Abstract
Objective
We evaluated how adolescents with or at risk of type 2 diabetes (T2DM) and their parent/guardians (parents) value health states associated with T2DM.
Methods
We interviewed overweight/obese (BMI≥85th percentile), 12–18 year-old adolescents with T2DM, prediabetes, or insulin resistance (IR) and a parent. The standard gamble (SG) method elicited preferences (utilities) for 7 hypothetical T2DM health states reported on a scale from 0 (dead) to 1 (perfect health). Adolescent’s current health was evaluated with the SG and Health Utilities Index (HUI).
Results
There were 70 adolescents and 69 parents. Adolescents were 67.1% female and 15.5±2.2 years old; 30% had T2DM, 30% prediabetes, and 40% IR. Almost half (48.6%) had a BMI>99th percentile. Parents (83% mothers) were 45.1±7.3 years old and 75% had at least some college/technical school education. Adolescents and parents rated T2DM with no complications treated with diet as most desirable (median [IQR]; adolescent 0.72 [0.54, 0.98]; parent 1.0 [0.88, 1.0]) and end stage renal disease as least desirable (adolescent 0.51 [0.31, 0.70]; parent 0.80 [0.65, 0.94]). However, adolescents’ utilities were significantly lower (p≤0.001) than parents for all health states assessed. Adolescents’ assessment of their current health with the SG and HUI were not correlated.
Conclusions
Adolescents with or at risk of T2DM rated treatments and sequelae of diabetes as significantly worse than their parents. These adolescent utilities should be considered in the evaluation of treatment strategies for youth with T2DM. Family-based programs for T2DM must also be prepared to address conflicting preferences in order to promote shared-decision making.
Keywords: Utilities, type 2 diabetes, adolescents, standard gamble, microvascular
The prevalence and incidence of type 2 diabetes (T2DM) among youth have increased (1). Overweight and obesity among U.S. children also rose between the 1960s and 1990s (2) producing generations of children at risk for metabolic complications including insulin resistance, prediabetes, and diabetes (3, 4). Similar to adults, treatment for T2DM in youth aims to normalize blood glucose and prevent development of microvascular and macrovascular complications (5). As such, diet and lifestyle changes are standard components of diabetes treatment and prevention, but pharmacologic management is often needed (5). Most adolescents with T2DM are treated with metformin, insulin, or a combination of the two with individualized therapy based on presentation and ongoing needs (5). However, adolescence is a vulnerable period for children. Recent work suggests that adolescents with T2DM demonstrate particular difficulty with adherence to diet and exercise (6) and have high rates of attrition from obesity management programs (7). Racial and ethnic disparities in glycemic control have also been observed with worse control seen among non-White youth with diabetes (6, 8, 9). Little is known about the preferences of adolescents with or at risk of T2DM and their families for diabetes treatments, how they value the risk for long term complications of diabetes (10, 11), or the cultural and family context that shapes these preferences.
Health state preferences (also known as utilities) are a measure of health-related quality of life that describe an individual’s perception about the “desirableness” or value of a health condition (12) in contrast to traditional measures of health-related quality of life, which describe the impact of a health condition on functional status in domains such as physical and emotional well-being (13). These preferences can be elicited with standardized measurement techniques (14) and can then be used in decision analyses and economic evaluations to guide recommended practice that considers health-related quality of life (15). For example, in cost-utility analysis, a form of cost-effectiveness analysis, utilities can be used to adjust remaining life expectancy for quality of life to calculate QALYs (quality-adjusted life-years) (14). Cost per QALYs gained as a result of an intervention or program can then guide decisions about use of resources (14). On an individual practice level, clarifying the preferences of adolescents can support patient-centered care and shared-decision making (16).
Health state preferences in adults, but not adolescents, with T2DM have been described (17–21). Importantly, Huang et al. have demonstrated that the cost-effectiveness of treatments for adults with T2DM is dependent on the assumptions made about patient preferences for these treatments (21). As preferences may change from childhood to adulthood (22–24), eliciting preferences from adolescents, where appropriate, has been recommended (22, 23, 25). The standard gamble, one method for directly eliciting preferences (14), has been used in adolescents with a number of chronic disorders (26–31). However, differences between adolescent and parent-proxy valuation of pediatric health states raise ongoing questions about how to approach economic evaluations for adolescent health and whose values should be considered (23, 25).
In this study, we sought to describe and compare the preferences of adolescents with or at risk of T2DM and their parents for key health states associated with T2DM and its treatments to inform treatment and prevention strategies. To our knowledge, this is the first study to directly elicit these utilities for this population. We hypothesized that significant differences would exist between child and parent preferences for key health states associated with T2DM and that race/ethnicity would be independently associated with adolescent and parent preferences.
Methods
Subjects
Subjects were adolescents between 12 and 18 years of age with BMI for age ≥85th percentile within the prior 2 years and T2DM, prediabetes, or insulin resistance along with a parent. Parent and child had to be fluent in English or Spanish. Adolescent were excluded for depression or other psychiatric disorders (other than attention deficit disorder); impaired cognitive skills or developmental delay if functioning below a 6th grade academic level by parent report; significant organ system illness; hospitalization within the prior 6 months for a non-diabetes-related chronic illness; pregnancy or planned pregnancy (for females); or parenthood. T2DM, prediabetes and insulin resistance diagnoses were clinical diagnoses based on documentation in the medical record. For pre-diabetes, the terms “pre-diabetes,” “impaired fasting glucose,” or “impaired glucose tolerance” were acceptable. Alternatively, laboratory data consistent with the prevailing American Diabetes Association criteria were also accepted. This included fasting blood glucose between 100 and 125 mg/dl and/or a 2 hour postprandial glucose in an oral glucose tolerance test (OGTT) between 140 and 199 mg/dl (32). For insulin resistance, the terms “insulin resistance,” “metabolic syndrome,” and “hyperinsulinemia” were accepted. Laboratory data were accepted as alternatives and included fasting insulin >16 uU/mL, insulin peak (post-OGTT) >150 uU/mL, or insulin levels at 120 minutes of OGTT >75 uU/mL (33–35). We did not require that laboratory criteria be met for all subjects as screening practices at sites varied, biochemical definitions for insulin resistance are not well established, and our goal was to identify patients perceived to be at heightened clinical risk of T2DM from the perspective of the patient and parent. Therefore, to be eligible, the parent also had to report awareness of the child’s diagnosis of T2DM or their risk for T2DM (for those with prediabetes or insulin resistance).
Subjects were recruited between April 2006 and December 2007 from programs at Children’s Hospital Boston and Joslin Diabetes Center treating adolescents with or at risk of T2DM. Potentially eligible patients were identified through clinicians, billing records or self-referral. Screening was in-person or by telephone. Those not initially indicating interest through clinicians or self-referral were sent a recruitment letter and opt-out postcard. A total of 143 patients completed screening. Of these, 99 (69.2%) were eligible and 80 (55.9%) were interested and enrolled. After enrollment and informed consent/assent, 10 subjects were withdrawn prior to interview because interviews could not be scheduled (N=4); they were no longer interested (N=3); or were subsequently found to be ineligible (N=3). The study was approved by the Institutional Review Boards of Children’s Hospital Boston and Joslin Diabetes Center.
Data Collection
An in-person parent interview was followed by an in-person child interview. Interviewers were fluent in English and Spanish. All interviews were audiorecorded, and a subset was reviewed by the first author for quality control. The adolescent’s most recent height and weight were abstracted from the clinical record to calculate BMI. Demographic data, family history, parent’s self-reported height and weight, and adolescent’s treatment regimen were collected during the parent interview.
Health Preference Assessment
Utilities, reported on a scale from 0 (dead) to 1 (perfect health), for seven hypothetical health states related to T2DM and its treatments were assessed by the standard gamble method. The health states included three health states with T2DM and no complications with varying treatments (dietary treatment only, oral medication and insulin treatment) and four health states focused on diabetes complications, all assuming treatment with insulin. All health states included the same standard description of diabetes self-management and routine diabetes health maintenance visits. Health state descriptions were developed based on literature review and clinical experience. Standard gamble surveys were professionally translated into Spanish using forward and back translation and piloted with 5 adolescent/parent dyads.
Adolescents valued their own current health over the prior 4 weeks and the T2DM health states. Parents valued the adolescent’s current health over the prior 4 weeks and the same T2DM health states for their children. Using the standard gamble method, the adolescent received a choice of remaining in the specified health state for the rest of his/her life or the option of a “magic potion” which had some chance (p) of curing his/her problem and leaving him/her in perfect health but with one side effect, a chance (1-p) that it may instead cause immediate painless death. The parent answered the same questions but with a “magic potion” with a chance (p) of either curing their child’s health state, leaving the child in perfect health, or a chance (1-p) of their child’s immediate painless death. Based on each response, interviewers utilized a written structured algorithm based on the bisection approach to determine the next gamble to offer in order to identify the respondent’s point of indifference, i.e., the point when the respondent was indifferent between the health state and the gamble (12). The value of the health state was then “p” at this point. For example, if the adolescent could not decide between a lifetime in the specified health state and accepting a “magic potion” with a 30% chance of his/her immediate painless death to avoid a lifetime in the specified health state, then the utility for that health state was 0.70 (i.e., 1–0.30). Interviewers confirmed any valuations offered by the respondent during the interview with a standard statement. After rating all health states, respondents were also given the opportunity to change their rating of prior health states. Health state descriptions and visual aids used to demonstrate the chances (p) were presented in a booklet during the interview (see appendix). At the end of the interview, interviewers rated the respondent’s comprehension of the standard gamble on a 4-point likert scale (1=very good, 4=limited). Standard gamble results considered invalid by the interviewer due to limited comprehension were excluded (N=3 adolescents, N=2 parents).
Various forms of bias may be introduced in the standard gamble interview process (12). To avoid anchoring bias, there were three starting points for the algorithm: a 25%, 50% or 75% chance of perfect health. To avoid an order effect, there were two orderings of the health states. Dyads were randomized to one of the 6 versions at the start of the interview. To avoid framing bias, health states were titled only with a letter rather than a medical description (e.g., Health State A) and both the positive and negative aspects of each gamble were presented (i.e., the chance of the child’s perfect health and of the child’s immediate painless death).
Utility for the adolescent’s current health over the prior 4 weeks was also self- and parent-proxy assessed using the interviewer-administered Health Utilities Index (HUI), a preference-based, generic measure of health-related quality of life (36). The HUI3 classification system, which includes the attributes of vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain, was then used to calculate the HUI3 multi-attribute utility score for the adolescent’s current health (36).
Analysis
Adolescent and parent utilities for the seven T2DM health states were measured by the standard gamble. Adolescent and parent utilities for the adolescent’s current health in the prior 4 weeks were measured with the standard gamble and the HUI3 multi-attribute utility score (36). Results are presented as medians with interquartile range. Comparison of the adolescent/parent dyad outcomes used the Wilcoxon signed-rank test. Differences in adolescent and parent preferences according to baseline characteristics were assessed using ANOVA, t-test, Pearson correlation or comparable non-parametric test, as appropriate. Spearman correlation coefficient compared adolescent and parent utilities for the adolescent’s current health assessed by standard gamble and HUI. There were 18 outcomes (i.e., 7 T2DM health states assessed by both adolescent and parent and the adolescent- and parent-reported assessments of the adolescent’s current health using both the standard gamble and HUI). Using a critical p value of ≤0.03, the expected number of type I errors is less than one-half.
BMI percentiles were based on the Centers for Disease Control and Prevention growth charts (37). BMI z-scores were calculated using EpiInfo™ version 3.3.2. SAS version 9.1 (Cary, NC) was used for analyses.
Results
Study Sample
Ten percent of the 69 parents completed the survey in Spanish, and all 70 adolescents completed the survey in English. Standard gamble and HUI analyses each included 66 adolescents and 65 parents. Consistent with the anticipated length of the full interviews, adolescents completed the interview in 33.7 ± 4.7 minutes and parents in 55.5 ± 14.7 minutes. Characteristics of the participants are summarized in Table 1. Almost half (48.6%) of the adolescents had a BMI>99th percentile. T2DM, prediabetes and insulin resistance were almost equally represented. For those with T2DM, 9.5% were treated with diet alone, 28.6% oral medication and diet, 9.5% insulin and diet, 38.1% oral medication, insulin and diet, 4.8% insulin alone, and 4.8% oral medication and insulin (4.8% unknown). Oral medication with or without diet was used among 19.1% of those with prediabetes and 14.3% of those with insulin resistance
Table 1.
Characteristic of Adolescent Subjects (N=70)
| Total N=70 |
Male N=23 |
Female N=47 |
|
|---|---|---|---|
| Age, yr | 15.5 ± 2.2 | 15.9 ± 2.0 | 15.3 ± 2.2 |
| Age at diagnosis, yr | 13.2 ± 2.6 | 13.4 ± 2.6 | 13.0 ± 2.6 |
| BMI z-score | 2.2 ± 0.6 | 2.4 ± 0.5 | 2.1 ± 0.6 |
| BMI Percentile | |||
| <95th | 13 (18.6) | 2 (8.7) | 11 (23.4) |
| 95th to 99th | 23 (32.9) | 7 (30.4) | 16 (34.0) |
| >99th | 34 (48.6) | 14 (60.9) | 20 (42.6) |
| Race/Ethnicity | |||
| Hispanic (any race) | 13 (18.6) | 3 (13.0) | 10 (21.3) |
| Non-Hispanic White | 28 (40.0) | 17 (73.9) | 11 (23.4) |
| Non-Hispanic Black | 20 (28.6) | 1 (4.4) | 19 (40.4) |
| Other/unknown | 9 (12.9) | 2 (8.7) | 7 (14.9) |
| Diagnosis | |||
| Type 2 Diabetes | 21 (30.0) | 5 (21.7) | 16 (34.0) |
| Prediabetes | 21 (30.0) | 6 (26.1) | 15 (31.9) |
| Insulin Resistance | 28 (40.0) | 12 (52.2) | 16 (34.0) |
| Family History of Diabetes | |||
| No/Unknown | 10 (14.3) | 6 (26.1) | 4 (8.5) |
| Yes | 60 (85.7) | 17 (73.9) | 43 (91.5) |
Data are mean ± standard deviation or N (%)
The parents (83% mothers) had an average age of 45.1±7.3 years and most had at least some college or technical school education (N=52, 75%). Parents had a mean BMI of 32.9±8.1 kg/m2, and almost a quarter had T2DM (N=16, 23%). On a 5-point Likert scale ranging from 1 (poor) to 5 (excellent), 29% (N=20) of parents rated their own current health as poor or fair and 13% (N=9) rated their health as excellent.
Adolescent vs. Parent Preferences for Type 2 Diabetes Health States
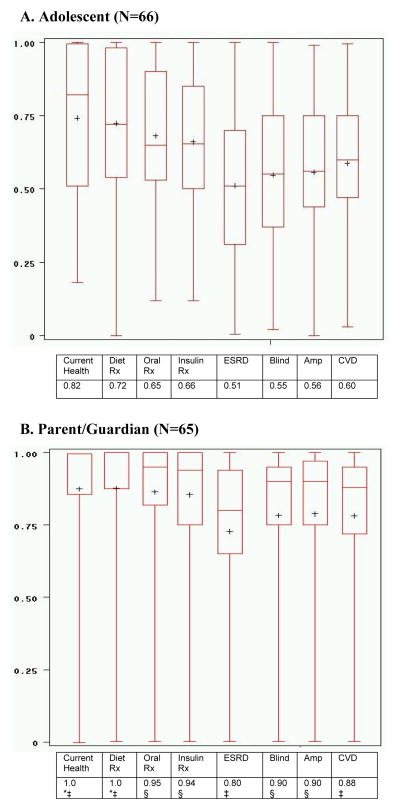
Adolescent and parent preferences for the seven T2DM health states and adolescent’s current health appear in Figure 1. Adolescents and parents rated T2DM with no complications treated with diet as the most desirable T2DM health state (median [IQR]; adolescent 0.72 [0.54, 0.98] vs. parent 1.0 [0.88, 1.0]) and end stage renal disease (ESRD) as the least desirable T2DM complication (adolescent 0.51 [0.31, 0.70] vs. parent 0.8 [0.65, 0.94]). Adolescents’ utilities were significantly lower (p≤0.001) than those of parents for all health states assessed. The range of utility scores was wide for both adolescents and parents although parent responses were skewed toward higher utilities, and adolescent utilities, particularly for complications, were near normally-distributed.
Figure 1. Type 2 Diabetes Health State Preferences by Standard Gamble: Adolescent vs. Parent/Guardian.
*N=64 due to missing data. Comparison of adolescent vs. parent/guardian ‡p≤0.001 §p<0.0001. For comparison within parent/adolescent dyads, 8 dyads (9 for current health) were excluded because either one or both of the standard gamble interviews were incomplete or considered invalid due to limited comprehension or improper administration. Diet Rx= type 2 diabetes with no complication and diet therapy; Oral Rx= type 2 diabetes with no complication and oral medication; Insulin Rx= type 2 diabetes with no complication and insulin; ESRD= type 2 diabetes with end stage renal disease; Amp= type 2 diabetes with amputation; Blind= type 2 diabetes with blindness; CVD= type 2 diabetes and heart disease. All complication health states assume treatment with insulin.
Adolescent and parent utilities for T2DM health states did not differ significantly by child’s age, race/ethnicity, or parent’s diagnosis of T2DM in bivariate analyses. However, adolescents with any family history of diabetes reported a significantly higher median utility for oral medication (0.70 [0.56, 0.91] vs. 0.50 [0.42, 0.56], p=0.001) and for insulin treatment (0.72 [0.54, 0.88] vs. 0.50 [0.45, 0.6], p=0.007) compared to those without a family history. Similarly, adolescents with parents who had at least some college/technical school education reported a significantly higher median utility for insulin (0.72 [0.60, 0.88] vs. 0.50 [0.24, 0.60], p=0.003) and for oral medication (0.72 [0.60, 0.91] vs. 0.54 [0.49, 0.62], p=0.005). Treatment with insulin or oral medication was not associated with a significant difference in the utility for insulin or oral medication, respectively, among either the adolescents or the parents. Increasing adolescent BMI (as z-score) was associated with higher adolescent utilities for blindness (p=0.03) and heart disease (p=0.02). After adjustment for the child’s diagnosis, adolescent BMI z-score was also associated with higher adolescent utility for amputation (p=0.03), and the other relationships with T2DM complications remained significant. While adolescent utilities and most parent utilities did not differ significantly by child’s diagnosis, we did find that parents’ utility for blindness was lower among the parents of adolescents with T2DM [T2DM mean (SD) 0.63 (0.52, 0.75) vs. prediabetes 0.84 (0.72, 0.95) vs. insulin resistance 0.84 (0.75, 0.94), p=0.02]. While parent utilities were not associated with the adolescent’s BMI, adjustment for adolescent’s BMI z-score strengthened the significance of the relationship between the adolescents’ diagnosis and the parents’ utility for blindness [T2DM 0.61 (0.49, 0.73) vs. prediabetes 0.85 (0.73, 0.96) vs. insulin resistance 0.85 (0.76, 0.94), p=0.006]. A similar relationship was observed with the parent utilities for ESRD, heart disease, and amputation although none achieved a p-value of 0.03. Parents’ preferences were not significantly different based on family history of diabetes, their educational status, or their self-rated health status.
Adolescent and Parent Utilities for Child’s Current Health
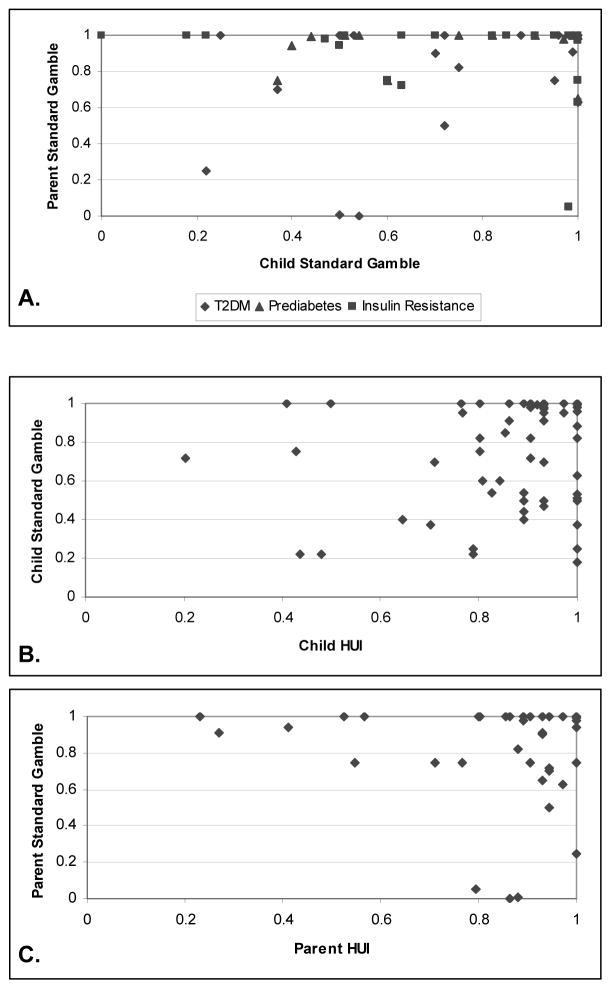
Adolescent- and parent-reported utility assessments for the child’s current health are compared in Figure 2. The adolescent’s self-assessed utility for current health was not correlated with the parent-proxy utility for the adolescent’s current health using the standard gamble (Figure 2A) and medians were significantly different (adolescent 0.82 [0.51, 1.0] vs. parent 1.0 [0.86, 1.0], p=0.001) (Figure 1). Among the adolescents, there was no correlation between their assessment of their current health based on the standard gamble and the HUI (Figure 2B). Similarly, there was no correlation between the parent-proxy assessments of the adolescent’s current health using these two methods (Figure 2C).
Figure 2. Evaluation of Adolescent’s Current Health.
A. Parent/Guardian Standard Gamble vs. Child Standard Gamble
N=61 dyads, r=0.22, p=0.09. Missing dyads due to incomplete or invalid standard gamble interviews (N=9). Symbols indicate child’s diagnosis in Figure 2A only.
B. Child Standard Gamble vs. Child Health Utilities Index
N=63, r=0.09, p=0.49. Missing points due to missing HUI data (N=3) or invalid standard gamble interviews (N=4).
C. Parent/Guardian Standard Gamble vs. Parent Health Utilities Index
N=60, r=0.23, p=0.08. Missing points due to missing HUI data (N=3), incomplete or invalid standard gamble interviews (N=5), or both (N=1).
Although the adolescents’ utility for their current health based on the standard gamble was not statistically different based on their diagnosis (p=0.23), ordering of utilities increased in the expected direction with the lowest utility for current health among adolescents with T2DM and the highest among those with insulin resistance (T2DM 0.72 [0.50, 0.96] vs. prediabetes 0.82 [0.51, 1.0] vs. insulin resistance 0.95 [0.63, 1.0]). A similar trend was observed for the parent-reported utility for their adolescent’s current health with the standard gamble.
Discussion
As the prevalence of T2DM among youth increases, a growing population of adolescents and young adults will be utilizing T2DM treatments and managing its chronic complications. In this study, we sought to describe how adolescents with and at risk of T2DM and their parents value these health states associated with T2DM. Using the standard gamble, we have derived adolescent utilities for T2DM health states that can be used in decision analysis models and economic analyses that incorporate health-related quality of life (15). Understanding how adolescents and their parents value treatments and future health risks related to T2DM can also facilitate shared decision-making (16). Standard gamble interviews have been performed with adolescents with a number of chronic health conditions (26–30, 38) but, to our knowledge, this is the first study to target youth with or at risk for T2DM.
On average, adolescents perceived T2DM complications and treatments to have significant negative impact on health-related quality of life. However, utilities for each health state were also quite heterogeneous. In the standard gamble, some adolescents were willing to accept high risks of death to avoid even life with T2DM free of complications and treated only with oral medication. These responses may in part reflect adolescent perspectives about mortality and attitudes toward risk (23, 39). In the National Longitudinal Study of Adolescent Health (Add Health), for example, 14.7% of adolescents reported at least a 50/50 chance that they would not live to age 35, and perception of a high risk for early death was associated with high risk behaviors and outcomes such as drug use and suicide (40).
Adolescents in this study rated all assessed chronic sequelae of diabetes significantly worse than their parents rated them. Based on median utilities, adolescents also ranked diabetes complications differently than their parents except that both identified ESRD as the least desirable T2DM complication. Few studies have compared child and parent-proxy utilities for the same health states, and the findings are heterogeneous (41). However, some using the standard gamble have demonstrated child-parent differences (28, 38). Adolescents capable of understanding the standard gamble task may provide a unique personal perspective, particularly with regard to the social and emotional impacts of health states, that may not be captured by a proxy-assessment (23, 25). Parent-proxy valuations may also be affected by the experiences of the parent (25). These issues underscore some of the potential problems with using parent-proxy preferences to guide medical decision-making for children and adolescents.
Among the most worrisome associations that we observed was the positive association between increasing adolescent BMI and positive perception of diabetes complications, suggesting that those with increasing risk of such complications may either not acknowledge this risk (i.e., denial), may be willing to accept this risk, or possibly perceive such outcomes to be unavoidable. The latter may make these adolescents less willing to take risks in the standard gamble. Prior work among adolescents with T2DM supports the presence of denial with respect to diabetes complications among some of these youth (42). Additional research from Add Health has also demonstrated that approximately one-third of obese adolescents were not perceived to be obese by either themselves or their parent (43). Given the heterogeneity in preferences we observed for the T2DM health states, especially for complications, these findings suggest that traditional prevention messages may not be uniformly successful in this population, and management discussions with every family likely need to be tailored to their individual needs and circumstances.
While we did not find differences based on race/ethnicity, the family context was found to influence adolescent perceptions of T2DM treatments. Specifically, a family history of diabetes was associated with higher utilities (i.e., more desirable perception) for medications to treat T2DM among adolescents. Given that children and adolescents with and at risk of T2DM are likely to come from families also challenged by obesity and T2DM (44, 45), it is not surprising that family history influences perceptions of care. Those with a family history may be willing to accept more aggressive treatments because of familiarity with and desire to avoid diabetes complications or these youth may view having diabetes as inevitable and their preference for more aggressive therapy may reflect their familiarity with treatments. Other studies have demonstrated higher utilities for health states among those familiar with the health state (20). Although our data do not allow us to differentiate between these explanations, focus groups with adolescents with T2DM have highlighted that when family members with diabetes have demonstrated poor self-management leading to complications, it has prompted adolescents to have greater focus on self-care behaviors (42). Rothman et al. have also shown that family history of diabetes was not associated with poor glycemic control in adolescents with T2DM (6). In contrast, family history of diabetes was not associated with parent-proxy preferences for adolescent health states in our study. In focus groups with parents of adolescents with T2DM, Mulvaney et al. found that family history of diabetes was also not clearly viewed as positive or negative with respect to its impact on adolescent diabetes self-care behaviors (46). However, in our sample, parent but not adolescent utilities for T2DM complications were influenced by the child’s diagnosis. Specifically, parents of adolescents with T2DM had significantly lower utility (i.e., less desirable perception) for blindness than parents of adolescents with either prediabetes or insulin resistance. As only 30% of the adolescents in our sample already had T2DM, these relationships warrant further exploration.
Differences observed between the standard gamble and HUI utilities for the adolescent’s current health further underscore the importance of understanding the source of utilities and their potential biases. The adolescent’s self-assessed utility for current health using the standard gamble and HUI were not correlated nor were the parent-proxy assessed utilities for the adolescent’s current health using the standard gamble and HUI. Discordant results between the standard gamble and the HUI among youth have been previously observed (26, 28, 47) and support the suggestion that there may be differences in the information or perspectives captured by these two methods (25). The more highly skewed results of the parent standard gamble utilities suggest that parents’ risk aversion (23), i.e., their unwillingness to risk their child’s death, may limit the usefulness of parent-proxy assessments of child utilities using the standard gamble when parents are required to risk the child’s death. The standard gamble valuations can also be influenced by the parent’s and the child’s valuation of death, illness and perfect health (29). While there is evidence to support the reliability of standard gamble measurements (27, 30), the stability of preferences over time remains a question warranting further study (14). In contrast, the HUI utility is a generic instrument based on answers to more objective questions describing domains of vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain (36). As parents are only able to report on what they observe, however, this may miss certain dimensions of health relevant to the child or limit the range of pediatric valuations of subjective aspects of the health experience (23, 25). The HUI utilities are also calculated based on the preferences reported by an adult referent population (36) that may not be representative of the population studied (23). Therefore, both methods have potential caveats.
In addition to the implications for clinical practice, our findings also have implications for policy and public health as we have demonstrated that adolescent preferences for T2DM health states differ from those of adults with diabetes (17, 20). Among adults with T2DM and mean age of 63 years, for example, Huang et al. found median utility scores of 0.25 for ESRD, 0.35 for blindness, 0.55 for lower extremity amputation, and 0.75 for angina (20). As health preferences may change over the life course (22, 23), using the preferences of adults with T2DM may not be appropriate for economic evaluations of treatment options during childhood. Further, as direct valuation of health states related to T2DM is feasible for adolescents, use of the standard gamble (or comparable method of direct preference elicitation) may be necessary to capture the breadth of the adolescent perspective.
Several limitations warrant comment. First, this was a convenience sample from two institutions in the Boston area including both adolescents at risk of T2DM in addition to those with T2DM. Therefore, the generalizability of our findings may be limited. Second, for some, the standard gamble may be cognitively challenging (23, 48, 49). However, we facilitated the interview by having a practice health state unrelated to diabetes as well as logic checks at the start of the interview, allowing the respondents to revise their earlier answers at the end of the survey, and providing survey guides to assist with explanation of the percentages. Further, fewer than 5% of standard gamble interviews were eliminated due to perceived comprehension difficulties. In addition, our parent sample, with 75% of adults having at least some college or technical school, and our adolescent sample, all functioning at a minimum of the 6th grade level, should not be more challenged than other adolescent (27) and adult (10) populations in which this methodology has been used. Our observed ranking of utilities for current health in the expected direction according to the adolescent’s diagnosis and the finding that health states with diabetes complications were viewed as less desirable than those without complications supports the face and construct validity of the standard gamble in this population.
The epidemic of childhood obesity and the earlier onset of T2DM in youth make understanding youth perceptions of disease treatment and outcomes critically important if management of young people with T2DM is to be successful. We found significant differences between the values of adolescents and parents for health states associated with T2DM as well as considerable heterogeneity in both groups. Family-based programs striving for a model of shared decision-making must be sufficiently flexible to communicate about the treatments and health risks related to T2DM and to then address the variable and potentially conflicting perspectives of adolescents and their parents.
Supplementary Material
Acknowledgments
The study was supported by the Centers for Disease Control and Prevention grant K01DP000089 (Rhodes, Ludwig, Prosser). Additional support to investigators included the Katherine Adler Astrove Youth Education Fund (Laffel); Maria Griffin Drury Fund (Laffel); National Institute of Diabetes and Digestive and Kidney Diseases grant K24DK082730 (Ludwig), and the New Balance Foundation (Rhodes). This work was published, in part, at the American Diabetes Association 68th Annual Scientific Sessions, June 6-10, 2008, San Francisco, CA. We would like to thank our research assistants Tessa Gonzalez AB, Kaitlin Rawluk BA, and Roula Zoghbi MPH for their work on this project; the Clinical Research Program at Children’s Hospital Boston for project management, database development and statistical support; and members of our Data and Safety Monitoring Board for their careful review and feedback.
Abbreviations
- T2DM
type 2 diabetes
- BMI
Body Mass Index
- IR
insulin resistance
- SG
standard gamble
- QALY
quality-adjusted life year
- HUI
Health Utilities Index
- IQR
interquartile range
- ESRD
end stage renal disease
Footnotes
Disclosure
Dr. Rhodes was formerly Chief Medical Officer for Pediatric Weight Management Centers, LLC’s Great Moves! Program, a company privately owned and operated in collaboration with the physicians of Children’s Hospital Boston. Dr. Rhodes provided contracted clinical and administrative services for the company but neither had nor has equity or other economic interest in the business. Dr. Rhodes also received salary support from an unrestricted, philanthropic grant from the New Balance Foundation. Dr. Laffel is on the Speaker’s Bureau for Sanofi-Aventis, Novo-Nordisk, Lilly, and Johnson&Johnson. She is also a consultant for Roche, Abbott, and Astra Zeneca. The remaining authors have no disclosures.
References
- 1.Mayer-Davis EJ, Bell RA, Dabelea D, D’Agostino R, Jr, Imperatore G, Lawrence JM, et al. The many faces of diabetes in American youth: type 1 and type 2 diabetes in five race and ethnic populations: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32 (Suppl 2):S99–101. doi: 10.2337/dc09-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. Jama. 2010;303(3):242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29(11):2427–32. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 4.Williams DE, Cadwell BL, Cheng YJ, Cowie CC, Gregg EW, Geiss LS, et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics. 2005;116(5):1122–6. doi: 10.1542/peds.2004-2001. [DOI] [PubMed] [Google Scholar]
- 5.Gahagan S, Silverstein J. Prevention and treatment of type 2 diabetes mellitus in children, with special emphasis on American Indian and Alaska Native children. American Academy of Pediatrics Committee on Native American Child Health. Pediatrics. 2003;112(4):e328. doi: 10.1542/peds.112.4.e328. [DOI] [PubMed] [Google Scholar]
- 6.Rothman RL, Mulvaney S, Elasy TA, VanderWoude A, Gebretsadik T, Shintani A, et al. Self-management behaviors, racial disparities, and glycemic control among adolescents with type 2 diabetes. Pediatrics. 2008;121(4):e912–9. doi: 10.1542/peds.2007-1484. [DOI] [PubMed] [Google Scholar]
- 7.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auslander WF, Thompson S, Dreitzer D, White NH, Santiago JV. Disparity in glycemic control and adherence between African-American and Caucasian youths with diabetes. Family and community contexts. Diabetes Care. 1997;20(10):1569–75. doi: 10.2337/diacare.20.10.1569. [DOI] [PubMed] [Google Scholar]
- 9.Petitti DB, Klingensmith GJ, Bell RA, Andrews JS, Dabelea D, Imperatore G, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155(5):668–72. e1–3. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll AE, Downs SM. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr. 2009;155(1):21–5. 25 e1–5. doi: 10.1016/j.jpeds.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Petrou S, Kupek E. Estimating preference-based health utilities index mark 3 utility scores for childhood conditions in England and Scotland. Med Decis Making. 2009;29(3):291–303. doi: 10.1177/0272989X08327398. [DOI] [PubMed] [Google Scholar]
- 12.Lenert L, Kaplan RM. Validity and interpretation of preference-based measures of health-related quality of life. Med Care. 2000;38(9 Suppl):II138–50. doi: 10.1097/00005650-200009002-00021. [DOI] [PubMed] [Google Scholar]
- 13.Cameron FJ. The impact of diabetes on health-related quality of life in children and adolescents. Pediatr Diabetes. 2003;4(3):132–6. doi: 10.1034/j.1399-5448.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 14.Bennet KJ, Torrance GW. Measuring Health State Preferences and Utilities: Rating Scale, Time Trade-Off, and Standard Gamble Techniques. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 15.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. Jama. 1996;276(14):1172–7. [PubMed] [Google Scholar]
- 16.Britto MT, DeVellis RF, Hornung RW, DeFriese GH, Atherton HD, Slap GB. Health care preferences and priorities of adolescents with chronic illnesses. Pediatrics. 2004;114(5):1272–80. doi: 10.1542/peds.2003-1134-L. [DOI] [PubMed] [Google Scholar]
- 17.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–43. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 18.Maddigan SL, Majumdar SR, Toth EL, Feeny DH, Johnson JA. Health-related quality of life deficits associated with varying degrees of disease severity in type 2 diabetes. Health Qual Life Outcomes. 2003;1(1):78. doi: 10.1186/1477-7525-1-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37) U.K Prospective Diabetes Study Group. Diabetes Care. 1999;22(7):1125–36. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 20.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478–83. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang ES, Shook M, Jin L, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new-onset diabetes. Diabetes Care. 2006;29(2):259–64. doi: 10.2337/diacare.29.02.06.dc05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenney ME, Campbell S. Measuring quality of life. Arch Dis Child. 1997;77(4):347–50. doi: 10.1136/adc.77.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prosser LA, Hammitt JK, Keren R. Measuring health preferences for use in cost-utility and cost-benefit analyses of interventions in children: theoretical and methodological considerations. Pharmacoeconomics. 2007;25(9):713–26. doi: 10.2165/00019053-200725090-00001. [DOI] [PubMed] [Google Scholar]
- 24.Eiser C. Children’s quality of life measures. Arch Dis Child. 1997;77(4):350–4. doi: 10.1136/adc.77.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrou S. Methodological issues raised by preference-based approaches to measuring the health status of children. Health Econ. 2003;12(8):697–702. doi: 10.1002/hec.775. [DOI] [PubMed] [Google Scholar]
- 26.Yi MS, Britto MT, Wilmott RW, Kotagal UR, Eckman MH, Nielson DW, et al. Health values of adolescents with cystic fibrosis. J Pediatr. 2003;142(2):133–40. doi: 10.1067/mpd.2003.51. [DOI] [PubMed] [Google Scholar]
- 27.Juniper EF, Guyatt GH, Feeny DH, Griffith LE, Ferrie PJ. Minimum skills required by children to complete health-related quality of life instruments for asthma: comparison of measurement properties. Eur Respir J. 1997;10(10):2285–94. doi: 10.1183/09031936.97.10102285. [DOI] [PubMed] [Google Scholar]
- 28.Brunner HI, Maker D, Grundland B, Young NL, Blanchette V, Stain AM, et al. Preference-based measurement of health-related quality of life (HRQL) in children with chronic musculoskeletal disorders (MSKDs) Med Decis Making. 2003;23(4):314–22. doi: 10.1177/0272989X03256008. [DOI] [PubMed] [Google Scholar]
- 29.Sung L, Young NL, Greenberg ML, McLimont M, Samanta T, Wong J, et al. Health-related quality of life (HRQL) scores reported from parents and their children with chronic illness differed depending on utility elicitation method. J Clin Epidemiol. 2004;57(11):1161–6. doi: 10.1016/j.jclinepi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman J, Aday LA, Begley CE, Ahn C, Lairson DR. Measuring health state preferences for hemophilia: development of a disease-specific utility instrument. Haemophilia. 2005;11(1):49–57. doi: 10.1111/j.1365-2516.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- 31.Yi MS, Britto MT, Sherman SN, Moyer MS, Cotton S, Kotagal UR, et al. Health values in adolescents with or without inflammatory bowel disease. J Pediatr. 2009;154(4):527–34. doi: 10.1016/j.jpeds.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association. Standards of Medical Care in Diabetes-2006. Diabetes Care. 2006;29(Supp 1):S4–S42. [PubMed] [Google Scholar]
- 33.Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137(9):959–65. doi: 10.1093/oxfordjournals.aje.a116768. [DOI] [PubMed] [Google Scholar]
- 34.Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23(2):171–5. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]
- 35.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003;88(6):2399–403. doi: 10.1210/jc.2003-030087. [DOI] [PubMed] [Google Scholar]
- 36.Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40(2):113–28. doi: 10.1097/00005650-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 38.Saigal S, Stoskopf BL, Feeny D, Furlong W, Burrows E, Rosenbaum PL, et al. Differences in preferences for neonatal outcomes among health care professionals, parents, and adolescents. Jama. 1999;281(21):1991–7. doi: 10.1001/jama.281.21.1991. [DOI] [PubMed] [Google Scholar]
- 39.Millstein SG, Halpern-Felsher BL. Perceptions of risk and vulnerability. J Adolesc Health. 2002;31(1 Suppl):10–27. doi: 10.1016/s1054-139x(02)00412-3. [DOI] [PubMed] [Google Scholar]
- 40.Borowsky IW, Ireland M, Resnick MD. Health status and behavioral outcomes for youth who anticipate a high likelihood of early death. Pediatrics. 2009;124(1):e81–8. doi: 10.1542/peds.2008-3425. [DOI] [PubMed] [Google Scholar]
- 41.Tarride JE, Burke N, Bischof M, Hopkins RB, Goeree L, Campbell K, et al. A review of health utilities across conditions common in paediatric and adult populations. Health Qual Life Outcomes. 2010;8:12. doi: 10.1186/1477-7525-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulvaney SA, Mudasiru E, Schlundt DG, Baughman CL, Fleming M, VanderWoude A, et al. Self-management in type 2 diabetes: the adolescent perspective. Diabetes Educ. 2008;34(4):674–82. doi: 10.1177/0145721708320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106(1 Pt 1):52–8. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 44.Pinhas-Hamiel O, Standiford D, Hamiel D, Dolan LM, Cohen R, Zeitler PS. The type 2 family: a setting for development and treatment of adolescent type 2 diabetes mellitus. Arch Pediatr Adolesc Med. 1999;153(10):1063–7. doi: 10.1001/archpedi.153.10.1063. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman FR, Hirst K, Linder B, Baranowski T, Cooper DM, Foster GD, et al. Risk factors for type 2 diabetes in a sixth- grade multiracial cohort: the HEALTHY study. Diabetes Care. 2009;32(5):953–5. doi: 10.2337/dc08-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulvaney SA, Schlundt DG, Mudasiru E, Fleming M, Vander Woude AM, Russell WE, et al. Parent perceptions of caring for adolescents with type 2 diabetes. Diabetes Care. 2006;29(5):993–7. doi: 10.2337/diacare.295993. [DOI] [PubMed] [Google Scholar]
- 47.Feeny D, Furlong W, Saigal S, Sun J. Comparing directly measured standard gamble scores to HUI2 and HUI3 utility scores: group- and individual-level comparisons. Soc Sci Med. 2004;58(4):799–809. doi: 10.1016/s0277-9536(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 48.Dolan P, Kind P. Inconsistency and health state valuations. Soc Sci Med. 1996;42(4):609–15. doi: 10.1016/0277-9536(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 49.Woloshin S, Schwartz LM, Moncur M, Gabriel S, Tosteson AN. Assessing values for health: numeracy matters. Med Decis Making. 2001;21(5):382–90. doi: 10.1177/0272989X0102100505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.