Summary
This protocol describes a recently developed strategy to generate 3D prostate organoid cultures from healthy mouse and human prostate (either bulk or FAC-sorted single luminal and basal cells), metastatic prostate cancer lesions and circulating tumour cells. Organoids derived from healthy material contain the differentiated luminal and basal cell types, whereas organoids derived from prostate cancer tissue mimic the histology of the tumour. The stepwise establishment of these cultures and the fully defined serum-free conditioned medium that is required to sustain organoid growth are outlined. Organoids established using this protocol can be used to study many different aspects of prostate biology, including homeostasis, tumorigenesis and drug discovery.
INTRODUCTION
The organoid technology holds great promise to study tissue homeostasis and cancer, but also for regenerative and personalized medicine1. Since the establishment of culture conditions for mouse small intestine2, similar culture protocols have been described for human small intestine and mouse and human colon, stomach, pancreas and liver3–9. These organoids can grow “indefinitely”, remain phenotypically and genetically stable and can be genetically modified using multiple genome editing systems10–13.
Development of the protocol to culture mouse and human prostate organoids
The prostate is a gland of the male reproductive system that produces seminal fluid. The pseudostratified epithelium present in the prostate consists out of luminal, basal and rare neuroendocrine cells. Androgen receptor (AR) signalling is essential for prostate development and homeostasis, as well as for prostate cancer initiation and progression. As generally acknowledged14,15, prostate (cancer) research has been hampered by the lack of suitable in vitro model systems. Although powerful in vivo models are available for prostate research, these are often expensive, time consuming, and technically challenging. Most in vitro research is performed using cell lines derived from neoplastic lesions and most of these do not have an intact AR signalling pathway, making them poor representatives of healthy prostate and prostate cancer tissue. The recent development of a testosterone-responsive prostate organoid culture system derived from primary prostate and advanced prostate cancer tissue16,17 opens new opportunities to study prostate homeostasis and cancer. This model was established by adapting and optimizing the culture conditions previously used to establish mouse and human small intestine and colon organoid cultures2,3. Based on literature, we added different compounds and growth factors to the “generic” organoid medium (containing epidermal growth factor (EGF), Noggin and R-spondin 1; ref. 2) enabling us to establish culture conditions supporting long-term growth of mouse and human prostate tissue and advanced prostate cancers. Using this culture system, we have shown that 1) both the luminal and basal lineages harbour multipotent progenitor cells and can be propagated for long-term, 2) organoids functionally recapitulate AR signalling, 3) organoids derived from prostate cancer mouse models recapitulate mouse phenotypes, 4) human prostate cancer-derived organoids genetically and phenotypically mimic the tumour where they were derived from16,17.
Comparison with other methods
Several other groups have demonstrated the in vitro growth of primary prostatic tissue. However, in contrast to our prostate organoid cultures, most of these models only support short-term growth, mainly support growth of basal cells, and do not allow for full luminal differentiation (making androgen responsiveness limited in these cultures)18–21. Moreover, these methods do not allow efficient growth of prostate cancer tissue. A protocol developed by Liu and colleagues enables indefinite growth of reprogrammed prostatic epithelial cells22. Interestingly, the Rho kinase inhibitor Y-27632 and the presence of feeder cells are essential in this system, possibly providing factors that are present in our defined prostate culture medium. Cells cultured under these conditions do not closely resemble the in vivo prostate, and androgen responsiveness is limited in this system. Finally, Chua et al. recently demonstrated a culture system that exclusively allows the growth of organoids from single luminal cells23, albeit at lower plating efficiency than reported with our method (0.2 – 0.3% described by Chua et al. versus 1 – 2% when using our method16). Under these conditions, organoids that resemble the in vivo prostate are formed. However, basal cell-derived organoids cannot be propagated for a prolonged time. Additionally, in contrast to our method, their medium is not fully defined. Possibly, the medium’s undefined additions (e.g. fetal calf serum) contain growth factors present in our defined medium. It has not yet been explored whether prostate cancer can be propagated under these conditions.
MATERIALS
Reagents
Collagenase Type II (Life Technologies, cat. no. 17101-015)
TrypLE Express (Life Technologies, cat. no. 12605-010)
Dulbeccos Modified Eagle Medium (DMEM; Life Technologies, cat. no. 31966)
Advanced DMEM/F12 (adDMEM/F12; Life Technologies, cat. no. 12634-034)
GlutaMAX 100× (Life Technologies, cat. no. 35050-068)
Penicillin-streptomycin (Life Technologies, cat. no. 15140-122)
Hepes (Life Technologies, cat. no. 15630-056)
Zeocin (Life Technologies, cat. no. R250-01)
Phosphate buffered saline
Matrigel, Growth Factor Reduced (GFR), Phenol Red-free (BD, cat. no. 356231)
B27 supplement 50× (Life Technologies, cat. no. 17504-044)
Nicotinamide (Sigma-Aldrich, cat. no. N0636)
N-acetylcysteine (Sigma-Aldrich, cat. no. A9165)
A83-01 (Tocris Bioscience, cat. no. 2939)
Y-27632 (Abmole Bioscience, cat. no. M1817)
Human FGF-10 (PeproTech, cat. no. 100-26)
Human FGF-2 (PeproTech, cat. no. 100-18B)
Human EGF (PeproTech, cat. no. AF-100-15)
Recombinant human Noggin (Peprotech, cat. no. 120-10C)
R-spondin 1-conditioned medium; home made from the 293T-HA-RspoI-Fc cell line24 (derived from Calvin Kuo lab), or recombinant R-spondin 1 protein (R&D Systems, cat. no. 4645-RS-025)
Prostaglandin E2 (Tocris Bioscience, cat. no. 2296)
SB202190 (Sigma-Aldrich, cat. no. S7076)
(DiHydro)Testosterone (5α-Androstan-17β-ol-3-one) (Sigma-Aldrich, cat. no. A8380)
Fetal bovine serum (Sigma-Aldrich, cat. no. F7524)
Deoxyribonuclease I (DNase I) from bovine pancreas (Sigma-Aldrich, cat. no. D5025)
DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride; Life Technologies, cat. no. D1306)
RosetteSep® Human CD45 Depletion Cocktail (Stem Cell Technologies, cat. no. 15122)
Ficoll-Paque™ PLUS (GE Healthcare Life Sciences, cat. no. 17-1440-02)
Recovery Cell Culture Freezing medium (Life Technologies, cat. no. 12648-010)
RNeasy mini kit (Qiagen, cat. no. 74104)
Reliaprep gDNA tissue miniprep system (Promega, cat. no. A2052)
GoScript Reverse Transcriptase (Promega, cat. no. A5003)
Oligo(dT) 15 Primer (Promega, cat. no. C1101)
Rec. RNasin RNase Inhibitor (Promega, cat. no. N2511)
Mouse and human material
Whole mouse prostate
Human prostate tissue piece (minimum size 1 mm3)
Human metastasis biopsy (minimum size 1 mm3)
Blood sample from patient with advanced prostate cancer (8 ml)
CRITICAL STEP Although it is preferred to use fresh material, we have been able to establish organoids from tissue that was stored overnight at 4°C in adDMEM/F12 (containing penicillin/streptomycin, 10 mM Hepes and GlutaMAX 100× diluted).
Antibodies
CD26-FITC conjugated antibody (anti-human 1:200, M-A261, eBioscience)
CD49f-alexa 647 conjugated antibody (anti-human/mouse 1:200, GoH3, BD Biosciences)
Cd49f-PE conjugated antibody (anti-human/mouse 1:200, GoH3, BD Biosciences)
Cd24-alexa 647 conjugated antibody (anti-mouse 1:200, 30-F1, eBioscience)
EQUIPMENT
Falcon tubes 15 ml
Falcon tubes 50 ml
5 ml polystyrene round-bottom tube with cell-strainer caps (Falcon)
Microcentrifuge tubes, 1.5 ml
37°C shaking platform
Plates 6-well (Greiner Bio-One, cat. no. 657 160)
Plates 12-well (Greiner Bio-One, cat. no. 665 180)
Plates 24-well (Greiner Bio-One, cat. no. 662 160)
Plates 48-well (Greiner Bio-One, cat. no. 677 180)
Cell culture dishes 100 × 20 mm (Greiner Bio-One, cat. no. 664 160)
Glasstic Slide with hemocytometer counting grid (Kova International, cat. no. 87144E)
Glass pasteur pipettes (VWR, cat. no. 612-1701)
Light microscope (Nikon, Eclipse TS100)
Dissection microscope (Leica, MZ75)
Dissection tools (NeoLab)
FACS (DaKo MoFlo)
Disposable scalpels (Swann-Morton, code 0501)
Centrifuge (Eppendorf, 5810R)
Centrifuge (Eppendorf, 5424)
CO2 incubator
Biosafety cabinet
CoolCell (BioCision)
Stericup-GP, 0.22 µm, polyethersulfone, 500 mL, radio-sterilized (Millipore, cat. no. SCGPU05RE)
REAGENT SETUP
Mouse prostate culture medium
Add 1.0 ml B27, 125.0 µl N-acetylcysteine (500 mM in PBS), 5.0 µl of EGF (0.5 mg/ml in PBS + 0.1% BSA), 2.0 µl A83-01 (5 mM in DMSO), 50.0 µl Noggin (100 µg/ml in PBS + 0.1% BSA), 50.0 µl R-spondin 1 (500 µg/ml in PBS + 0.1% BSA or 10% conditioned medium), 50.0 µl dihydrotestosterone (1 µM in ethanol) and top up to 50 ml with adDMEM/F12 (containing penicillin/streptomycin, 10 mM Hepes and GlutaMAX 100× diluted). After passaging, Y-27632 is added to the culture medium (e.g. add 5.0 µl of 100 mM to 50 ml mouse prostate culture medium).
Human prostate culture medium
Add 1.0 ml B27, 500 µl nicotinamide (1 M in PBS), 125.0 µl N-acetylcysteine (500 mM in PBS), 0.5 µl of EGF (0.5 mg/ml in PBS + 0.1% BSA), 5.0 µl A83-01 (5 mM in DMSO), 50.0 µl Noggin (100 µg/ml in PBS + 0.1% BSA), 50.0 µl R-spondin 1 (500 µg/ml in PBS + 0.1% BSA or 10% conditioned medium), 50.0 µl dihydrotestosterone (1 µM in ethanol), 5.0 µl FGF2 (50 µg/ml in PBS + 0.1% BSA), 5.0 µl FGF10 (0.1 mg/ml in PBS + 0.1% BSA), 5.0 µl prostaglandin E2 (10 mM in DMSO), 16.7 µl SB202190 (30 mM in DMSO) and top up to 50 ml with adDMEM/F12 (containing penicillin/streptomycin, 10 mM Hepes and GlutaMAX 100× diluted). After passaging, Y-27632 is added to the culture medium (e.g. add 5.0 µl of 100 mM to 50 ml human prostate culture medium).
CRITICAL STEP The culture media should not be stored for longer than 2 weeks at 4°C.
CRITICAL STEP We have never seen any difference in organoid establishment, maintenance and morphology when using prostate culture medium containing either R-spondin 1-conditioned medium or recombinant R-spondin 1.
Preparation and storage of growth factor stocks
| R-spondin 1 medium | See Box 1 |
| N-Acetylcysteine | Dissolve 81.5 mg per ml H2O to prepare a 400× 500mM stock solution. Store at −20°C. |
| FGF-10 | Dissolve 500 µg in 5 ml PBS + 0.1% BSA to prepare a 10000× 0.1 mg/ml stock solution. Store at −20°C. |
| Nicotinamide | Dissolve 1.2 g in 10 ml PBS to prepare a 100× 1M stock solution. Store at −20°C. |
| Human EGF | Dissolve 1 mg in 2 ml PBS + 0.1% BSA to prepare a 10.000× 0.5mg/ml stock solution. Store at −20°C. |
| Rec human Noggin | Dissolve 100 µg in 1 ml of adDMEM/F12 (containing penicillin/streptomycin, 10 mM Hepes and GlutaMAX (adDMEM/F12 +/+/+)) to prepare a 1000× stock solution. Store at 4°C. |
| Y-27632 | Dissolve 50 mg in 1.5 ml H2O to prepare a 10.000× 100mM stock solution. Store at −20°C. |
| SB202190 | Dissolve 25 mg in 2.75 ml DMSO to prepare a 30mM 3000× stock solution. Store at −20°C. |
| A83-01 | Dissolve 10 mg in 950 µl DMSO to get a 25mM 50000× stock solution. Store at −20°C. |
| B27 | Provided as 50× stock solution. Store at −20°C. |
| FGF2 | Dissolve 50 µg in 100 µl 5 mM Tris pH 7.6 (0.5 mg/ml). Dilute to a 10000× 50 µg/ml stock solution by adding 900 µl of PBS + 0.1% BSA to the 100 µl 0.5 mg/ml solution. Store at −20°C. |
| PGE2 | Dissolve 10 mg in 2.84 ml DMSO to prepare a 10000× 10mM stock solution. Store at −20°C. |
| DHT | Dissolve 1 mg in 3.44 ml 100% ethanol to obtain a 1 mM solution. Dilute 1000× in 100% ethanol to prepare a 1000× 1 µM stock solution. Store at −20°C. |
BOX 1. Preparation of R-spondin 1-conditioned medium.
Culture 293T-HA-RspoI-Fc cell line24 in DMEM + 10% FBS + penicillin/streptomycin + zeocin (300 µg/ml) in 175 cm2 flasks until confluency.
Passage confluent flask (split ratio ~ 1:6) and grow cells in DMEM + 10% FBS + penicillin/streptomycin, without zeocin.
When cells reach confluency (after 3 – 4 days), replace DMEM + 10% FBS + penicillin/streptomycin medium with adDMEM/F12 +/+/+.
After one week, harvest the medium in 50 ml Falcon tubes.
Centrifuge at 1500 rpm for 5 min.
-
Pass the medium through a Stericup-GP, 0.22 µm filter.
CRITICAL STEP Step 5 and 6 are included to remove any 293T cells from the R-spondin 1-conditioned medium.
Store the R-spondin 1-conditioned medium at −20 °C.
Collagenase Type II
Dissolve 5 mg Collagenase Type II in 1 ml of adDMEM/F12 +/+/+ to make a 5 mg/ml solution. Add Y-27632 to a final concentration of 10 µM and dihydrotestosterone at a final concentration of 1 nM.
CRITICAL STEP Collagenase 5 mg/ml solution is freshly prepared.
Blocking solution
Add 2.5 ml FBS to 47.5 ml of adDMEM/F12 +/+/+ to get a 5% blocking solution. Add Y-27632 to a final concentration of 10 µM and dihydrotestosterone at a final concentration of 1 nM.
Staining solution
Add 25.0 µl FBS to 49.975 ml of adDMEM/F12 +/+/+ to get a 0.05% blocking solution. Add Y-27632 to a final concentration of 10 µM and dihydrotestosterone at a final concentration of 1 nM.
PROCEDURE
Establishment of mouse prostate organoid cultures (timing 2.5 h)
-
1
Sacrifice male mouse at minimally 8 weeks of age (maximum tested 2 years).
-
2
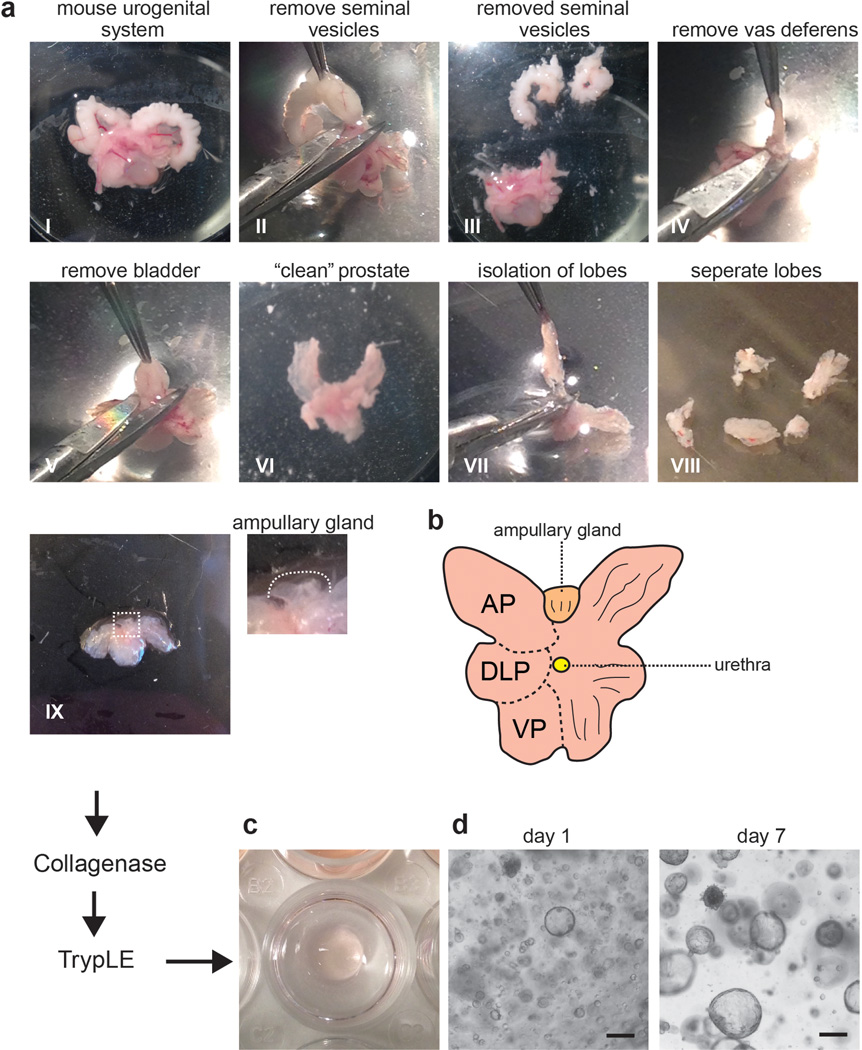
Isolate the urogenital system (Fig. 1a I).
-
3
Remove seminal vesicles by breaking/cutting blood vessels and connective tissue and making an incision at the base of the urethra (Fig. 1a II, III; for a detailed isolation protocol of the murine prostate see25).
-
4
Remove the vas deferens by cutting it near the prostate (Fig. 1a IV).
-
5
Remove the bladder by cutting it near the base of the urethra (Fig. 1a V).
-
6
Remove remaining vesicles and fat tissue by gentle cutting (Fig. 1a VI).
-
7
Remove urethra; carefully pull the prostate lobes, so they are no longer attached to the urethra (Fig. 1a VII).
CRITICAL STEP The ampullary gland is not considered part of the prostate. The gross anatomy is very similar to prostate. The ampullary gland is located between the two lobes of the anterior prostate (Fig. 1a IX, b). Do not isolate this part.
-
8
Isolate each lobe individually (anterior prostate (AP), ventral prostate (VP), dorsolateral prostate (DLP)), or continue with the whole prostate (Fig. 1a VII, VIII, 1b).
-
9
Mince the prostate (lobes) in small pieces (~ 1 mm3) in a 10 cm culture dish using a scalpel.
-
10
Digest the prostate in 5 mg/ml Collagenase II with 10 µM Y-27632 in a 15 ml Falcon tube for 1 – 1.5 h at 37°C on a shaking platform. Use 1 ml of 5 mg/ml Collagenase II per ~ 50 mg minced tissue.
-
11
Wash once by topping up to 10 ml with adDMEM/F12 +/+/+.
-
12
Centrifuge at 150 g for 5 min at 4°C.
-
13
Aspirate supernatant and resuspend pellet in 1 ml TrypLE with 10 µM Y-27632 and digest for approximately 15 min at 37°C.
CRITICAL STEP Pipet up and down with a P1000 pipet every 5 min to ensure efficient digestion.
TROUBLESHOOTING
-
14
Wash once by toping up to 10 ml with adDMEM/F12 +/+/+ and centrifuge at 150 g for 5 min at 4°C.
-
15
Aspirate supernatant and place digested tissue in ice-cold Matrigel (Matrigel protein concentration ~75%). Pipette up and down 5 – 10 times to mix.
CRITICAL STEP Work quickly to ensure that Matrigel does not solidify before plating.
CRITICAL STEP Do not dilute the Matrigel too much to ensure efficient plating.
-
16
Count cells using hemocytometer and plate 20,000 cells in a 40 µl drop in the middle of one well of a 24-well dish (Fig. 1c, Table 2). On average one prostate will yield 25 drops.
CRITICAL STEP Tissue culture plates should be pre-warmed (overnight at 37°C).
-
17
Place the dish into the 37°C incubator for 15 min to allow the Matrigel to solidify.
CRITICAL STEP Place the plate upside down in the incubator to prevent adherence to the plate bottom.
-
18
Gently pipette 500 µl of pre-warmed (37 °C) mouse prostate culture medium plus 10 µM Y-27632 into each well.
-
19
Refresh medium every 2 – 3 days.
-
20
After 7 days Y-27632 can be removed from the medium.
Figure 1. Establishment of mouse prostate organoid cultures.
A) Overview of the isolation of the prostate from the mouse urogenital system (for a detailed isolation protocol see25). The procedure refers to pictures I – IX.
B) Schematic representation of the anatomy of the mouse prostate. AP, anterior prostate; DLP, dorsolateral prostate; VP, ventral prostate.
C) Example of how to plate the matrigel disc in a well of a tissue culture plate.
D) Representative pictures of organoids growing from mouse prostate tissue 1 and 7 days after plating.
Scale bars, 100 micron.
Table 2.
Plate format and culture medium volumes used for organoid culturing.
| Plate | Matrigel volume | Number of Matrigel discs | Medium |
|---|---|---|---|
| 96-well | 10 µl | 1 | 100 µl |
| 48-well | 20 µl | 1 | 250 µl |
| 24-well | 40 µl | 1 | 500 µl |
| 12-well | 40 µl | 3–5 | 1000 µl |
| 6-well | 40 µl | 10–15 | 2000 µl |
Passaging mouse prostate organoid cultures (timing 30 min)
-
21
After approximately 7 days (Fig. 1d), harvest organoids (e.g. 1 well 24-well dish) in the remaining culture medium and transfer to a 15 ml Falcon tube.
TROUBLESHOOTING
-
22
Dissociate organoids by trituration with a fire-polished glass pipette. The glass pipette should have an opening of about 0.5 – 1 mm after polishing.
-
23
Pipet up and down 15 – 20 times.
-
24
Add 5 ml ice-cold adDMEM/F12 +/+/+ to dissolve residual Matrigel.
-
25
Centrifuge at 150 g for 5 min at 4°C.
-
26
Aspirate supernatant.
-
27
Resuspend pellet in 160 µl Matrigel (split ratio 1:4) and plate a drop of 40 µl Matrigel into the middle of one well of a 24-well dish (Table 2).
-
28
Place the dish into the 37°C incubator for 15 min to allow the Matrigel to solidify.
CRITICAL STEP Place the plate upside down in the incubator to prevent adherence to the plate bottom.
-
29
Gently pipette 500 µl of pre-warmed (37 °C) mouse prostate culture medium into each well. Trituration with a fire-polished glass pipette breaks down organoids into clumps of cells (TrypLE treatment gives high percentage of single cells). Y-27632 enhances outgrowth of single cells after plating. Therefore, addition of Y-27632 to the culture medium is only required when organoids are passaged using TrypLE.
-
30
Refresh medium every 2 – 3 days.
Establishment of human prostate organoid cultures (timing 20 h)
-
31
Mince human prostate tissue in small pieces (~ 1 – 5 mm3, Fig. 2b) in a 10 cm culture using a scalpel.
-
32
Digest the tissue overnight in 5 mg/ml Collagenase II with 10 µM Y-27632 in a 15 ml Falcon tube at 37°C on a shaking platform. Use 1 ml of 5 mg/ml Collagenase II per ~ 50 mg minced tissue.
-
33
Wash once by topping up to 10 ml with adDMEM/F12 +/+/+.
-
34
Centrifuge at 200 g for 5 min at 4°C.
-
35
Resuspend pellet in 1 ml TrypLE with 10 µM Y-27632 and digest for approximately 15 min at 37°C.
CRITICAL STEP Pipet up and down every 5 min to ensure efficient digestion (P1000 pipet).
-
36
Wash once by topping up to 10 ml with adDMEM/F12 +/+/+.
-
37
Centrifuge at 200 g for 5 min at 4°C.
-
38
Aspirate supernatant and place digested tissue in ice-cold Matrigel and pipette up and down 5 – 10 times to mix.
CRITICAL STEP Work quickly to ensure that Matrigel does not solidify before plating.
CRITICAL STEP Do not dilute the Matrigel to much to ensure efficient plating.
-
39
Count cells using hemocytometer and plate approximately 20,000 cells in a 40 µl drop in the middle of one well of a 24-well dish.
CRITICAL STEP Tissue culture plates should be pre-warmed (overnight at 37°C).
-
40
Place the dish into the 37°C incubator for 15 min to allow the Matrigel to solidify.
CRITICAL STEP Place the plate upside down in the incubator to prevent adherence to the plate bottom.
-
41
Gently pipette 500 µl of pre-warmed (37 °C) human prostate culture medium plus 10 µM Y-27632 into each well.
-
42
Refresh medium every 2 – 3 days.
-
43
After 7 days, remove Y-27632 from the medium.
CRITICAL STEP Human organoids are split 1:2 every 1 – 2 weeks. This is dependent on density and whether the organoids are luminal- or basal-derived. For instance, the day 7 basal-derived and the day 14 luminal-derived organoids depicted in Fig. 2b are of the size and density to be passaged. Preferred method of splitting for human organoids is with TrypLE. If organoids are small, but the density is high, do not split with TrypLE, but instead use a fire-polished pipette as described in step 22, 23.
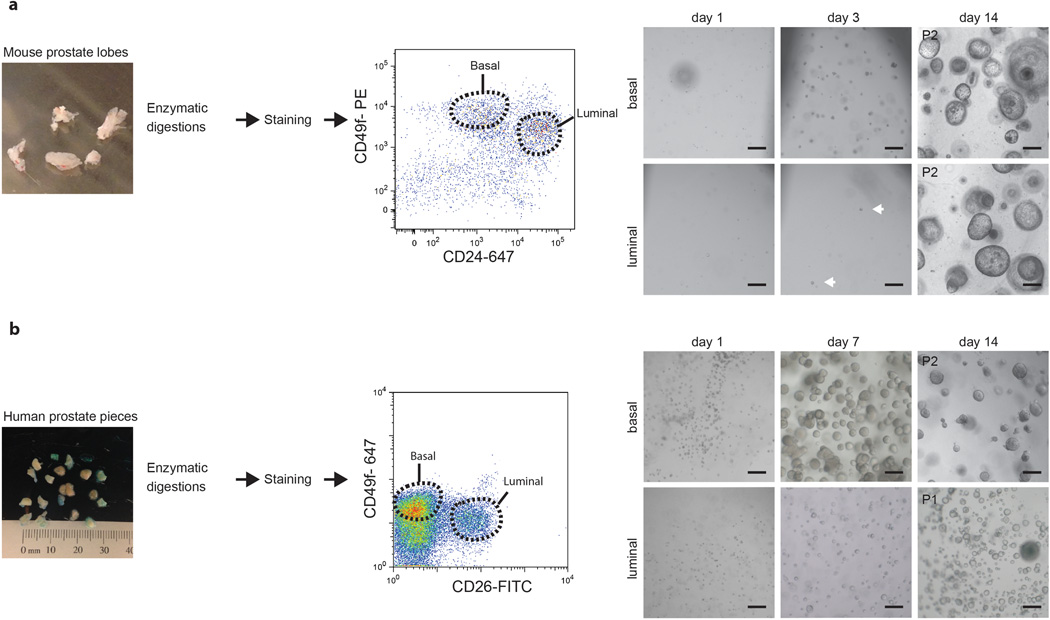
Figure 2. Establishment of mouse and human luminal and basal cell-derived prostate organoid cultures.
A) Overview of the establishment of luminal and basal-cell derived mouse prostate organoid cultures using FACS. Cells are sorted based on expression of Cd24 (luminal cells) and Cd49f (basal cells). Scale bars, 100 micron.
B) Overview of the establishment of luminal and basal-cell derived human prostate organoid cultures using FACS. Cells are sorted based on expression of CD26 (luminal cells) and CD49f (basal cells). Scale bars, 100 micron.
Passaging human prostate organoid cultures (timing 30 min)
-
44
After approximately 14 days, harvest organoids (e.g. 1 well 24-well dish) in the remaining culture medium and transfer to a 15 ml Falcon tube.
TROUBLESHOOTING
-
45
Dissociate organoids enzymatically using TrypLE with 10 µM Y-27632 on a shaking platform for 5 min at 37 °C.
-
46
Inactivate TrypLE by adding adDMEM/F12 +/+/+ containing 5% FBS.
-
47
Centrifuge at 200 g for 5 min at 4°C.
-
48
Aspirate supernatant.
-
49
Resuspend pellet in 80 µl Matrigel (split ratio 1:2) and plate 40 µl drops into the middle of one well of a 24-well dish (total 4 wells).
-
50
Place the dish into the 37°C incubator for 15 min to allow the Matrigel to solidify.
CRITICAL STEP Place the plate upside down in the incubator to prevent adherence to the plate bottom.
-
51
Gently pipette 500 µl of pre-warmed (37 °C) human prostate culture medium plus 10 µM Y-27632 into each well.
-
52
Refresh medium every 2 – 3 days. After 7 days, remove Y-27632 from the medium.
Establishment of luminal and basal derived cultures (timing 4 h)
-
53
Enzymatically digest mouse (Fig. 2a) or human (Fig. 2b) prostate tissue as described in step 1 – 13 and step 31 – 35, respectively.
-
54
Wash digested single cells in 10 ml blocking solution in a 15 ml Falcon tube.
-
55
Centrifuge at 200 g for 5 min at 4°C.
-
56
Aspirate supernatant.
-
57
Resuspend cells in 1 ml blocking solution.
-
58
Pass resuspended cells through cell-strainer of a polystyrene round-bottom tube.
-
59
Block cells in blocking solution on ice for 15 min.
-
60
Centrifuge at 200 g for 5 min at 4°C and aspirate supernatant.
CRITICAL STEP Keep cells aside (about one tenth of total) for unstained negative control and single color staining controls for FACS. Use the rest for the staining protocol as described from step 61.
-
61Stain in 500 µl staining solution for 60 min on ice and in the dark with the following antibodies:
- Human
- CD26-FITC conjugated antibody (M-A261, 1:200)
- CD49f-alexa 647 conjugated antibody (GoH3, 1:200)
- Mouse
- CD24-alexa 647 conjugated antibody (30-F1, 1:200)
- CD49f-PE conjugated antibody (GoH3, 1:200)
-
62
Wash twice with 10 ml adDMEM/F12 +/+/+ plus Y-27632 (10 µM) plus dihydrotestosterone (1 nM).
-
63
Centrifuge at 200 g for 5 min at 4°C.
-
64
Resuspend cells in staining solution (no antibody added)
-
65
Add DAPI (1.0 µg/ml final concentration).
-
66
Add DNaseI (0.5 – 1.0 Units/µl final concentration).
TROUBLESHOOTING
-
67
Isolate cells using FACS (Fig. 2). On average, 200 mg of prostate tissue gives approximately 150,000 luminal cells and 600,000 basal cells.
-
68
Plate cells as described in step 16 – 18 and step 39 – 41 (Fig. 2).
TROUBLESHOOTING
Establishing organoids from prostate cancer metastasis biopsies (timing 2h)
-
69
Mince human advanced prostate cancer biopsy tissue (minimum size ~ 1 mm3) in small pieces (~ 1 – 5 mm3, Fig. 2b) using a scalpel.
-
70
Digest the tissue in 5 mg/ml Collagenase II with 10 µM Y-27632 in a 15ml Falcon tube for 1 h at 37°C. Use 1 ml of 5 mg/ml Collagenase II per ~ 50 mg minced tissue.
-
71
Wash once with 10 ml adDMEM/F12 +/+/+.
-
72
Centrifuge at 200 g for 5 min at 4°C.
-
73
Resuspend pellet in 1 ml TrypLE with 10 µM Y-27632 and digest for approximately 10 min at 37°C.
CRITICAL STEP Pipet up and down every 5 min to ensure digestion (P1000 pipet).
-
74
Wash once with 10 ml adDMEM/F12 +/+/+.
-
75
Centrifuge at 200 g for 5 min at 4°C.
-
76
Repeat wash (step 74–75).
-
77
Place digested tissue in ice-cold Matrigel and pipette up and down 5 – 10 times to mix.
CRITICAL STEP Work quickly to ensure that Matrigel does not solidify before plating.
CRITICAL STEP Do not dilute the Matrigel to much to ensure efficient plating.
-
78
Count cells using hemocytometer and plate approximately 50,000 cells in a 40 µl drop into the middle of one well of a 24-well dish (Table 2).
CRITICAL STEP Tissue culture plates should be pre-warmed (overnight at 37°C).
CRITICAL STEP Seed the prostate cancer cells at high density.
-
79
Place the dish into the 37°C incubator for 15 min to allow the Matrigel to solidify.
CRITICAL STEP Place the plate upside down in the incubator to prevent adherence to the plate bottom.
-
80
Gently pipette 500 µl of pre-warmed (37 °C) human prostate culture medium plus 10 µM Y-27632 into each well.
-
81
Refresh medium every 2 – 3 days and check organoid growth using a light microscope.
CRITICAL STEP Keep 10 µM Y-27632 in the medium.
-
82
Keep Y-27632 in the medium until the first passage of the prostate cancer organoids.
Establishing and maintenance of circulating prostate tumour cells (CTC)
-
83
Collect 8 ml blood from patient with advanced prostate cancer.
CRITICAL STEP Total CTC number should be ~ 50 in 8ml blood (CTC count performed in clinic using cell search circulating tumor cell kit (www.cellsearchctc.com)).
-
84
Incubate blood with 400 µl RosetteSep® Human CD45 Depletion Cocktail for 20 min at room temperature.
-
85
Deplete red and white blood cells using Ficoll-Paque.
-
86
Wash once with adDMEM/F12 +/+/+.
-
87
Place cells in 30 µl ice-cold Matrigel and pipette up and down 5 – 10 times to mix.
CRITICAL STEP Work quickly to ensure that Matrigel does not solidify before plating.
CRITICAL STEP Do not dilute the Matrigel to much to ensure efficient plating.
-
88
Plate circulating tumor cells in a 30 µl drop into the middle of one well of a 24-well dish.
CRITICAL STEP Tissue culture plates should be pre-warmed (overnight at 37°C).
-
89
Place the dish into the 37°C incubator for 15 min to allow the Matrigel to solidify.
CRITICAL STEP Place the plate upside down in the incubator to prevent adherence to the plate bottom.
-
90
Gently pipette 500 µl of pre-warmed (37 °C) human prostate culture medium plus 10 µM Y-27632 into each well.
-
91
Refresh medium every 2 – 3 days and check organoids grow every 2 days.
CRITICAL STEP Keep 10 µM Y-27632 in the medium.
-
92
Keep Y-27632 in the medium, until the first passage of the prostate cancer organoids.
Cryopreservation of mouse and human prostate organoids (timing 30 min)
-
93
Aspirate medium from Matrigel disc.
-
94
Resuspend the Matrigel disc in 1 ml of TrypLE using P1000 pipette.
-
95
Transfer suspension to a 15 ml Falcon tube.
-
96
Incubate 5–10 min at 37 °C. If needed pipet up and down to break up organoid structures after incubation.
-
97
Add 10 ml of ice-cold adDMEM/F12 +/+/+.
-
98
Centrifuge at 300 g for 5 min at 4 °C.
-
99
Resuspend one 24-well of organoids in 500 µl of Recovery Cell Culture Freezing medium. Freeze cells using a CoolCell (BioCision) or comparable method.
To verify that the organoids are indeed derived from prostate tissue, (Q-) RT-PCR can be performed for expression of prostate-specific genes (see Anticipated Results and ref. 16). Additionally, to confirm the growth of prostate tumor organoids, WGS can be performed to analyze mutation spectra17. Below we describe the procedures to isolate RNA, produce cDNA and isolate genomic DNA from organoids.
RNA isolation and cDNA production from prostate organoids
-
100
Aspirate prostate culture medium from Matrigel disc.
-
101
Harvest organoids (at least 50 µl of Matrigel) directly in 350 µl RLT buffer (addition of β-mercaptoethanol is not essential).
-
102
Incubate at room temperature for 15 min on a shaking platform.
-
103
Add 350 µl 70% ethanol and mix by pipetting.
-
104
Transfer mixture to a RNeasy column (Qiagen).
-
105
Centrifuge for 30 s at 8000 g and discard flow-through.
-
106
Add 700 µl Buffer RW1.
-
107
Centrifuge for 30 s at 8000 g and discard flow-through.
-
108
Add 500 µl Buffer RPE.
-
109
Centrifuge for 30 s at 8000 g and discard flow-through.
-
110
Add 500 µl Buffer RPE.
-
111
Centrifuge for 2 min at 8000 g and discard flow-through.
-
112
Place column in clean collection tube and centrifuge for 1 min at full speed.
-
113
Elute RNA with 30 µl RNase-free H2O.
TROUBLESHOOTINGComponents Amount (µl) RNA from step 113 (100 – 500 ng) × Oligo(dT)15 1.0 RNase-free H2O to 5.0 -
114Incubate for 5 min at 70°C and place tube on ice
Components Amount (µl) GoScript 5× Reaction Buffer 4.0 25mM MgCl2 4.0 10mM PCR Nucleotide Mix 1.0 Rec RNasin Ribonuclease Inhibitor 0.5 GoScript Reverse Transcriptase 1.0 RNase-free H2O to 15.0 -
115
Add 15.0 µl reverse transcription mix to 5.0 µl RNA/oligo(dT) mix
-
116Perform reverse transcription using following incubations:
Step Temperature Time 1 25°C 5 min 2 42°C 60 min 3 70°C 15 min -
117
Produced cDNA can be used for subsequent (Q-) RT-PCR using standard protocols.
Genomic DNA isolation from organoids
-
118
Harvest organoids (at least 50 µl of Matrigel) in culture medium and transfer into 1.5 ml microcentrifuge tube.
-
119
Centrifuge 5 min at 3000 rpm.
-
120
Aspirate supernatant.
-
121
Resuspend pellet in 160 µl PBS.
-
122
Add 20 µl Proteinase K (PK) solution.
-
123
Mix by vortexing thoroughly.
-
124
Incubate 56°C for 1 h; vortex every 15 min.
-
125
Add 20 µl RNaseA solution.
-
126
Mix by vortexing thoroughly.
-
127
Incubate 56°C for 10 min.
-
128
Add 250 µl Binding Buffer (BBA) and mix by vortexing.
-
129
Transfer solution to a ReliaPrep Binding Column.
-
130
Centrifuge for 1 min at maximum speed and discard flow-through.
-
131
Add 500 µl of Column Wash Solution (CWD) to the column.
-
132
Centrifuge 2 min at maximum speed and discard flow-through.
-
133
Repeat step 131 and 132 for a total of 3 times.
-
134
Place the column in a clean 1.5 ml microcentrifuge tube
-
135
Add 50 µl of nuclease-free H2O to elute genomic DNA from the column
-
136
Centrifuge for 1 min at maximum speed. Flow-through contains genomic DNA, which should be stored at −20°C (long-term).
TROUBLESHOOTING
| Step | Problem | Possible reasons | Solution |
|---|---|---|---|
| 13 | Big tissue pieces remaining after digestion |
Inefficient digestion | Increase digestion time. The duration of the enzymatic digestion is variable and dependent on the initial tissue size. Pipet up and down with a fire- polished glass pipette after digestion. |
| 21 + 44 | No/few/small organoids appearing |
Inactive/less active growth factors in culture medium. No ROCK inhibitor inhibitor (Y-27632) added to medium. |
Change medium every 2 – 3 days. Make fresh medium to ensure that the growth factors in the culture medium are active. Absence of the ROCK inhibitor (Y- 27632) will greatly decrease the efficiency of organoid outgrowth. |
| 66 | Cell suspension remains viscous after DNaseI addition |
Presence of high concentration of genomic DNA in the suspension. |
Add more DNaseI to the suspension. |
| 68 | High percentage of contaminating (non-prostate) cells (e.g. immune cells) in sorted population |
The tissue was not properly dissected. |
Sort out epithelial cells by co-staining for an epithelial marker (e.g. Epcam). Moreover, non-epithelial cells do not grow under the described prostate culture conditions. |
| 113 | No/low yield from RNA isolation |
Amount of organoids used as input was too low. Organoids were not efficiently lysed. |
Use more organoids for the isolation. Snap-freeze samples in liquid nitrogen after step 102 for more efficient lysis |
ANTICIPATED RESULTS
The protocol describes an efficient method for establishing organoid cultures from mouse and human prostate tissue. The efficiency of establishing these cultures is > 95%. The efficiency of organoid establishment from advanced prostate cancers is significantly lower (~15 – 20%) mainly because of the small amount of input material. After sorting, basal cells have an organoid-forming capacity of approximately 70% (of which >95% are solid), whereas approximately 1 – 2% of sorted luminal cells give rise to organoids (>95% are cystic). To reach these efficiencies and to be able to maintain growth “indefinitely” it is essential to use medium that is not stored for more than two weeks and to use well-tested and stored growth factors and chemical compounds. Prostate organoids can be genetically modified10,16 and can thus be used to study the involvement of genes in prostate homeostasis and cancer. We have not been successful in growing organoids derived from primary prostate cancers, most probably due to overgrowth by normal prostate epithelium present within each sample.
For mouse and human organoid culture protocol, small organoids can be detected within 2 – 3 days after plating. Mouse organoids are generally cystic, whereas unsorted newly established human organoid cultures will mainly consist out of solid basal cell-derived organoids during the initial passages. After 5 – 7 days, small cystic organoids can be observed from sorted luminal cells. The morphology of organoids derived from advanced prostate cancer patients can vary greatly between patients and – due to tumour heterogeneity – even within cultures derived from the same patient17.
To confirm that the organoids are indeed derived from prostate tissue, expression of prostate-specific genes like prostate specific antigen (PSA) can be determined. Luminal-specific marker (androgen receptor, cytokeratin 8, cytokeratin 18, Probasin, PSA) and basal-specific marker (p63, cytokeratin 5) expression analysis will confirm the presence of both lineages in the established cultures.
For samples derived from advanced prostate cancer, growth speed and morphology are highly variable. Confirmation of the cancerous origin of the organoids can be achieved by genomic analysis either by whole genome sequencing or comparative genomic hybridization. Moreover, urogenital sinus mesenchyme (UGSM) recombination assays (described in detail in ref. 26), where single prostate organoid cells can be mixed with mesenchymal cells derived from the urogenital sinus of mouse embryos and placed under the kidney capsule, can be performed to confirm that healthy or tumour organoids can produce prostate glands or neoplastic growth in vivo, respectively.
Human material for organoid cultures
Approval for this study was obtained by the ethics committee of the University Medical Centre Utrecht and Memorial Sloan-Kettering Cancer Center Institutional Review Board. All patients provided informed consent.
Mouse material for organoid cultures
All procedures were performed in compliance with local animal welfare laws and guidelines.
Table 1.
Overview of culture medium components for mouse and human prostate organoids.
| Factor | Mouse organoids | Human organoids |
|---|---|---|
| B27 | 50× diluted | 50× diluted |
| N-acetylcysteine | 1.25 mM | 1.25 mM |
| EGF | 50 ng/ml | 5 ng/ml |
| Noggin | 100 ng/ml | 100 ng/ml |
| R-spondin 1 | 500 ng/ml or 10% conditioned medium |
500 ng/ml or 10% conditioned medium |
| A83-01 | 200 nM | 500 nM |
| FGF10 | 10 ng/ml | |
| FGF2 | 5 ng/ml | |
| Prostaglandin E2 | 1 µM | |
| Nicotinamide | 10 mM | |
| SB202190 | 10 µM | |
| DHT | 1 nM | 1 nM |
| Y-27632* | 10 µM | 10 µM |
, Y-27632 is only added to the medium during establishment of the culture and after passaging the organoids using TrypLE.
Acknowledgments
We would like to thank members of the contributing labs for support. We are grateful for support from the following: The Netherlands Organisation for Scientific Research (NWO-ZonMw) VENI grant to J.D. (91614138), Prostate Cancer Foundation (W.R.K., C.L.S, Y.C.), and from the CancerGenomics.nl (NWO Gravitation) program.
Footnotes
Author contributions
J.D., W.R.K., Y.C., C.S. and H.C. conceived the study. J.D., W.R.K., D.G. and E.D. wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, et al. Single Lgr5 stem cell build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Jung P, et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 5.Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Huch M, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013a;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huch M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013b;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boj S, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huch, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo BK, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H. Controlled gene expression in primary Lgr5 organoid cultures. Nat. Methods. 2011;9:81–83. doi: 10.1038/nmeth.1802. [DOI] [PubMed] [Google Scholar]
- 11.Schwank G, Andersson-Rolf A, Koo BK, Sasaki N, Clevers H. Generation of BAC transgenic epithelial organoids. PLoS One. 2013;8:e76871. doi: 10.1371/journal.pone.0076871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwank G, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Drost J, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 14.Pienta KJ, et al. The current status of preclinical prostate cancer animal models. Prostate. 2008;68:629–639. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toivanen R, Taylor RA, Pook DW, Ellem SJ, Risbridger GP. Breaking through a roadblock in prostate cancer research: an update on human model systems. J. Steroid Biochem. Mol. Biol. 2012;131:122–131. doi: 10.1016/j.jsbmb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Karthaus WR, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159:163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao D, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- 19.Garraway IP, et al. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate. 2010;70:491–501. doi: 10.1002/pros.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niranjan B, Lawrence MG, Papargiris MM, Richards MG, Hussain S, Frydenberg M, Pedersen J, Taylor RA, Risbridger GP. Primary culture and propagation of human prostate epithelial cells. Methods Mol. Biol. 2013;945:365–382. doi: 10.1007/978-1-62703-125-7_22. [DOI] [PubMed] [Google Scholar]
- 21.Hӧfner T, et al. Defined conditions for the isolation and expansion of basal prostate progenitor cells of mouse and human origin. Stem Cell Reports. 2015;4:503–518. doi: 10.1016/j.stemcr.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua CW, et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 2014;16:951–961. doi: 10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KA, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 25.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nat. Protocols. 2010;5:702–713. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc. Natl. Acad. Sci. USA. 2003;100:11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]