, Jones MK, Karst SM. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog. 2013;9(9):e1003592. doi: 10.1371/journal.ppat.1003592. [DOI] [PMC free article] [PubMed] [Google Scholar]