Abstract
Plasma levels of the inflammatory biomarker high sensitivity C-reactive protein (hsCRP) predict vascular risk with an effect estimate as large as that of total or HDL cholesterol. Further, randomized trial data addressing hsCRP have been central to understanding the anti-inflammatory effects of statin therapy and have consistently demonstrated on-treatment hsCRP levels to be as powerful a predictor of residual cardiovascular risk as on-treatment levels of LDL cholesterol. Yet, while hsCRP is clinically useful as a biomarker for risk prediction, most mechanistic studies suggest that CRP itself is unlikely to be a target for intervention. Moving upstream in the inflammatory cascade from CRP to IL-6 to IL-1 provides novel therapeutic opportunities for atheroprotection that focus on the central IL-6 signaling system and ultimately on inhibition of the IL-1β producing NLRP3 inflammasome. Cholesterol crystals, neutrophil extracellular traps (NETs), atheroprone flow, and local tissue hypoxia activate the NLRP3 inflammasome. As such, a unifying concept of hsCRP as a downstream surrogate biomarker upstream IL-1β activity has emerged. From a therapeutic perspective, small ischemia studies show reductions in acute phase hsCRP production with the IL-1 receptor antagonist anakinra and the IL-6 receptor blocker tocilizumab. A phase IIb study conducted among diabetic patients at high vascular risk indicates that canakinumab, a human monoclonal antibody that targets IL-1β, markedly reduces plasma levels of IL-6, hsCRP, and fibrinogen with no change in atherogenic lipids. Canakinumab in now being tested as a method to prevent recurrent cardiovascular events in a randomized trial of 10,065 post-myocardial infarction patients with elevated hsCRP that is fully enrolled and due to complete in 2017. Clinical trials employing alternative anti-inflammatory agents active against the CRP/IL-6/IL-1 axis including low dose methotrexate and colchicine are being explored. If successful, these trials will close the loop on the inflammatory hypothesis of atherosclerosis and serve as examples of how fundamental biologic principles can be translated into personalized medical practice.
Keywords: cytokines, inflammation, atherosclerosis, clinical trials, prevention
Introduction
Vascular inflammation plays important roles in plaque initiation, progression, and the process of sudden fibrous cap rupture that triggers local thrombosis and onset of hypoxia related myocardial damage (1). Recent evidence suggests that a wide array of cell types in the monocyte and macrophage lines are involved in atherothrombosis, as are specific cytokines, chemokines, and adhesion molecules that relate to vascular function (2). Yet, despite accumulating evidence, interest in moving beyond LDL cholesterol to target the inflammatory process itself has only recently garnered significant investigative support (3). Part of this hesitation relates to the fact that the clinical expression of the inflammation hypothesis of atherothrombosis has relied on assays for high sensitivity C-reactive protein (hsCRP) as a biomarker of vascular risk. While hsCRP is clinically proven as a method to predict vascular risk and to enhance event rates in clinical trials, CRP itself is unlikely to provide an effective target for intervention. Thus, clinical investigation has sequentially moved upstream, first to interleukin-6 (IL-6) and then to interleukin-1 (IL-1) seeking more promising targets for anti-inflammatory atheroprotection. On the basis of robust pathophysiologic, genetic, and phase II trial data, large scale outcome trials directly targeting the central IL-6 signaling pathway as well as the upstream IL-1β producing NLRP3 inflammasome are underway. In this review, epidemiologic, genetic, experimental, and clinical evidence supporting this upstream movement from CRP to IL-6 to IL-1 are described, as is the unifying concept of hsCRP as a downstream biomarker for IL-1β activity.
The Evidence for C-Reactive Protein (CRP): Strong Positive Associations with Atherothrombotic Disease in Primary and Secondary Prevention, Neutral Data for Causality
CRP is a nonglycosylated circulating pentraxin composed of five identical subunits arranged with pentameric symmetry. First described by Tillett and Francis in 1930 at the Rockefeller University, the concept of CRP functioning as an “acute phase reactant” was developed by Macleod, Avery and McCarty in the 1940’s (4-6). By the 1980’s, work by Kushner, Pepys, and others had established that the bulk of circulating CRP was produced by hepatocytes under regulatory control from circulating cytokines, in particular IL-6 (7). With a circulating half-life of approximately 19 hours, the plasma concentration of CRP is largely determined by synthetic rate.
While a few case reports from the 1950’s suggested elevated levels of CRP following acute myocardial infarction, cardiovascular interest in CRP re-emerged in the 1990’s with reports from several groups describing increased CRP among those with ongoing ischemia, unstable angina, and chronic atherosclerotic disease (8-11). However, because CRP levels increase following a variety of inflammatory stimuli (including myocardial ischemia), these important studies could not address whether CRP elevations preceded the onset of vascular disease. That controversial issue was settled by data from the prospective Physicians Health Study (PHS) which, in 1997, published evidence demonstrating that levels of CRP measured with a “high sensitivity assay” were elevated decades before first ever acute ischemic events (12)(Figure 1). This study also demonstrated that those at future risk for vascular events had stable elevations of hsCRP over long periods of time; that the anti-inflammatory agent aspirin was significantly more effective in preventing first ever heart attacks when taken by those with elevated levels of hsCRP; and that effects were additive to that of total and HDL cholesterol but limited to arterial atherosclerotic events (including peripheral arterial disease, stroke, and sudden cardiac death) but not deep vein thrombosis (13,14). It is important in retrospect to recognize that the PHS did not indicate that CRP itself was causal for atherosclerosis since other inflammatory biomarkers measured in that study including sICAM-1, IL-6, and fibrinogen also predicted future vascular risk, as did the alternative inflammatory pentraxin serum amyloid A. These data were, however, consistent with early observations of thermal heterogeneity in rupture prone plaques and hence contributed to the emerging concepts that both local and systemic inflammation were relevant for acute infarction (15).
Figure 1.
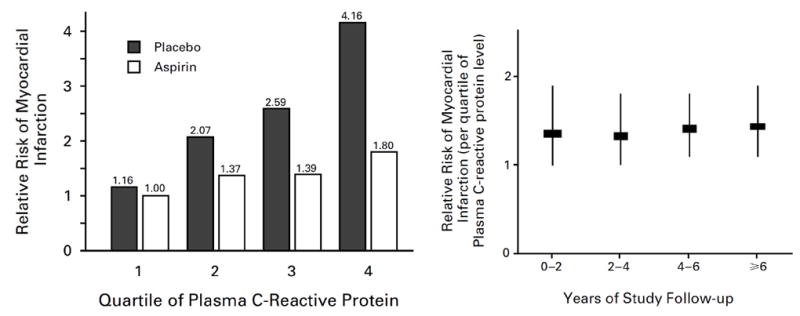
Relationship of baseline plasma levels of hsCRP to risks of future myocardial infarction, stroke, and cardiovascular death in the prospective Physicians’ Health Study among those randomly allocated to aspirin or placebo (left). Risk estimates associated with elevated hsCRP levels are stable over long periods of time (right). Adapted from N Engl J Med 1997;336:973-9.
The prospective PHS data in apparently healthy men was rapidly replicated in apparently healthy women (16). Then, with the availability of standardized commercial assays for hsCRP, more than 50 prospective cohorts worldwide would perform critical replications in multiple varied patient groups. By 2010, these data had been carefully brought together in a meta-analysis conducted by the Emerging Risk Factor Consortium. In that overview encompassing more than 160,000 individuals with 1.3 million person years of follow-up, each standard deviation increase in log normalized hsCRP associated with a multi-variate adjusted relative increase in risk of 1.37 for future coronary heart disease (95%CI 1.27-1.48) and 1.55 (95%CI 1.37-1.76) for future cardiovascular mortality (17). Importantly, the magnitude of effect for hsCRP was at least as large as that for total cholesterol, HDL cholesterol, and blood pressure (Figure 2). The effects of hsCRP on vascular risk are linear across a broad range of values. Levels of hsCRP < 1, 1 to 3, and > 3 mg/L connote lower, average, and higher relative vascular risk in the context of other traditional risk factors.
Figure 2.
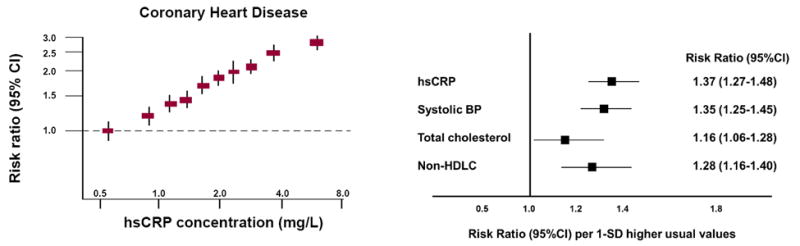
Meta-analysis of the relationship of hsCRP levels in healthy individuals to future risks of coronary heart disease and vascular deaths (left). The magnitude of cardiovascular risk associated with a one standard deviation change in hsCRP is at least as large as that associated with a similar change in systolic blood pressure, total cholesterol, or non-HDL cholesterol (right). Adapted from Lancet 2010;375:132-40.
Many clinicians elect to use the hsCRP containing Reynolds Risk Score (www.reynoldsriskscore.org) in daily practice as this global risk algorithm consistently outperforms those based on traditional Framingham covariates (18). In a direct head to head comparison of risk scores including the new ACC/AHA pooled cohort model that was performed within the prospective Multi-Ethnic Study of Atherosclerosis (MESA), the Reynolds Risk Score had the largest C-statistic (indicating superior discrimination) and the best match between predicted and observed event rates (indicating superior calibration)(19).
Were hsCRP only a risk marker for atherothrombosis, it is unlikely that clinical guidelines worldwide would come to endorse its use in “intermediate risk” populations. That acceptance derived from further evidence that there was a specific therapy – statins – that could be recommended to those with elevated hsCRP even when LDL cholesterol levels were already low. The hypothesis underlying that claim came from initial observations in the Cholesterol and Recurrent Events (CARE) trial indicating that statins lowered hsCRP in an LDL independent manner and that the relative risk reductions attributable to statin therapy were greater among those with elevated hsCRP (20). This observation, subsequently corroborated in the AFCAPS/TexCAPS, REVERSAL, PROVE IT, and A to Z trials (21-24), led to the clinical concept of “dual goals” for statin therapy in which greatest clinical benefits were seen for those who not only reduced LDL below 70 mg/dL but who also reduced hsCRP below 2 mg/L (25). Recent analyses from the IMPROVE-IT trial reiterate the fact that on-treatment hsCRP levels are as important a predictor or recurrent events as on-treatment levels of LDL cholesterol (26).
Ultimately, the 18,000 patient JUPITER primary prevention trial would show that rosuvastatin 20 mg reduces by half the rate of first ever heart attack and stroke among those with initially low levels of LDLC but elevated levels of hsCRP (27). As in earlier studies, on treatment levels of hsCRP in JUPITER proved to be as important for predicting recurrent disease as were on treatment levels of LDLC, and the magnitude of initial hsCRP elevation was directly related to the magnitude of efficacy attributable to statin initiation (28). Because those in JUPITER started with low levels of LDLC, JUPITER also provided the first evidence from a major contemporary trial that on treatment levels of LDLC below 25 to 30 mg/dL was likely to be safe, critical data for the development of PCSK9 inhibitors.
While the above data established hsCRP as a powerful risk biomarker for first and recurrent events, they do not establish CRP as a causal agent for atherothrombosis. CRP is predominantly produced in the liver as a primary acute phase reactant and plays a role in complement activation and in innate immune function. CRP can also be produced by inflammatory cells in localized inflammation, albeit at concentrations less likely to have systemic effects. For example, beyond hepatic production, inflammatory cytokines have been shown to stimulate CRP production in human coronary artery smooth muscle cells and in human adipocytes (29,30). In other work, CRP has been found to have direct pro-inflammatory and pro-thrombotic effects on human endothelial cells (31), partially through increases in plasminogen activator inhibitor expression and decreased prostacyclin release (32-34). Increased thrombosis after arterial injury has also been reported in human CRP transgenic mice (35) which, when crossed with apo-E deficient mice, resulted in strains with accelerated aortic atherosclerosis (36). Other mouse studies, however, did not find evidence of a role for CRP in atherosclerotic development (37,38).Further, some human infusion studies suggesting more direct effects on atherothrombotic pathways are difficult to interpret due to possible contamination of early CRP preparations with bacterial lipopolysaccharide (39,40). In complementary recent studies for my group (using an anti-sense oligonucleotide targeted to CRP production)(41) and from Mark Pepys’ group (using pharmaceutical grade CRP infusions)(42), no upstream effects on systemic inflammation were observed in direct response to alterations in CRP production. These neutral data for causality are consistent with population based Mendelian Randomization genetic studies which have confirmed the clinical utility of hsCRP as a biomarker, but did not support direct causation (43,44).
Ongoing controversy regarding causal roles for CRP do not diminish the clinical utility of hsCRP as a diagnostic test in primary and secondary prevention, an issue that has recently been reviewed elsewhere (45). hsCRP has also proven effective as an enrichment criterion for secondary prevention trials seeking to enhance vascular risk. Current guidelines in the United States, Europe, and Canada endorse hsCRP ascertainment for those at “intermediate risk” or where there is uncertainty about the utility of statin therapy.
Moving Partially Upstream to Interleukin-6 (IL-6): Positive Associations with Disease and Partial Links to Causality
If CRP is a downstream biomarker for atherothrombosis, what do comparable data for the upstream “secondary messenger” cytokine IL-6 show? First, like hsCRP, IL-6 levels measured in apparently healthy populations also predict future vascular risk; this observation was initially made in men in 2000 (46), confirmed in women (16), and subsequently reproduced in more than 25 prospective epidemiologic cohorts worldwide. Second, also in parallel with hsCRP, meta-analysis performed by the Emerging Risk Factors Collaboration would eventually demonstrate that for each SD increase in log IL-6, there is a 25 percent increase in risk of future vascular events (RR 1.25, 95%CI 1.19-1.32)(47)(Figure 3). Third, like hsCRP, IL-6 levels have been shown to correlate with endothelial dysfunction, arterial stiffness, extent of sub-clinical atherosclerosis, and are similarly predictive of incident type 2 diabetes (48-51). There is no clinically approved assay for IL-6, however, and measurement in clinical settings is more difficult than for hsCRP due to issues of circadian variation, short half-life, post-prandial effects, and assay stability.
Figure 3.
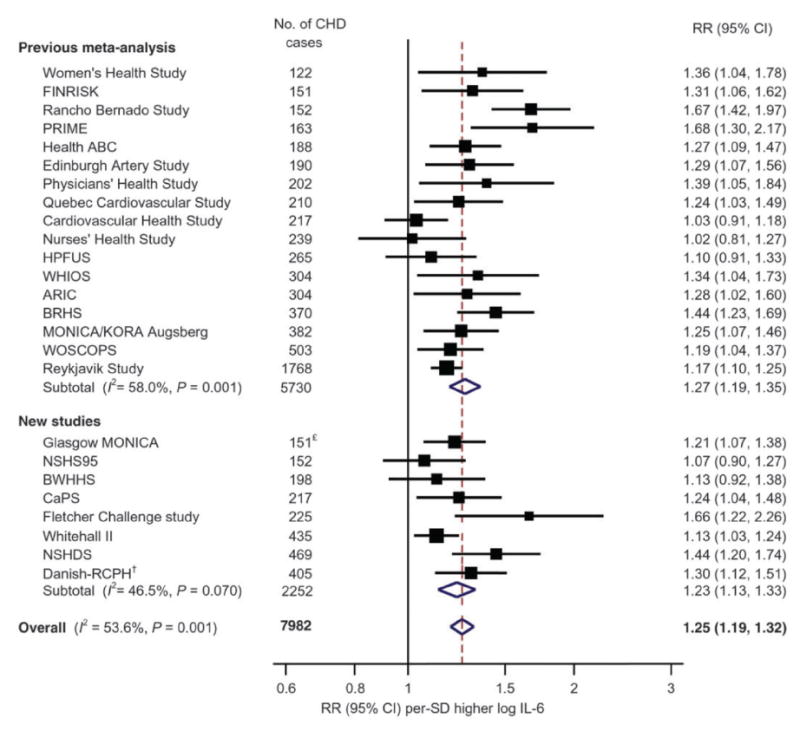
Relationship of plasma levels of IL-6 to future risks of cardiovascular disease in 25 prospective epidemiologic cohorts. Overall, for each SD increase in log IL-6, there is a 25 percent increase in risk of future vascular events (95%CI 1.19-1.32). Adapted from Eur Heart J 2014;35:578-89.
Where IL-6 differs substantively from CRP are in its links to causal pathways related to atherothrombosis. While IL-6 is the primary cytokine leading to hepatic CRP production, upstream Il-6 signaling has also been linked to plaque initiation and destabilization (52,53), to microvascular flow dysfunction (54), and to adverse outcomes in the setting of acute ischemia (55). In contrast to CRP, IL-6 is highly upregulated at the site of coronary occlusion in patients with ST segment elevation myocardial infarction (56). This latter observation is of particular interest as IL-6 (but not CRP) can be produced by cardiac myocytes under conditions of local hypoxia in the viable border zone of reperfused infarctions (57). These data are consistent with the concept that downstream CRP synthesis is largely secondary to IL-6 induced stimulation.
Perhaps the most persuasive data suggesting a direct role for IL-6 signaling in atherosclerosis derives from Mendelian Randomization studies that, again in contrast for those done earlier for CRP, do show evidence suggestive of causality. Broadly, Mendelian Randomization studies take advantage of the random assortment of alleles that occurs at conception and then seeks to link specific genetic polymorphisms both to a measured intermediate phenotype (such as hsCRP) and to a defined clinical outcome (such as myocardial infarction or stroke). In elegant studies from two independent consortia that have used this strategy, polymorphism in the IL-6 signaling pathway at rs2228145 and rs7529229 was found to concordantly associate with lifetime lower levels of hsCRP as well as lifetime lower levels of vascular risk (58.59)(Figure 4). These data suggest that, on a genetic segregation basis, vascular risk varies widely due to heritable differences in IL-6 signaling. Since heritable differences in IL-6 signaling influence both hsCRP and rates of vascular events, we can more strongly infer a causal relationship between IL-6 and vascular disease on this basis. As noted, this positive upstream data for IL-6 signaling provides a counterpoint to earlier null Mendelian Randomization studies of downstream hepatic acute phase reactants including both CRP and fibrinogen.
Figure 4.
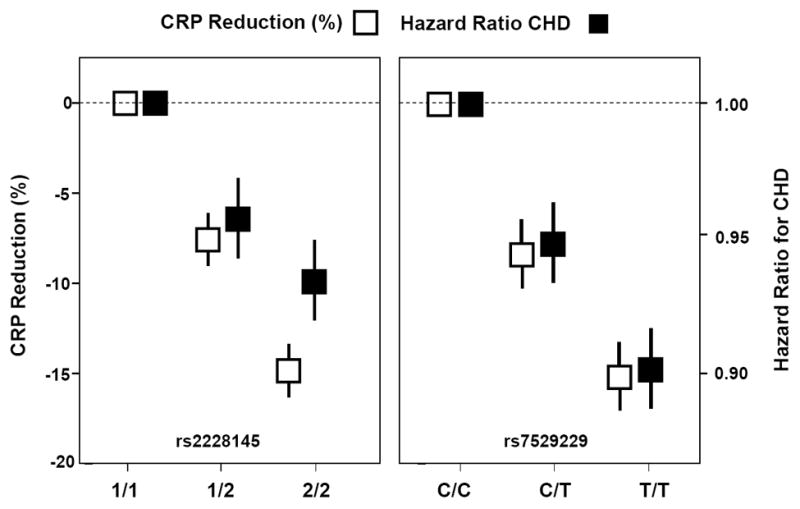
Mendelian Randomization studies demonstrate that polymorphism in the IL-6 signaling pathway at rs2228145 and rs7529229 concordantly associate with both lifetime lower levels of hsCRP and lifetime lower risks of coronary heart disease. Adapted from Lancet 2012;379:1214-24 and Lancet 2012;379:1205-13.
Enthusiasm for IL-6 targeting as a direct target for atheroprotection is tempered by counter balancing issues. First, in the same meta-analysis indicating similar risk signals for IL-6 as for CRP, elevations of IL-18, TNF, MMP-9, and Lp-PLA2 were also observed (47). Thus, as with hsCRP, these data suggest that moving further upstream beyond IL-6 may be needed for anti-inflammatory approaches to atheroprotection. Second, as IL-6 functions primarily as a secondary signaling cytokine, it is uncertain whether direct inhibition of IL-6 would lead to desired effects on vascular disease or have the specificity needed for therapeutic use; as reviewed elsewhere, these concerns in part reflect distinctions between auto-inflammatory disorders (driven primarily by monocytes and macrophages) as compared to autoimmune disorders (driven primarily by T cells and adaptive immunity). Third, IL-1 levels largely drive IL-6 signaling. Yet, many of the drivers of IL-1 production through the NLRP3 inflammasome that are directly related to atherothrombosis do not on their own impact upon IL-6.
Despite these reservations, clinical trials of IL-6 inhibition with agents such as tocilizumab (a humanized anti-IL-6 receptor antibody) are under serious consideration. Preliminary data from a single dose study of tocilizumab in non-ST elevation myocardial infarction showed this approach to reduce area under the CRP curve and to have a directionally similar effect on area under the troponin T curve, but this latter effect was not statistically significant (60)(ClinicalTrial.gov NCT01491074). The ENTRACTE study is an ongoing randomized open-label trial comparing tocilizumab to the TNF-inhibitor etanercept on the rate of vascular events among patients with moderate to severe rheumatoid arthritis (ClinicalTrials.gov NCT01331837). In this study, rheumatoid arthritis patients aged 50 years and older with inadequate clinical response to at least one non-biologic disease modifying agent and a history of coronary disease are being followed prospectively for vascular events. Because all ENTRACTE participants have symptomatic rheumatoid arthritis and thus are in need of active anti-inflammatory therapy, there is no placebo group in this trial.
A further potential limitation of direct IL-6 inhibition is that this approach may upregulate apolipoprotein B leading to an increase in LDL cholesterol. Initial tocilizumab studies in rheumatoid arthritis patients suggested that this effect was dose-dependent, potentially unrelated to inflammatory status, and thus a significant limiting factor in the development of IL-6 receptor blockade for atherosclerosis (61,62). However, whether or not this increase in LDL is more than a reverse acute phase effect remains controversial. Partially to address this issue, several surrogates of vascular risk were evaluated in the recent MEASURE trial evaluating IL-6 receptor blockade in rheumatoid arthritis (63). In this study of 132 patients treated with tocilizumab or placebo for 24 weeks, total cholesterol, LDL cholesterol, and triglycerides increased by 12, 28, and 11 percent respectively among those allocated to tocilizumab. Yet, HDL-associated serum amyloid A content decreased with tocilizumab and the apoB to Apo A1 ratio remained stable over time. As such, an argument can be made that these changes may not be pro-atherogenic. Further, if putative changes in LDL cholesterol associated with IL-6 receptor blockade can be controlled with high dose statin therapy, this approach may be viable. On the other hand, toxicity in terms of infection and potential reactivation of tuberculosis could reduce enthusiasm for agents such as tocilizumab. As will be reviewed below, current clinical data do not suggest that these untoward effects are as prevalent in association with specific partial inhibition of IL-1.
Moving Fully Upstream to Interleukin-1 (IL-1): Can a Causal Pathway be Proven and a Therapeutic Target Validated?
If CRP is conceived as a downstream biomarker and IL-6 as a secondary signaling cytokine, then it is not surprising that the upstream IL-1 signaling pathway has emerged as a major target for immune modulation and atherothrombotic protection. IL-1 is the “apical” pro-inflammatory mediator in both acute and chronic inflammation and among the most powerful inducers of innate immunity (64,65). IL-1 induces both its own production (an issue in several auto-inflammatory disorders) as well as the synthesis and expression of multiple secondary inflammatory mediators including IL-6.
Two genetically coded proteins, IL-1α and IL-1β, bind to the type 1 IL-1 receptor. IL-1α is largely membrane bound and thus plays predominantly a local rather than systemic role. By contrast, IL-1β is the primary circulating form of IL-1 but is produced as a precursor (pro-IL-1β) that is cleaved following activation of the NLRP3 inflammasome by caspase-1 to produce the active cytokine under a variety of inflammatory stimuli (66). As reviewed by Dinarello (67), the active form of IL-1β can result in autocrine, paracrine, and endocrine effects and thus is hypothesized to be involved in a broad spectrum of “auto-inflammatory” disorders in which monocyte-macrophage lines are the critical dysfunctional cells that promote pathologic inflammation (64). This is an important distinction from classical “auto-immune” disorders in which T cells are the critical driver of the inflammatory response.
There is considerable genetic influence on IL-1β production and rare inherited disorders such as Muckle Wells syndrome, cryopyrin-associated periodic syndrome (CAPS), and neonatal-onset multisystem inflammatory disease (NOMID) are associated with overproduction of IL-1β. These disorders typically present with periodic fever, neutropenia, fatigue, myalgia, elevated CRP levels, and in severe cases with joint deformation and developmental disability (68.69). Importantly, as intervention with canakinumab (an anti- IL-1β antibody), anakinra (an IL-1 receptor antagonist), and rilonocept (an IL-1 trap) all improve symptoms in these overproduction syndromes, it can be inferred that the critical culprit is IL-1β rather than IL-1α (64,70-72).
As IL-1β levels cannot be reliably measured in plasma, there are no comparable epidemiologic studies relating IL-1β to cardiovascular risk as there are for hsCRP and IL-6. However, abundant experimental and pathologic data have long implicated IL-1β in atherogenesis. Early work in the 1980s showed that IL-1 can induce leucocyte adhesion in vascular endothelial cells, lead to procoagulant activity, and serve as a mitogen for human vascular smooth muscle cell (73-75). In mouse knockout models, deficiency of IL-1β is associated with reduced lesion formation (76,77). By contrast, in cholesterol fed porcine models, exposure to exogenous IL-1β increases intimal medial thickening (78,79). In humans, atherosclerotic lesions have been shown to contain IL-1β (80) and polymorphisms in the IL-1 receptor antagonist gene correlate with rates of restenosis and local atherosclerotic progression (81,82).
Equally important, multiple factors known to associate with atherosclerosis have recently been found to activate the crucial IL-1β producing NLRP3 inflammasome (Figure 5). In 2010, two groups demonstrated that cholesterol crystals can serve as endogenous danger signals that when engulfed by inflammatory monocytes can directly trigger the NLRP3 inflammasome; these data provide a critical linkage between cholesterol deposition and a systemic pro-inflammatory state (83,84). In 2013, Xiao and colleagues reported that atheroprone oscillatory flow activates sterol regulatory element binding protein 2 (SREBP2) in endothelium and subsequently also induces the NLRP3 inflammasome (85). In 2014, Folco and colleagues reported that hypoxia potentiates IL-1β expression in human macrophages, again suggesting a direct pro-inflammatory effect on atherogenesis secondary to NLRP3 activation (86). Most recently, in mid-2015, Warnatsch and colleagues reported that cholesterol crystals interact with neutrophils to trigger the release of neutrophil extracellular traps (NETs) which prime macrophages to produce the precursor pro- IL-1β (87). NETs are comprised of extracellular released DNA fibers that form “netlike” entities which bind bacteria and platelets and exert multiple cytotoxic effects. In the process of “NETosis”, neutrophils expel cytosolic and nuclear material, a suicidal act that can lead to acute thrombosis and is related to several pro-atherosclerotic processes (88). Extracellular chromatin is injurious in ischemia reperfusion and correlates in man with the extent of underlying atherosclerosis (89). In oncologic settings, cancer associated NETosis is associated with increased deep vein thrombosis and pulmonary embolism. Diabetes, a major cardiovascular risk factor, has also been reported to prime neutrophils to undergo NETosis (90).
Figure 5.
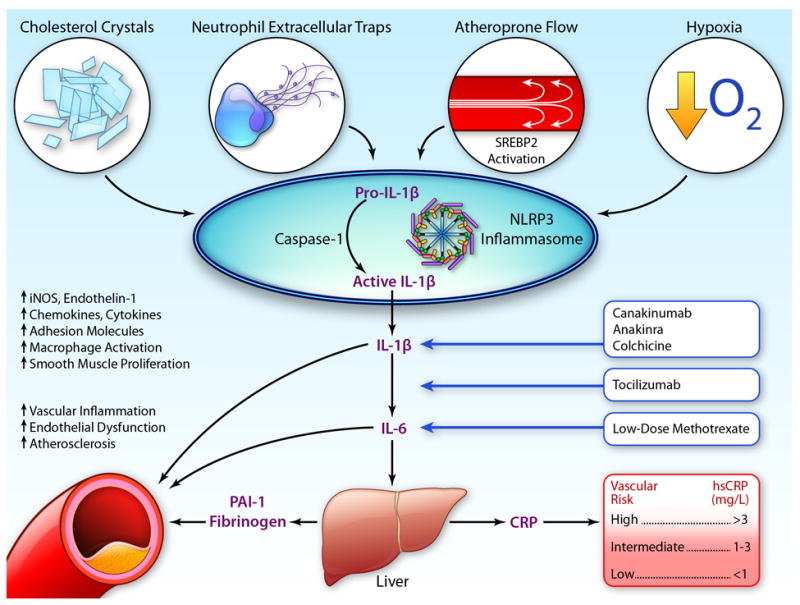
Activation of the NLRP3 inflammasome by cholesterol crystals, neutrophil extracellular traps, hypoxia, and atheroprone flow result in production of pro-IL-1β to IL-1β with consequent downstream effects on IL-6 and CRP, as well as increased vascular atheroma. Potential targets for intervention include canakinumab, anakinra, tocilizumab, methotrexate, and colchicine. PAI-1 = plasminogen activator inhibitor-1; SREBP2 = sterol regulatory binding protein 2. (Illustration credit: Ben Smith).
The central role played by the inflammasome has made inhibition of the IL-1 pathway an attractive theoretical target for atheroprotection (3,91,92) and several agents that target IL-1 activity are currently available (Table) . In a phase II study of 182 patients with non-ST elevation acute coronary syndrome, Morton and colleagues have shown that 14 days of treatment with the IL-1 receptor antagonist (IL-1Ra) anakinra significantly reduces the area under the CRP release curve, confirming that IL-1 drives CRP elevation during acute ischemia (93). Similarly, two pilot studies performed by Abbate et al in ST segment elevation myocardial infarction reported that anakinra reduces the magnitude of ischemia driven CRP release (94,95). However, anakinra leads to dual IL-1α and IL-1β inhibition which may not be optimal for atheroprotection or provide the best safety balance between IL-1 activation and inhibition. In a Mendelian Randomization study of the IL1RN gene (that encodes endogenous IL-1Ra), IL-1Ra raising alleles were associated with lower levels of IL-6 and CRP and reduced rates of rheumatoid arthritis, but also with an increase in myocardial infarction and abdominal aortic aneurysm (96). Interpretation of this study is complex, however, as there was no genetic method to differentiate IL-1α from IL-1β activity (97).
Table 1.
Characteristics of IL-1 inhibitors.
| Name | Mechanism | Blockade
|
FDA Approval | Dose | Route | Frequency | ||
|---|---|---|---|---|---|---|---|---|
| IL-1a | IL-1B | IL-1Ra | ||||||
| Anakinra | Receptor antagonist | Yes | Yes | No | Rheumatoid Arthritis | 100 mg | SC | Daily |
| Rinalocept | IL-1 trap | Yes | Yes | Yes | CAPS | 160 mg | SC | Weekly |
| Canakinumab | IL-1b antibody | No | Yes | No | CAPS | 150 mg | SC | 3 Months |
| Gevokizumab | IL-1b antibody | No | Yes | No | --- | 0.3 mg/kg | IV | Monthly |
CAPS = cryopyrin associated periodic syndromes.
In contrast to anakinra, canakinumab is a fully human monoclonal antibody targeting IL-1β and thus provides a highly specific method to address whether IL-1β inhibition can improve cardiovascular outcomes without alteration of IL-1α. Canakinumab is an approved agent for the treatment of Muckle Wells syndrome and CAPS, and has also shown activity in the settings of diabetes and gout. In a phase IIb trial conducted among 556 diabetics with high vascular risk, canakinumab produced dose-dependent reductions exceeding 50 percent for IL-6 and CRP as well as having a smaller effect on circulating fibrinogen (Figure 6)(98). In that trial, canakinumab had no effect on LDL or HDL, though a small increase in triglycerides was observed. Moreover, single doses of canakinumab were shown to inhibit inflammasome mediated IL-1β, IL-6, and CRP production for a period of several months demonstrating that long-term inflammatory inhibition could be achieved if canakinumab was given only 3 to 4 times annually. This is important since chronic inhibition of inflammation may be crucial to atherosclerotic protection. As canakinumab leaves IL-1α function intact, this approach should have reduced infectious risk when given long-term; in contrast to IL-6 or TNF inhibitors, IL-1β inhibition with canakinumab does not appear to reactivate tuberculosis nor cause increased infectious risk among those with HIV.
Figure 6.
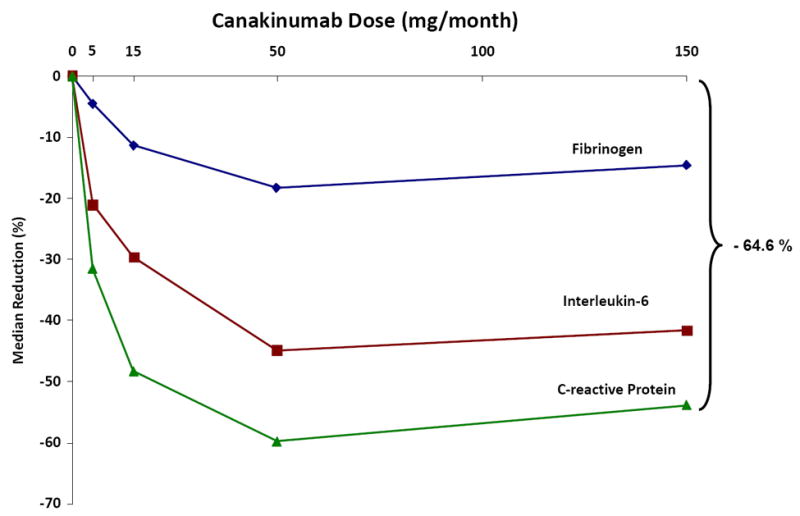
Dose dependent effects of canakinumab at 4 months for CRP, IL-6, and fibrinogen among 556 diabetic patients at high risk for vascular disease. Adapted from Circulation 2012;116:2739-48.
Partly on the basis of these phase II data, the large scale Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) was launched in 2011 to address whether IL-1β inhibition with SC canakinumab every 3 months as compared to placebo can reduce recurrent cardiovascular event rates in stable coronary artery disease patients who remain at high inflammatory risk due to a persistent elevation of hsCRP (99). Enrollment in CANTOS was limited to those with hsCRP > 2 mg/L for three important reasons. First, absolute event rates are enhanced in the trial as the anticipated median hsCRP of the study group should be roughly 4 mg/L despite treatment with an aggressive prevention regimen including statins. Second, by pre-screening for elevated hsCRP, the trial protocol limits canakinumab exposure to those with inflammation, a step which should improve safety and tolerability. Third, as noted above, IL-1β levels cannot reliably be measured in plasma. Thus, CANTOS trial is effectively using hsCRP as a surrogate for enhanced IL-1β activity.
With 10,065 post-myocardial infarction patients enrolled worldwide, CANTOS is an event driven trial due to complete in 2017 when approximately 1400 cases of myocardial infarction, stroke, or cardiovascular death have accrued. The trial is testing three doses of canakinumab against placebo and is powered to detect a 20 percent relative risk reduction in hard cardiovascular events (Figure 7). Canakinumab directly inhibits the IL-1β to IL-6 to CRP axis with no effect on LDL cholesterol; thus, CANTOS will be the first large scale test of the inflammation hypothesis of atherothrombosis. Prior work with anakinra and canakinumab have shown modest effects on HbA1c through similar anti-inflammatory pathways. As diabetes is often considered an inflammatory disease (100), rates of incident diabetes and progression of diabetes are critical secondary endpoints of the trial. Further, as canakinumab has been hypothesized to reduce metastatic disease in part through alteration of adhesion molecule function, incident cancers are also being tracked closely (101); this latter issue is collinear with interests in inhibition of IL-1 and innate immune function as a potential therapeutic tool in the oncology community (102). A logical extension of the CANTOS program will be to evaluate IL-1β inhibition in the setting of acute ischemia; very recent data in mice parabiosis models has shown that IL-1β contributes to bone marrow activation after acute myocardial infarction and that neutralizing IL-1β with a murine analogue of canakinumab can inhibit this process in a manner favoring infarct healing (103). Anticipated side effects from canakinumab include an increased risk of infection and thus any potential benefits on vascular events must exceed this potential hazard. Pre-specified analyses within CANTOS include effect modification by on-treatment levels of IL-6 measured in fresh plasma.
Figure 7.
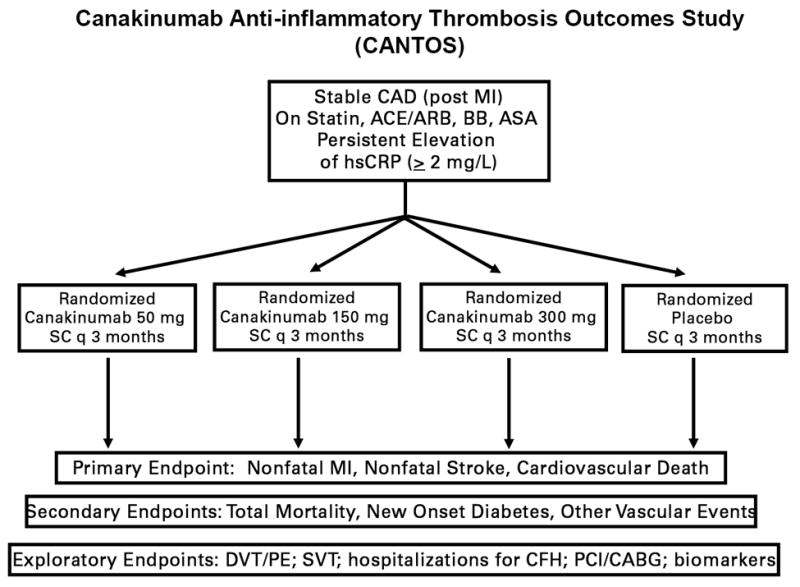
Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Adapted from Am Heart J 2011;162:597-605.
In addition to CANTOS, the cardiovascular community is actively engaged in trials of alternative agents that impact the central CRP, IL-6, IL-1 axis. As one example, the United States National Heart, Lung, and Blood Institute has funded a 7,000 patient hard outcomes trial evaluating low dose methotrexate (15-20 mg weekly) as compared to placebo in aggressively treated secondary prevention patients (104). In observational studies, low dose methotrexate is associated with reduced cardiovascular event rates among those with rheumatoid arthritis and psoriatic arthritis and in animal models low dose methotrexate has been shown to reduce lesion formation. As a second example, the anti-inflammatory agent colchicine (which primarily functions as a microtubule inhibitor) is also known to have effects on the NLRP3 inflammasome and can reduce IL-1β expression (105,106). In a pilot study of those with ST elevation myocardial infarction, 5 days of colchicine reduced area under the CK-MB curve as well as infarct size defined by late gadolinium enhancement using cardiac magnetic resonance imaging (107). Most importantly, in an open label randomized trial, colchicine was found to reduce cardiovascular event rates (108). This provocative observation requires testing in formal double blind settings.
Several outstanding recent reviews have addressed the basic immunology underlying atherothrombotic progression and the mechanisms of specific drug response (2, 109-111. In concert with this work, the translational research community has come a long distance since studies in the mid 1990’s first linked biomarkers of inflammation to future vascular risk. Close collaboration between clinical, epidemiologic, and bench investigators has now led to randomized outcome trials targeting the CRP, IL-6, IL-1 pathway. If successful, these trials will close the loop on the inflammatory hypothesis of atherosclerosis and serve as examples of how fundamental biologic principles can be translated into personalized medical practice.
Acknowledgments
Disclosures. Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital, Harvard Medical School that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to Seimens and AstraZeneca, and has received investigator initiated research grant support from the National Heart Lung and Blood Institute, Novartis, Astra-Zeneca, the Doris Duke Charitable Foundation, the Leducq Foundation, and the Donald W Reynolds Foundation to perform work relevant to the topics discussed in this manuscript.
Sources of Funding. The work described in this manuscript is supported by investigator initiated research grants to Dr Ridker from Novartis and AstraZeneca; the Leducq Foundation, the American Heart Association, and the National Heart Lung and Blood Institute (HL101422-03).
Abbreviations and Acronyms
- AFCAPS/TexCAPS
Airforce/Texas Coronary Atherosclerosis Prevention Study
- CANTOS
Canakinumab Anti-Inflammatory Thrombosis Outcomes Study
- CAPS
cryopyrin-associated periodic syndrome
- CARE
Cholesterol and Recurrent Events
- hsCRP
high sensitivity C-reactive protein
- IL-1Ra
Interleukin 1 receptor antagonist
- JUPITER
Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
- MESA
Multi-Ethnic Study of Atherosclerosis
- NETS
neutrophil extracellular traps
- NLRP3
NOD-like receptor family pyrin domain containing 3
- NOMID
neonatal-onset multisystem inflammatory disease
- PAI-1
plasminogen activator inhibitor-1
- PHS
Physicians Health Study
- SREBP2
sterol regulatory binding protein 2
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–6. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–91. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillett W, Francis T. Serological reactions in pneumonia with non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macleod CM, Avery OT. The Occurrence during Acute Infections of a Protein Not Normally Present in the Blood : Ii. Isolation and Properties of the Reactive Protein. J Exp Med. 1941;73:183–90. doi: 10.1084/jem.73.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarty M. The Occurrence during Acute Infections of a Protein Not Normally Present in the Blood : Iv. Crystallization of the C-Reactive Protein. J Exp Med. 1947;85:491–8. doi: 10.1084/jem.85.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker PM. C-reactive protein: eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clin Chem. 2009;55:209–15. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- 8.de Beer FC, Hind CR, Fox KM, Allan RM, Maseri A, Pepys MB. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J. 1982;47:239–43. doi: 10.1136/hrt.47.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65:168–72. doi: 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 10.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 11.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–6. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 15.Casscells W, Hathorn B, David M, Krabach T, Vaughn WK, McAllister HA, Bearman G, Willerson JT. Thermal detection of cellular infiltrates in living atherosclerotic plaques: possible implications for plaque rupture and thrombosis. Lancet. 1996;347:1447–51. doi: 10.1016/s0140-6736(96)91684-0. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 17.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, Rossouw JE, Wassertheil-Smoller S, Ridker PM. Comparison of the Framingham and Reynolds Risk scores for global cardiovascular risk prediction in the multiethnic Women’s Health Initiative. Circulation. 2012;125:1748–56. S1–11. doi: 10.1161/CIRCULATIONAHA.111.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–75. doi: 10.7326/M14-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–44. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM., Jr Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O’Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 24.Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–8. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45:1644–8. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 26.Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better ouctomes in IMPROVE-IT. Circulation. 2015;132 doi: 10.1161/CIRCULATIONAHA.115.018381. DOI 10.1161. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 29.Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 30.Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–3. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–8. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 32.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 33.Singh U, Deveraj S, Jialal I. C-reactive protein decreases tissue plasminogen activator activity in human aortic endothelial cells. Evidence that C-reactive protein is a procoagulant. Arterioscler Thromb Vasc Biol. 2005;25:2216–21. doi: 10.1161/01.ATV.0000183718.62409.ea. [DOI] [PubMed] [Google Scholar]
- 34.Venugopal SK, Devaraj S, Jialal I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation. 2003;108:1676–8. doi: 10.1161/01.CIR.0000094736.10595.A1. [DOI] [PubMed] [Google Scholar]
- 35.Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, Fay WP, Simon DI, Edelman ER. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–5. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 36.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–55. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 37.Trion A, de Maat MP, Jukema JW, van der Laarse A, Maas MC, Offerman EH, Havekes LM, Szalai AJ, Princen HM, Emeis JJ. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:1635–40. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- 38.Hirschfield GM, Gallimore JR, Kahan MC, Hutchinson WL, Sabin CA, Benson GM, Dhillon AP, Tennent GA, Pepys MB. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2005;102:8309–14. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–91. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- 40.Anand SS, Yusuf S. C-reactive protein is a bystander of cardiovascular disease. Eur Heart J. 2010;31:2092–6. doi: 10.1093/eurheartj/ehq242. [DOI] [PubMed] [Google Scholar]
- 41.Noveck R, Stroes ES, Flaim JD, Baker BF, Hughes S, Graham MJ, Crooke RM, Ridker PM. Effects of an antisense oligonucleotide inhibitor of C-reactive protein synthesis on the endotoxin challenge response in healthy human male volunteers. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane T, Wassef N, Poole S, Mistry Y, Lachmann HJ, Gillmore JD, Hawkins PN, Pepys MB. Infusion of pharmaceutical-grade natural human C-reactive protein is not proinflammatory in healthy adult human volunteers. Circ Res. 2014;114:672–6. doi: 10.1161/CIRCRESAHA.114.302770. [DOI] [PubMed] [Google Scholar]
- 43.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, Coin L, Ashby D, Tzoulaki I, Brown IJ, Mt-Isa S, McCarthy MI, Peltonen L, Freimer NB, Farrall M, Ruokonen A, Hamsten A, Lim N, Froguel P, Waterworth DM, Vollenweider P, Waeber G, Jarvelin MR, Mooser V, Scott J, Hall AS, Schunkert H, Anand SS, Collins R, Samani NJ, Watkins H, Kooner JS. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 45.Ridker PM. A test in context: critical evaluation of high sensitivity C-reactive protein (hsCRP) J Am Coll Cardiol. 2015 doi: 10.1016/j.jacc.2015.11.037. in press. [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 47.Kaptoge S, Seshasai SR, Gao P, Freitag DF, Butterworth AS, Borglykke A, Di Angelantonio E, Gudnason V, Rumley A, Lowe GD, Jorgensen T, Danesh J. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35:578–89. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esteve E, Castro A, Lopez-Bermejo A, Vendrell J, Ricart W, Fernandez-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30:939–45. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- 49.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–22. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 50.Lee WY, Allison MA, Kim DJ, Song CH, Barrett-Connor E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo Study) Am J Cardiol. 2007;99:99–102. doi: 10.1016/j.amjcard.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 51.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 52.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 53.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJ, Drexler H. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 54.Guo F, Dong M, Ren F, Zhang C, Li J, Tao Z, Yang J, Li G. Association between local interleukin-6 levels and slow flow/microvascular dysfunction. J Thromb Thrombolysis. 2014;37:475–82. doi: 10.1007/s11239-013-0974-0. [DOI] [PubMed] [Google Scholar]
- 55.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–13. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 56.Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, Sutsch G, Roffi M, Neidhart M, Eberli FR, Tanner FC, Gobbi S, von Eckardstein A, Luscher TF. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation. 2005;111:1355–61. doi: 10.1161/01.CIR.0000158479.58589.0A. [DOI] [PubMed] [Google Scholar]
- 57.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–51. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 58.Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O’Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleveland O, et al. Effect of a single dose of the interleukin-6 receptor antagonist on inflammation and troponin release in patients with Non-STEMI. ESC Abstract, London, 2015. doi: 10.1093/eurheartj/ehw171. [DOI] [PubMed] [Google Scholar]
- 61.Kawashiri SY, Kawakami A, Yamasaki S, Imazato T, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, Nakamura H, Ida H, Origuchi T, Ueki Y, Eguchi K. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int. 2011;31:451–6. doi: 10.1007/s00296-009-1303-y. [DOI] [PubMed] [Google Scholar]
- 62.Strang AC, Bisoendial RJ, Kootte RS, Schulte DM, Dallinga-Thie GM, Levels JH, Kok M, Vos K, Tas SW, Tietge UJ, Muller N, Laudes M, Gerlag DM, Stroes ES, Tak PP. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis. 2013;229:174–81. doi: 10.1016/j.atherosclerosis.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 63.McInnes IB, Thompson L, Giles JT, Bathon JM, Salmon JE, Beaulieu AD, Codding CE, Carlson TH, Delles C, Lee JS, Sattar N. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694–702. doi: 10.1136/annrheumdis-2013-204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–23. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 67.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–12. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, Anderson JP, Wanderer AA, Firestein GS. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–85. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, Weinstein SP, Belomestnov P, Yancopoulos GD, Stahl N, Mellis SJ. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–52. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 72.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–25. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 73.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–23. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–11. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Libby P, Warner SJ, Friedman GB. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988;81:487–98. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 77.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y, Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–73. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 78.Chamberlain J, Gunn J, Francis S, Holt C, Crossman D. Temporal and spatial distribution of interleukin-1 beta in balloon injured porcine coronary arteries. Cardiovasc Res. 1999;44:156–65. doi: 10.1016/s0008-6363(99)00175-3. [DOI] [PubMed] [Google Scholar]
- 79.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97:769–76. doi: 10.1172/JCI118476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 81.Kastrati A, Koch W, Berger PB, Mehilli J, Stephenson K, Neumann FJ, von Beckerath N, Bottiger C, Duff GW, Schomig A. Protective role against restenosis from an interleukin-1 receptor antagonist gene polymorphism in patients treated with coronary stenting. J Am Coll Cardiol. 2000;36:2168–73. doi: 10.1016/s0735-1097(00)01014-7. [DOI] [PubMed] [Google Scholar]
- 82.Francis SE, Camp NJ, Dewberry RM, Gunn J, Syrris P, Carter ND, Jeffery S, Kaski JC, Cumberland DC, Duff GW, Crossman DC. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation. 1999;99:861–6. doi: 10.1161/01.cir.99.7.861. [DOI] [PubMed] [Google Scholar]
- 83.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JY. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–42. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Folco EJ, Sukhova GK, Quillard T, Libby P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res. 2014;115:875–83. doi: 10.1161/CIRCRESAHA.115.304437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–20. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nahrendorf M, Swirski FK. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–8. doi: 10.1126/science.aac7801. [DOI] [PubMed] [Google Scholar]
- 89.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer BL. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032–40. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–9. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation. 2008;117:2577–9. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 92.Abbate A, Dinarello CA. Anti-inflammatory therapies in acute coronary syndromes: is IL-1 blockade a solution? Eur Heart J. 2015;36:337–9. doi: 10.1093/eurheartj/ehu369. [DOI] [PubMed] [Google Scholar]
- 93.Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377–84. doi: 10.1093/eurheartj/ehu272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377 e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 95.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The Interleukin I Gentics Consortium. Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2015;3:243–53. doi: 10.1016/S2213-8587(15)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herder C, Donath MY. Interleukin-1 receptor antagonist: friend or foe to the heart? Lancet Diabetes Endocrinol. 2015;3:228–9. doi: 10.1016/S2213-8587(15)00035-2. [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T on behalf of the CANTOS Pilot Investigative Group. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen. A phase IIb randomized placebo controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 99.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 100.Donath MY, Schoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 101.Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–29. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Sagar HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting interleukin-1β reduces leucocyte production after acute myocardial infarction. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.016160. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Everett BM, Pradhan AD, Solomon DH, Paynter N, MacFadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 106.Martinez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, Celermajer DS, Patel S. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, Sianos G, Goudevenos J, Alexopoulos D, Pyrgakis V, Cleman MW, Manolis AS, Tousoulis D, Lekakis J. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.017611. in press. [DOI] [PubMed] [Google Scholar]
- 108.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–10. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 109.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 110.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodeling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hilgendorf I, Swirski FK, Robbins CS. Monocyte fate in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:272–9. doi: 10.1161/ATVBAHA.114.303565. [DOI] [PubMed] [Google Scholar]


