Abstract
Marine n-3 polyunsaturated fatty acids alter cardiac phospholipids and prevent cardiac pathology in rodents subjected to pressure overload. This approach has not been evaluated in humans or large animals with hypertension-induced pathological hypertrophy. We evaluated docosahexaenoic acid (DHA) in old female dogs with hypertension caused by 16 weeks of aldosterone infusion. Aldosterone-induced hypertension resulted in concentric LV hypertrophy and impaired diastolic function in placebo treated dogs. DHA supplementation increased DHA and depleted arachidonic acid in cardiac phospholipids, but did not improve LV parameters compared to placebo. Surprisingly, DHA significantly increased serum aldosterone concentration and blood pressure compared to placebo. Cardiac mitochondrial yield was decreased in placebo treated hypertensive dogs compared to normal animals, which was prevented by DHA. Extensive analysis of mitochondrial function found no differences between DHA and placebo groups. In conclusion, DHA did not favorably impact mitochondrial or LV function in aldosterone hypertensive dogs.
Keywords: Fish oil, n3 polyunsaturated fatty acids, heart failure, mitochondrial permeability transition
Introduction
Despite aggressive treatment with current therapies, left ventricular (LV) dysfunction and heart failure remain a major public health problem due to poor quality of life, frequent hospitalization and death. New therapeutic approaches are needed to prevent the development of heart failure, and slow or reverse progression of established dysfunction. Recent clinical studies suggest that treatment with a mixture of the marine n3 polyunsaturated fatty acids (n3 PUFA) docosahexaenoic acid (DHA; 22:6n3) and eicosapentaenoic acid (EPA; 20:5n3) can improve left ventricular function[1,2] and modestly reduce clinical events[3] in heart failure patients with a low LV ejection fraction. Treatment with a mixture of DHA and EPA in animal models of chronic LV dysfunction found beneficial effects in mouse and rat models of pressure overload-induced cardiac pathology [4–8]. On the other hand, improved cardiac structure and function is not a consistent finding, as various formulations of DHA and EPA alone or in combination did not improve survival or cardiac function in studies in rodents with arterial pressure overload[9] or myocardial infarction[10], in cardiomyopathy hamsters [11], or in dogs following myocardial infarction[12].
The cardioprotective mechanisms of marine n3 PUFA in chronically dysfunctional myocardium are not well established, but appear to be dependent upon changes in the phospholipid fatty-acyl composition of cardiac membranes, and the resultant effects on key cellular processes [13,14]. Supplementation with only DHA may be optimal to elicit beneficial effects on cardiac function, as DHA is more readily incorporated into cardiac membranes than EPA [15,16]. Further, DHA increased both EPA and DHA in cardiac membrane phospholipids, but treatment with EPA alone does not increase DHA [9,11,17], as there is low enzymatic capacity for EPA elongation to DHA in heart [18]. Thus the increase in total DHA+EPA, and parallel decrease in arachidonic acid are greater with equivalent doses of DHA than with EPA. We found that rats treated with DHA alone had greater resistance to Ca2+-induced mitochondrial permeability transition, a catastrophic event characterized by collapse of membrane potential, cessation of ATP production and activation of cell death pathways[17]. Extrapolating these results to humans is difficult. When consuming a typical low DHA diet rats have a high DHA content in cardiac membranes (~8–10% of total phospholipid fatty-acyl content) while levels humans and dogs are much lower (1–2%) [12,19–21]. Thus it is important to evaluate the effects of DHA in a large animal model of heart failure prior to embarking on studies in patients.
The goal of the present investigation was to evaluate the impact of long term treatment with a clinically relevant dose of DHA on cardiac phospholipid composition, and mitochondria and LV structure and function in dogs subjected to chronic hypertensive stress. Specifically, we assessed the effects of treatment with purified DHA on cardiac membrane fatty-acyl composition, mitochondrial function and susceptibility to stress-induced permeability transition, and LV wall thickness, chamber volume and function. Studies were performed in mature female beagles (8 years old) subjected to 16 weeks of aldosterone infusion. We have previous shown that this results in hypertension, concentric LV hypertrophy and diastolic dysfunction [22]. We hypothesized that treatment with DHA would have no effect on arterial blood pressure, but would increase DHA content and deplete n6 PUFA in cardiac membranes, enhance mitochondrial resistance to Ca2+-induced permeability transition, and improve LV function compared to placebo treated dogs.
Methods
Experimental Design
All procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals (NIH publication No. 85–23), and were approved by the University of Maryland Institutional Animal Care and Use Committee, where all the animal work was conducted. Three groups of older adult female beagles (8 years old retired breeders) were studied. An untreated group (n=8) was compared to dogs treated with aldosterone for 16 weeks to induce hypertension and given either placebo (n=7) or DHA (n=8). Animals were purchased from a commercial vendor (Covance Research Products Inc, Cumberland, VA, USA), and were ovariectomized two to six weeks prior to delivery to our facility. All terminal procedures were completed over a five week period by the same personnel. Dosing and measurements performed with the investigators blinded to treatment. Mitochondrial and cardiovascular function data from the two control groups (untreated dogs and dogs subjected to aldosterone-induced hypertension with placebo treatment) were previously reported [22]. In the present investigation these animals serve as the controls for the DHA treated group.
Dogs were housed in pairs and fed commercial dog food (Pedigree, Harlan Laboratories, Inc, Frederick, MD, USA). After 3–5 days of acclimation arterial blood pressure was measured in the tail using an oscillometric automated blood pressure cuff (PetMAP, Tampa, FL, USA) in conscious dogs, as previously described in detail [22]. Mean arterial pressure (MAP) was calculated as diastolic pressure + (pulse pressure/3).
Induction of Hypertension with Infusion Aldosterone
A battery powered programmable infusion pump (iPRECIO model #MK02-V2, Data Sciences International, St. Paul, MN, USA) was implanted in the neck to deliver a constant infusion of aldosterone into the jugular vein (D-aldosterone (Sigma Aldrich, St. Louis, MO, USA) at 30 μg • kg−1 • day−1 in 15% ethanol, 50% DMSO, and 35% water (10 mg of aldosterone/mL). Pump implantation was performed under sterile conditions under general anesthesia using propofol (4 to 6 mg/kg i.v.) and inhaled isoflurane (1.5–3.0%) to effect, with local infiltration with bupivacaine (approximately 2 mL of 0.25%), as previously described[22]. This dose of aldosterone was selected based on pilot studies that found it was well tolerated and resulted in stable hypertension out to 8 weeks of treatment (data not shown). Sixteen dogs initiated treatment with aldosterone, but one placebo treated dog developed an infection at the site of pump implantation and was discontinued, thus data are reported for 15 animals. The untreated normotensive control animals were not instrumented. The aldosterone treated dogs were assigned to treatment with either placebo (2.00 g corn oil/day given in three opaque gelatin capsules containing 0.66 g each) (n=7) or DHA (1.80 g DHA/day, with each capsule containing 0.66 g of 90% DHA ethylester (KD Pharma, Bexbach, Germany). Placebo and DHA capsules were made in accordance with Good Manufacturing Practice (GMP) requirements in the University of Maryland School of Pharmacy GMP facility. Capsule purity and stability were verified by analysis using the official USP compendial method for Omega-3-Acid Ethyl Esters Capsules; this assay used gas chromatography with flame ionization detection (data not shown). The storage conditions for the stability studies were in accordance with ICH conditions. We estimate that this dose of DHA is equivalent to 4 g/day in humans, based on the assumption that the typical energy requirement of a 12 kg dog is ~920 kcal/day [23] and that 1.8 g of DHA has 16.2 kCals (9 kCals/g DHA), accounting for 1.8% of energy intake. Assuming a human energy intake of 2000 kcals/day, 1.8% of energy intake would be 36 kcals from DHA, or 4 g/day in humans. Animals were dosed once each day in the morning, and investigators were blinded to treatment.
Echocardiography was performed 7 to 3 days prior to the terminal study (MyLab 30CV, Esaote North America, Inc, Indianapolis, IN, USA, with a 3.5–1.6 MHz probe). In the aldosterone treated dogs an echocardiographic exam was also performed 7 to 2 days prior to the pump instrumentation. All measurements were performed in a right lateral decubitus position without sedation or restraint, as previously described [22]. Echocardiograms were read by two investigators blinded to treatment, and then averaged. LV wall thickness was taken as the sum of anterior and posterior wall thickness at end diastole, and relative LV wall thickness as LV wall thickness/end diastolic diameter.
Blood samples were drawn from a superficial forelimb vein drawn prior to implantation of the infusion pump and initiation of treatment, at 8 weeks of treatment, and at 7 to 2 days prior to the terminal procedure. All samples were drawn between 11:00 and 14:00 without fasting. After 16 weeks of aldosterone infusion and treatment with placebo or DHA a terminal procedure was performed to measure left ventricular pressure and remove the heart for analysis. Dogs were anesthetized as described above, and a 5-F high fidelity manometer-tip catheter (Millar Instruments, Houston, TX, USA) was advanced into the LV from the left carotid artery. The animal was euthanized by increasing the isoflurane to 5% for 1 minute, rapidly performing a left side thoracotomy, severing the superior vena cava, and removing the heart. The total time for removal of the heart was <90 seconds from the initial incision. Myocardium for mitochondrial isolation was taken from the anterior LV free wall and placed in ice cold buffer. Tissue from the lateral LV free wall was fixed in embedding medium (Tissue-Tek O.C.T. Compound, Sakura) for subsequent histological analysis and frozen at −80°C. The residual LV and right ventricle (RV) tissue was carefully dissected and weighed for assessment of LV and RV mass.
Mitochondria Isolation and Measurements
Isolation of myocardial SSM and IFM was done using a protocol modified from Palmer et al. and Rosca et al [24,25], as recently described in detail [22]. Mitochondrial respiration was assessed with glutamate+malate (20 and 10mM), pyruvate+malate (20 and 10 mM), palmitoylcarnitine (40μM), or succinate (20mM + 7.5μM rotenone) as substrates. State 4 was measured with and without the addition of oligomycin (5μg/mL), and the respiratory control ratio (RCR) was calculated using State 4 without oligomycin.
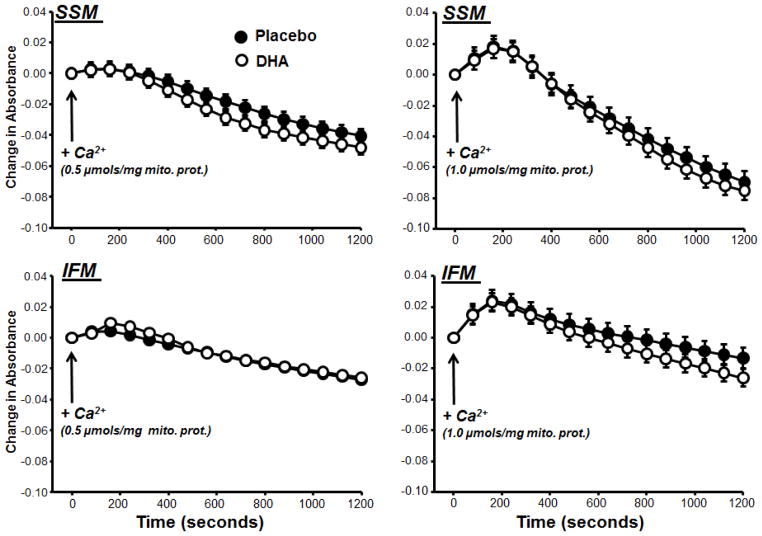
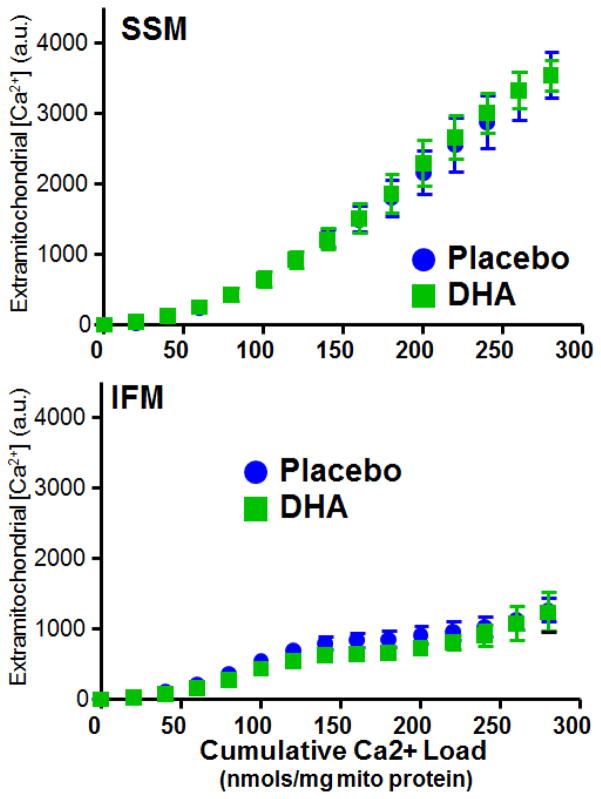
Ca2+-induced mitochondrial permeability transition was evaluated in SSM and IFM using two previously described methods [9,17,22,26]. First, mitochondrial swelling was assessed by following the change in absorbance at 540 nm after the addition of a single bolus of Ca2+. Second, the capacity for Ca2+ uptake was used as an index of resistance to mitochondrial permeability transition. Extramitochondrial [Ca2+] was measured with Calcium Green-5N (750nM) during progressive additions of 20 nmols Ca2+/mg mitochondrial protein. Both of these assays were performed at 37°C, and have been described in detail [22]. Mitochondrial membrane potential and size were measured with flow cytometry using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol carbocyanine iodide (JC-1) and MitoTracker Green FM, respectively, as previously described [22,27,28].
Tissue and Serum Analysis
The fatty-acyl composition of phospholipids was analyzed in myocardial samples from the LV lateral free wall using gas chromatography coupled with mass spectroscopy, as previously described [29]. Histological analysis of LV samples for extracellular fibrosis, myocyte cross-sectional area and capillary density was assessed as previously described [30]. Serum aldosterone was measured by ELISA (Cayman Chemical).
Statistical Analysis
Values are shown as mean ± standard error. The three groups were compared using a 1-way ANOVA with a post hoc t-test using the Bonferroni correction. The effect of DHA treatment on tail cuff blood pressure in the aldosterone treated dogs was assessed by a 2-way ANOVA for repeated measures. Comparison of echocardiographic data from baseline to 15 weeks was performed with a 2 way ANOVA for repeated measures. A p-value of <0.05 was considered significant.
RESULTS
Cardiac Phospholipid Fatty-acyl Composition
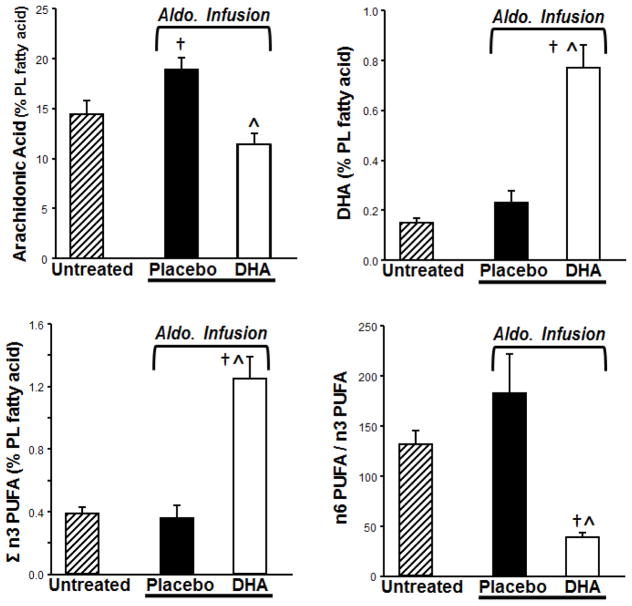
DHA treatment significantly altered myocardial phospholipid fatty acyl composition. DHA and EPA (20:5n3) and the sum of n3 PUFA (Σn3 PUFA) in cardiac phospholipids were increased by DHA treatment compared to placebo (Figure 1, Table 1). This was accompanied by a DHA-induced depletion of arachidonic acid (20:4n6) from cardiac phospholipids. The most abundant n6 PUFA, linoleic acid (18:2n6), was not different among groups, but the Σn6 PUFA was significantly decreased in the DHA treated group compared to placebo (Table 1). Together this resulted in a significant fall in the Σn6PUFA/Σn3PUFA ratio (Figure 1). The magnitude of the increase in total n3 PUFA was relatively modest in absolute terms (0.9% of total fatty acids) compared to the fall in arachidonic acid (7% of total fatty acids), which implies that other fatty acyl groups replaced arachidonic acid. There was a trend for higher saturated and monounsaturated fatty acids in DHA treated animals, but there was not a statistically significant increase in any specific fatty acid (Table 1). Thus the DHA-induced decrease in arachidonic acid was due to distributed replacement by a variety of long chain saturated and monunsaturated fatty acids, and was not targeted to a single fatty acid moiety.
Figure 1.
Effects of treatment with DHA on the fatty-acyl content of myocardial phospholipids. †p<0.05 compared to the untreated group. ^ <0.05 compared to the placebo treated group.
Table 1.
Myocardial phospholipid fatty acid composition.
| Untreated | Aldosterone Infusion
|
||
|---|---|---|---|
| Placebo | DHA | ||
| C14:0 | 0.12±0.01 | 0.10±0.01 | 0.14±0.01 |
| C16:0 | 16.6±0.5 | 16.9±0.7 | 18.9±1.0 |
| C16:1 | 0.23±0.02 | 0.20±0.01 | 0.23±0.01 |
| C18:0 | 13.7±0.4 | 12.7±0.4 | 13.6±0.5 |
| C18:1n9 | 18.7±0.8 | 17.5±0.7 | 19.2±1.3 |
| C18:1n7 | 1.93±0.08 | 1.61±0.04† | 1.68±0.08 |
| C18:2n6 | 33.8±0.6 | 31.7±0.8 | 33.6±1.1 |
| C18:3n3 | 0.11±0.01 | BQL | BQL |
| C20:3n6 | 0.08±0.01 | 0.07±0.01 | 0.10±0.01 |
| C20:5n3 | 0.06±0.004 | 0.06±0.02 | 0.41±0.07*† |
| C22:5n3 | 0.09±0.01 | 0.06±0.01 | 0.05±0.01 |
| Σn6 | 48.4±0.9 | 50.6±1.3 | 45.1±1.7* |
| ΣSat | 30.4±0.4 | 29.7±0.7 | 32.6±1.0 |
| ΣMUFA | 20.9±0.9 | 19.3±0.7 | 21.1±1.4 |
p<0.01 compared to placebo,
p<0.05 compared to untreated old dogs.
Myocardial phospholipids from placebo treated dogs subjected to aldosterone-induced hypertension had higher content of arachidonic acid (20:4n6) and lower vaccenic acid (18:1n7) compared to untreated age-matched dogs (Table 1, Figure 1). This effect was not observed in DHA treated dogs.
DHA Effects on Serum Aldosterone and Blood Pressure
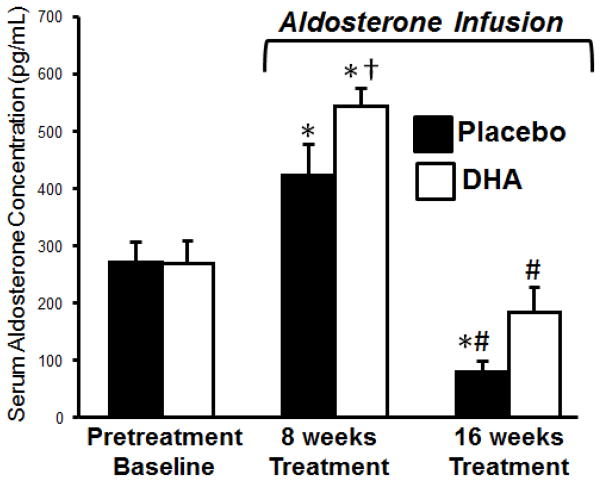
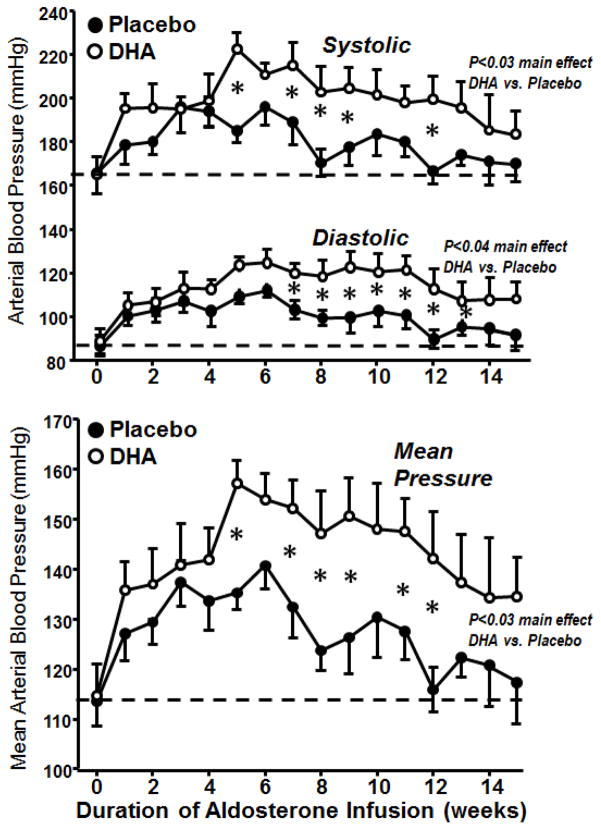
No difference was observed in baseline serum aldosterone concentrations between the placebo and DHA treated groups (Figure 2), and were similar to those we previously reported from untreated young and old beagles (290±21 and 235±28 pg/mL, respectively)[22]. Tail cuff blood pressure measurements acquired in conscious unrestrained dogs prior to aldosterone infusion were similar in the DHA and placebo treated groups (Figure 3). We previously reported that infusion of aldosterone caused an increase in both serum concentration of aldosterone and in blood pressure at 8 weeks [22]. This was followed by a decline in aldosterone concentration to below baseline values and a return in blood pressure to near pretreatment values by 15 weeks, suggesting that there was an adaptive increase in aldosterone clearance induced by aldosterone infusion [22]. In the present investigation we were surprised to observe that treatment with DHA significantly potentiated the increase in serum aldosterone at 8 weeks (Figures 2), and the increase in arterial blood pressure from 5 to 12 weeks of aldosterone infusion (Figure 3). Serum aldosterone decreased from 8 to 16 weeks, as previously reported [22], and there was a strong trend for higher aldosterone concentration with DHA than placebo treatment at 16 weeks (p=0.06).
Figure 2.
Serum aldosterone concentration. *<0.05 compared to baseline within treatment group. #Compared to 8 weeks within treatment group. †p<0.05 compared to the untreated group at 8 weeks. There was a strong trend for high aldosterone concentration at 16 weeks with DHA treatment compare to placebo (p=0.064).
Figure 3.
Arterial blood pressure measured by the tail cuff method plotted as a function of the duration of aldosterone infusion. *p<0.05 between placebo and DHA treated groups.
Cardiac Mass and Function
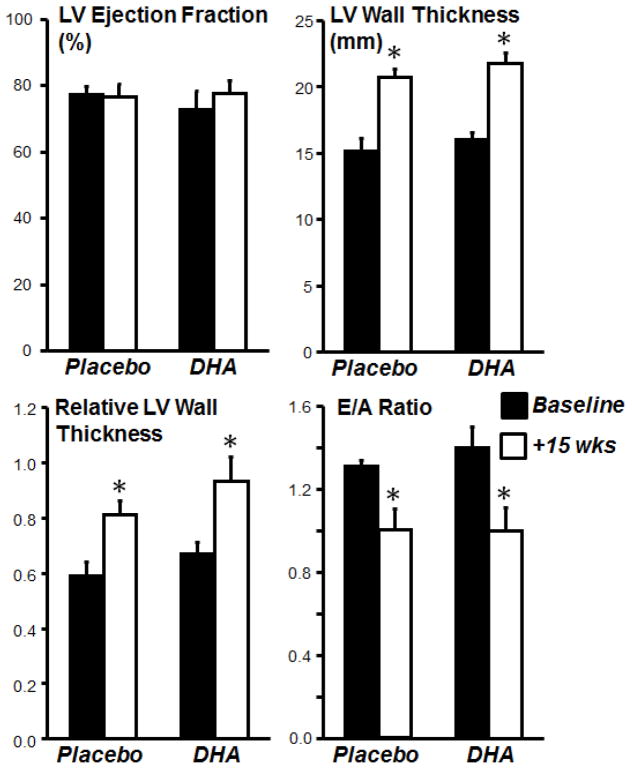
Body mass was similar among groups (Table 2). LV, RV and LV+RV masses were not significantly different among groups, though there was a trend for a greater LV mass with aldosterone infusion (Table 1). Echocardiographic measurements showed that LV chamber diameter and ejection fraction were not different between placebo and DHA treated groups either before or after treatment (Figure 4, Table 2). On the other hand, LV wall thickness was significantly increased by aldosterone infusion in both placebo and DHA treated group, indicating that aldosterone infusion induced LV concentric cardiac hypertrophy that was not prevented by DHA. The E/A ratio decreased to the same extent with aldosterone infusion in both placebo and DHA groups, suggesting that DHA was ineffective at preventing deterioration of LV diastolic function. On the other hand, it is interesting to note that despite the increase in blood pressure and circulating aldosterone in the DHA treated group, LV hypertrophy was not aggravated.
Table 2.
Body and heart mass, and cardiac function. Echocardiographic measurements were performed in conscious animals. LV pressure was assessed by catheterization in anesthetized animals just prior to euthanasia.
| Untreated | ALDOSTERONE TREATED | ||
|---|---|---|---|
| +Placebo | +DHA | ||
| Body Mass (kg) | 11.2±0.5 | 12.0±0.8 | 12.1±0.7 |
| LV mass (g) | 52.4±3.5 | 61.2±4.7 | 63.4±2.8 |
| RV mass(g) | 18.6±1.6 | 19.6±1.3 | 20.3±1.0 |
| LV mass + RV mass (g) | 70.9±5.0 | 80.8±5.6 | 83.7±3.6 |
| LV mass/body mass (g/kg) | 4.66±0.22 | 5.11±0.22 | 5.33±0.34 |
| RV mass/body mass | 1.65±0.10 | 1.66±0.10 | 1.70±0.10 |
| Echocardiographic Measurements | |||
| EDD (mm) | 24.6±0.9 | 25.9±1.5 | 24.3±1.6 |
| ESD (mm) | 15.1±0.8 | 16.4±0.9 | 14.4±1.1 |
| FS (%) | 0.39±0.02 | 0.39±0.03 | 0.41±0.03 |
| EDV (mL) | 16.0±1.6 | 19.2±3.7 | 16.2±2.9 |
| ESV (mL) | 3.8±0.6 | 4.2±0.8 | 3.5±0.8 |
| LV ejection fraction (%) | 76.6±2.3 | 76.6±3.8 | 77.8±3.8 |
| LV Wall Thickness (anterior + posterior free wall (mm) | 17.1±1.0 | 20.7±0.7† | 21.8±0.7† |
| E/A ratio | 1.25±0.03 | 1.00±0.04† | 1.00±0.04† |
| Left Ventricle Pressure | |||
| Heart Rate (beats/min) | 113±5 | 114±7 | 115±4 |
| LV peak systolic pressure (mmHg) | 102±6 | 92±5 | 98±6 |
| LV end diastolic pressure (mmHg) | 3.7±0.4 | 4.0±0.5 | 4.6±0.3 |
| LV maximum dP/dt (mmHg/s) | 1740±196 | 1472±79 | 1823±117 |
| LV minimum dP/dt (mmHg/s) | −1834±185 | −1773±182 | −1845±128 |
p<0.05 compared to untreated dogs.
Figure 4.
Echocardiographic assessment of left ventricular (LV) function taken at baseline before treatment (black bars) and after 15 weeks of treatment (open bars) with either placebo or DHA in dogs subjected to concurrent aldosterone-induced hypertension. E/A, peak rapid filling velocity (E)-to-peak atrial filling velocity (A) ratio. *p<0.05 compared to pretreatment within placebo or DHA treated groups.
LV pressure in anesthetized dogs showed no difference among normal untreated dogs and the aldosterone treated animals for heart rate, LV Peak systolic and end diastolic pressure, and maximum and minimum dP/dt (Table 2).
Histological assessment of LV myocardium found no increase in extracellular fibrosis, myocyte cross sectional area and capillary density with infusion of aldosterone in the placebo treated group, as previously published [22]. Analysis of the DHA treated group showed no effect on any of these parameters (myocyte cross sectional area of 528±29 μm2), volume fraction of interstitial fibrosis of 9.8±0.7%, and capillary density of 2154±128 capillaries/mm2, which are similar to values obtained from the placebo treated group (546±28 μm2, 10.0±0.6%, and 2106±92 capillaries/mm2, respectively).
Mitochondrial Parameters
Compared to normal untreated dogs, the placebo treated group with aldosterone infusion had a significant 18% decrease in total mitochondrial yield (Table 3). Treatment with DHA prevented the decrease in yield, as the DHA group was not different from either the untreated group or placebo treated dogs (Table 3). The yields for SSM and IFM were not statistically different among groups.
Table 3.
Mitochondrial morphology and membrane potential (ΔΨm) assessed by flow cytometry.
| Mitochondrial Yield (mg protein/g wet tissue) | Untreated | Aldosterone Infusion
|
|
|---|---|---|---|
| +Placebo | +DHA | ||
| Total Yield | 19.2±0.9 | 15.8±1.0† | 18.4±0.8 |
| Subsarcolemmal mitochondria (SSM) | 8.4±0.7 | 7.2±0.6 | 6.6±0.9 |
| Interfibrillar mitochondria (IFM) | 10.8±1.0 | 9.2±0.6 | 11.2±0.5 |
| SSM | |||
| Mitochondrial Diameter (AU) | 399±7 | 453±16† | 441±9† |
| Mitochondrial Internal Complexity (AU) | 85.9±0.9 | 94.2±4.1 | 89.6±3.4 |
| ΔΨm (JC-1 aggregate/monomer) | 5.9±0.7 | 6.8±0.4 | 6.0±0.8 |
| IFM | |||
| Mitochondrial Diameter (AU) | 364±8 | 395±7† | 386±7 |
| Mitochondrial Internal Complexity (AU) | 80.1±0.8 | 85.5±1.5 | 82.1±1.8 |
| ΔΨm (JC-1 aggregate/monomer) | 4.5±0.3 | 4.5±0.5 | 4.9±0.4 |
p<0.05 compared to untreated normal dogs.
Flow cytometer analysis showed no differences in mitochondrial diameter, internal complexity, or membrane potential between DHA and placebo groups in either SSM or IFM (Table 3). Mitochondrial diameter was increased in SSM in both DHA and placebo groups compared to untreated normal dogs. In the placebo treated group the mitochondrial diameter of IFM was increased compared to the healthy normal group, while DHA treated dogs were no different from either group. No difference among groups were observed for internal complexity or membrane potential for either SSM or IFM (Table 3)
Overall, mitochondrial respiration was minimally affected by aldosterone-induced hypertension and treatment with DHA (Table 4). In SSM there were no differences in State 3 respiration. State 4 respiration in SSM measured with either succinate+rotenone with or without oligomycin was significantly higher in the placebo treated group than in the untreated normal dogs, while the DHA treated group was not statistically different from either group (Table 4). The RCR was significantly lower in the placebo treated group compared to normal healthy dogs with pruvate+malate or succinate+rotenone, while the DHA treated group was not different from either the placebo or untreated normal groups. There were no significant differences in any parameters in IFM.
Table 4.
Cardiac mitochondrial yields and respiration in dogs untreated dogs, and dogs infused with aldosterone for 16 weeks and treated with placebo or DHA.
| SSM | Untreated | Aldosterone Infusion
|
|
|---|---|---|---|
| +Placebo | +DHA | ||
| State 3 | |||
| Glutamate + Malate | 198±11 | 199±12 | 222±26 |
| Pyruvate + Malate | 137±26 | 129±14 | 143±8 |
| Palmitoylcarnitine | 148±13 | 159±16 | 149±9 |
| Succinate + Rotenone | 269±14 | 268±14 | 259±12 |
| State 4 (no Oligomycin) | |||
| Glutamate + Malate | 32.4±4.1 | 44.7±5.8 | 41.7±1.8 |
| Pyruvate + Malate | 39.5±6.6 | 48.4±5.2 | 46.2±1.5 |
| Palmitoylcarnitine | 33.8±3.2 | 48.6±6.3 | 41.7±2.0 |
| Succinate + Rotenone | 105±5 | 139±12* | 114±4 |
| State 4 (+ Oligomycin) | |||
| Glutamate + Malate | 7.5±1.5 | 8.5±1.8 | 10.2±2.3 |
| Pyruvate + Malate | 18.2±9.8 | 27.5±5.1 | 23.8±1.7 |
| Palmitoylcarnitine | 9.8±1.9 | 14.5±3.9 | 12.3±1.7 |
| Succinate + Rotenone | 37.8±3.3 | 61.0±8.0* | 47.8±2.5 |
| RCR | |||
| Glutamate + Malate | 6.6±0.7 | 4.9±0.6 | 5.5±0.5 |
| Pyruvate + Malate | 3.6±0.2 | 2.7±0.2† | 3.1±0.2 |
| Palmitoylcarnitine | 4.5±0.5 | 3.5±0.4 | 3.6±0.3 |
| Succinate + Rotenone | 2.6±0.1 | 2.0±0.1† | 2.3±0.1 |
| ADP:O | |||
| Glutamate + Malate | 2.7±0.2 | 2.4±0.1 | 2.6±0.1 |
| Pyruvate + Malate | 3.2±0.3 | 2.9±0.3 | 2.8±0.2 |
| Palmitoylcarnitine | 2.4±0.2 | 2.4±0.1 | 2.3±0.1 |
| Succinate + Rotenone | 1.7±0.3 | 1.5±0.1 | 1.5±0.1 |
| IFM | |||
| State 3 | |||
| Glutamate + Malate | 227±27 | 242±18 | 247±19 |
| Pyruvate + Malate | 203±22 | 176±13 | 221±16 |
| Palmitoylcarnitine | 225±20 | 238±26 | 254±22 |
| Succinate + Rotenone | 340±36 | 376±23 | 385±18 |
| State 4 (no Oligomycin) | |||
| Glutamate + Malate | 36.4±5.1 | 54.4±4.8 | 43.8±3.4 |
| Pyruvate + Malate | 62.8±8.3 | 66.5±8.6 | 77.9±4.9 |
| Palmitoylcarnitine | 49.5±6.0 | 65.5±7.4 | 60.1±3.5 |
| Succinate + Rotenone | 129±13 | 160±14 | 153±14 |
| State 4 (+ Oligomycin) | |||
| Glutamate + Malate | 7.5±1.5 | 15.9±3.3 | 11.9±1.1 |
| Pyruvate + Malate | 41.4±8.0 | 51.2±11.4 | 57.4±6.9 |
| Palmitoylcarnitine | 21.9±5.5 | 30.1±9.0 | 29.6±3.0 |
| Succinate + Rotenone | 65.9±7.5 | 91.7±11.7 | 88.1±7.4 |
| RCR | |||
| Glutamate + Malate | 6.5±0.5 | 5.2±0.7 | 5.8±0.4 |
| Pyruvate + Malate | 3.3±0.2 | 2.8±0.2 | 2.8±0.1 |
| Palmitoylcarnitine | 4.8±0.5 | 3.7±0.4 | 4.2±0.2 |
| Succinate + Rotenone | 2.6±0.2 | 2.4±0.1 | 2.6±0.1 |
| ADP:O | |||
| Glutamate + Malate | 2.2±0.1 | 2.4±0.1 | 2.6±0.2 |
| Pyruvate + Malate | 2.8±0.3 | 3.0±0.3 | 2.7±0.1 |
| Palmitoylcarnitine | 2.5±0.1 | 3.0±0.3 | 2.4±0.1 |
| Succinate + Rotenone | 1.4±0.1 | 1.4±0.1 | 1.5±0.1 |
P<0.05 and
p<0.01 compared to untreated normal dogs.
Discussion
There has recently been much interest in treating heart failure with purified formulations of marine n3 PUFA. Positive effects were observed in small clinical trials that assessed LV function, clinical class and/or exercise tolerance with high doses of mixtures of DHA and EPA (1.7 to 3.4 g/day) [1,2,31]. The GISSI-HF trial showed that a low dose (0.85 g/d) of a 55:45 mixture of DHA+EPA decreased the occurrence of the combined endpoint of death plus hospitalization over 4 years[3]. These studies focused on patients with established heart failure and a low LV ejection fraction. Approximately half of heart failure patients have a normal LV ejection fraction but diastolic dysfunction and congestion. This “heart failure with preserved ejection fraction” is more common in patients with a history of hypertension and LV hypertrophy [32–34]. Studies in rodents with aortic constriction and LV hypertrophy, which recapitulates key aspects of heart failure with preserved ejection fraction, found that treatment with a mixture of DHA and EPA attenuated LV remodeling and pathology in mice and rats [4–8]. This suggests that this approach may be effective for preventing and treating heart failure with preserved ejection fraction.
The present investigation assessed the ability of a clinically relevant dose of DHA to alter cardiac phospholipid fatty acyl composition and mitochondria function, and prevent development of cardiac dysfunction and pathology in a large animal model of chronic hypertensive stress. We elected to study purified DHA without EPA, as DHA is more readily incorporated into cardiac phospholipids than EPA, and is metabolized to EPA which is also incorporated into phospholipids. Treatment with DHA increased the ΣDHA+EPA 3-fold and significantly depleted arachidonic acid from 19% of the total phospholipid fatty acyl content to 11%, indicating a clear effect on cardiac membrane composition. Despite these significant changes, the increase in LV wall thickness and development of diastolic dysfunction were not prevented.
While our main hypothesis was not supported, we made two serendipitous findings that merit further investigation: 1) treatment with DHA increased serum aldosterone concentration and arterial blood pressure in aldosterone infused animals, and 2) LV structure and function were not worsened in the DHA group despite exposure to persistently higher blood pressures and aldosterone levels. We were surprised to see that DHA significantly increased aldosterone concentration and arterial blood pressure compared to placebo. Aldosterone is primarily removed by the liver and kidneys, thus these finding suggest the possibility that DHA impairs aldosterone clearance by these organs. Since marine n3 PUFA supplements high in DHA are frequently taken by patients with hypertension and/or heart failure, a similar augmentation in aldosterone and blood pressure would be disadvantageous in this population. This might partially explain the mixed results of high doses of n3 PUFA in cardiovascular clinical trials [13]. Additional investigation is needed to determine the mechanism(s) for these observations and if a similar response occurs in patients. It is important to keep in mind that studies in normotensive individuals and patients with hypertension found that supplementation with DHA has a modest but significant blood pressure lowering effect [16,35], thus we expected to see the DHA treatment would have either no effect or modestly lower blood pressure. In any case, the elevated blood pressure in this study complicates the interpretation of the potential therapeutic effects of DHA. The LV of DHA treated dogs was exposed to a significantly greater afterload than the placebo treated group from ~5 to ~14 weeks, yet there was not greater LV wall thickening or a larger fall in the E/A ratio. This suggests that DHA might have been protective if the rise in blood pressure and aldosterone had been matched between groups.
Compared to previously published reports on phosopholipid fatty acyl composition in LV myocardium in dogs or humans, we found lower levels of n3 PUFA in the absence of treatment, but a robust response to treatment with DHA. Studies in humans by Harris et al reported higher myocardial DHA and EPA content under conditions of low n3 PUFA intake (ΣDHA+EPA of 1.7%) [19], and an increase to 2.9% after 6 months of treatment with 0.4 g DHA + 0.6 g EPA per day. Billman et al reported a ΣDHA+EPA of 0.82% in mixed bred dogs on a low n3 PUFA diet, which after three months of treatment with either 0.84, 1.7 or 3.4 g/day of a 55:45 mixture of DHA+EPA increased to 4.1%, 4.9% and 7.2% [12]. We observed 0.36% for the ΣDHA+EPA in our placebo group, which increased to 1.25% in the DHA treated group (1.8 g/day). This comparison of the changes in DHA and EPA with the available data from LV samples from humans and dog suggests that our increase in phospholipid DHA+EPA was less than expected. The differences with the study by Billman et al could be due to differences in dog strain, age, sex and hormonal status. In the present study we used 8 year old ovariectomized beagles who were retired breeders, while they used 2 to 3 year old mixed bred dogs of both sexes, suggesting that perhaps a beneficial effect would be seen with DHA treatment in young dogs with hypertension-induced cardiac dysfunction. The analytical methods employed may have also contributed to the observed differences, as we used mass spectrometery for detection combined with isotope-labeled standards for quantification, not a flame ionization detector as in the dog and human studies discussed above. In any case, we saw a clear effect of DHA treatment on n3 PUFA and arachidonic acid in cardiac phospholipids in our model system (Figure 1). A higher dose of DHA would have elicited greater changes in cardiac phospholipids, however we gave a dose that was equivalent to about 4g/day in humans, which is at the high end of what has previously be used in patients.
In previous studies in rats and cardiomyopathic hamsters we observed that DHA supplementation improved resistance to Ca2+-induced mitochondrial permeability transition[9–11,17]. In contrast, we found no changed in resistance to Ca2+-induced mitochondrial permeability transition in the present study. Our previous work suggest that this effect is due to the absolute increase DHA in cardiac mitochondrial membranes, and not to DHA-induced depletion of arachidonic acid [26], thus the lack of effect in the present study may be the result of the modest absolute increase in DHA in cardiac membranes. It is also important to note that in previous work in rodents suggests that greater susceptibility to stress-induced mitochondrial permeability transition is not a predictor of poorer outcome in heart failure [11,36]. In addition, it is important to note that we studied isolated mitochondria to allow highly controlled evaluation of function, however future studies should considering evaluating mitochondrial function in intact cardiomyocyte, as this would avoid potential damage and bias inherent in the isolation procedure.
The results of the present study provide the impetus for future studies in this area. First, it is possible that a higher dose of DHA might have elicited a beneficial effect due to greater changes in membrane phospholipids and more pronounced physiological effects. Second, we did not assess the effects of pre-treatment with DHA before initiation of hypertension as we did in a previous study showing efficacy in rats with aortic constriction[5]. This approach is more likely to provide protection than concurrent initiation DHA treatment with the onset of hypertension, as was done in the present investigation. Third, we evaluated DHA and did not assess EPA, as our previous work in rodents strongly suggest that cardiac benefits are strongly associated with the extent that DHA and EPA increase and arachidonic acid decreases in cardiac phospholipids. On the other hand, EPA supplementation may be cardioprotective in dogs via alterations in systemic factors such as anti-inflammatory effects, thus one cannot rule out that treatment with EPA may be beneficial in dogs with heart failure. Lastly, it is important to consider evaluation of DHA in non-pharmacological large animal models of heart failure that recapitulate key aspect of clinical heart failure, such as the aortic constriction, renal injury or coronary microembolization models.
In summary, DHA supplementation increased DHA and depleted arachidonic acid in cardiac phospholipids in dogs with hypertension caused by aldosterone infusion. Cardiac mitochondrial yield was decreased in placebo treated dogs compared to normal animals, and DHA treatment prevented this effect. On the other hand, it did not enhance mitochondrial resistance to stress-induce permeability transition or improve mitochondrial respiration. Finally, DHA did not prevent LV wall thickening or the decline in the E/A ratio. Thus DHA was not protective in a dog model of aldosterone-induced hypertensive cardiomyopathy.
Figure 5.
The change in absorbance at 540 nm in SSM (upper panel) and IFM (Lower Panel) following addition of either 0.5 or 1.0 μmol Ca2+/mg mitochondrial protein. There were no significant differences between treatment groups.
Figure 6.
Extramitochondrial Ca2+ concentration plotted as a function of cumulative Ca2+ load for SSM (upper panel) and IFM (Lower Panel) from the LV. There were no significant differences between treatment groups.
Acknowledgments
This work was supported by the National Institutes of Health grant numbers HL074237 and HL110731. The authors wish to thank Ramesh Dandu and Seon Hepburn for assistance with the capsule manufacturing and analysis.
Footnotes
Disclosures
William Stanley is the inventor on a pending US patent application filed by the University of Maryland for the use of DHA for the treatment of heart failure.
Reference List
- 1.Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am Heart J. 2011;161:915–919. doi: 10.1016/j.ahj.2011.02.011. S0002-8703(11)00164-5 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei CL. Effects of n-3 Polyunsaturated Fatty Acids on Left Ventricular Function and Functional Capacity in Patients With Dilated Cardiomyopathy. J Am Coll Cardiol. 2011;57:870–879. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Gissi-Hf I. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 4.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Shea KM, Chess DJ, Khairallah RJ, Hecker PA, Lei B, Walsh K, des Rosiers C, Stanley WC. omega-3 Polyunsaturated fatty acids prevent pressure overload-induced ventricular dilation and decrease in mitochondrial enzymes despite no change in adiponectin. Lipids Health Dis. 2010;9:95. doi: 10.1186/1476-511X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Shearer GC, Chen Q, Healy CL, Beyer AJ, Nareddy VB, Gerdes AM, Harris WS, O’Connell TD, Wang D. Omega-3 Fatty Acids Prevent Pressure Overload-Induced Cardiac Fibrosis Through Activation of Cyclic GMP/Protein Kinase G Signaling in Cardiac Fibroblasts. Circulation. 2011;123:584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLennan PL, Abeywardena MY, Dallimore JA, Raederstorff D. Dietary fish oil preserves cardiac function in the hypertrophied rat heart. Br J Nutr. 2011;108:645–654. doi: 10.1017/S0007114511005915. [DOI] [PubMed] [Google Scholar]
- 9.Khairallah RJ, O’Shea KM, Brown BH, Khanna N, des Rosiers C, Stanley WC. Treatment with docosahexaenoic acid, but not eicosapentaenoic acid, delays Ca2+-induced mitochondria permeability transition in normal and hypertrophied myocardium. J Pharmacol Exp Ther. 2010;335:155–162. doi: 10.1124/jpet.110.170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, des Rosiers C, Kristian T, Murphy RC, Fiskum G, Stanley WC. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009;47:819–827. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvao TF, Khairallah RJ, Dabkowski ER, Brown BH, Hecker PA, O’Connell KA, O’shea KM, Sabbah HN, Rastogi S, Daneault C, des Rosiers C, Stanley WC. Marine n3 Polyunsaturated Fatty Acids Enhance Resistance to Mitochondrial Permeability Transition in Heart Failure, but Do Not Improve Survival. Am J Physiol Heart Circ Physiol. 2013;73:H12–H21. doi: 10.1152/ajpheart.00657.2012. ajpheart.00657.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billman GE, Nishijima Y, Belevych AE, Terentyev D, Xu Y, Haizlip KM, Monasky MM, Hiranandani N, Harris WS, Gyorke S, Carnes CA, Janssen PM. Effects of dietary omega-3 fatty acids on ventricular function in dogs with healed myocardial infarctions: in vivo and in vitro studies. Am J Physiol Heart Circ Physiol. 2010;298:H1219–H1228. doi: 10.1152/ajpheart.01065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. S0735-1097(11)03131-7 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Stanley WC, Khairallah RJ, Dabkowski ER. Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2012;15:122–126. doi: 10.1097/MCO.0b013e32834fdaf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sergiel JP, Martine L, Raederstorff D, Grynberg A, Demaison L. Individual effects of dietary EPA and DHA on the functioning of the isolated working rat heart. Can J Physiol Pharmacol. 1998;76:728–736. doi: 10.1139/cjpp-76-7-8-728. [DOI] [PubMed] [Google Scholar]
- 16.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 17.Khairallah RJ, Sparagna GC, Khanna N, O’shea KM, Hecker PA, Kristian T, Fiskum G, des RC, Polster BM, Stanley WC. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta. 2010;1797:1555–1562. doi: 10.1016/j.bbabio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Rat heart cannot synthesize docosahexaenoic acid from circulating alpha-linolenic acid because it lacks elongase-2. J Lipid Res. 2008;49:1735–1745. doi: 10.1194/jlr.M800093-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 20.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–1228. doi: 10.1093/ajcn/85.5.1222. [DOI] [PubMed] [Google Scholar]
- 21.Crawford MA. Fatty-acid ratios in free-living and domestic animals. Possible implications for atheroma. Lancet. 1968;1:1329–1333. doi: 10.1016/s0140-6736(68)92034-5. [DOI] [PubMed] [Google Scholar]
- 22.Asemu G, O’Connell KA, Cox JW, Dabkowski ER, Xu W, Ribeiro RF, Jr, Shekar KC, Hecker PA, Rastogi S, Sabbah HN, Hoppel CL, Stanley WC. Enhanced resistance to permeability transition in interfibrillar cardiac mitochondria in dogs: effects of aging and long-term aldosterone infusion. Am J Physiol Heart Circ Physiol. 2013;304:H514–H528. doi: 10.1152/ajpheart.00674.2012. ajpheart.00674.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rainbird A, Kienzle E. Studies on the energy requirement of dogs depending on breed and age. Kleintierpraxis. 1990;35:149–158. [Google Scholar]
- 24.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 25.Rosca MG, Vazquez EC, Kerner J, Parland W, Chandler MP, Stanley WC, Sabbah HN, Hoppel CL. Cardiac mitochondria in coronary microembolization-induced heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khairallah RJ, Kim J, O’Shea KM, O’Connell KA, BBH, Galvao TDRC, Polster BM, Hoppel CL, Stanley WC. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One. 2012;7:e34402. doi: 10.1371/journal.pone.0034402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papanicolaou KN, Ngoh GA, Dabkowski ER, O’Connell KA, Ribeiro RF, Stanley WC, Walsh K. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol. 2012;302:H167–H179. doi: 10.1152/ajpheart.00833.2011. ajpheart.00833.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol. 2010;299:H529–H540. doi: 10.1152/ajpheart.00267.2010. ajpheart.00267.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelinas R, Thompson-Legault J, Bouchard B, Daneault C, Mansour A, Gillis MA, Charron G, Gavino V, Labarthe F, des Rosiers C. Prolonged QT interval and lipid alterations beyond {beta}-oxidation in very long-chain acyl-CoA dehydrogenase null mouse hearts. Am J Physiol Heart Circ Physiol. 2011;301:H813–H823. doi: 10.1152/ajpheart.01275.2010. ajpheart.01275.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation. 2000;102:1990–1995. doi: 10.1161/01.cir.102.16.1990. [DOI] [PubMed] [Google Scholar]
- 31.Xin W, Wei W, Li X. Effects of fish oil supplementation on cardiac function in chronic heart failure: a meta-analysis of randomised controlled trials. Heart. 2012;98:1620–1625. doi: 10.1136/heartjnl-2012-302119. heartjnl-2012-302119 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 33.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 35.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 36.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. 43171 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]