Abstract
ATP-insensitive KATP channel mutations cause neonatal diabetes mellitus (NDM). To explore the mechanistic etiology, we generated transgenic mice carrying an ATP-insensitive mutant KATP channel subunit. Constitutive expression in pancreatic β-cells caused neonatal hyperglycemia and progression to severe diabetes and growth retardation with loss of islet insulin content and β-cell architecture. Tamoxifen-induced expression in adult β-cells led to diabetes within 2-weeks, with similar secondary consequences. Diabetes was avoided by transplantation of normal islets under the kidney capsule, before induction. Moreover, the endogenous islets maintained normal insulin content and secretion in response to sulfonylureas, but not glucose, consistent with reduced ATP sensitivity of β-cell KATP channels. In NDM, transfer to sulfonylurea therapy is less effective in older patients. This may result from poor glycemic control or lack of insulin, since glibenclamide treatment prior to tamoxifen-induction prevented diabetes and secondary complications in mice, but failed to halt disease progression after diabetes had developed.
Keywords: Neonatal, Diabetes, KATP, transgenic, pancreas, mice, transplantation
INTRODUCTION
In the pancreatic β-cell, ATP-sensitive K+ (KATP) channels play a critical role in coupling membrane excitability to glucose-stimulated insulin secretion, thereby maintaining blood glucose within a narrow physiologic range. Increase in glucose metabolism leads to elevated intracellular [ATP]:[ADP], closure of KATP channels, and membrane depolarization, inducing voltage-dependent Ca2+-entry which, in turn triggers insulin secretion. Conversely, decrease in the metabolic signal opens KATP channels and suppresses the electrical trigger of insulin secretion. Sulfonylurea drugs, widely used in diabetes treatment, promote insulin secretion by binding to the regulatory sulfonylurea receptor-1 (SUR1) subunit and inhibiting KATP current, thus inducing insulin secretion (Ashcroft and Gribble, 1999; Nichols, 2006).
Mutations that result in “overactive” KATP channels should decrease membrane excitability and impair glucose sensing by the β-cell. Reduced insulin secretion and a diabetic phenotype is predicted, and genetic studies have now identified KATP channel mutations as the commonest cause of Neonatal Diabetes Mellitus (NDM) in humans (Gloyn et al., 2004b; Hamilton-Shield, 2007; Polak and Cave, 2007; Sperling, 2006; Vaxillaire et al., 2004). Predicting the human disease, we previously demonstrated that mice constitutively expressing ATP-insensitive β-cell KATP channels develop profound neonatal diabetes (Koster et al., 2000). These mice die shortly after birth, precluding detailed analysis of disease progression. We have now generated a novel inducible KATP ‘gain-of-function’ transgenic mouse by insertion of an ATP-insensitive Kir6.2 construct into the Rosa26 locus under Cre-recombinase control. These mice permit selective induction of transgene expression in any tissue of interest, thereby modeling tissue-specific expression of NDM mutations, when crossed into appropriate Cre expressing mice. By crossing with Rat-Insulin-Promoter driven Cre-recombinase expressing (Rip-Cre) (Herrera, 2002) or tamoxifen-inducible Pdx1PBCreER™ (Pdx-Cre) (Zhang et al., 2005) mice, we generated pancreatic specific Rip-Cre/Rosa26-Kir6.2[K185Q,ΔN30] double transgenic (Rip-DTG) or Pdx-Cre/Rosa26-Kir6.2[K185Q,ΔN30] double transgenic (Pdx-DTG) mice. Rip-DTG and Pdx-DTG mice develop severe glucose intolerance around weaning or within two weeks after tamoxifen injection, respectively, and progress to severe diabetes. These mice survive with undetectably high blood sugars through adulthood, but demonstrate growth retardation, profound loss of β-cell mass and dramatic reduction of total insulin content. The secondary progression of the disease is completely avoided by syngeneic islet transplantation or early onset sulfonylurea therapy. These results have important implications for understanding human NDM progression, and therapeutic possibilities.
RESULTS
β-cell expression of ATP-insensitve channels
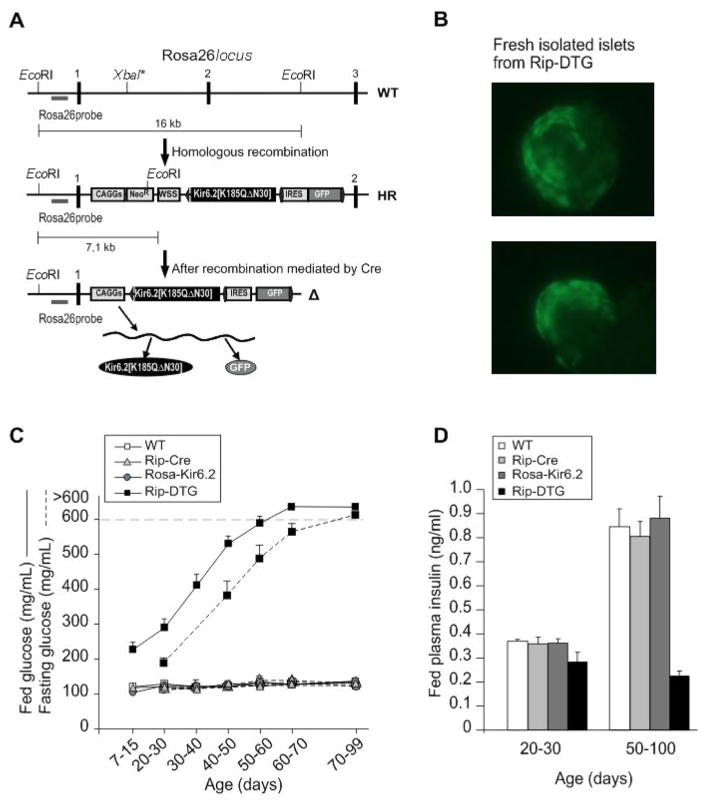
NDM mutations in KATP channels all cause loss of ATP sensitivity, either by reduced ATP affinity (mutations in the ATP binding site), or allosterically by enhancing open probability. By combining an ATP binding site mutation (K185Q) with an allosteric gating mutation (truncation of the N-terminal 30 amino acids, ΔN30) we have generated channels with significantly lowered ATP-sensitivity (Koster et al., 1999b). This Kir6.2[K185Q,ΔN30] was targeted to the Rosa26 locus in ES cells, using a Neo/WSS cassette which leads to expression of both the Kir6.2[K185Q,ΔN30] protein and GFP via an internal ribosome entry site (IRES), upon Cre-mediated recombination. Correctly targeted ES cells were injected into CB20 blastocysts. We obtained several chimeras, and successful germline transmission was obtained in one line (Fig. 1A). Rosa26-Kir6.2[K185Q,ΔN30] (Rosa26-Kir6.2) transgenic mice were first crossed with rat insulin promoter driven Cre-recombinase (Rip-Cre) mice in order to drive transgene expression in β-cells. Rip-Cre/Rosa26-Kir6.2[K185Q,ΔN30] double transgenic mice (Rip-DTG) were born at the expected frequency and even though these mice develop a dramatic diabetic phenotype over time, neonatal lethality was avoided, with 100% survival into adulthood. Consistent with transgene expression, β-cell-specific green fluorescence was present in islet cells from Rip-DTG mice, but not in the 3 control genotypes (WT, Rip-Cre or Rosa26-Kir6.2, Fig. 1B).
Figure 1. Induced expression of ATP-insensitive Kir6.2 subunits leads to development of severe diabetes in Rip-DTG mice.
(A) Generation of mice with inducible Kir6.2[K185Q,ΔN30] expression. The targeting strategy for generation of a Cre-inducible Kir6.2[K185Q,ΔN30] transgene in the ROSA26 locus is shown. ES cells were transfected with the depicted vector, and homologous recombinants were detected by Southern Blot (not shown). Correctly targeted ES cells were injected into blastocysts. (B) Cre-mediated recombination of the Neo/WSS cassette leads to expression of the Kir6.2[K185Q,ΔN30] protein but also to expression of a GFP via an internal ribosome entry site (IRES). Freshly isolated islets from Rip-Cre/Rosa26-Kir6.2[K185Q,ΔN30] (Rip-DTG) animals show diffuse green fluorescence in the core of the islet, reflecting transgene expression in β-cells. (C) Fed (solid) and fasted (dashed) blood glucose levels from Rip-DTG and control (WT, Rip-Cre and Rosa26-Kir6.2[K185Q,ΔN30] (Rosa-Kir6.2)) littermates over time (n=8–10 mice in each group, mean ± SEM). (D) Fed plasma insulin from all 4 genotype mice at 20–30 day-old and 50–100 day-old.
Progressive neonatal diabetes in Rip-DTG mice
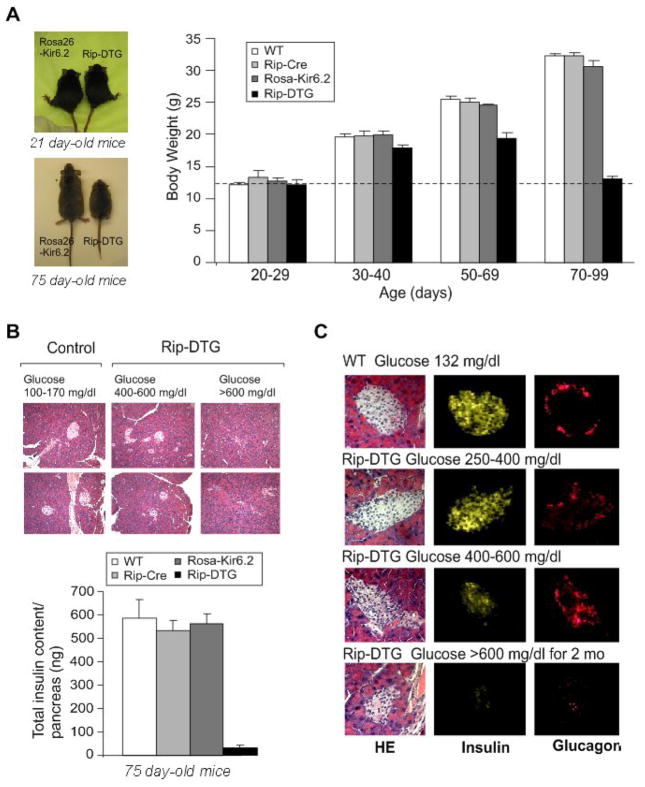
In contrast to our previous report on constitutively ‘overactive KATP‘ transgenic mice, no neonatal lethality was seen in RIP-DTG mice, although blood glucose was consistently >200 mg/dl, within the first two weeks, and there was a progressive elevation of fed and fasted glucose through-weaning, reaching unmeasurable high levels (>600 mg/dl) at around 2 months (Fig. 1C). Concomitant with the developing hyperglycemia, plasma insulin levels were decreased at early stages and dropped close to the level of detection (0.18 ng/ml) at late stages (Fig. 1D). Importantly, none of these symptoms were present in control littermates (Fig. 1), indicating no aberrant expression of the Rosa26-Kir6.2[K185Q,ΔN30], nor detrimental effects of RIP-Cre expression. Neonatal Rip-DTG mice had normal body weight. However, significant growth retardation accompanied the subsequent progression of the disease (Fig. 2A, ~50% reduction in body weight compared with control littermates by about 3 months, paralleled by similar reduction in pancreatic weight, such that pancreatic-weight/body-weight ratio remained normal, data not shown). However, the insulin content of the pancreas and the islet mass dropped precipitously over this time (to less than 10%) in Rip-DTG mice, with no changes in control littermates (Fig. 2B).
Figure 2. Growth retardation accompanied by dramatic changes in islet morphology and hormone content in diabetic Rip-DTG animals in late stages of diabetes.
(A) (left) Photographs of Rip-DTG mice and single transgenic littermates at 21 days and 75 days. (right). Body weight as a function of age for Rip-DTG, WT and single genotype littermates (n = 12–15 in each case). (B) (top) Low magnification (20x) pancreatic sections from Rip-DTG and control animals stained with hematoxylin-eosin and (lower) pancreatic insulin content as a function of age for Rip-DTG, WT and single genotype littermates (mean ± SEM, n = 4–8 mice in each case). (C) Hematoxylin-eosin staining (left), insulin (middle) and glucagon (right) immunostaining of pancreatic paraffin sections from WT control (top) and Rip-DTG mice at early (middle) and late stages of disease (below).
Immuno-histochemical analyses were performed on sectioned pancreata. Low magnification images of Hematoxylin-Eosin stained sections indicate reduced number of islets per pancreas in Rip-DTG (by ~50 % in moderate to >90% in severe diabetic mice, Fig. 2B). High magnification images (Fig. 2C) suggest reduced islet size with time, although islet integrity is maintained. Immunostaining for insulin and glucagon confirms relatively normal distribution of both insulin-containing β–cells and glucagon-containing α-cells in early stages of the disease (Fig. 2C, upper panels). However α-cell infiltration, evidenced by glucagon staining in the core of the islet, together with reduced insulin immunofluorescence was observed in later stages of the disease, and at the latest stages (glucose >600 mg/dl for > 3 weeks), both insulin-and glucagon-staining essentially disappears (Fig. 2C, middle and lower panels).
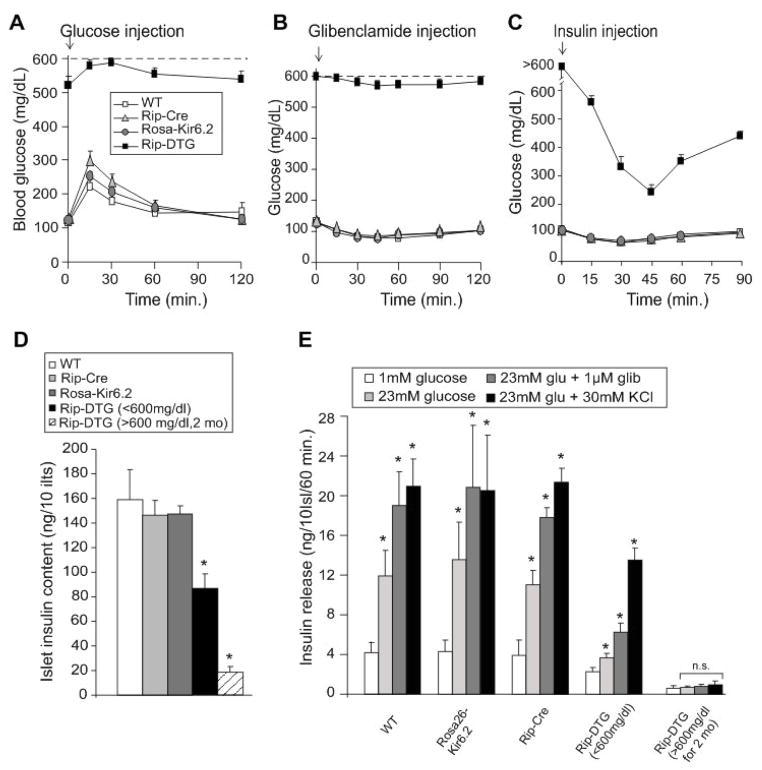
Consistent with the elevated blood glucose levels, Rip-DTG animals over 6 weeks old showed impaired, but not completely absent, responses to glucose and glibenclamide injection (Fig. 3A, B), although direct comparison to littermate controls is difficult, given the elevated glucose baselines. Similarly, insulin sensitivity is not readily comparable between Rip-DTG and other genotypes, but dramatic glucose lowering was observed in response to insulin in Rip-DTG animals (Fig. 3C). These mice reiterate a profound diabetes, but the time course is slower than that which we observed with constitutive Rip expression (Koster et al., 2000), potentially a result of incomplete expression of the transgenes, or perhaps slow induction of the Cre-lox system.
Figure 3. Insulin sensitivity and secretion in Rip-DTG and control littermate mice.
(A) Intraperitoneal glucose tolerance test on fasted WT, Rip-DTG and single transgenic littermate mice at 6 weeks of age. Blood [glucose] versus time following injection of 1.5 g/kg glucose (mean + SEM, n= 8–11 animals in each group). (B, C) Response of fed WT, Rip-DTG and single transgenic littermate mice at 6 weeks of age to single i.p. injection of glibenclamide (1.0 mg/g, B) or response of 6 hours fasted mice to i.p. injection of insulin (0.5 mU/g, C) (mean + SEM, n= 6–10 animals in each group). (D) Insulin content of isolated islets from WT, Rip-DTG and single transgenic littermate mice, at 6 weeks of age, and Rip-DTG also determined at ~3 months of age (glucose >600 mg/dl for > 2 months, dashed bar) (mean ± s.e.m., n= 5–7 animals in each case). (E) Glucose-dependent insulin secretion from isolated islets as in D. Batches of 10 islets were statically pre-incubated with low glucose and then incubated at different glucose concentrations (with or without glibenclamide and K+) for 1hour. Insulin release was measured in the media by radioimmunoassay (n=5–7 mice in each group, samples done in triplicates). Significant differences *p<0.05 between test and WT mice (D) or 1 mM glucose (E) are indicated.
Glucose-dependent insulin secretion is lostin Rip-DTG mice
Consistent with the overt diabetes and drastic loss of pancreatic insulin content and islet insulin staining in adult Rip-DTG mice, measured insulin content was negligible in isolated RIP-DTG islets from animals with blood glucose >600 mg/dl (Fig. 3D), and there was negligible insulin secretion from these islets, in response to glucose, glibenclamide, or KCl (Fig. 3E). However insulin content of islets isolated from animals with blood glucose between 400–600 mg/dl was still ~60% of control (Fig. 3D). Glucose-dependent secretion from these islets was negligible, as predicted for the expected KATP overactivity, but there was a significant response to glibenclamide, and release in response to K+ depolarization was essentially normal, indicating no impairment of secretory process downstream of the electrical signal, at this stage (Fig. 3E).
Similar diabetic progression following induction of ROSA-Kir6.2 in adults
The above results demonstrate that Rip-Cre-induced expression of the Kir6.2[K185Q,ΔN30] transgene reiterates the expected primary consequences of ATP-insensitive KATP channels, but also shows that this secondarily leads to a dramatic loss of insulin content and unresponsivity to sulfonylureas, that is not naively predicted. In patients with NDM it has been observed that the ability to transfer to sulfonylurea therapy is greater when the transfer is attempted at a young age (Pearson et al., 2006), and that older adults, even with relatively less severe mutations, cannot always transfer (e.g. Masia et al., 2007). The profound secondary loss of insulin content that we observe in Rip-DTG animals underlies a gradual loss of glibenclamide sensitive secretion (Fig. 3E), leading us to suggest that the same secondary progression may contribute to the failure to transfer to sulfonylureas in older adults (see below).
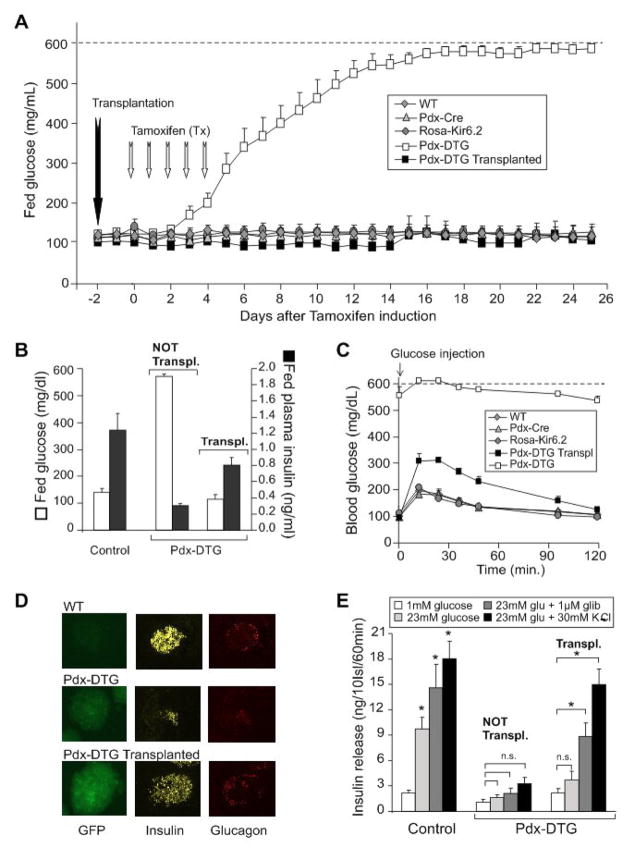
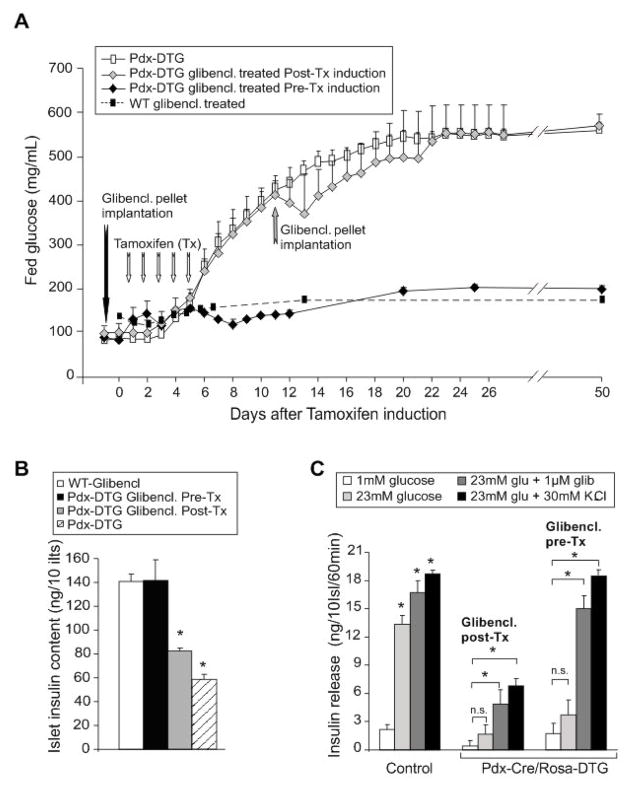
What is the cause of the secondary loss of insulin content? ‘Glucotoxicity’, as a result of the profound systemic hyperglycemia, or low circulating insulin levels, are likely mechanisms, leading to death of β-cells (Poitout and Robertson, 2002), and we thus hypothesize that maintenance of normoglycemia/normal insulin levels might protect against this secondary consequence. One way to test this is to transplant healthy normal islets into the transgenic animals prior to the development of diabetes. However, this is not a trivial procedure in neonatal animals, and we have therefore utilized a second induction system that permits temporal control of the onset of transgene induction. Tamoxifen-inducible Pdx-Cre mice were crossed with Rosa26-Kir6.2[K185Q,ΔN30] transgenic mice, resulting in animals in which the transgene was expected to be activated in Pdx-expressing islet cells, only following tamoxifen injection. As shown in Fig. 4, non-injected Pdx-Cre/Rosa26-Kir6.2[K185Q,ΔN30] double transgenic (Pdx-DTG) mice maintained normal blood glucose as adults, as well as normal glucose tolerance (not shown). To induce expression, Pdx-DTG and littermate control mice were injected daily with tamoxifen (50 μg/g body weight) for 5 days. Within two days of the onset of injections, blood glucose began to rise and reached unmeasurable levels in ~2 weeks, in Pdx-DTG animals, with no effects on single transgenic or wild type littermates (Fig. 4A). Thus the NDM disease phenotype can be readily induced in adulthood, following transgene induction. Moreover, the secondary consequences appear to be as severe as when the disease is initiated in the neonatal period, since, as shown in Fig. 4D, islet insulin content essentially disappears in Pdx-DTG within 3 weeks of tamoxifen induction.
Figure 4. Rapid development of severe diabetes in tamoxifen-induced Pdx-DTG mice, and complete rescue by syngeneic transplant of normal islets.
(A) Fed blood glucose levels from Pdx-DTG and control (WT, Pdx-Cre and Rosa-Kir6.2) littermate mice following onset of tamoxifen induction (at 6 weeks of age, n = 6–8 mice in each group). Transplantation of 300 syngeneic islets from single transgene or control littermates was performed in a sub-set of Pdx-DTG animals (n=5, black squares) two days before the onset of tamoxifen induction. (B) Blood glucose and plasma insulin levels in fed Pdx-DTG (transplanted and non-transplanted as indicated) and control littermate mice, 3 weeks following tamoxifen induction (n=5–8 mice in each group). (C) Intraperitoneal glucose tolerance test on Pdx-DTG (transplanted, black squares and non-transplanted, white squares) and control littermate mice. Blood [glucose] versus time following injection of 1.5 g/kg glucose (mean + SEM, n= 4–5 animals in each group). (D) Intrinsic GFP fluorescence of representative freshly isolated islets (left), as well as insulin (middle) and glucagon (right) immunostaining of pancreatic paraffin sections from WT (top) and from transplanted and non-tranplanted Pdx-DTG mice at 3 weeks following induction (below). (E) Glucose-dependent insulin secretion from isolated islets. Batches of 10 islets were statically pre-incubated with low glucose and then incubated at different glucose concentrations (with or without glibenclamide or K+) for 1 hour. Released insulin was measured by radioimmunoassay. n=4–7 mice in each group, samples done in triplicates. Significant differences *p<0.05 between test and 1 mM glucose control are indicated.
Maintenance of normoglycemia completely prevents secondary consequences of NDM
In order to test whether it is the chronic hyperglycemia/hypoinsulinemia that leads to the β-cell loss in DTG animals, we attempted to maintain normoglycemia by transplanting Pdx-DTG mice with 300 syngeneic islets under the kidney capsule, 2 days prior to onset of tamoxifen induction (Montana et al., 1993). As shown in Fig. 4A, this resulted in a dramatic avoidance of diabetes, and transplanted mice maintained normoglycemia and near normal plasma insulin levels for the ensuing 3 weeks (Fig. 4B), at which point the animals were sacrificed and islets isolated. Just prior to termination, glucose tolerance tests were performed (Fig. 4C). Transplanted Pdx-DTG animals showed mild glucose intolerance, but still returned to basal glucose within ~2 hours.
Most significantly, the endogenous islets from transplanted animals failed to develop any of the secondary morphological changes or loss of insulin content. By three weeks following induction, islet insulin content was essentially normal, as was α- and β-cell distribution within the islets (Fig. 4D). Consistent with the drastic loss of insulin content in Rip-DTG animals, there was negligible insulin secretion from non-transplanted, tamoxifen-induced Pdx-DTG islets (Fig. 4E). In endogenous islets from transplanted Pdx-DTG mice, there was also no glucose-stimulated insulin secretion, as expected. However, these islets retained essentially normal secretion in response to K+ depolarization, and significant release in response to the sulfonylurea glibenclamide (Fig. 4E). It thus appears that maintenance of normoglycemia does indeed lead to avoidance of all secondary consequences of the diabetes. As discussed below, these findings have implications for the progress of human NDM and for the potential benefits of maintaining good glycemic control through early sulfonylurea intervention.
Sulfonylurea treatment prevents secondary consequences
It is now clear that sulfonylurea drugs can effectively trigger insulin secretion in NDM patients, even after long-standing disease, although it is also the general finding that dose requirements tend to increase with length of disease, and in some cases, sulfonylurea therapy is ineffective. We therefore asked whether sulfonylurea therapy can protect these mice from hyperglycemia and the secondary consequences of diabetes. To achieve chronic dosing of sulfonylureas, adult Pdx-DTG mice were implanted with slow-release glibenclamide pellets under the skin (Remedi and Nichols, 2008), two days before onset, or 6 days after cessation, of tamoxifen induction. In Pdx-DTG mice treated with glibenclamide before tamoxifen induction, fed glucose and islet insulin content remained essentially normal at 60 days post-induction (Fig. 5). These results dramatically illustrate the effectiveness of sulfonylureas to trigger release of endogenous insulin and protect against secondary consequences of the diabetes. Moreover, isolated islets from these mice show normal responses to K+ depolarization and to glibenclamide, but do not respond to glucose, exactly as expected (Fig. 5C). Critically, however, glibenclamide treatment was ineffective when initiated following the development of diabetes (glucose ~400 mg/dl): blood glucose continued to rise to above 600 mg/dl (Fig. 5A) and, at 60 days, insulin content was significantly reduced (Fig. 5B) and insulin release substantially reduced (Fig. 5C). It is important to note that glucose had already risen to quite high (~400 mg/dl) levels at pellet implantation in the latter animals but, as discussed below, the finding that glibenclamide therapy is ineffective at this stage may have important implications for the therapeutic outcome in NDM patients with long-standing or poorly controlled disease.
Figure 5. Rescue of diabetes and its secondary consequences in Pdx-DTG mice treated with sulfonylureas.
(A) Fed blood glucose levels from Pdx-DTG treated with glibenclamide pre- and post-tamoxifen induction respect to controls (WT- glibenclamide treated and Pdx-DTG untreated mice). At 6 weeks of age, Pdx-DTG mice were implanted with slow-release glibenclamide pellets 1-day before (black diamonds) or following onset of tamoxifen induction (grey diamonds) (n = 4 mice in each group). Dashed line shows response of WT mice to glibenclamide implantation (from Remedi and Nichols, 2008) (B) Insulin content of isolated islets from glibenclamide treated WT and Pdx-DTG mice 60 days after tamoxifen induction (mean ± s.e.m., n= 4 animals in each group). (C) Glucose-dependent insulin secretion from isolated islets. Batches of 10 islets were statically pre-incubated with low glucose and then incubated at different glucose concentrations (with or without glibenclamide or K+) for 1 hour. Released insulin was measured by radioimmunoassay. n=3 mice in each group, samples done in triplicates, in Pdx-DTG animals, implanted with glibenclamide pellets (before (pre-) or after (post-) induction, or control littermates. Significant differences *p<0.01 between test and 1 mM glucose control are indicated.
Reduced ATP sensitivity of KATP currents in Pdx-DTG β-cell membranes
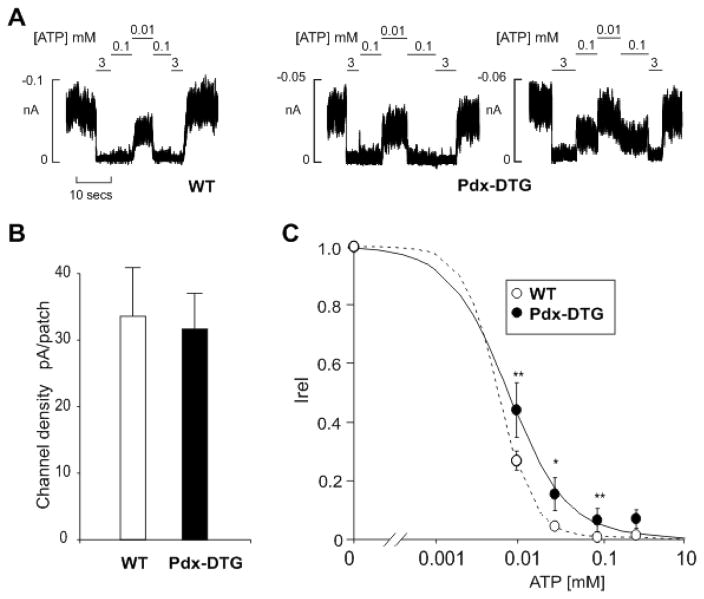
In recombinant expression, Kir6.2[K185Q,ΔN30] mutant channels show marked (~100-fold) reduction of ATP sensitivity (Koster et al., 1999b). In all NDM-associated Kir6.2 mutations, the underlying molecular phenotype is also reduction of ATP sensitivity of expressed channels, although loss of ATP-sensitivity has not been demonstrated in NDM β-cells. In the disease case, the mutation is heterozygous (as are the DTG mice), and so the expected channel phenotype in β-cells should be generated by mixed expression with wild type subunits, which typically results in much weaker shifts in ATP sensitivity (Gloyn et al., 2004b). Consistent with this, endogenous β-cell KATP channels isolated from non-diabetic transplanted Pdx-DTG adult mice displayed a mild but significant decrease in ATP sensitivity relative to channels from control littermate membranes (Fig. 6A, C). Importantly, there is higher activity in the millimolar ATP range, consistent with a causal basis for the observed disease. It is noteworthy that the overall KATP density is not different in the double transgenic case (Fig. 6B), excluding gross overexpression of KATP subunits as an artefactual basis for the diabetes in non-transplanted animals, and implicating the critical effect of reduced ATP sensitivity in the disease mechanism.
Figure 6. Reduced ATP-sensitivity, but normal density, of KATP currents in primary β-cells from transplanted Pdx-DTG animals.
(A) Representative currents recorded from inside-out membrane patches from WT and Pdx-DTG pancreatic β-cells at −50 mV in K-INT solution (see Experimental Procedures). Membrane patches were exposed to different ATP concentrations as shown. (B) KATP channel density and (C) steady-state dependence of membrane current on [ATP] relative to current in zero ATP [Irel]) for KATP channels from wild-type and Pdx-DTG β-cells. Data points represent means + SEM, n= 3 animals (15–30 patches). The fitted lines in C correspond to least squares fits of the Hill equation, with the H (Hill coefficient), and K ½ values allowed to vary. For WT, H= 2, K 1/2= 6 μM (dashed line) and for Pdx-Cre/Rosa-DTG, H= 1.2, and K 1/2= 8 μM (solid line). Significant differences *P < 0.05 and **P < 0.01 from transgenic respect to control cells.
Glucagon secretion and content are lost in severely diabetic mice
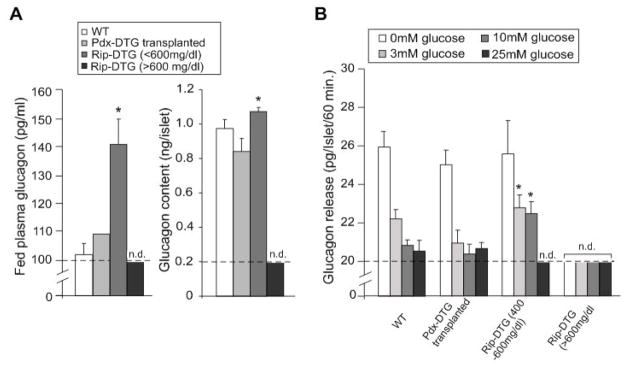
Both Rip and Pdx-1 driven transgenes should be expressed primarily in β-cells of the adult mice, but it is possible that either aberrant expression in α-cells, or lack of paracrine effects of insulin secretion on α-cell function, could affect glucagon content and release in these animals. To examine this, we determined glucagon levels, as well as islet glucagon content and glucose-dependent glucagon release. In early stages of disease, Rip-DTG mice showed a significant increase in plasma glucagon, and enhanced glucagon release at higher glucose levels, possibly due to the lack of inhibitory effect of insulin on α-cells, or enhanced glucagon content in isolated islets (Figs. 7A, 2C). However, at late stages of the disease, plasma glucagon, as well as islet glucagon content and release, were undetectable (Fig. 7A), consistent with the absence of glucagon staining in isolated islets from severely diabetic mice (Fig. 2C). Importantly, endogenous islets from transplanted Pdx-DTG animals show essentially normal glucagon content and release (Fig. 7), consistent with the profound loss of α-cells in the untreated Rip-DTG islets being due to hyperglycemia/hypoinsulinemia and further confirming that maintenance of normoglycemia does indeed lead to avoidance of all secondary consequences of diabetes, including pancreatic α-cell loss.
Figure 7. Glucagon content and secretion in Rip-DTG diabetic mice.
(A) Plasma glucagon and glucagon content per islet from WT, Rip-DTG and syngeneically transplanted Pdx-DTG mice determined at ~3.5 months of age (mean ± s.e.m., n= 3 animals in each case). Plasma glucagon and glucagon content were determined by radioimmunoassay (see Experimental Procedures). Significant differences *p<0.05 between test and Wt are indicated. (B) Glucose-dependent glucagon secretion from isolated islets as in A. Batches of 10 islets were statically incubated at different glucose concentrations for 1 hour. Glucagon release was measured in the media by radioimmunoassay (n=3 mice in each group, samples done in triplicates). Significant differences *p<0.05 between test and same glucose concentration from controls are indicated.
DISCUSSION
Physiological overactivity of KATP channels as cause of NDM
Closure of KATP in response to glucose metabolism couples membrane excitability to β-cell insulin secretion. KATP mutations that cause loss of ATP sensitivity are thus expected to maintain the membrane in a hyperpolarized state with hypo-secretion of insulin. We previously generated transgenic mice constitutively expressing mutant KATP channels with reduced ATP-sensitivity, under rat insulin promoter control (Koster et al., 2000). These mice were profoundly diabetic, with marked reduction in serum insulin levels and dramatic elevation of blood glucose and ketone (D-3-hydroxybutyrate) levels as neonates, and essentially all mice died within the first week (Koster et al., 2000). These mice predicted subsequent genetic studies which demonstrate that similar mutations, located throughout both the pore-forming Kir6.2 subunits and the regulatory SUR1 subunits of the KATP channel underlie permanent and transient neonatal diabetes mellitus (NDM) in humans (Gloyn et al., 2004a; Gloyn et al., 2004b; Gloyn et al., 2005; Hattersley and Ashcroft, 2005; Massa et al., 2005; Sagen et al., 2004; Vaxillaire et al., 2004). In all cases, the identified mutations cause a loss of ATP-sensitivity of recombinant KATP channels, and are predicted to cause reduced excitability of the β-cell and hence inhibition of insulin secretion, in vivo (Gloyn et al., 2004b; Koster et al., 2007; Koster et al., 2005; Masia et al., 2007; Proks et al., 2006; Proks et al., 2005; Tammaro et al., 2006). This realization has led to dramatic change in therapeutic options, and many patients have now successfully transferred from injected insulin therapy to sulfonylurea tablets (Gloyn et al., 2006; Hattersley and Ashcroft, 2005; Koster et al., 2007; Masia et al., 2007; Pearson et al., 2006). Sulfonylurea sensitivity of channel activity also tends to be mechanistically coupled to ATP sensitivity (Koster et al., 1999a; Reimann et al., 1999), such that many of these disease mutations also reduce channel sensitivity to sulfonylureas (Koster et al., 2005), which may contribute to the generally observed requirements for high doses of sulfonylureas in treatment of NDM patients (Hattersley and Ashcroft, 2005).
KATP channel overactive mice as a model of human NDM
However, successful transfer from insulin to sulfonylureas has not proven possible in all cases; generally transferability seems negatively correlated with the patient age (Hattersley and Ashcroft, 2005; Hattersley and Pearson, 2006), and there are several examples of successful transfer of an infant, but not of the parent afflicted with the same mutation (Pearson et al., 2006). This might suggest that secondary loss of insulin availability may be a problem over the course of the disease. In order to assess islet function in isolation, and to assess long-term consequences and mechanisms of NDM suitable animal models are required. The constitutively expressing ATP-insensitive Kir6.2 transgenic animals that we first generated (Koster et al., 2000) reiterate essential features of human NDM, but neonatal death precluded detailed study. In order to overcome these limitations, we have developed an inducible model utilizing a Cre-induction strategy. The Rosa26-Kir6.2[K185Q,ΔN30] transgene incorporates two mutations that result in significant loss of ATP sensitivity both by disruption of the ATP binding site (K185Q) and by increase in intrinsic open state stability (ΔN30) (Koster et al., 1999b), and thus reiterates essential features of NDM mutations in recombinant channels.
Blood sugar is elevated within the first few days of life in Rip-DTG mice (Fig. 1), but contrary to our previous constitutive KATP channel transgenic mice, glucose does not become unmeasurably high until after weaning, and neonatal death is avoided (Figs. 1C, D). Consistent with the subsequent progression from mild to marked hyperglycemia, serum insulin levels decrease over time, and are undetectable after ~2 months, by which time the mice show significant growth retardation. At this stage, the animals are profoundly intolerant to glucose and glibenclamide injections, although blood glucose lowering is still observed in response to insulin injections, suggesting that insulin sensitivity is still normal. Pdx-DTG mice remain essentially normal through adulthood, unless the transgene is induced by tamoxifen injection. Following tamoxifen induction there is an accelerated but otherwise identical development of diabetes to that seen in Rip-DTG, with glucose beginning to rise within two days, and becoming unmeasurable by two weeks (Fig. 4). The similar progression of the disease with two distinct activator transgenes is an important control and argues against any aberrant effects of the Rip-Cre or Pdx-Cre expression per se.
Morphologically, isolated islets appear essentially normal at early stages of the disease in both Rip-Cre and Pdx-Cre mice (Fig. 2C), with normal distribution of insulin and glucagon. However, dramatic changes are seen as the disease progresses, with drastic loss of islet cells as well as reduced number of islets per pancreas (assessed by immunocytochemistry), in both DTG models (Figs. 2C, 4D). At these late stages, whole body growth retardation is evident, with parallel reduction of pancreatic weight, suggesting an overall growth inhibition as a result of the profound insulinopenia. Isolated islets from both DTG animals showed dramatic loss of insulin content, and failed to secrete insulin in response to glucose, glibenclamide, or K+ depolarization (Figs.3,4). Notably however, islets isolated from Rip-DTG mice did respond to high K+ depolarization at early stages of the disease, reflecting maintained insulin content (Fig. 3), and indicating that the insulin secretory machinery, downstream of the electrical signal, is intact at this stage.
An unanticipated finding of the present study was that glucagon content initially increases in diseased islets and subsequently falls to undetectable levels, with concomitant changes in glucagon secretion and blood levels (Fig. 7). Relevant data from NDM patients is not available, although Type-2 diabetic patients can show hyperglucagonemia, and Type-1 patients can progress to lack of glucagon response to hypoglycemia (Bansal and Wang, 2008; Brown et al., 2008; Del Prato and Marchetti, 2004). At this juncture, we can only speculate as to likely mechanisms. Early hyperglucagonemia may well be a consequence of lack of inhibitory effects of paracrine secretion of insulin (Bansal and Wang, 2008; Rorsman et al., 2008) but, at later stages, islet death resulting from systemic hyperglycemia/hypoinsulinism leads to loss of both insulin-containing β-cells and glucagon-containing α-cells.
Maintenance of normoglycemia prevents secondary complications
Mechanistically, the secondary loss of islet cells could be the result of an intrinsic process, potentially a result of chronic hyperpolarization and low intracellular [Ca2+], or it could result from the systemic hyperglycemia or lack of insulin that is present. By transplanting syngeneic islets into the kidney capsule prior to tamoxifen-induction in Pdx-DTG animals we have demonstrated that the latter explanation is correct (Fig. 4). Even though the transgene is expressed at high level (indicated by GFP fluorescence), these animals are completely spared from diabetes (which incidentally seems to rule out a paracrine mechanism by which the diseased islets secrete an agent that acts extracellularly to kill β-cells), and maintain normal islet morphology and insulin content, over the time course that would otherwise lead to complete loss of insulin content (Fig. 5B). In these animals, the diseased endogenous islets retain normal insulin secretion in response to KCl, and near normal response to glibenclamide (Fig. 5B), yet glucose-dependent secretion is nevertheless essentially absent, consistent with the expected consequences of ATP-insensitivity of membrane KATP channels. Since islet viability is unaffected in these mice, we were able to assess the functional properties of endogenous β-cell KATP channels. Consistent with mixed expression of transgenic and wild type subunits, β-cell KATP channel density was essentially normal, but there was a significant reduction of ATP sensitivity (Fig. 6). By increasing KATP activity at any given glucose concentration, this will correspondingly inhibit secretion in response to glucose, but not in response to K+ or glibenclamide.
The transplantation results clearly show that secondary loss of insulin content and secretion is a consequence of systemic diabetes. Patients with NDM typically first present with extremely high glucose levels, and glucose control on insulin therapy is subsequently frequently poor. We speculate that exposure to episodic hyperglycemia may lead to similar secondary progression in human NDM patients as we observe in DTG mice. Such a progression may underlie the finding that the ability to transfer to sulfonylureas (i.e. to trigger endogenous insulin secretion) declines with duration of the disease (Pearson et al., 2006). This is further supported by the clear demonstration that early sulfonylurea intervention in the Pdx-DTG mice can completely prevent diabetes and secondary complications following subsequent tamoxifen induction, whereas glibenclamide is ineffectual once the disease has developed and insulin content has been lost (Fig. 5).
EXPERIMENTAL PROCEDURES
Generation of Rosa26-Kir6.2[K185Q,ΔN30] mice
Fig. 1 shows the targeting strategy we used for generation of a Cre-inducible Kir6.2[K185Q,ΔN30] transgene (Koster et al., 2000) in the Rosa26 locus. ES cells were transfected with the depicted vector, and homologous recombinants were detected by Southern Blot. Correctly targeted ES cells were injected into blastocysts. We obtained several chimeras, and successful germline transmission into offspring was confirmed by PCR. Note that Cre-mediated recombination of the Neo/WSS cassette leads to expression of the Kir6.2[K185Q,ΔN30] protein but also to expression of a GFP via an internal ribosome entry site (IRES), therefore GFP can be used to detect successful recombination.
We generated a Rosa26 locus targeting vector in which CAG-promoter driven expression of the Kir6.2[K185Q,ΔN30] mutant and IRES-GFP is prevented by a loxP-flanked Stop-cassette. This vector was transfected into V6.5 ES cells that were screened for correct integration by standard Southern blot methods. Correctly targeted ES cells were used to generate chimeras, which were backcrossed to C57/Bl6 background and examined for germline transmission. All Rosa26-Kir6.2[K185Q,ΔN30] mice were heterozygous for the Kir6.2[K185Q,ΔN30] transgene.
Blood glucose and plasma Insulin and glucagon
All experiments were performed in compliance with the relevant laws and institutional guidelines, and were approved by the Washington University Animal Studies Committee. Tail blood, in fed/fasting conditions was assayed for glucose content using the Glucometer Elite XL described above. Plasma insulin and glucagon were measured using a Rat Insulin enzyme-linked immunoabsorbent assay kit (ELISA Kit) and glucagon radioimmunoassay (RIA), respectivelly.
Glucose- and Insulin-tolerance tests
Intraperitoneal Glucose Tolerance Test was performed in 12 hr. fasted mice by injection of a bolus of glucose (1.5 g/kg body weight). For Insulin Tolerance Test, mice were injected with 0.5 U insulin/Kg body weight following 5-hr fast. Blood was taken at different times (indicated in figures) and assayed for glucose content as above.
Glibenclamide-sensitivity test
Mice were intraperitoneally injected with glibenclamide (30 μg/Kg body weight). Only double transgenic mice (diabetic) were also injected with higher doses of glibenclamide (100 and 400 μg/Kg body weight). Blood was taken at different times and assayed for glucose content as above.
Total insulin content per pancreas
Whole pancreas was removed and disrupted in homogenization buffer containing a mixture of protease, phosphatase and kinase inhibitors using a Polytron© homogenizer. Samples were centrifuged at low-speed and supernatants were used to measure insulin content. Rat Insulin radioimmunoassay according to manufacturer’s procedure (Linco Inc., St. Charles, MO) was used to determine total insulin content per pancreas.
Islet isolation
Mice were anesthetized with halothane© (0.2 ml) and killed by cervical dislocation. The bile duct was cannulated and perfused with Hank’s balanced salt solution (Sigma Corp., St. Louis, MO) containing collagenase (0.3 mg/ml, Collagenase Type XI, Sigma Corp., St. Louis, MO). Pancreata were removed and digested for 5 min at 37°C, hand shaken and washed 3 times in cold Hank’s solution. Islets were isolated by hand under a dissecting microscope and pooled islets were maintained in CMRL-1066 culture medium (GIBCO) supplemented with fetal calf serum (FCS, 10%), penicillin (100 units/ml), and streptomycin (100 μg/ml).
Insulin and glucagon release
Following overnight incubation in low glucose (5.6 mM) CMRL-1066 medium, islets (10 per well in 12 well plates) were pre-incubated in glucose-free CMRL-1066 plus 3 mM glucose (only for insulin secretion), then incubated for 60 min at 37°C in CMRL-1066 plus different [glucose], 1 μM glibenclamide, or 30 mM KCl, as indicated. After the incubation period, the medium was removed and assayed for insulin or glucagon release. Islets were disrupted using ethanol-HCl extraction and sonicated on ice prior to estimation of insulin and glucagon content. Experiments were repeated in triplicate. Insulin and glucagon secretion and content were measured by RIA as above.
Immunohistological Analysis
Pancreata were fixed in 10% formalin, and paraffin embedded for serial sectioning (5μm thick). Hematoxylin-Eosin staining was carried out as described previously (Remedi et al., 2004). For immunofluorescence, pancreatic sections were incubated overnight at 37°C with guinea pig anti-insulin primary antibody or guinea pig anti-glucagon primary antibody (1:250 or 1:500, respectively; Linco Research, St. Charles, MO). Primary antibodies were detected by incubating for 1.5 hr. at 25°C with anti-guinea pig secondary antibody conjugated with the Alexa™ 488 fluorescent dye (Molecular Probes, Eugene, OR) (Remedi et al., 2004).
Islet transplantation
We followed protocols developed by Dr. Bonner-Weir and colleagues (Montana et al., 1993) to transplant islets isolated from anesthetized mice as above, under the kidney capsule of recipients on the day of isolation. Briefly, islets were aspirated into the tip of a Hamilton syringe (Fisher Inc.) and then injected into a plastic transplant tubing attachment with a beveled cut end. Recipient mice were anesthetized with Avertin © (0.25 mg/g mouse body weight), and opened just above the kidney. The kidney was exposed and a small incision was made in the kidney capsule using a 23g needle. The tip of the transplant tubing was inserted under the capsule and 300 islets per transplant were implanted. The tubing was removed, the opening in the capsule was cauterized, and the animal was sutured and allowed to recover.
Chronic sulfonylurea treatment
Glibenclamide (Glyburide) pellets at the concentration of 2.5 mg per 60-day release were obtained from Innovative Research of America (Sarasota, FL). Six-week old mice were anesthetized with Avertin © (0.25 mg/g mouse body weight). The skin on the lateral side of the neck of the animal was lifted and pellets were implanted under the skin of the neck using a stainless steel precision trochar.
Analysis of KATP channel activity
Patch-clamp experiments of dispersed pancreatic β-cells were made at room temperature, in a chamber that allowed rapid exchange of bathing solution. The pipette (extracellular) and bath (cytoplasmic) solution (Kint) used in excised patch experiments had the following composition: 140 mM KCl, 1 mM EGTA, 10 mM HEPES, pH 7.3 (all chemicals purchased from Sigma-Aldrich, St. Louis, MO). ATP (dipotassium salt) was stored as a 100 mM stock in Kint solution, with the pH adjusted to 7.3. Stocks were stored at −20 °C, and diluted into working concentrations on each experimental day. Currents were recorded from excised membrane patches at a holding potential of −50 mV, filtered at 1 kHz, digitized at 5 kHz and stored directly on computer hard drive using Clampex v.9 software (Molecular Devices, Sunnyvale, CA). The ATP dose-response was quantified by fitting the raw data with a Hill equation:
| (Eq.1) |
where Irel is the current relative to that in the absence of ATP, [ATP] is the ATP concentration, K1/2,ATP is the half-maximal inhibitory ATP concentration, and H is the Hill coefficient.
Statistics
Data are presented as mean ± SEM. Differences among the 4 genotypes were tested using analysis of variance (ANOVA) and post-hoc Duncan’s test. When only two groups were compared, unpaired t-tests were used to assess significance. Differences were assumed to be significant in each case if p <0.05, unless indicated; and non-significant differences are not indicated.
Acknowledgments
We are extremely grateful to Drs. Pedro Herrera (Department of Genetic Medicine and Development, University of Geneva Medical School, Geneva, Switzerland), and Maureen Gannon (Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, Tennessee), for providing us with the Rip-Cre and Pdx-Cre mice, respectively. We are also very grateful to Jennifer Lock, Jeff Thorne, and Dr. Susan Bonner-Weir for instruction in the technique of islet transplantation. This work was supported by a NIH grant (DK69445) to CGN and reagent support was provided by the Washington University DRTC (DK20579).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease.[comment] Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab. 2008;295:E751–761. doi: 10.1152/ajpendo.90295.2008. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care. 2008;31:1403–1404. doi: 10.2337/dc08-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prato S, Marchetti P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm Metab Res. 2004;36:775–781. doi: 10.1055/s-2004-826163. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Cummings EA, Edghill EL, Harries LW, Scott R, Costa T, Temple IK, Hattersley AT, Ellard S. Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 Gene encoding the Kir6.2 subunit of the beta-cell potassium adenosine triphosphate channel. Journal of Clinical Endocrinology & Metabolism. 2004a;89:3932–3935. doi: 10.1210/jc.2004-0568. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Diatloff-Zito C, Edghill EL, Bellanne-Chantelot C, Nivot S, Coutant R, Ellard S, Hattersley AT, Robert JJ. KCNJ11 activating mutations are associated with developmental delay, epilepsy and neonatal diabetes syndrome and other neurological features. European Journal of Human Genetics. 2006;14:824–830. doi: 10.1038/sj.ejhg.5201629. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JMCL, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JPH, Sumnik Z, van Rhijn A, Wales JKH, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT. Activating Mutations in the Gene Encoding the ATP-Sensitive Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes. N Engl J Med. 2004b;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJG, Shield JPH, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925–934. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- Hamilton-Shield JP. Overview of neonatal diabetes. Endocrine Development. 2007;12:12–23. doi: 10.1159/000109601. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, Pearson ER. Minireview: pharmacogenetics and beyond: the interaction of therapeutic response, beta-cell physiology, and genetics in diabetes. Endocrinology. 2006;147:2657–2663. doi: 10.1210/en.2006-0152. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Defining the cell lineages of the islets of Langerhans using transgenic mice. International Journal of Developmental Biology. 2002;46:97–103. [PubMed] [Google Scholar]
- Koster JC, Cadario F, Peruzzi C, Colombo C, Nichols CG, Barbetti F. The G53D Mutation in Kir6.2 (KCNJ11) is Associated with Neonatal Diabetes and Motor Dysfunction in Adulthood that is Improved with Sulfonylurea Therapy. Journal of Clinical Endocrinology and Metabolism. 2007 Dec 11;:328–336. doi: 10.1210/jc.2007-1826. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005;54:2645–2654. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- Koster JC, Sha Q, Nichols CG. Sulfonylurea and K(+)-channel opener sensitivity of K(ATP) channels. Functional coupling of Kir6.2 and SUR1 subunits. Journal of General Physiology. 1999a;114:203–213. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster JC, Sha Q, Shyng S, Nichols CG. ATP inhibition of KATP channels: control of nucleotide sensitivity by the N-terminal domain of the Kir6.2 subunit. Journal of Physiology. 1999b;515:19–30. doi: 10.1111/j.1469-7793.1999.019ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masia R, Koster JC, Tumini S, Chiarelli F, Colombo C, Nichols CG, Barbetti F. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;56:328–336. doi: 10.2337/db06-1275. [DOI] [PubMed] [Google Scholar]
- Massa O, Iafusco D, D’Amato E, Gloyn AL, Hattersley AT, Pasquino B, Tonini G, Dammacco F, Zanette G, Meschi F, Porzio O, Bottazzo G, Crino A, Lorini R, Cerutti F, Vanelli M, Barbetti F Early Onset Diabetes Study Group of the Italian Society of Pediatric E Diabetology. KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Human Mutation. 2005;25:22–27. doi: 10.1002/humu.20124. [DOI] [PubMed] [Google Scholar]
- Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. Journal of Clinical Investigation. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:471–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT Neonatal Diabetes International Collaborative G. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations.[see comment] New England Journal of Medicine. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- Polak M, Cave H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet Journal Of Rare Diseases. 2007;2:12. doi: 10.1186/1750-1172-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Human Molecular Genetics. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- Proks P, Girard C, Haider S, Gloyn AL, Hattersley AT, Sansom MS, Ashcroft FM. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Reports. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Tucker SJ, Proks P, Ashcroft FM. Involvement of the n-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J Physiol (Lond) 1999;518:325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi MS, Koster JC, Markova K, Seino S, Miki T, Patton BL, McDaniel ML, Nichols CG. Diet-Induced Glucose Intolerance in Mice With Decreased {beta}-Cell ATP-Sensitive K+ Channels. Diabetes. 2004;53:3159–3167. doi: 10.2337/diabetes.53.12.3159. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Nichols CG. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic beta-cells. PLoS Med. 2008;5:e206. doi: 10.1371/journal.pmed.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Salehi SA, Abdulkader F, Braun M, Macdonald PE. K(ATP)-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab. 2008;19:277–284. doi: 10.1016/j.tem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Sagen JV, Raeder H, Hathout E, Shehadeh N, Gudmundsson K, Baevre H, Abuelo D, Phornphutkul C, Molnes J, Bell GI, Gloyn AL, Hattersley AT, Molven A, Sovik O, Njolstad PR. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53:2713–2718. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- Sperling MA. The genetic bases of neonatal diabetes mellitus. Pediatric Endocrinology Reviews. 2006;4:71–75. [PubMed] [Google Scholar]
- Tammaro P, Proks P, Ashcroft FM. Functional effects of naturally occurring KCNJ11 mutations causing neonatal diabetes on cloned cardiac KATP channels. Journal of Physiology. 2006;571:3–14. doi: 10.1113/jphysiol.2005.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaxillaire M, Populaire C, Busiah K, Cave H, Gloyn AL, Hattersley AT, Czernichow P, Froguel P, Polak M. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 2004;53:2719–2722. doi: 10.2337/diabetes.53.10.2719. [DOI] [PubMed] [Google Scholar]
- Zhang H, Fujitani Y, Wright CV, Gannon M. Efficient recombination in pancreatic islets by a tamoxifen-inducible Cre-recombinase. Genesis: the Journal of Genetics & Development. 2005;42:210–217. doi: 10.1002/gene.20137. [DOI] [PubMed] [Google Scholar]