Abstract
Objective
Energy expenditure (EE) and measures of inflammation increase with adiposity, and this obesity-induced chronic and subclinical inflammation was extensively reported to be a cause of insulin resistance. However, whether subclinical inflammation has a role in increasing EE, which may be at the cost of developing insulin resistance, is not clear.
Methods
We investigated the association between circulating white blood cell count (WBC) in a population of Native Americans (n=243) with measurement of EE in a respiratory chamber, and in a subset of the same population (n=34), with gene expression measures of inflammation in subcutaneous abdominal adipose tissue (SAAT). All subjects were healthy on oral glucose tolerance test. Statistically, nonnormally distributed variables were logarithmically transformed before analyses to approximate normal distributions.
Results
WBC was associated with 24-h EE adjusted for age, sex, fat-free mass, and fat mass (r=0.13, P=0.04). In SAAT, tumor necrosis factor-α (TNF-α), shown as log10-transformed TNF-α (r=0.36, P=0.05), and plasminogen activator inhibitor-1 (PAI-1), shown as log10-transformed PAI-1 (lPAI-1; r=0.41, P=0.02), expressions were also positively correlated with adjusted 24-h EE. lPAI-1 was also correlated with adjusted sleep EE (r=0.34, P=0.07).
Conclusions
In conclusion, circulating markers of inflammation (WBC) and markers of inflammation within adipose tissue (TNF-α and PAI-1) are positively associated with EE, indicating a role of chronic subclinical inflammation in the regulation of metabolic rate.
Introduction
Measures of inflammation increase with increasing adiposity, and they have been implicated in the metabolic consequences of increasing adiposity (1–4). However, there is evidence that some mediators of inflammation may also serve to signal a brake on further weight gain. For instance, tumor necrosis factor-α (TNF-α) has been identified as causal in cancer-related cachexia (5–7). In addition, over-expression of the cytokine TNF-α in mice leads to worsening insulin action, but also to reduced adiposity (8). In humans, lower concentration of the anti-inflammatory adipocytokine, adiponectin, is associated with higher resting energy expenditure (EE) (9), and transgenic over-expression of adiponectin increases adiposity in leptin-deficient mice (10).
To further characterize the association of measures of inflammation with EE, we investigated the association of circulating white blood cell count (WBC), which is increased with adiposity and predicts diabetes (11), as a predictor of EE measured in a respiratory chamber in Pima Indians. In a subset of these individuals, we also obtained samples of adipose tissue, and therefore, examined the association of the expression of inflammatory markers from subcutaneous abdominal adipose tissue (SAAT) with EE.
Subjects and methods
Subjects
In an ongoing longitudinal study to identify risk factors for the development of type 2 diabetes and obesity, 243 and 34 (in a subset group) adult Pima Indians (at least three-fourth Pima or closely related Tohono O'odham Indians) participated. All the subjects reported that they were nonsmokers, not taking any medication, and were in good health, as determined by medical history, physical examination, and routine laboratory testing. Three days after admission and after a 12-h overnight fast, a 2-h 75-g oral glucose tolerance test was performed to identify and exclude subjects with diabetes. The 1999 World Health Organization criteria were used. For the study protocol, subjects were admitted to the Clinical Research Unit of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in Phoenix, AZ, USA, for 8–15 days and were provided a standard weight-maintaining diet containing 50% of calories as carbohydrate, 30% as fat, and 20% as protein for at least 3 days before fat biopsy. The study was approved by the NIDDK Institutional Review Board and the Gila River Indian Community Tribal Council. Before participation, written informed consent was obtained.
Methods
WBC and dual-energy X-ray absorptiometry scan
Total WBC was measured after an overnight fast on the day of admission (between 0800 and 1000 h). WBC was measured in the central laboratory of Phoenix Indian Medical Center by an automated cell counter (Cell-DYN Sapphire; Abbott Diagnostics). Reliability coefficients, based on blind replicate control data, ranged from 0.96 to 1.00 (11). Body composition was assessed by dual-energy X-ray absorptiometry (DPX-l; Lunar, Madison, WI, USA).
Respiratory chamber
EE was measured in a respiratory chamber as described previously (12). Briefly, volunteers entered the chamber at 0745 h after an overnight fast and remained therein for 23 h. Meals were provided at 0800, 1130, and 1700 h, and an evening snack was provided at 2000 h. The rate of EE was measured continuously, calculated for each 15-min interval, and then averaged for the 24-h interval (24EE). Sleeping metabolic rate was defined as the average EE of all the 15-min periods between 2330 and 0500 h during which spontaneous physical activity (assessed by motion radar) was <1.5%.
Fat biopsy, morphological analysis
As reported previously (13), after an overnight fast, subcutaneous adipose tissue was obtained by percutaneous needle biopsy of periumbilical fat depots using a 15-gauge needle through skin after anesthetizing with 1% lidocaine. Samples were immediately frozen in liquid nitrogen. Adipose tissue samples were fixed for 1200– 1600 h at room temperature in zinc–formalin fixative (Anatech, Battle Creek, MI, USA) and were embedded in paraffin. As described previously, five micron sections, cut at 50-micron intervals, were mounted on charged glass slides, deparaffinized in xylene, and stained for expression of CD68 as done previously (Caltag, Burlingame, CA, USA) (14). For each individual tissue block, four different 20× fields from each of the five different sections were analyzed independently by two blinded evaluators. The total number of nuclei and the number of nuclei of CD68-expressing cells were counted for each field. The fraction of macrophage cells for each sample was calculated as the sum of the number of nuclei of CD68-expressing cells divided by the total number of nuclei in sections of a sample.
Real-time quantitative PCR
Total RNA was extracted from frozen adipose tissue (∼50 mg) using a commercially available acid-phenol reagent (Trizol; Invitrogen Inc). First-strand cDNA was synthesized using Superscript III reverse transcriptase and random hexamer primers as described in the manufacturer's protocol (Invitrogen Inc). Samples of cDNA were diluted 1:25 in nuclease-free water (Qiagen Inc). PCR amplification mixtures (20 μl) contained 10 μl of 2× PCR SYBR Green I Quantitect Master Mix (Qiagen Inc.), 0.4 μl of 25 μM reverse and forward primer mix, and 11.6 μl of diluted cDNA template. Real-time quantitative PCR was carried out using the DNA Engine Opticon (Bio-Rad) instruments with the following cycling parameters: polymerase activation: 15 min, 95 °C; amplification for 40 cycles: 15 s, 94 °C; 20 s, 58 °C; 20 s, 72 °C. After amplification, melting curve analysis was performed as described in the manufacturer's protocol (Qiagen Inc). The control gene, casein kinase-1d (CSNK1d) (13), was used to normalize simple load and reactions and for calculation of Δ−ΔCt values from which relative expression values were determined (arbitrary units). Human primer orientation and sequences were as follows: CSNK1d: forward, 5′-AGGAGAAGAGGTTGCCATCAAG-3′, and reverse, 5′-TCCATCACCATGACGTTGTAGTC-3′; TNF-α: forward, 5′-TGCTTGTTCCTCAGCCTCTTC-3′, and reverse, 5′-GCTTGTCACTCGGGGTTCG-3′; plasminogen activator inhibitor-1 (PAI-1): forward, 5′-CTGGTTCTGCCCAAGTTCTCC-3′, and reverse, 5′-CCACAAAGAGGAAGGGTCTGTC-3′; monocyte chemo-attractant protein-1 (MCP-1): forward, 5′-CAATCAATGCCCCAGTCACC-3′, and reverse, 5′-GAATCCTGAACCCACTTCTGC-3′; macrophage migration inhibitory factor (MIF): forward, 5′-GTTCATCGTAAACACCAACGTG-3′, and reverse, 5′-CCGCGTTCATGTCGTAATAGTT-3′; adiponectin: forward, 5′-CCAGGAAACCACGACTCAAG-3′, and reverse, 5′-TAGGCACCTTCTCCAGGTTCTC-3′; CD68: forward, 5′-GCTACATGGCGGTGGAGTACAA-3′, and reverse, 5′-ATGATGAGAGGCAGCAAGATGG-3′; CD11b: forward, 5′-GAGTCCAACGCTAATGTCAAGG-3′, and reverse, 5′-CCCGTAGAGAACAGCATCACAC-3′; and CSF1R: forward, 5′-GCTCAACCTCAAAGTCATGGTG-3′, and reverse, 5′-GAAGGTGTGCCTGTATGTGTCC-3′.
Statistical analysis
Statistical analyses were performed using SAS software (SAS version 8.2, SAS Institute, Inc., Cary, NC, USA). Throughout the article, the data are expressed as means±s.d. Normality of the data was tested by the Shapiro–Wilk test. Nonnormally distributed variables were logarithmically transformed before analyses to approximate normal distributions. If normal distribution was not achieved by logarithmic transformation, nonparametric tests were used. Student's t-test or Wilcoxon test was used for sex comparison for parametric and nonparametric variables. Pearson's correlation was used to test the relationships between variables. Linear regression analysis was used to adjust 24EE and sleep EE for age, sex, fat mass (FM), and fat-free mass (FFM), and to model the effect of inflammatory markers. An interaction term was included to test whether the association differed by sex. Level of statistical significance was set at P<0.05.
Results
Anthropometric, metabolic characteristics, and measures of inflammation are summarized in Table 1. As expected, body mass index (BMI) and percent body fat (Pfat) were higher and height and unadjusted 24EE were lower in women (data not shown); the rest of the variables did not differ by sex.
Table 1.
Subject characteristics of white blood cell count (WBC) and subset group. Data are means±s.d.
| WBC group | Cytokine group | |
|---|---|---|
| n | 243 | 34 |
| M/F | 155/88 | 20/14 |
| Age (years) | 33 (17–58) | 30 (18–44) |
| Height (cm) | 168.1 (7) | 166.7 (7) |
| BMI (kg/m2) | 33.7 (8) | 30.6 (9) |
| Pfat (%) | 32.1 (8) | 30.6 (9) |
| NGT/IGT | 173/70 | 24/10 |
| 24EE (kcal/24 h) | 2375 (407) | 2289 (395) |
| Sleep EE (kcal/24 h) | 1682 (281) | 1608 (312) |
| WBC (×1000 cells/mm3) | 7.85 (1.5) | 7.9 (1.2) |
| ITNF-α (AU) | – | −3.78 (0.6) |
| IPAI-1 (AU) | – | −3.18 (0.8) |
| IMCP-1 (AU) | – | −1.57 (0.6) |
| IMIF (AU) | – | −1.81 (0.8) |
| IAdiponectin (AU) | – | 5.05 (0.58) |
| CD68+ cell (AU) | – | 0.27 (0.11) |
| ICD68 | – | −0.13 (0.36) |
| ICSF1R (AU) | – | 0.11 (0.4) |
| ICD11b (AU) | – | −1.26 (0.42) |
M/F, male and female; BMI, body mass index; Pfat, percent body fat; NGT/IGT, normal glucose tolerance test and impaired glucose tolerance test; WBC, white blood cell count; lTNF-α, lPAI-1, lMCP-1, lMIF, ladiponectin, lCD68, lCSF1R, lCD11b, log10-transformed tumor necrosis factor-α, plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, macrophage migration inhibitory factor, adiponectin, CD68, CSF1R, CD11b; CD68+ cell, the fraction of nuclei of CD68-positive cells by immunohistochemistry; AU, arbitrary unit.
Circulating WBC and EE
WBC correlated with age (r=−0.16, P<0.01), BMI (r=0.29, P<0.01), Pfat (r=0.31, P<0.01), and fasting insulin (r=0.27, P<0.01). WBC was also associated with unadjusted 24EE (r=0.17, P<0.01) and sleep EE (r=0.14, P=0.03). After adjustment for age, sex, FM, and FFM, only 24EE remained significantly associated with WBC (r=0.13, P=0.04; Fig. 1). There was no evidence of an interaction between WBC and sex (P=0.7).
Figure 1.
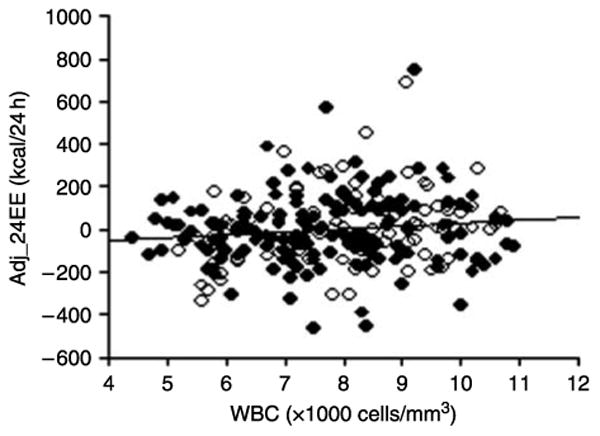
Correlation of 24-h energy expenditure with WBC. Adjusted 24-h energy expenditure is positively correlated with white blood cell count (r=0.13, P=0.04). Adj_24EE, 24-h energy expenditure adjusted for age, sex, fat-free mass, and fat mass. WBC, white blood cell count. ○ = female, ● = male.
Expression of SAAT TNF-α and PAI-1 and EE
As shown in Table 2, TNF-α, PAI-1, and MCP-1 expressions were correlated with BMI, while those of MIF, and adiponectin were not. As reported previously, adipose tissue macrophage content assessed by CD68+ cell and gene expression of CD68, CSF1R, and CD11b were correlated with BMI and Pfat (13). TNF-α, PAI-1, and MCP-1 expressions were correlated with unadjusted 24EE and sleep EE, but adiponectin and MIF expressions were not. Markers of macrophage content of adipose tissue were also not significantly associated with EE. After adjustment for confounders, expressions of TNF-α and PAI-1 were still significantly correlated with 24EE (r=0.36, P=0.05; r=0.41, P=0.02; Fig. 2A and B). The interaction term TNF-α×sex was significant (P=0.05), and when analyzed by sex, the association between TNF-α expression and adjusted 24EE was much stronger in men (r=0.70, P=0.01). The interaction term PAI-1× sex was not significant. After adjustment for the same covariates, TNF-α expression was not associated with sleep EE (r=0.23, P=0.23), and the association of sleep EE with PAI-1 expression was attenuated (r=0.34, P=0.07; Fig. 2C).
Table 2.
Correlation of measures of subcutaneous abdominal adipose tissue inflammatory markers with body mass index, percent body fat, and energy expenditure (EE).
| BMI R | Pfat R | 24EE R | Sleep EE R | |
|---|---|---|---|---|
| lTNF-α | 0.42* | 0.20 | 0.43* | 0.40* |
| IPAI-1 | 0.37* | 0.09 | 0.50† | 0.47† |
| IMCP-1 | 0.50† | 0.32 | 0.40* | 0.34* |
| lMIF | 0.03 | −0.15 | 0.13 | 0.06 |
| IAdiponectin | −0.2 | −0.19 | −0.25 | −0.21 |
| CD68+ cell | 0.25 | 0.46* | 0.08 | 0.02 |
| ICD68 | 0.43† | 0.46† | 0.21 | 0.28 |
| ICSF1R | 0.54† | 0.61† | 0.18 | 0.26 |
| ICD11b | 0.61† | 0.66† | 0.31 | 0.34 |
P<0.05,
P<0.01.
Figure 2.
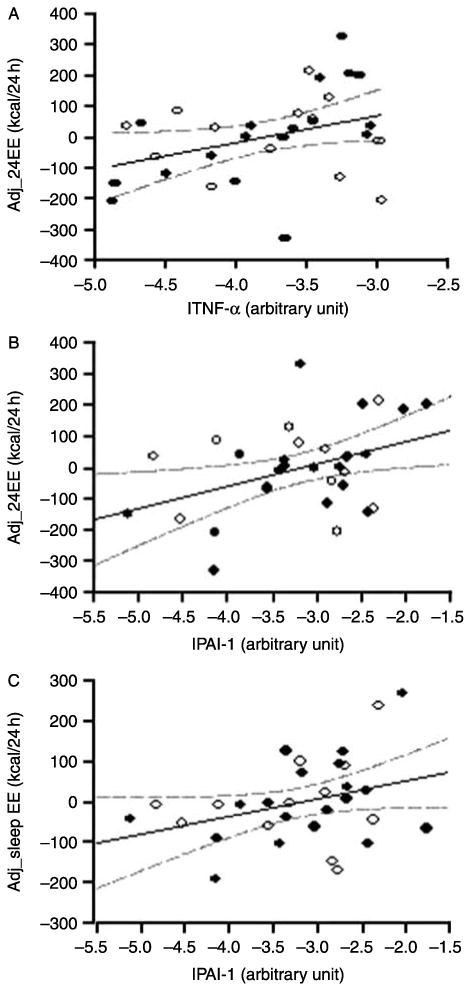
Correlation of energy expenditure with TNF-α and PAI-1. Adjusted 24-h energy expenditure is positively correlated with TNF-α (r=0.36, P=0.05) (A) and PAI-1 (r=0.41, P=0.02) (B), and adjusted sleeping energy expenditure is positively correlated with PAI-1 (r=0.34, P=0.07) (C). Adj_24EE, 24-h energy expenditure adjusted for age, sex, fat-free mass, and fat mass; Adj_sleep, sleeping energy expenditure adjusted for age, sex, fat-free mass, and fat mass. lTNF-α, lPAI-1 (log10-transformed tumor necrosis factor-α and plasminogen activator inhibitor-1). ○ = female, ● = male.
Discussion
In this study, we found a positive correlation between circulating WBC and SAAT-secreted TNF-α and PAI-1 with adjusted 24EE. For TNF-α expression, the association was different by sex, showing a much stronger association in men than in women. Our findings indicate that subclinical inflammation may have a role in modulating the increased EE seen with increasing adiposity.
Inflammation has been implicated previously as playing a role in EE. In healthy humans, TNF-α infusions have been shown to increase resting EE (15). In mice, specific over-expression of TNF-α in adipose tissue decreased local insulin sensitivity, while reducing body fat (8). Administration of TNF-α to rats also leads to an increase in thermogenic activity in BAT (16). In more severe pathologic conditions such as chronic infections, arthritides, and cancer-related cachexia, circulating TNF-α has been implicated in the associated wasting syndrome (17, 18). Moreover, patients with collagen vascular disease tended to gain weight over time when treated with TNF-α antagonists (19, 20). How PAI-1 may affect EE is less clear. In contrast to our results, PAI-1 knockout mice have increased EE (21). In the context of our study, PAI-1 may be serving as a marker for overall increased subacute inflammation within adipose tissue. It should be noted that our results are also consistent with those of a previous analysis done by our group, demonstrating a negative association between fasting adiponectin concentrations, an anti-inflammatory adipocytokine, and resting EE (9). Lack of significance regarding the association of adiponectin with EE in the current study was very likely due to the decreased number of biopsy samples compared with the plasma samples in the former study.
The mechanisms by which inflammation increases EE are not completely clear. TNF-α has a direct central effect on thermogenesis (22), but it seems unlikely that TNF-α in tissue would have a direct central effect. Whether inflammatory mediators might directly affect peripheral sympathetic nervous system (SNS) activity, thus modulating EE, is unclear. SNS activity is a positive independent predictor of both 24EE and resting EE in Caucasians, but not, however, in Pima Indians (23). In cross-sectional studies, SNS activity is positively associated with adiposity, indicating that increased SNS activity could play a role in the defense of further weight gain. However, reduced SNS activity is a prominent feature of rodent models of obesity (24), and lower SNS activity predicts future weight gain in Pima Indians (25). Furthermore, the evidence that SNS activity is positively associated with inflammation is conflicting (26–28). Increased hepatic glucose production (HGO), declining insulin action, and increasing fasting insulin and free fatty acid concentrations have been identified as determinants of EE, particularly in those with impaired glucose regulation (29). Higher WBC also predicts declines in insulin action (11). Thus, another possible explanation could be that subclinical inflammation leads to reduced insulin action and increased HGO, which then causes increased EE. Increased body temperature could be another mechanism linking inflammation to higher EE (30).
The association between circulating WBC and 24EE was modest compared to those seen with the adipose tissue measurements. This may be due to an indirect effect of WBC on EE either via circulating intermediates (such as TNF-α, interleukin-6 (IL-6), or other cytokines) or via factors such as insulin action and HGO. Alternatively, a common intermediate could affect both WBC and EE. For instance, IL-6 may have a separate role in both WBC differentiation (28) and EE (31). Unfortunately, we did not have sufficient remaining adipose tissue or appropriately stored measurement of IL-6 to perform these measurements on this group. The diurnal variation in WBC could also confound the association with EE. However, this is unlikely in this case, as all samples were drawn during fasting in the morning. Although only automated differentials were available, no specific white cell lineage (such as monocytes) explained the association with EE (data not shown).
Since FM itself is an independent determinant of EE (12), local factors that increase the metabolic capacity of adipose tissue or directly affect insulin action (as mediators of the increase in EE) may explain the relatively stronger association with EE than that seen with circulating factors. However, as noted above, the precise mechanism is not clear. For TNF-α expression, we did find that the association with EE differed by sex, with it being much stronger in men. Given the small number of women in the study, we cannot clearly discern whether this represents a true sex difference in response to inflammation or may be secondary to differences when the biopsies were done in relationship to the menstrual cycle (not documented in our study).
It was known that TNF-α, PAI-1, MCP-1, IL-6 and IL-1 induce insulin resistance in vitro and in vivo, and white blood cells predict diabetes independent of adiposity. Furthermore, the severity of obesity-associated insulin resistance from NGT to diabetes paralleled the degree of thermogenesis. Our current finding in healthy population potentially implies that enhanced thermo-genesis induced by chronic and subclinical inflammation might be an early and underlining indication of developing insulin resistance.
In conclusion, we found a modest positive association between circulating WBC and adjusted 24EE in healthy nondiabetic Pima Indians. In a subgroup of these individuals, we also found an association between adjusted 24EE and TNF-α and PAI-1 expressions in adipose tissue. Thus, chronic subclinical inflammation may have a role in upward regulation of metabolic rate with increased adiposity.
Acknowledgments
We thank Joy Bunt and Carol Massengill and the nursing staff of the National Institutes of Health (NIH) Clinical Unit.
Funding: This study was supported by the Intramural Research Program of the NIH, NIDDK. Most of all, we thank the volunteers for their participation in the study.
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. Journal of Clinical Investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Grimble RF. Inflammatory status and insulin resistance. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5:551–559. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard L, Mauriege P, Vohl MC, Bouchard C, Perusse L. Plasminogen-activator inhibitor-1 polymorphisms are associated with obesity and fat distribution in the Quebec Family Study: evidence of interactions with menopause. Menopause. 2005;12:136–143. doi: 10.1097/00042192-200512020-00006. [DOI] [PubMed] [Google Scholar]
- 5.Argiles JM, Lopez-Soriano J, Busquets S, Lopez-Soriano FJ. Journey from cachexia to obesity by TNF. FASEB Journal. 1997;11:743–751. doi: 10.1096/fasebj.11.10.9271359. [DOI] [PubMed] [Google Scholar]
- 6.Spiegelman BM, Hotamisligil GS. Through thick and thin: wasting, obesity, and TNF alpha. Cell. 1993;73:625–627. doi: 10.1016/0092-8674(93)90243-j. [DOI] [PubMed] [Google Scholar]
- 7.Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987;50:555–563. doi: 10.1016/0092-8674(87)90028-6. [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNF alpha in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143:1502–1511. doi: 10.1210/endo.143.4.8715. [DOI] [PubMed] [Google Scholar]
- 9.Pannacciulli N, Bunt JC, Ortega E, Funahashi T, Salbe AD, Bogardus C, Krakoff J. Lower total fasting plasma adiponectin concentrations are associated with higher metabolic rates. Journal of Clinical Endocrinology and Metabolism. 2006;91:1600–1603. doi: 10.1210/jc.2005-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PI. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Journal of Clinical Investigation. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 12.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, Jr, Krakoff J. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Poll T, Romijn JA, Endert E, Borm JJ, Buller HR, Sauerwein HP. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. American Journal of Physiology. 1991;261:E457–E465. doi: 10.1152/ajpendo.1991.261.4.E457. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell NJ. Cytokines and thermogenesis. International Journal of Obesity and Related Metabolic Disorders. 1993;17:S98–S101. [PubMed] [Google Scholar]
- 17.Balkwill FR, Smyth JF. Interferons in cancer therapy: a reappraisal. Lancet. 1987;2:317–319. doi: 10.1016/s0140-6736(87)90901-9. [DOI] [PubMed] [Google Scholar]
- 18.Evans DA, Jacobs DO, Wilmore DW. Tumor necrosis factor enhances glucose uptake by peripheral tissues. American Journal of Physiology. 1989;257:R1182–R1189. doi: 10.1152/ajpregu.1989.257.5.R1182. [DOI] [PubMed] [Google Scholar]
- 19.Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. American Journal of Clinical Nutrition. 2006;84:1463–1472. doi: 10.1093/ajcn/84.6.1463. [DOI] [PubMed] [Google Scholar]
- 20.Saraceno R, Schipani C, Mazzotta A, Esposito M, Di Renzo L, De Lorenzo A, Chimenti S. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacological Research. 2008;57:290–295. doi: 10.1016/j.phrs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughman DE, Fogo AB. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 22.Amaral ME, Barbuio R, Milanski M, Romanatto T, Barbosa HC, Nadruz W, Bertolo MB, Boschero AC, Saad MJA, Franchini KG, Velloso LA. Tumor necrosis factor-alpha activates signal trans-duction in hypothalamus and modulates the expression of pro-inflammatory proteins and orexigenic/anorexigenic neuro-transmitters. Journal of Neurochemistry. 2006;98:203–212. doi: 10.1111/j.1471-4159.2006.03857.x. [DOI] [PubMed] [Google Scholar]
- 23.Saad MF, Alger SA, Zurlo F, Young JB, Bogardus C, Ravussin E. Ethnic differences in sympathetic nervous system-mediated energy expenditure. American Journal of Physiology. 1991;261:E789–E794. doi: 10.1152/ajpendo.1991.261.6.E789. [DOI] [PubMed] [Google Scholar]
- 24.Young JB, Landsberg L. Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. American Journal of Physiology. 1983;245:E148–E154. doi: 10.1152/ajpendo.1983.245.2.E148. [DOI] [PubMed] [Google Scholar]
- 25.Tataranni PA, Young JB, Bogardus C, Ravussin E. A low sympathoadrenal activity is associated with body weight gain and development of central adiposity in Pima Indian men. Obesity Research. 1997;5:341–347. doi: 10.1002/j.1550-8528.1997.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 26.Sayk F, Vietheer A, Schaaf B, Wellhoener P, Weitz G, Lehnert H, Dodt C. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2008;295:R891–R898. doi: 10.1152/ajpregu.90444.2008. [DOI] [PubMed] [Google Scholar]
- 27.Diakakis GF, Parthenakis FI, Patrianakos AP, Koukouraki SI, Stathaki MI, Karkavitsas NS, Vardas PE. Myocardial sympathetic innervation in patients with impaired glucose tolerance: relationship to subclinical inflammation. Cardiovascular Pathology. 2008;17:172–177. doi: 10.1016/j.carpath.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Aso Y, Wakabayashi S, Nakano T, Yamamoto R, Takebayashi K, Inukai T. High serum high-sensitivity C-reactive protein concentrations are associated with relative cardiac sympathetic overactivity during the early morning period in type 2 diabetic patients with metabolic syndrome. Metabolism. 2006;55:1014–1021. doi: 10.1016/j.metabol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999;48:2197–2203. doi: 10.2337/diabetes.48.11.2197. [DOI] [PubMed] [Google Scholar]
- 30.Tarner IH, Muller-Ladner U, Uhlemann C, Lange U. The effect of mild whole-body hyperthermia on systemic levels of TNF-alpha, IL-1beta, and IL-6 in patients with ankylosing spondylitis. Clinical Rheumatology. 2008;28:397–402. doi: 10.1007/s10067-008-1059-x. [DOI] [PubMed] [Google Scholar]
- 31.Rush EC, Plank LD, Yajnik CS. Interleukin-6, tumour necrosis factor-alpha and insulin relationships to body composition, metabolism and resting energy expenditure in a migrant Asian Indian population. Clinical Endocrinology. 2007;66:684–690. doi: 10.1111/j.1365-2265.2007.02801.x. [DOI] [PubMed] [Google Scholar]


