Abstract
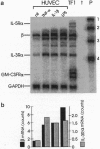
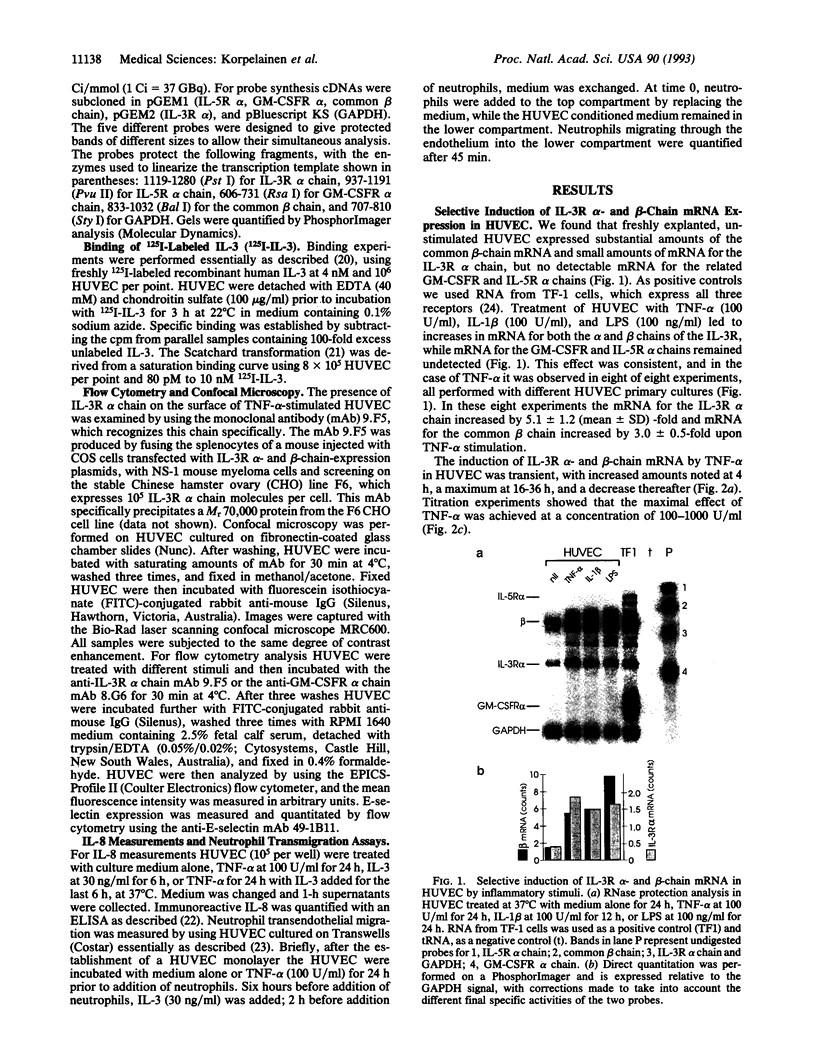
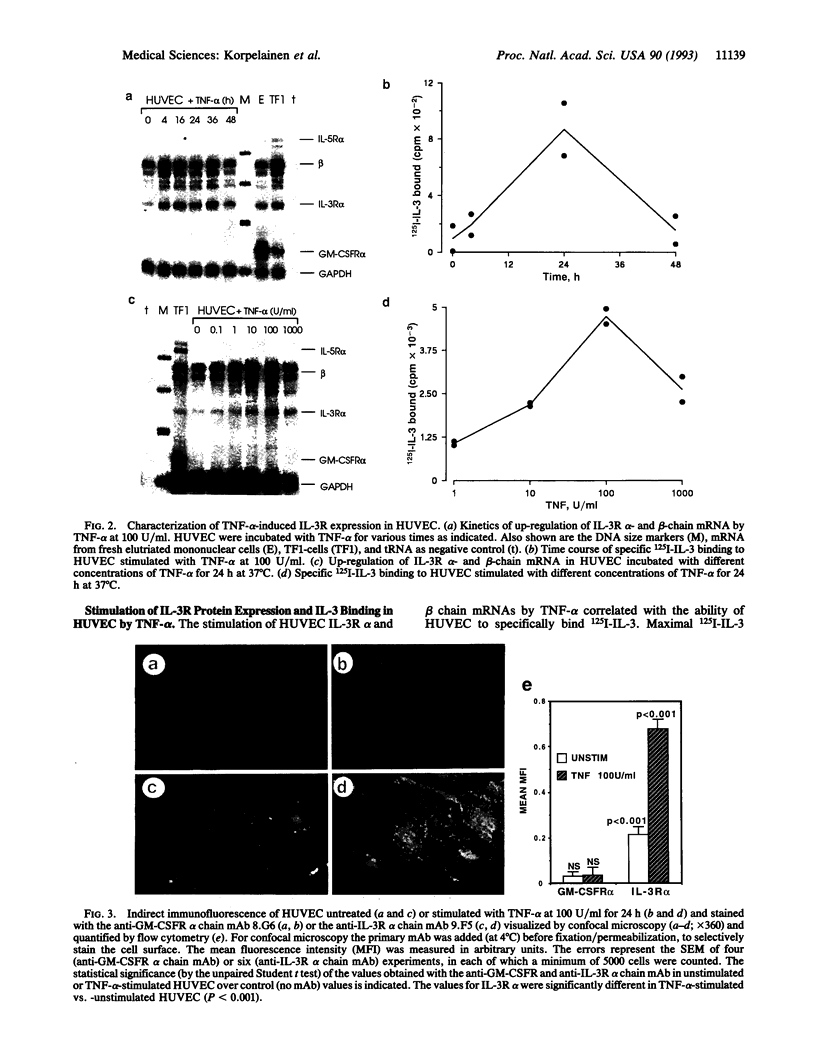
Interleukin (IL)-3 stimulates hemopoiesis in vitro. However, IL-3 is not normally found in bone marrow, raising doubts as to the in vivo role of IL-3. We have found that human umbilical vein endothelial cells (HUVEC) express functional high-affinity receptors for IL-3 after stimulation with tumor necrosis factor alpha (TNF-alpha), IL-1 beta, or lipopolysaccharide, and that this receptor is involved in inflammatory phenomena. TNF-alpha caused time- and dose-dependent up-regulation of mRNA for the IL-3 receptor alpha and beta chains, with maximal effects occurring 16-36 h after stimulation with TNF-alpha at 100 units/ml. Induction of mRNA correlated with protein expression on the cell surface as judged by monoclonal antibody staining and by the ability of HUVEC to specifically bind 125I-labeled IL-3. Scatchard analysis under optimal conditions of TNF-alpha stimulation revealed approximately 1500 IL-3 receptors per cell, which were of a high-affinity class (Kd = 500 pM) only. In contrast to a previous report, receptors for granulocyte-macrophage colony-stimulating factor could not be detected. IL-3 binding to TNF-alpha-activated HUVEC enhanced IL-8 production, E-selection expression, and neutrophil transmigration. The selective induction of a functional IL-3 receptor on endothelial cells suggests that, beyond hemopoiesis, IL-3 may have an important role in chronic inflammation and in allergic diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows L. J., Piper P. J., Lindley I. D., Westwick J. Intraperitoneal injection of human recombinant neutrophil-activating factor/interleukin 8 (hrNAF/IL-8) produces a T cell and eosinophil infiltrate in the guinea pig lung. Effect of PAF antagonist WEB2086. Ann N Y Acad Sci. 1991;629:422–424. doi: 10.1111/j.1749-6632.1991.tb38004.x. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Defilippi P., Turrini F., Sanavio F., Edgell C. J., Aglietta M., Arese P., Mantovani A. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989 Feb 2;337(6206):471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Coutinho L. H., Spooncer E., Heyworth C. M., Daniel C. P., Schiro R., Chang J., Allen T. D. Stromal cells in haemopoiesis. Ciba Found Symp. 1990;148:76–95. doi: 10.1002/9780470513880.ch6. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Cleland L. G., Gamble J. R., Lopez A. F. IL-3 and granulocyte-macrophage colony-stimulating factor stimulate two distinct phases of adhesion in human monocytes. J Immunol. 1990 Jul 1;145(1):167–176. [PubMed] [Google Scholar]
- Gamble J. R., Elliott M. J., Jaipargas E., Lopez A. F., Vadas M. A. Regulation of human monocyte adherence by granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7169–7173. doi: 10.1073/pnas.86.18.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Wiebauer K., Filipowicz W. Analysis of pre-mRNA processing in transfected plant protoplasts. Methods Enzymol. 1990;181:148–161. doi: 10.1016/0076-6879(90)81117-d. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Haak-Frendscho M., Arai N., Arai K., Baeza M. L., Finn A., Kaplan A. P. Human recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3 cause basophil histamine release. J Clin Invest. 1988 Jul;82(1):17–20. doi: 10.1172/JCI113567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Ying S., Varney V., Gaga M., Durham S. R., Moqbel R., Wardlaw A. J., Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991 Mar 1;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Miyajima A. Functional reconstitution of the human interleukin-3 receptor. Blood. 1992 Jul 1;80(1):84–90. [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Takaku F., Miyajima A. IL-1 up-regulates the expression of cytokine receptors on a factor-dependent human hemopoietic cell line, TF-1. Int Immunol. 1991 Jun;3(6):571–577. doi: 10.1093/intimm/3.6.571. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Dyson P. G., To L. B., Elliott M. J., Milton S. E., Russell J. A., Juttner C. A., Yang Y. C., Clark S. C., Vadas M. A. Recombinant human interleukin-3 stimulation of hematopoiesis in humans: loss of responsiveness with differentiation in the neutrophilic myeloid series. Blood. 1988 Nov;72(5):1797–1804. [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Lyons A. B., Tapley P. M., To L. B., Park L. S., Clark S. C., Vadas M. A. Human interleukin-3 inhibits the binding of granulocyte-macrophage colony-stimulating factor and interleukin-5 to basophils and strongly enhances their functional activity. J Cell Physiol. 1990 Oct;145(1):69–77. doi: 10.1002/jcp.1041450111. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Elliott M. J., Woodcock J., Vadas M. A. GM-CSF, IL-3 and IL-5: cross-competition on human haemopoietic cells. Immunol Today. 1992 Dec;13(12):495–500. doi: 10.1016/0167-5699(92)90025-3. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991 Oct 25;254(5031):529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- Niemeyer C. M., Sieff C. A., Mathey-Prevot B., Wimperis J. Z., Bierer B. E., Clark S. C., Nathan D. G. Expression of human interleukin-3 (multi-CSF) is restricted to human lymphocytes and T-cell tumor lines. Blood. 1989 Mar;73(4):945–951. [PubMed] [Google Scholar]
- Orazi A., Cattoretti G., Schiró R., Siena S., Bregni M., Di Nicola M., Gianni A. M. Recombinant human interleukin-3 and recombinant human granulocyte-macrophage colony-stimulating factor administered in vivo after high-dose cyclophosphamide cancer chemotherapy: effect on hematopoiesis and microenvironment in human bone marrow. Blood. 1992 May 15;79(10):2610–2619. [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Postmus P. E., Gietema J. A., Damsma O., Biesma B., Limburg P. C., Vellenga E., de Vries E. G. Effects of recombinant human interleukin-3 in patients with relapsed small-cell lung cancer treated with chemotherapy: a dose-finding study. J Clin Oncol. 1992 Jul;10(7):1131–1140. doi: 10.1200/JCO.1992.10.7.1131. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Smith W. B., Gamble J. R., Clark-Lewis I., Vadas M. A. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991 Jan;72(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Van Zee K. J., DeForge L. E., Fischer E., Marano M. A., Kenney J. S., Remick D. G., Lowry S. F., Moldawer L. L. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991 May 15;146(10):3478–3482. [PubMed] [Google Scholar]