Abstract
Purpose
Ferritin is an iron storage protein that is generally cytoplasmic. However, in embryonic avian corneal epithelial (CE) cells, the authors previously observed that the ferritin was predominantly nuclear. They also obtained evidence that this ferritin protects DNA from oxidative damage by UV light and hydrogen peroxide and that the nuclear localization involves a tissue-specific nuclear transporter, termed ferritoid. In the present investigation, the authors have determined additional properties of the nuclear ferritoid-ferritin complexes.
Methods
For biochemical characterization, a combination of molecular sieve chromatography, immunoblotting, and nuclear-cytoplasmic fractionation was used; DNA binding was analyzed by electrophoretic mobility shift assay.
Results
The CE nuclear ferritin complex has characteristics that differentiate it from a “typical” cytoplasmic ferritin, including the presence of ferritin and ferritoid subunits; a molecular weight of approximately 260 kDa, which is approximately half that of cytoplasmic ferritin; its iron content, which is below our limits of detection; and its ability to bind to DNA.
Conclusions
Within CE cell nuclei, ferritin and ferritoid are coassembled into stable complex(es) present in embryonic and adult corneas. Thus, ferritoid not only serves transiently as a nuclear transporter for ferritin, it remains as a component of a unique ferritoid-ferritin nuclear complex.
Iron is essential for life in all eukaryotes and most prokaryotes; however, free iron (Fe2+), in excess, can exacerbate oxidative damage through the Fenton reaction, which generates hydroxyl radicals, the most energetic and deleterious reactive oxygen species (ROS).1–3 Therefore, iron-sequestering proteins such as ferritin have evolved as one of the cellular mechanisms of detoxification.4–7
Although it was generally believed that the subcellular localization of ferritin is exclusively cytoplasmic, recent studies have reported cells with ferritin in a nuclear location. For tissues in vivo, these include avian embryonic corneal epithelium (CE) and nucleated red blood cells.8,9 In developing rats, these include the brain.10 For cells in culture, these include astrocytoma and glial cell lines and cells subjected to iron overloading and other pathologic conditions.11–13 Several functions for nuclear ferritin have been suggested. In CE cells, we have considerable evidence that the nuclear ferritin affords protection from UV-and H2O2-induced damage to DNA.14–16 In other cell types, nuclear ferritin has also been suggested to protect DNA and, in addition, to provide iron for nuclear enzymes and to regulate the initiation of transcription.11,12,17 Similarly, for the nuclear transport of ferritin, at least two mechanisms have been suggested. One, in CE cells, involves a tissue-specific nuclear transporter protein for ferritin and another, in astrocytoma cells, involves posttranslational modifications of the ferritin H-chain.18,19
Cytoplasmic mammalian ferritin complexes are heteropolymers composed of two types of subunits, H and L, assembled in different ratios to form a 24-mer supramolecular complex capable of sequestering approximately 4500 atoms of iron.20,21 In addition, the cytoplasmic ferritin complex has been reported to associate with nonferritin proteins that deliver iron to the ferritin core22 and others that are involved in the subcellular distribution of ferritin.8,23 However, in avian species, only the H-subunit has been detected. In chicken CE cells, we have previously identified a novel protein, ferritoid, that binds to ferritin and translocates it into the nucleus. Ferritoid consists of two domains. One ferritin-like domain is involved in its binding to ferritin, and the other domain has a consensus SV40-type nuclear localization signal that is responsible for the nuclear transport.24 Other than this, however, little was known concerning the association between ferritoid and ferritin, such as the type of complexes formed between these two components, the subcellular localization(s) of these complexes, and whether they are transient—that is, present only during the transport process—or whether, once formed, they remain stable. In addition, if the ferritoid-ferritin complexes are stable, do they have unique characteristics/properties that distinguish them from other multimeric ferritin complexes? In the present study we have determined certain of the characteristics of the nuclear ferritoid-ferritin complexes.
Methods
Corneal Epithelium Tissue and Cell Culture
Chicken embryos of embryonic day (E) 8 to E1725 were used. Adult chicken eyes were from PelFreeze Biologicals (Brown Deer, WI). Corneal epithelia (CE) were obtained by treatment with 0.5% dispase in PBS (4°C, 1 hour).26 For CE cell cultures, epithelia were digested with 0.25% trypsin at 37°C for 5 minutes, and the cells were cultured as described earlier.9
Protein Lysates Enriched for Ferritoid and Ferritin
Tissue lysates were enriched for the ferritin supramolecular complexes using a heat treatment procedure (adapted from Mete et al.27). Frozen CE tissue from four dozen corneas was thawed on ice for 30 minutes and then was resuspended in 200 µL of 50 mM HEPES buffer, pH 7.4, and homogenized by sonication on ice (2 × 10 seconds). Samples were then heated to 70°C for 10 minutes, cooled on ice for 30 minutes, and centrifuged twice at 16,000 rpm for 30 minutes each. The supernatant, enriched with the ferritoid and ferritin complexes, was collected and stored at −20°C.
Size Exclusion Chromatography
Total tissue lysates (extracted with either RIPA or HEPES buffer followed by sonication for 2 × 10 seconds) or lysates enriched for ferritoid and ferritin by heating at 70°C for 10 minutes were fractionated by size exclusion chromatography on a gel filtration column (Superdex 200 HR 10/30; Pharmacia, Alameda, CA) in 140 mM NaCl in phosphate buffer, pH 7.4, 0.02% Triton X-100. Absorbance at 280 nm was monitored, and 0.5-mL fractions were collected. The molecular weight standard curve was generated with the use of gel filtration calibration kits (Sigma, St. Louis, MO; containing 2000 kDa blue dextran, 200 kDa cytochrome c, 150 kDa carbonic anhydrase, 66 kDa bovine albumin, 29 kDa alcohol dehydrogenase, and 12.5 kDa β amylase). The fractions were analyzed further by SDS-PAGE followed by silver or Coomassie staining (Invitrogen, Carlsbad, CA) for protein detection or by Western blot analysis.
Antibodies
The antibody used was an anti–chicken ferritin mouse monoclonal antibody, 6D11.28
Western Blot Analysis and Immunoprecipitation
Fractions collected from the gel filtration column (Superdex 200; Pharmacia) were incubated with anti–ferritin 6D11 antibody for 1 hour at 4°C with rocking, and ferritin and associated proteins were coimmunoprecipitated with 20 µL protein agarose (A/G+; EMD Biosciences, San Diego, CA). Proteins separated by 12% or 15% SDS-PAGE were transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Western blot analysis for ferritoid was performed using a biotinylated IgY anti–ferritoid antibody (1:10,000 dilution in 1.25% nonfat dry milk in PBS containing 0.025% Tween-20), followed by incubation with horseradish peroxidase (HRP)-conjugated streptavidin (1:5000 dilution; Sigma).29 In the blots, ferritin was identified with the 6D11 antibody (1 µg/mL used at 1:500 dilution), followed by incubation with secondary anti–mouse HRP-conjugated antibody (Pierce, Rockford, IL) and detection using a chemiluminescent substrate (Denville Scientific, Metuchen, NJ).
Indirect Immunofluorescence
Fixation of anterior eyes, tissue sectioning, and indirect immunofluorescence were performed as described.29 The monoclonal anti–ferritin antibody was used as the undiluted hybridoma supernatant, and the biotinylated polyclonal IgY antibody against ferritoid was used as a 1:250 dilution. The secondary antibody for ferritin was FITC-conjugated goat anti–mouse IgG (Pierce), and for the biotinylated ferritoid it was TRITC-conjugated avidin (NeutrAvidin; Molecular Probes, Eugene, OR). DNA was visualized by Hoechst staining. Confocal images were obtained using a laser scanning microscope and detector (LSM510; Zeiss, Thornwood, NY).
Detection of Iron
Iron was analyzed with the use of an iron stain kit (Accustain; Sigma) at a final concentration of 10 mM potassium ferrocyanide followed by visual evaluation.
Lactate Dehydrogenase Activity Assay
Enzymatic activity of lactate dehydrogenase (LDH) was detected by colorimetric assay based on conversion of the tetrazolium salt WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonyl)-2H-tetrazolium, monosodium salt] to a formazan dye by LDH (LDH-Assay kit; BioVision, Mountain View, CA).
Subcellular Fractionation of CE Cells
A cytoplasmic fraction from cultured CE cells was isolated using 0.1% liquid surfactant (Igepal [Sigma]; polyethylene glycol octylphenol ether) lysis buffer, followed by centrifugation. To obtain a nuclear fraction, the resultant pellet was solubilized in RIPA buffer.
Quantitative RT-PCR
Quantitative real-time PCR was performed with a PCR kit (QuantiTect SYBR Green; Qiagen, Valencia, CA). Primer sequences for ferritin, ferritoid, and β-actin have been described.29
Results
Analysis of Supramolecular Complexes of Ferritoid and Ferritin in CE Cells
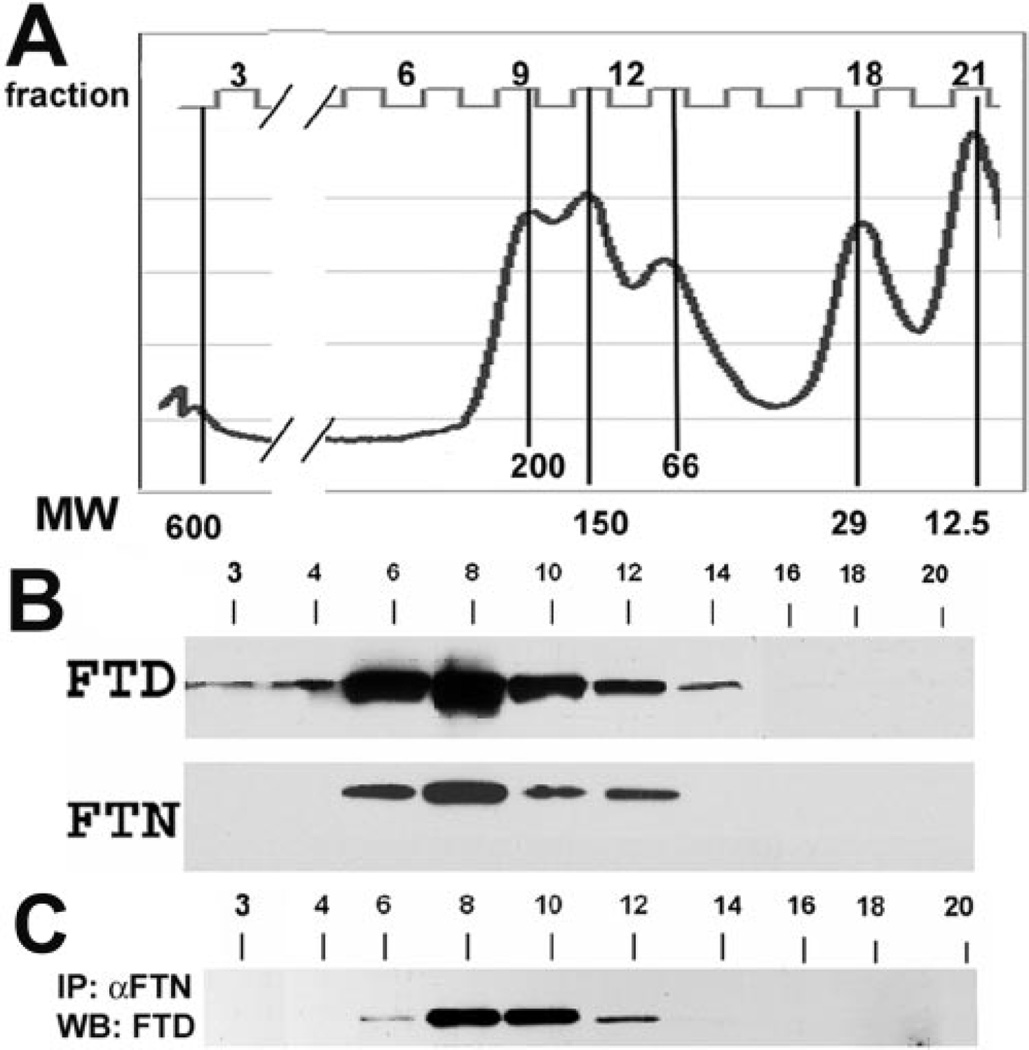
To characterize the endogenous supramolecular forms of the ferritoid-ferritin complexes in CE cells, we used size exclusion chromatography on a column (Superdex 200 HR 10/30; Pharmacia). This procedure has been used previously.30 to determine that the size of the avian cytoplasmic ferritin complex from liver is approximately 470 kDa, similar to that of a “typical” cytoplasmic ferritin from other species. The column is capable of fractionating components ranging from 12.5 kDa to 600 kDa, as can be seen in the curve of molecular weight standards (Fig. 1A). With this fractionation procedure, we analyzed the sizes of the ferritin and ferritoid complexes in extracts of embryonic CE tissue prepared by sonication in HEPES buffer. These analyses showed detectable levels of ferritoid in fractions with molecular masses ranging from 50 kDa to more than 470 kDa (Fig. 1B, fractions 3–14). Most of the ferritoid eluted in fractions 6 to 12 (~100–320 kDa), which were in a Poisson distribution, constituting a single peak whose median fraction (8) was 260 kDa. We therefore referred to the complex(es) in this peak as the 260-kDa fraction. Lesser amounts of ferritoid were in fractions 3 and 4 (~390–480 kDa) and fractions 12 to 14 (~50–100 kDa).
Figure 1.
Gel filtration chromatography of proteins extracted with HEPES buffer from E15 embryonic CE tissue. (A) Calibration curve showing the molecular weight makers in kilodaltons, and the corresponding numbers of the fractions collected and subsequently analyzed for ferritoid (FTD) and ferritin (FTN) by denaturing SDS-PAGE, followed by Western blot analysis. (B) Western blot analysis of the fractions probed for FTD and FTN after denaturing SDS-PAGE. (C) Immunoprecipitation of the fractions with anti–FTN antibody followed by Western blot analysis for FTD.
The fractions were also subjected to Western blot analysis for ferritin, and again the strongest signal was in fractions 6 to 12 (100–320 kDa; Fig. 1B, FTN). The presence of ferritoid and ferritin in the same fractions suggested that these components might have occurred within the same complex(es). To examine this further, each fraction was immunoprecipitated with the anti–ferritin antibody, and the precipitated material was then examined for ferritoid by Western blot analysis. These analyses (Fig. 1C) showed the highest levels of ferritoid that coimmunoprecipitated with ferritin to be in fractions 8 to 10 (~180–260 kDa), with less in fractions 6 (320 kDa) and 12 (100–150 kDa). These results suggest that in CE cells, ferritoid and ferritin exist together chiefly as a supramolecular complex of approximately 260 kDa, which is approximately half the size of a typical ferritin complex (>470 kDa).
Subcellular Localization of the Ferritoid-Ferritin Complexes
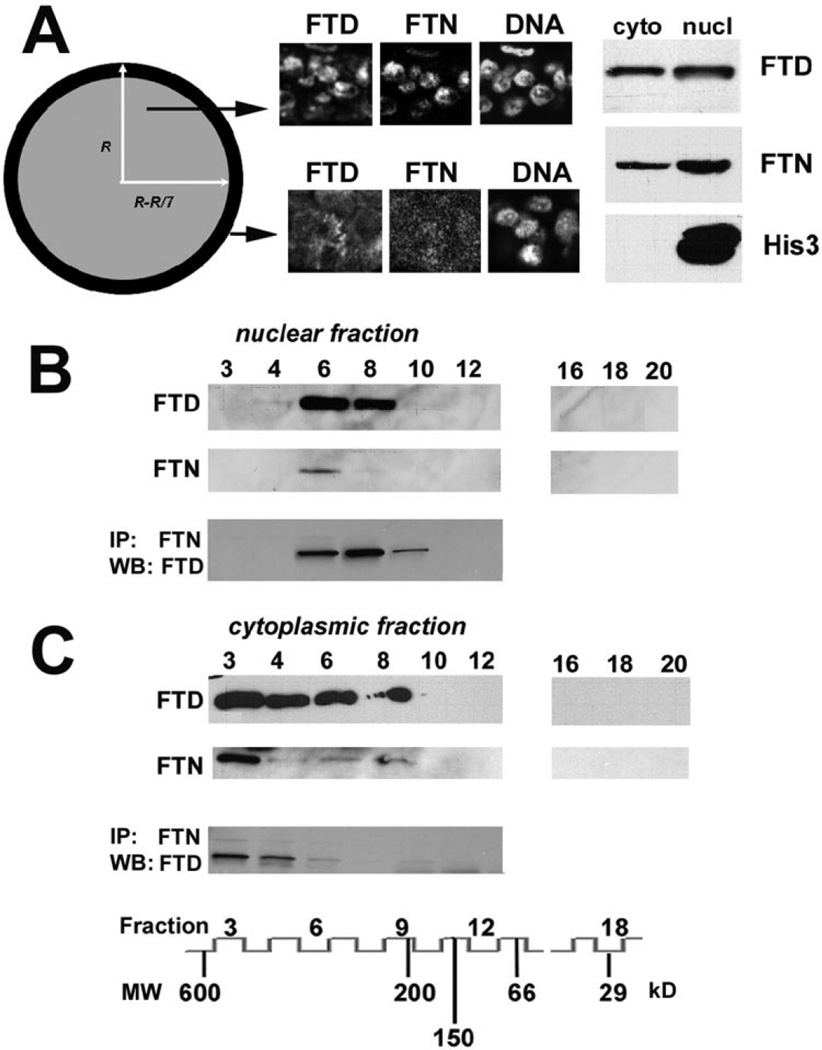
To analyze biochemically the nuclear and cytoplasmic ferritoid-ferritin complexes, we performed subcellular fractionation. Importantly, in the CE, the spatial localization of the ferritoid-ferritin complexes is not homogeneous.29 As depicted in Figure 2A, and as shown in the accompanying confocal micrographs (for ferritoid, ferritin, and nuclear DNA), the ferritoid and ferritin of the completely differentiated cells in the central region (gray area) are predominantly in a nuclear location. However, in the cells undergoing differentiation at the periphery of the cornea (black region), the ferritoid and ferritin are cytoplasmic. The area of the peripheral ring is approximately 40% that of the central region (calculated using the formula in the Fig. 2 legend). Biochemical analyses of CE tissue include cells from both regions. Western blot analyses of the cytoplasmic and nuclear fractions were consistent with immunohistochemical observations in showing both fractions to contain ferritoid and ferritin; levels of both proteins were higher in the nuclear fraction (Fig. 2A).
Figure 2.
(A) Schematic diagram of the cornea depicting the central area (gray) in which the ferritin (FTN) and ferritoid (FTD) of CE cells have a predominantly nuclear localization and the peripheral ring (black) in which FTN and FTD are cytoplasmic, as shown in the accompanying immunoconfocal micrographs for FTD and FTN, and for DNA (by Hoechst staining). The area of the ring is approximately 40% of the central region [π(r2 − r − r/7)2 versus π(r − r/7)2]. Also shown are Western blot analyses for cytoplasmic (cyto) and nuclear (nucl) fractions using antibodies against FTD, FTN, and histone 3 (His3). (B, C) Subcellular localization of the FTD-FTN complexes. Analyses of the nuclear (B) and cytoplasmic (C) extracts of cultured CE cells, separated by gel filtration chromatography, followed by immunoprecipitation for FTN (IP/FTN) and Western blot for FTD (WB/FTD). The fraction number and molecular weight (MW) of each marker is shown at the bottom.
Of note, the cytoplasmic fraction of CE cells obtained with liquid surfactant (Igepal; Sigma) lysis contained no nuclear material, as determined in Western blot analysis by an absence of the nuclear marker, histone 3 (Fig. 2A, cyto, His3). However, the nuclear fraction had minor cytoplasmic contamination, as determined by enzymatic assay for cytoplasmic LDH, which showed less than 5% of total activity to be with the nuclear fraction (data not presented).
Next, the cytoplasmic and nuclear fractions were further fractionated by gel filtration chromatography, and the collected fractions were analyzed for ferritoid and ferritin by Western blot. For the nuclear material (Fig. 2B, nuclear fraction), the highest levels of ferritoid and ferritin were in fractions 6 to 8 (260–320 kDa), suggesting again an association between the two proteins in a supramolecular complex approximately half the size of a typical ferritin. This was confirmed by coimmunoprecipitation analyses in which immunoprecipitates with the ferritin antibody also contained ferritoid (by Western blot analysis; Fig. 2B).
Conversely, in the cytoplasmic extract (Fig. 2C, cytoplasmic fraction), the predominant fraction for ferritin was fraction 3 and ferritoid eluted in fractions 3 to 8, with the greatest amount of material in fraction 3 (>470 kDa), a molecular weight corresponding to a typical ferritin composed of 24 subunits.8,30,31 Coimmunoprecipitation analysis detected a ferritoid-ferritin complex of approximately 470 kDa (Fig. 2C). These results indicate that the cytoplasmic complexes of ferritin in CE cells are similar in size to the “standard” form of ferritin. However, unlike the typical chicken ferritin from liver, which is a homopolymer of H-chains,8 the cytoplasmic ferritin complex in CE cells also contains ferritoid subunits.
It should be noted that precise determination of the molecular sizes of ferritin complexes was achievable only through size exclusion chromatography used in this study. Previously, it was observed in analyses of typical cytoplasmic liver ferritin that the 470-kDa complex can dissociate into 240-kDa units under certain experimental conditions, such as in the presence of SDS in the running buffer used in polyacrylamide gel electrophoresis performed under nondenaturing conditions.31 This may explain why previous studies on ferritin isolated from chicken CE cells and nucleated erythrocytes, both of which have nuclear and cytoplasmic complexes of ferritin, did not detect two types of complexes.8,9 However, the experimental conditions used in this study preserve the 470-kDa cytoplasmic complex intact, in agreement with previous studies,30 and distinguish it from the 260-kDa nuclear complex. The 260-kDa complex, detected here, is the authentic nuclear complex of CE cells, as confirmed by its presence in the detergent-free HEPES-based cell extracts.
Characterization of the Ferritoid-Ferritin–Enriched Cellular Material
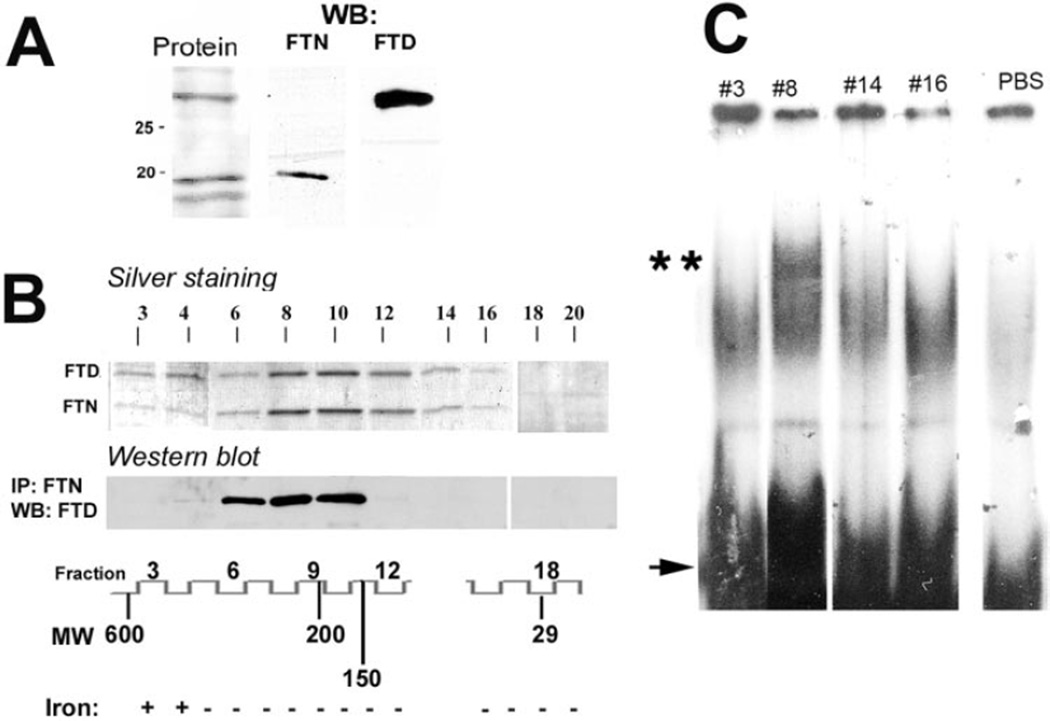
Our previous results suggest that the nuclear ferritin in CE cells protects their DNA from the UV light– and hydrogen peroxide– induced damage and that this protection involves attenuation of the toxic affects of iron.9,14 Of potential interest, the size of the nuclear ferritoid-ferritin complexes (240–260 kDa) is similar to that of certain ferritin-like bacterial proteins termed the DNA-binding proteins of starvation (Dps), which bind to DNA and are inherently low in iron (see Discussion). We therefore examined whether the CE ferritoid-ferritin complexes also have these properties. For these analyses, to decrease the possibility of the results being affected by irrelevant proteins, we used lysates enriched for ferritin proteins by heat treatment27,32 (described in Materials and Methods). For CE cells, this procedure generated lysates that consisted of two major bands by SDS-PAGE, one of 19 kDa and another of 30 kDa. These were identified by Western blot analysis as, respectively, ferritin and ferritoid (Figs. 3A, B) and by mass spectrometry (data not presented). Only a few minor contaminating proteins were present, as determined by Coomassie staining (Fig. 3A, Protein).
Figure 3.
(A) Denaturing SDSPAGE of the heat-enriched ferritoidferritin (FTD-FTN) fraction of a CE tissue extract, stained with Coomassie. Left: molecular weights. Also shown are Western blots (WB) for FTN and FTD. (B) Gel filtration chromatography of the heat-enriched material, followed by SDS-PAGE visualized by silver staining (upper, FTD and FTN). Lower: Western blot detection of FTD in the material immunoprecipitated with the anti–FTN antibody. Positions of molecular weight markers and fraction numbers are shown at the bottom. Also shown are the results of iron detection (± signs) in the fractions. (C) Electrophoretic mobility shift assay of column fractions 3, 8, 14, and 16. Double asterisks: shift in DNA mobility indicative of protein binding. Arrow: free probe.
The size distribution of the ferritoid-ferritin complexes in the heat-enriched CE lysates was determined by protein silver staining of the fractions collected from the column (Superdex 200 HR 10/30 [Pharmacia]; Fig. 3B). Ferritoid and ferritin coeluted from the column in fractions 3 to 16 (>470 kDa to ~40 kDa), with the median fraction again 8 (~260 kDa). Coassembly of the ferritoid and ferritin within the same molecular complex(s) in fractions 6 to 10 was confirmed by coimmunoprecipitation of the fractions with the ferritin antibody, followed by Western blot analysis with the ferritoid antibody (Fig. 3B, Western blot). This size distribution is essentially the same as was seen with total lysates produced by sonication of CE tissue in HEPES buffer (described earlier and shown in Fig. 1B). Thus, not only do the analyses with these two different methods of preparation confirm one another, they also show that the heat enrichment procedure does not disrupt the structure of the ferritoid-ferritin complexes, which makes them suitable for analysis in the DNA binding assay.
An additional observation from these analyses is that ferritin and ferritoid are present in fractions 14 and 16 (~40–66 kDa). However, in these fractions, they do not coimmunoprecipitate with one another, which raises the possibility that these fractions consist of homodimers of ferritin or ferritoid (see Discussion).
DNA Binding by Ferritoid-Ferritin Complexes
To assay for DNA binding, we used an electrophoretic mobility shift assay. For this, the heat-enriched ferritoid-ferritin was fractionated by gel filtration chromatography, and the fractions were incubated with a mixture of DNA oligonucleotides consisting of six different double-stranded, randomly chosen 23-mers. This was followed by separation of the DNA/protein complexes by polyacrylamide electrophoresis. Fractions analyzed were the cytoplasmic >470-kDa ferritoid-ferritin complex (fraction 3); the nuclear 260-kDa complex (fraction 8); and the low molecular–weight putative homodimers, and trimers of ferritoid and ferritin, with molecular weights of 50 to 66 kDa (fractions 14 and 16). Shifted complexes were seen only in fraction 8 (Fig. 3C, double asterisk), corresponding to the nuclear ferritoid-ferritin complexes. Neither the cytoplasmic ferritoid-ferritin complexes nor the low molecular–weight putative oligomers of ferritin or ferritoid showed detectable DNA binding. Therefore, this DNA-binding activity of the nuclear ferritoid-ferritin complex may be another property involved in the protection of DNA (see Discussion).
Presence of Iron in the Ferritoid-Ferritin Complexes
We also analyzed the column fractions for the presence of iron (Fig. 3B, designated by the ± signs below the diagram with fraction numbers). Iron was detected only in fractions 3 and 4, corresponding to the cytoplasmic ferritin complex; it was not detectable in any of the lower molecular weight complexes (neither those corresponding to the nuclear ferritoid-ferritin complexes nor those corresponding to the low molecular weight oligomers). This result agrees with an absence of detectable iron in CE tissue examined immunochemically by Prussian blue staining (our unpublished observations, 1997) and suggests that the nuclear ferritin of CE cells is very low in iron and perhaps is an apoferritin form (see Discussion).
Ferritoid and Ferritin in Adult CE
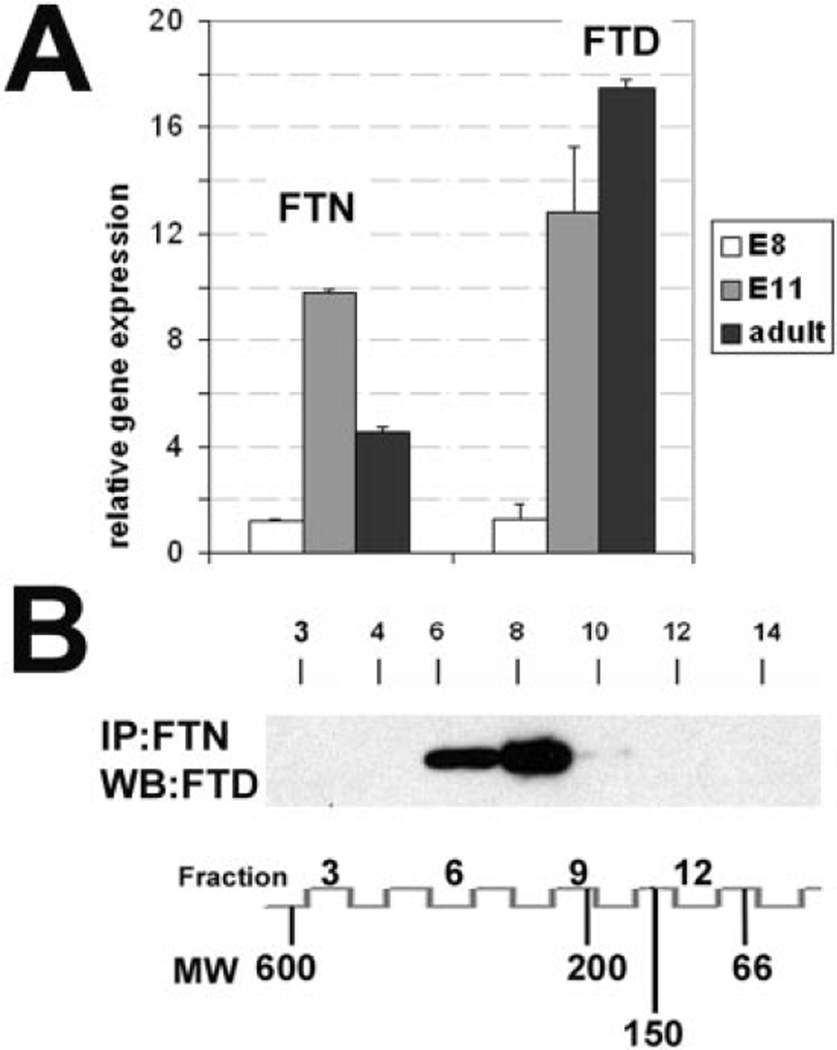
One of the functions we propose for nuclear ferritin is the prevention of UV damage to DNA; this would require that ferritin and ferritoid also be present in the adult CE (when such protection would be needed). We therefore analyzed by real-time PCR (Fig. 4A) the expression of both components in the adult cornea and compared these to E8 tissue (a time when mRNA levels for both genes are very low and neither protein is detected29) and E11 corneas (when both components are being produced). Results show that the upregulation of both components observed at E11 extends into the adult. We also analyzed the supramolecular form of the ferritin and ferritoid in adult CE tissue. This was determined by column chromatography of HEPES lysates followed by coimmunoprecipitation with the anti–ferritin antibody and Western blot analysis for ferritoid. Results (Fig. 4B) showed that the ferritoid-ferritin complexes in the adult CE resemble those in the embryonic cornea, eluting as a single peak in fractions 6 to 8 (260–320 kDa).
Figure 4.
(A) Expression and assembly of ferritin (FTN) and ferritoid (FTD) in CE from E8 and E11 and in the adult tissue (analyzed by quantitative RT-PCR and normalized to β-actin). (B) Coimmunoprecipitation analysis of protein fractions collected by gel filtration chromatography of the HEPES extracts from adult chicken CE. Western blot detection of FTD (WB/FTD) in the material immunoprecipitation with the anti–FTN antibody (IP/FTN). The fraction numbers and corresponding molecular weight (MW) markers are shown at the bottom.
Discussion
Cytoplasmic ferritin is a ubiquitous protein that, depending on metabolic requirements, can either sequester or release iron; thus, it plays central roles in iron metabolism. Equally important, the sequestration of free iron provides a potent mechanism for preventing oxidative damage through inhibition of the formation of Fenton reaction– generated free radicals. Recently, additional ferritins with more restricted tissue distributions have been identified in nuclei and mitochondria. Mitochondrial ferritin has been characterized as a separate gene product.33,34 Its distribution is restricted to tissues with high metabolic activity and oxygen consumption, suggesting that it may play a role in protecting mitochondria from iron-induced damage.35 Similarly, nuclear ferritins have been suggested to prevent oxidative damage (see the Introduction), but their structures and biochemical characteristics have remained largely unknown.
The work presented here demonstrates that the nuclear ferritin complex of avian CE cells has novel characteristics. Structurally, it includes ferritoid, its molecular weight (260 kDa) is approximately half that of a typical cytoplasmic ferritin, and its iron content is significantly lower than that of cytoplasmic ferritin. Functionally, it is capable of binding to DNA.
Spatially juxtaposing the ferritoid-ferritin complex to DNA may contribute to protecting DNA from ROS-mediated damage14–16 by providing maximum capability of the ferritin subunit, through its ferroxidase activity, to sequester free iron that otherwise could associate with the DNA. Such local iron sequestration would be most effective at inhibiting damage from the hydroxyl radicals close to DNA because the highly reactive hydroxyl radicals act only over a very short distance. Although another study has suggested that a typical ferritin can bind to DNA,12 the results presented here show that for CE cells the cytoplasmic complex has significantly lower DNA-binding activity than the nuclear complex. One possibility for this is that the ferritoid component of the nuclear complex promotes or stabilizes binding to DNA, or it does both. Potentially, this could be mediated by a 32-amino acid, N-terminal domain that is present in ferritoid but not in ferritin. This N-terminal domain resembles the N-terminal tail of the bacterial Dps, which themselves are ferritin-like dodecameric proteins that are approximately the size (240 kDa) of the CE nuclear ferritin, bind to DNA, and protect DNA from damage. Both ferritoid and the Dps have two lysine residues that, in the Dps, have been shown to be involved in the interaction with DNA.36 Availability of the ferritoid N-terminal domain for DNA binding may require the high proportion of ferritoid present in the CE nuclear complexes. It is even possible that in the CE nuclear complex it is the high ratio of ferritoid to ferritin subunits that limits the size of this complex through steric interactions. However this remains to be determined.
By further analogy to the mechanisms of DNA protection proposed for the bacterial Dps36,37 for CE cells, the very low, essentially nondetectable iron content of the nuclear ferritoid-ferritin complex may be important. Possibly this inherently low iron content renders the molecule especially effective in sequestering elevated free iron, when it occurs, as has been demonstrated for the Dps from Listeria innocua,38 which endogenously contains only 5 to 10 atoms of iron per complex, at least in the crystalline form of the molecule used for x-ray crystallography. However, it has the capability of rapidly incorporating iron, up to its maximum of 500 Fe/molecule. For the CE nuclear ferritin, its relative capability for sequestering free iron remains to be directly determined. Further, low iron content in endogenous nuclear ferritin may be beneficial under oxidative stress because studies in other cell types suggest that high iron ferritin may exacerbate oxidative damage through the release of stored iron.39–41
Concerning the stoichiometry of the ferritoid and ferritin in the CE nuclear complex, several possibilities are consistent with the currently available information. By column chromatography, the major amount of material elutes as a single peak, which we have referred to as 260 kDa for the molecular weight of its median fraction. However, the entire peak is composed of several fractions (10–6) that span molecular weights from 200 to 320 kDa. In these fractions, ferritoid and ferritin are present in approximately equal amounts, as detected by silver staining of gels (Fig. 3B), suggesting that within the nuclear complex, the ratio between ferritin and ferritoid may vary, at most, from 2:1 to 1:2. If correct, this suggests that each putative dodecameric complex may contain four to eight ferritoid subunits.
Additional information suggests that, within the complex, ferritoid and ferritin subunits may be arranged as homodimers because in the lowest molecular weight fractions from the columns (fractions 14–16) we have detected putative homodimers. That these are dimers is suggested by their molecular weights, from approximately 40 kDa (for dimeric ferritin) to approximately 66 kDa (for dimeric ferritoid). That they are homodimers is likely because they do not coimmunoprecipitate with one another.
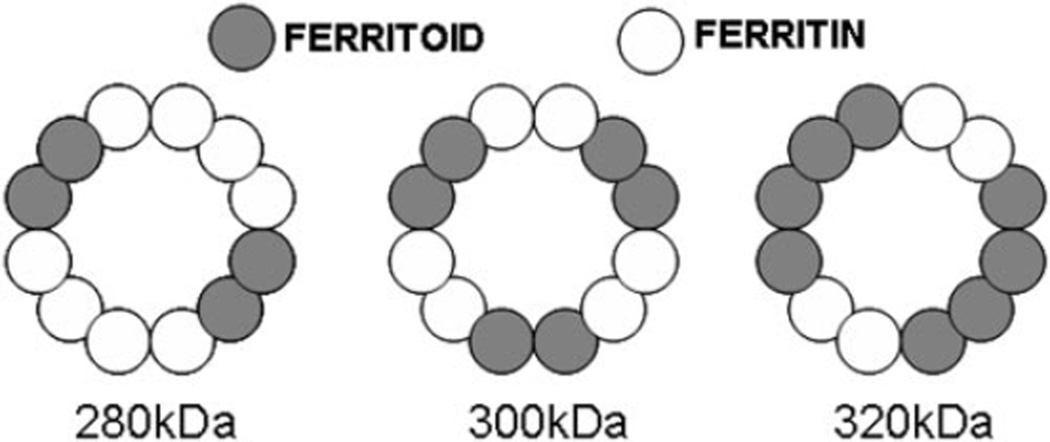
If these homopolymers are the subunits that undergo further assembly to form the dodecameric nuclear ferritoid-ferritin complexes, which may contain the four to eight ferritoid subunits described, the possible models for the complexes are those depicted in Figure 5.
Figure 5.
Proposed models of dodecameric nuclear complexes composed of homodimeric subunits of ferritin and ferritoid in ratios of 2:1 (280 kDa), 1:1 (300 kDa), and 1:2 (320 kDa).
Conversely, the cytoplasmic ferritin complex of CE cells is the size of a typical 24-mer ferritin complex, such as that of chicken tissue,8 and it shows no detectable binding to DNA compared with the nuclear complex. However, it does appear to contain some ferritoid, which distinguishes it from the cytoplasmic ferritin complexes of other tissues. This observation suggests that the presence of ferritoid per se does not confer nuclear transport of ferritin and that other types of regulation are also likely to be involved.
Acknowledgments
Supported by National Institutes of Health Grant EY13127.
Footnotes
Disclosure: M.V. Nurminskaya, None; C.J. Talbot, None; D.I. Nurminsky, None; K.E. Beazley, None; T.F. Linsenmayer, None
References
- 1.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 2.Henle ES, Luo Y, Gassmann W, Linn S. Oxidative damage to DNA constituents by iron-mediated Fenton reactions: the deoxyguanosine family. J Biol Chem. 1996;271:21177–21186. [PubMed] [Google Scholar]
- 3.Luo Y, Henle ES, Linn S. Oxidative damage to DNA constituents by iron-mediated Fenton reactions: the deoxycytidine family. J Biol Chem. 1996;271:21167–21176. [PubMed] [Google Scholar]
- 4.Shimmura S, Suematsu M, Shimoyama M, Tsubota K, Oguchi Y, Ishimura Y. Subthreshold UV radiation-induced peroxide formation in cultured corneal epithelial cells: the protective effects of lactoferrin. Exp Eye Res. 1996;63:519–526. doi: 10.1006/exer.1996.0142. [DOI] [PubMed] [Google Scholar]
- 5.Pappa A, Estey T, Manzer R, Brown D, Vasiliou V. Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem J. 2003;376:615–623. doi: 10.1042/BJ20030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theil EC. The ferritin family of iron storage proteins. Adv Enzymol. 1990;63:421–449. doi: 10.1002/9780470123096.ch7. [DOI] [PubMed] [Google Scholar]
- 7.Mertz JR, Theil EC. Subunit dimers in sheep spleen apoferritin: the effect on iron storage. J Biol Chem. 1983;258:11719–11726. [PubMed] [Google Scholar]
- 8.Infante AA, Infante D, Chan MC, et al. Ferritin associates with marginal band microtubules. Exp Cell Res. 2007;313:1602–1614. doi: 10.1016/j.yexcr.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Cai CX, Birk DE, Linsenmayer TF. Ferritin is a developmentally-regulated nuclear protein of avian corneal epithelial cells. J Biol Chem. 1997;272:12831–12839. doi: 10.1074/jbc.272.19.12831. [DOI] [PubMed] [Google Scholar]
- 10.Cheepsunthorn P, Palmer C, Connor JR. Cellular distribution of ferritin subunits in postnatal rat brain. J Comp Neurol. 1998;400:73–86. [PubMed] [Google Scholar]
- 11.Pountney D, Trugnan G, Bourgeois M, Beaumont C. The identification of ferritin in the nucleus of K562 cells, and investigation of a possible role in the transcriptional regulation of adult β-globin gene expression. J Cell Sci. 1999;112:825–831. doi: 10.1242/jcs.112.6.825. [DOI] [PubMed] [Google Scholar]
- 12.Thompson KJ, Fried MG, Ye Z, Boyer P, Connor JR. Regulation, mechanisms and proposed function of ferritin translocation to cell nuclei. J Cell Sci. 2002;115:2165–2177. doi: 10.1242/jcs.115.10.2165. [DOI] [PubMed] [Google Scholar]
- 13.Surguladze N, Thompson KM, Beard JL, Connor JR, Fried MG. Interactions and reactions of ferritin with DNA. J Biol Chem. 2004;279:14694–14702. doi: 10.1074/jbc.M313348200. [DOI] [PubMed] [Google Scholar]
- 14.Cai CX, Birk DE, Linsenmayer TF. Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol Biol Cell. 1998;9:1037–1051. doi: 10.1091/mbc.9.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsenmayer TF, Cai CX, Millholland JM, et al. Nuclear ferritin in corneal epithelial cells: tissue-specific nuclear transport and protection from UV-damage. Prog Retin Eye Res. 2005;24:139–159. doi: 10.1016/j.preteyeres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Cai CX, Ching A, Lagace C, Linsenmayer T. Nuclear ferritin-mediated protection of corneal epithelial cells from oxidative damage to DNA. Dev Dyn. 2008;237:2676–2683. doi: 10.1002/dvdy.21494. [DOI] [PubMed] [Google Scholar]
- 17.Broyles RH, Belegu V, DeWitt CR, et al. Specific repression of β-globin promoter activity by nuclear ferritin. Proc Natl Acad Sci U S A. 2001;98:9145–9150. doi: 10.1073/pnas.151147098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surguladze N, Patton S, Cozzi A, Fried MG, Connor JR. Characterization of nuclear ferritin and mechanism of translocation. Biochem J. 2005;388:731–740. doi: 10.1042/BJ20041853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Luo C, Mines M, Zhang J, Fan GH. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281:37616–37627. doi: 10.1074/jbc.M607266200. [DOI] [PubMed] [Google Scholar]
- 20.Treffry A, Harrison PM. The functional role of isoferritins. Biochem Soc Trans. 1980;8:656–657. doi: 10.1042/bst0080656. [DOI] [PubMed] [Google Scholar]
- 21.Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Ann Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- 22.Shiau CE, Lwigale PY, Das RM, Wilson SA, Bronner-Fraser M. Robo2-Slit1 dependent cell-cell interactions mediate assembly of the trigeminal ganglion. Nat Neurosci. 2008;11:269–276. doi: 10.1038/nn2051. [DOI] [PubMed] [Google Scholar]
- 23.Hasan MR, Morishima D, Tomita K, Katsuki M, Kotani S. Identification of a 250 kDa putative microtubule-associated protein as bovine ferritin: evidence for a ferritin-microtubule interaction. Eur J Biochem. 2005;272:822–831. doi: 10.1111/j.1742-4658.2004.04520.x. [DOI] [PubMed] [Google Scholar]
- 24.Millholland JM, Fitch JM, Cai CX, Gibney EP, Beazley KE, Linsenmayer TF. Ferritoid, a tissue-specific nuclear transport protein for ferritin in corneal epithelial cells. J Biol Chem. 2003;278:23963–23970. doi: 10.1074/jbc.M210050200. [DOI] [PubMed] [Google Scholar]
- 25.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 26.Spurr SJ, Gipson IK. Isolation of corneal epithelium with dispase II or EDTA: effects on the basement membrane zone. Invest Ophthalmol Vis Sci. 1985;26:818–827. [PubMed] [Google Scholar]
- 27.Mete A, Van Zeeland YRA, Vaandrager AB, Van Dijk JE, Marx JJM, Dorrestein GM. Partial purification and characterization of ferritin from, the liver and intestinal mucosa of chickens, turtledoves and mynahs. Avian Pathol. 2005;34:430–434. doi: 10.1080/03079450500267908. [DOI] [PubMed] [Google Scholar]
- 28.Zak NB, Linsenmayer TF. Monoclonal antibodies against developmentally regulated corneal antigens. Dev Biol. 1983;99:373–381. doi: 10.1016/0012-1606(83)90287-7. [DOI] [PubMed] [Google Scholar]
- 29.Beazley KE, Nurminskaya M, Talbot CJ, Linsenmayer TF. Corneal epithelial nuclear ferritin: developmental regulation of ferritin and its nuclear transporter ferritoid. Dev Dyn. 2008;237:2529–2541. doi: 10.1002/dvdy.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J, Han J, Dunn MA. Effect of dietary aluminum on tissue nonheme iron and ferritin levels in the chick. Toxicology. 2000;142:97–109. doi: 10.1016/s0300-483x(99)00119-5. [DOI] [PubMed] [Google Scholar]
- 31.Passaniti A, Roth TF. Purification of chicken liver ferritin by two novel methods and structural comparison with horse spleen ferritin. Biochem J. 1989;258:413–419. doi: 10.1042/bj2580413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos Benito FF, Martin Mateo MC. Isolation, purification and characterization of spleen ferritin of Gallus domesticus L. Comp Biochem Physiol B. 1983;74:643–645. doi: 10.1016/0305-0491(83)90243-2. [DOI] [PubMed] [Google Scholar]
- 33.Drysdale J, Arosio P, Invernizzi R, et al. Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol Dis. 2002;29:376–383. doi: 10.1006/bcmd.2002.0577. [DOI] [PubMed] [Google Scholar]
- 34.Bou-Abdallah F, Santambrogio P, Levi S, Arosio P, Chasteen ND. Unique iron binding and oxidation properties of human mitochondrial ferritin: a comparative analysis with human H-chain ferritin. J Mol Biol. 2005;347:543–554. doi: 10.1016/j.jmb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Santambrogio P, Biasiotto G, Sanvito F, Olivieri S, Arosio P, Levi S. Mitochondrial ferritin expression in adult mouse tissues. J Histochem Cytochem. 2007;55:1129–1137. doi: 10.1369/jhc.7A7273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant RA, Filman DJ, Finkel SE, Kolter R, Hogle JM. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat Struct Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- 37.Ilari A, Stefanini S, Chiancone E, Tsernoglou D. The dodecameric ferritin from Listeria innocua contains a novel intersubunit ironbinding site. Nat Struct Biol. 2008;7:38–43. doi: 10.1038/71236. [DOI] [PubMed] [Google Scholar]
- 38.Bozzi M, Mignogna G, Stefanini S, et al. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. doi: 10.1074/jbc.272.6.3259. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Yoshida T, Okamoto K, Hirai S. Dopamine and DOPA cause release of iron from ferritin and lipid peroxidation of liposomes. NeuroReport. 1999;10:1883–1887. doi: 10.1097/00001756-199906230-00016. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal R, Sharma PK, Rao GS. Release of iron from ferritin by metabolites of benzene and superoxide radical generating agents. Toxicology. 2001;168:223–230. doi: 10.1016/s0300-483x(01)00412-7. [DOI] [PubMed] [Google Scholar]
- 41.Double KL, Maywald M, Schmittel M, Riederer P, Gerlach M. In vitro studies of ferritin iron release and neurotoxicity. J Neurochem. 1998;70:2492–2499. doi: 10.1046/j.1471-4159.1998.70062492.x. [DOI] [PubMed] [Google Scholar]