Abstract
Recent data have shown that lyso-Gb3, the deacylated derivative of globotriaosylceramide (Gb3), is possibly involved in the pathogenesis of Fabry disease (FD) and might be a clinically useful biomarker of its metabolic load. To test this hypothesis, we assayed Gb3 and lyso-Gb3 and related analogs in plasma and/or urine samples of 12 clinically well-characterized subjects carrying several different GLA variant alleles associated with a wide range of residual α-galactosidase A activities. Urinary Gb3 was measured by HPLC–MS/MS; plasma and urinary lyso-Gb3 and related analogs were measured by UPLC–MS/MS.
Individual profiles of Gb3 and lyso-Gb3 and related analogs closely correlated with the phenotypic data for each subject, discerning the classical FD patient from the two patients carrying cardiac variants as well as these from all the others without FD. The lyso-Gb3 analog at m/z 836 was found at increased levels only in patients manifesting clinically severe heart disease, irrespective of the pathogenicity of the GLA variant they carried. This finding suggests that this lyso-Gb3 analog might be an earlier biomarker of progressive heart disease, non-specific of the FD cardiomyopathy. The possibility that urinary Gb3 is a specific marker of kidney involvement in FD deserves further study.
Keywords: Fabry disease, α-galactosidase A, GLA gene, biomarker, Gb3, lyso-Gb3 and analogs
1. INTRODUCTION
Fabry disease (FD) (OMIM no. 301500) is a glycosphingolipidosis caused by mutations affecting the X-linked GLA gene [1, 2], which codes for lysosomal alphα-galactosidase A (α-Gal, EC 3.2.1.22). Deficient α -Gal activity leads to lysosomal accumulation of glycosphingolipids (GSL), particularly globotriaosylceramide (Gb3) and its deacylated derivative, globotriaosylsphingosine (lyso-Gb3), which are the pathologic hallmarks of FD. The involvement of vascular smooth muscle cells and endothelia of cardiomyocytes and of kidney epithelial cells is critical for the development of the late, but clinically nonspecific, cerebrovascular, cardiac and renal complications of FD. However, the pathogenic mechanisms leading to stroke, to left ventricular hypertrophy (LVH) and cardiomyopathy, and to progressive chronic kidney disease (CKD), which are the major causes of morbidity and mortality in adult FD patients, remain unclear. Specifically, the pathophysiology of the systemic vasculopathy that characterizes FD, the homeostatic regulatory pathways affected by lysosomal GSL accumulation and the molecular mediators involved in these processes are still poorly understood [3–6].
In males, the clinical severity of FD broadly correlates with the level of in vitro α-Gal residual enzyme activity (REA) measured in leukocytes or plasma [7]. The classical multisystemic early onset phenotype, presenting with acroparesthesias, angiokeratomas and impaired sweating, which is more typically observed in young boys and adolescents, is caused by α-Gal mutations usually with ≤1% REA [7]. Mutations with REA in the range of >1–10% of normal are associated with organ-limited, later-onset renal and/or cardiac phenotypes. Heterozygous females present more variable clinical phenotypes and are usually less severely affected than the hemizygous males [6, 8], showing no clear-cut genotype-phenotype correlations, including with REA. Furthermore, the distribution of α -Gal enzyme activities measured in heterozygous females with pathogenic GLA mutations partially overlap those observed in healthy females, potentially leading to many false negative diagnoses [9]. This is, at least in part, attributable to the metabolic mosaicism created by random X chromosome inactivation in females [10]. As a major consequence of this phenomenon, genetic molecular testing is mandatory for a conclusive diagnosis of FD in females, while in males the laboratory measurement of α-Gal enzyme activity is highly reliable both for diagnostic and screening purposes [2, 11].
Specific treatment for FD, by enzyme replacement therapy (ERT) with genetically-engineered human α-Gal preparations (Agalsidase-alfa, Replagal from Shire; and Agalsidase-beta, Fabrazyme from Genzyme, A Sanofi Division) has been in clinical use for more than a decade now [12, 13]. The extremely high cost of ERT, the questions regarding the cost-effectiveness of ERT for FD [14, 15], the efficacy of different dosing regimens [16, 17], and the impact of anti-agalsidase antibodies in classically affected males treated with ERT [18], make it urgent to identify biomarker(s) that reliably allow the clinical monitoring of the response to ERT and dose individualization, without the need for invasive diagnostic procedures (e.g. heart or kidney biopsies).
Although Gb3 is the major GSL accumulated in FD patients, and its quantitation in plasma and urine has been used for laboratory monitoring of disease progression [17], the correlation between clinical symptoms of FD and levels of Gb3 is inconsistent: [17, 19, 20] for instance, asymptomatic children with absent or very low α -Gal REA sometimes show abnormally high plasma Gb3 levels, while clinically affected females generally have plasma Gb3 levels within the normal range. On the other hand, urine Gb3 excretion, expressed as the Gb3/creatinine concentration ratio, significantly correlated with gender, genotype, and ERT status of FD patients [21], and the measurement of urine Gb3 concentration has been suggested as a potential marker of disease severity and monitoring response to treatment in FD males [22], as well as for high-risk screening [23]. However, other investigators have questioned the usefulness of Gb3 as a biomarker in FD [24]. Although the decrease in left ventricular mass (LVM) observed in response to agalsidase treatment correlates with the decline of plasma Gb3 levels only in women [18], the reduction in plasma and urinary Gb3 in the first year of ERT predicts the hazard of developing white matter lesions and stroke in both genders [18].
Lyso-Gb3 has recently been proposed as a key pathogenic mediator of the onset and progression of some of FD complications [25]. In vitro, lyso-Gb3 promotes the proliferation of smooth muscle cells [25], a biological effect that might contribute to the increase of carotid intima-media thickness, in the absence of atherosclerotic lesions, observed in adult FD patients [26], For monitoring the effect of ERT upon LVM and the risk of cerebrovascular complications of FD, measurements of plasma lyso-Gb3 levels were performed, as well as plasma Gb3 [18]. Furthermore, while urine from healthy controls contained no detectable lyso-Gb3, increases in urinary lyso-Gb3/creatinine ratio significantly correlated with the concentrations of Gb3, types of GLA mutation, gender and ERT status [27].
More than 750 unequivocally pathogenic GLA mutations have already been described [Human Gene Mutation Database; last accessed at http://www.hgmd.cf.ac.uk, March 1, 2015], most of which are private to single families. However, the GLA sequence variants most frequently identified in newborn screenings and in case studies of patients with potential late complications of FD, are novel neutral variants or exonic and intronic variants of unknown pathogenic significance (VUPS) [28]. In the absence of definite biomarkers of GSL tissue load in FD patients, the interpretation of equivocal genotyping findings should be supported by robust clinical and histopathological data, as well as co-segregation studies within families, in order to avoid false assumptions of pathogenicity [28, 29].
Clear-cut increases in plasma lyso-Gb3 or Gb3 concentrations have been acknowledged as an ancillary diagnostic criterion of FD [28, 29]. Moreover, the discovery and analysis of analogs of lyso-Gb3 in urine [30–33] and in plasma [34–37] have shown to be interesting biomarkers for FD patients. In fact, urinary lyso-Gb3 analogs were specifically increased in FD patients with cardiac variant mutations, in whom urinary Gb3 and lyso-Gb3 was normal, particularly in children [33]. We hypothesized that the measurement of lyso-Gb3 and related analogs in plasma and urine might be helpful for the differential diagnosis of GLA neutral variants and VUPS. To test this hypothesis, we have measured lyso-Gb3 and related analogs in urine [32] and in plasma [37] samples in clinically well-characterized patients carrying several different GLA variant alleles, associated with a wide range of α-Gal REA.
2. SUBJECTS, MATERIALS AND METHODS
2.1. Case ascertainment, consent and protocol clinical evaluations
The majority of the subjects selected for this study were patients who either presented with clinical phenotypes warranting the differential diagnosis of FD, or with decreased α -Gal enzyme activity on dried blood spots (DBS), collected to screen for FD among adult patients presenting with LVH, CKD or stroke of unclear aetiology. The remaining cases were healthy individuals identified during family screening of selected patients. Patients were originally diagnosed at specialized clinics at the university, tertiary care hospital (São João Hospital Centre, Porto, Portugal), or referred thereto from other hospitals, for expert clinical and genetic evaluation. The blood and urine samples required for the Gb3- and lyso-Gb3-related biomarker assays were collected after obtaining specific informed consent from each enrolled subject.
Baseline evaluation included a detailed clinical history and physical examination, as well as a review of the available relevant analytical and echocardiographic data, paying particular attention to possible dermatological (hypohidrosis, angiokeratomas), neuropathic (acroparesthesias), cerebrovascular (transient ischaemic attack, stroke), cardiac (arrhythmias, LVH, ischaemic heart disease) and renal (albuminuria, proteinuria, azotaemia) manifestations of FD. All patients were referred to the ophthalmology clinic for slit-lamp examination, to screen for the typical corneal dystrophy of FD (i.e., cornea verticillata) [23]. The final diagnosis was classified according to previously suggested criteria [28]. With the exception of an anuric patient, who had been on renal replacement therapy (RRT) by haemodialysis (HD) for several years, the plasma creatinine concentration (pCr), the urinary albumin/creatinine ratio (UACR) and urinary sediments were evaluated in all individuals, to diagnose and stage CKD according to the guidelines of the “Kidney Disease: Improving Global Outcomes (KDIGO)” initiative [38]. The glomerular filtration rate was estimated (eGFR) from pCr by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [39].
2.2. GLA gene sequence analysis, laboratory assessment of α-galactosidase activity and bioinformatic analyses
GLA gene mutations and sequence variants were identified by direct sequencing of polymerase chain reaction (PCR) or reverse transcription-PCR (RT-PCR) products, using a conventional Sanger method [40]. The blood sample processing and analytical protocols used for GLA genotyping have been previously reported [40]. GLA sequence changes are described according to the most recent recommendations of the Human Genome Variation Society [HGVS; http://www.hgvs.org/mutnomen/recs-prot.html].
The α-Gal enzyme activity in plasma, leukocytes or whole blood (DBS) was measured by standard fluorometric assays, using 4-methylumbelliferyl-α-D-galactopyranoside as the fluorescent substrate, and N-acetyl-D-galactosamine as an inhibitor of α-N-acetylgalactosaminidase activity [41]. Whenever necessary for proper interpretation of the genotyping data, the diagnosis of α-Gal deficiency in patients originally identified by decreased enzyme activity on DBS testing was confirmed on a plasma or leukocyte α -Gal assay. In vitro demonstration of marked α-Gal deficiency on a plasma or leukocyte assay was considered as diagnostic in hemizygous males, while the finding of a pathogenic GLA mutation was needed for confirmation of FD diagnosis in heterozygous females [2, 11].
To assist in the prediction of the possible impact of amino acid substitutions caused by novel missense GLA variants on the structure and function of α-Gal, bioinformatic tools were used: “MutationTaster2” (accessed at http://www.mutationtaster.org/), “PolyPhen-2” (accessed at http://genetics.bwh.harvard.edu/pph2/) and SIFT (accessed at http://sift.jcvi.org). Variants were modelled onto the structure of wild-type α-Gal, and the effect of the mutation on the α-Gal structure was analyzed for the changes in chemical environment around the substituted amino-acid residue.
2.3. Clinical and genetic characterization of the study cohort
The relevant demographic, clinical and genetic data from 12 individuals enrolled in this study are summarized in Table 1. None of them reported current or past history of acroparesthesias or of hypohidrosis, or had typical FD angiokeratomas on physical examination of the skin, and only patient (case I) showed cornea verticillata on slit-lamp ophthalmological examination.
Table 1.
Demographic, clinical, biochemical and genetic characteristics of study subjects.
| Case identifier |
Gender | Age at genetic diagnosis |
Age at sampling* |
GLA gene variant |
α-Galactosidase activity |
(normal range) |
Clinical phenotype leading to the genetic diagnosis |
Definite diagnosis |
|---|---|---|---|---|---|---|---|---|
| I | F | 22 | 32 | p.Arg220Ter | (L) 25 nmol/h/mg | (36–80) | Family screening. Left ventricular non- compaction. |
classical |
| II | M | 63 | 64 | p.Phe113Leu | (S) 0.84 pmol/h/spot |
(8.75–15.6) | Hypertrophic cardiomyopathy. | non-classical |
| III | M | 52 | 53 | p.Asn215Ser | (L) 4 nmol/h/mg | (36–80) | Hypertrophic cardiomyopathy. | non-classical |
| IV-P | F | 26 | 37 | p.Arg118Cys | (L) 44 nmol/h/mg | (36–80) | Angiokeratomas. | no FD |
| IV-Fa | M | 53 | 63 | p.Arg118Cys | (L) 25 nmol/h/mg | (22–73) | Family screening: father of case IV-P. | no FD |
| V-P | F | 33 | 38 | p.Arg118Cys | (L) 38 nmol/h/mg | (36–80) | Stroke. | no FD |
| V-Mo | F | 71 | 74 | p.Arg118Cys | ND | Family screening: mother of case V-P. | no FD | |
| VI | F | 39 | 44 | p.Arg118Cys | (L) 40 nmol/h/mg | (36–80) | Stroke. | no FD |
| VII | F | 47 | 49 | p.Asp83Asn | (L) 45 nmol/h/mg | (36–80) | Stroke. | no FD |
| VII-Br¶ | M | 45 | 45 | p.Asp83Asn | (L) 73 nmol/h/mg (P) 13 nmol/h/ml |
(36–80) (6–19) |
Family screening: brother of case VII-P. | no FD |
| VIII | M | 36 | 38 | p.Asn228Ser | (P) 9 nmol/h/ml | (6–19) | Dilated cardiomyopathy; end-stage renal disease. |
no FD |
| IX-P | M | 20 | 30 | c.−10C>T | (L) 68.4 nmol/h/mg |
(79–130) | Stroke. | no FD |
| IX-Si | F | 23 | 34 | c.−10C>T | (L) 65.2 nmol/h/mg |
(79–130) | Family screening: sister of case IX-P. | no FD |
Case identifiers: roman numerals refer to individual pedigrees, P = proband; Fa = proband’s father; Mo = proband’s mother; Si = proband’s sister; Br = proband’s brother.
Gender identifiers: M = male / F = female. Ages reported in full years.
Age at the sampling of plasma and urine for this study.
Not included in the biomarker profile study.
Missense exonic GLA gene sequence variants amino acid code: Arg = arginine; Phe = phenylalanine; Leu = leucine; Asn = asparagine; Ser = serine; Cys = cysteine; Asp = aspartate; Ter = premature stop codon. C>T = cytosine to thymine transition.
(L) leukocyte assay; (P) plasma assay; (S) dried blood spot assay. ND: not determined. FD: Fabry disease.
Patient I was screened for FD at the age of 22 years and found to be heterozygous for the nonsense GLA mutation p.Arg220Ter (or R220X) that she inherited from her affected mother. The patient’s leukocyte α-Gal activity was moderately decreased (see Table 1), within the usual range for FD females [1], but all her affected male relatives had <1% REA, in agreement with the known association of this mutation with the classical FD phenotype [1, 42]. At 30 years of age, she was diagnosed with an unusual morphological presentation of FD cardiomyopathy, as reported elsewhere [43]. At age 32 years, she remained clinically asymptomatic and with normal UACR.
In patient II, LVH was first noticed at the age of 58 years, in the workup of atypical acute chest pain, but its cause was attributed to long-standing high blood pressure. The diagnosis of a cardiac variant of FD was not recognized until 6 years later, when a screening DBS α -Gal assay showed low REA (≈7% of the normal average) and GLA genotyping revealed hemizygosity for mutation p.Phe113Leu (or F113L), which is associated with mild/attenuated cardiac phenotypes of FD [1, 42]. At age 64 years, the patient’s echocardiogram showed severe concentric LVH (maximum left ventricular wall thickness = 26 mm), with systolic anterior motion of the mitral valve; his renal function was normal (pCr = 0.84 mg/dl), total proteinuria was quantified as 90 mg/day and the microscopic examination of urinary sediments did not show microscopic haematuria; and no significant vascular or white matter abnormalities were identified on the computed tomography scan of the brain.
The diagnosis of FD in patient III was made at the age of 52 years, on the electron-microscopy (EM) examination of a cardiac biopsy, which had been obtained for the differential diagnosis of severe LVH (maximum left ventricular wall thickness = 19 mm). The histopathological diagnosis was confirmed by the demonstration of low REA α -Gal on a leukocyte assay (≈7% of the normal average) and the identification of the pathogenic GLA missense mutation p.Asn215Ser (or N215S), which is characteristically associated with a late-onset cardiac variant of FD [1, 42]. The patient reported history of poorly controlled severe hypertension since the age of 38 years. Eight years later, on the screening for additional cardiovascular risk factors following an acute coronary syndrome, he was diagnosed with one-vessel coronary heart disease, type 2 diabetes mellitus (T2DM) with non-proliferative diabetic retinopathy, and combined hyperlipidaemia. Finally, at the age of 50 years, he had suffered a haemorrhagic stroke resulting in severe right hemiparesis. A permanent pacemaker was implanted at age 52 years, for treatment of advanced atrioventricular block. At baseline evaluation for systemic complications of FD, had CKD stage 3 (pCr = 1.7 mg/dl; GFR = 45 ml/min/1.73 m2) with UACR of 329 mg/g and normal urinary sediments, but refused kidney biopsy for the differential diagnosis of the aetiology of his kidney disease.
Patient IV-P was found to be heterozygous for the GLA VUPS p.Arg118Cys (or R118C) on the diagnostic workup of multiple angiokeratomas affecting the buttocks and upper thighs that she had first noticed at the age of 22 years. Clinical details are described elsewhere [44]. Her father (case IV-Fa) was identified by cascade screening. Patients V-P and VI, who are heterozygous for the same GLA VUPS, were diagnosed in a prospective FD case-finding study among incident adult stroke patients aged up to 55 years [45]. Patient V-P’s mother (case V-M), was identified on cascade screening. Cases IV-Fa and V-Mo have been thoroughly assessed for manifestations of FD but showed no major target organ complications attributable to the disease, respectively in their 7th and 8th decade of life [46]. The available clinical, biochemical and histopathology data from individuals carrying the GLA p.Arg118Cys variant strongly suggest that it does not co-segregate with FD clinical phenotypes in a highly penetrant Mendelian fashion, but might rather be a modulator of the multifactorial risk of cerebrovascular disease [46].
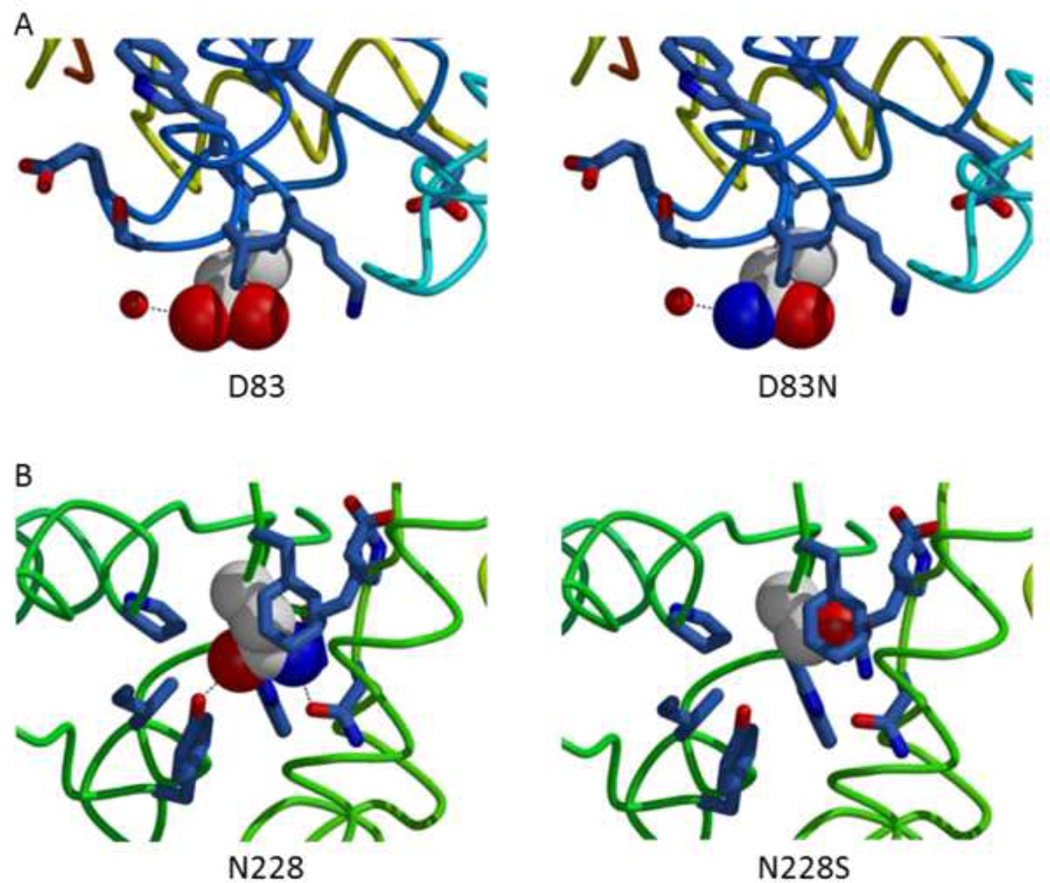
Patient VII, a 47-year-old female with history of T2DM and hypertension, was screened for FD for having suffered a cryptogenic ischaemic stroke. Her α -Gal activity on a DBS assay was below the normal range and the subsequent GLA genotyping identified a novel missense variant, p.Asp83Asn (or D83N), consistently predicted to be non-pathogenic change on bioinformatic analyses. The p.Asp83Asn substitution leads to a small change on the surface of the α-Gal protein and is not expected to cause major perturbations to the structure (see Figure 1A). On family screening, one of her brothers, aged 45 years, was found to be hemizygous for the same GLA variant but showed no manifestations whatsoever of FD, and had normal plasma and leukocyte α -Gal activities (Table 1). Their mother had died at age 55 years, from lung cancer, with no history of any health problems attributable to FD.
Figure 1.
The alphα-galactosidase A (α-Gal) structure analyzed for the changes in chemical environment around the substituted amino-acid residue.
A The p.Asp83Asn (D83N) substitution leads to a small change on the surface of the α-Gal protein.
B The p.Asn228Ser (N228S) substitution leads to loss of two internal hydrogen bonds and the introduction of a packing defect in the center of the hydrophobic core of the α-Gal protein.
Patient VIII, a 38-year-old man with end-stage CKD of unclear aetiology and history of severe hypertension, who had been on regular HD treatment since the age of 36 years, was eventually screened for FD because of co-existent dilated cardiomyopathy. After the finding of subnormal α-Gal activity on a DBS assay, GLA sequencing identified a novel missense variant, p.Asn228Ser (or N228S). The p.Asn228Ser substitution leads to loss of two internal hydrogen bonds and the introduction of a packing defect in the center of the hydrophobic core of the α-Gal protein and thus expected to cause a significant perturbation to the structure (see Figure 1B). Although this variant was consistently predicted to be a pathogenic change also on bioinformatic analyses, a confirmatory enzyme assay in plasma showed α-Gal activity within the normal range, excluding FD as the cause of the patient’s cardiac dysfunction.
Patient IX-P was originally screened for FD in the etiologic investigation of recurrent ischaemic stroke in adolescence, as reported elsewhere [40]. His leukocyte α -Gal activity was slightly, but consistently below the normal reference range, and the only variation identified on sequencing all the GLA exons and exon-intron boundaries was the presence of a single nucleotide polymorphism (SNP) of the 5’-untranslated region (5’UTR) of exon 1, resulting from a cytosine-to-thymine transition at position −10 in the cDNA (c.-10C>T). On family screening, his healthy sister (case IX-Si), his healthy brother and their healthy mother were all found to be carriers of the same SNP [40]. At the age of 34 years, case IX-Si remained in good health. The GLA c.−10C>T SNP is identified by the reference number rs2071225 at the SNP database of the National Centre for Biotechnology Information (dbSNP; National Library of Medicine, NCBI; Bethesda, MD, U.S.A.; http://www.ncbi.nlm.nih.gov/snp) and has been reported from several ethnically different populations [40, 47–49], with minor allele frequencies of 0.03 in the Portuguese [50] and 0.05 in Britons [47].
2.4. Blood and urine sample collection, processing, and shipment for laboratory analysis of lyso-Gb3 and related analogs
Venous blood and urine samples were collected at a routine clinical visit, before ERT was eventually started. No urine sample could be obtained from patient VIII due to his anuric condition.
Lyso-Gb3 and analogs were quantified in plasma and urine samples, whereas Gb33 was quantified only in urine. All analogs of lyso-Gb3 in urine and in plasma were structurally elucidated and characterized as modified sphingosine moieties [30, 36]. For plasma separation, blood was drawn by phlebotomy into a 6.0 mL BD Vacutainer® K2EDTA tube (BD – Becton, Dickinson and Company, catalog number 367864; Plymouth, United Kingdom). Blood samples were immediately centrifuged at 2250 g for 15 minutes, at 4 °C, and the plasma was pipetted into 2 mL plastic vials and kept frozen at −80 °C until shipment to the laboratory. The urine specimens were collected from the midstream void into plastic 150 mL sterile urine collection cups (Deltalab, catalog reference 409726; Barcelona, Spain). Immediately after collection, the urine cup was vortexed and an aliquot of the well-mixed urine was pipetted into a 15 mL plastic tube and stored at −20 °C until shipment to the laboratory. The plasma and urine samples were sent to the laboratory by overseas express courier, in temperature-controlled packaging units, at −20 °C.
2.5. Quantification of lyso-Gb3 and related analogs in plasma and in urine, and of Gb3 in urine
Urinary Gb3 was measured by high performance liquid chromatography coupled to tandem mass spectrometry (Alliance-Quattro Micro MS/MS; Waters Corporation, Milford, MA, United States of America) according to a method previously published [21]. Lyso-Gb3 and related analogs in urine and in plasma were measured by ultraperformance liquid chromatography coupled to a tandem mass spectrometry system (Acquity-UPLC I-Class Xevo TQ-S MS/MS; Waters) [32, 37]. Creatinine concentrations were quantified according to a method of isotope dilution using an ultraperformance liquid chromatography coupled to an electrospray time-of-flight (UPLC-ESI-TOF) mass spectrometry system [30]. The levels of all the urinary biomarkers were expressed as a ratio to creatinine to normalize the concentration of urine [51].
3. RESULTS
The results of biomarker quantification in plasma and urine samples of 12 subjects enrolled in this study are presented in Table 2.
Table 2.
Plasma and urinary tandem mass spetrometry biomarker results of the 12 studied subjects carrying GLA gene variants.
| Case identifier |
Gender | GLA gene variant |
Plasma biomarkers [nmol/L] | Urinary biomarkers [µg/mmol Cr] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyso-Gb3 m/z 786 |
Analog 758 |
Analog 784 |
Analog 802 |
Analog 804 |
Analog 820 |
Analog 836 |
Gb3 | Lyso-Gb3 m/z 786 |
Analog 758 |
Analog 774 |
Analog 784 |
Analog 800 |
Analog 802 |
Analog 820 |
Analog 836 |
|||
| I | F | p.Arg220Ter | 7,45 | 0,01 | 0,06 | 0,02 | 1,55 | 0,75 | 0 | 36 | 67 | 10 | 35 | 22 | 23 | 168 | 85 | 44 |
| II | M | p.Phe113Leu | 22,17 | 0,04 | 0,20 | 0,13 | 3,07 | 2,76 | 0,35 | 116 | 13 | 35 | 259 | 50 | 74 | 474 | 168 | 101 |
| III | M | p.Asn215Ser | 11,05 | 0,26 | 4,00 | 2,98 | 2,21 | 2,28 | 0,20 | 8 | 16 | 1 | 77 | 14 | 32 | 227 | 116 | 97 |
| IV-P | F | p.Arg118Cys | 0,38 | 0,01 | 0 | 0 | 0 | 0,07 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 9 | 15 | 45 |
| IV-Fa | M | p.Arg118Cys | 0,37 | 0 | 0 | 0 | 0 | 0,19 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| V-P | F | p.Arg118Cys | 0,47 | 0 | 0 | 0 | 0 | 0,16 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 24 |
| V-Mo | F | p.Arg118Cys | 0,61 | 0 | 0 | 0 | 0 | 0,30 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 31 |
| VI | F | p.Arg118Cys | 0,37 | 0 | 0 | 0 | 0 | 0,20 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 4 | 6 | 20 |
| VII | F | p.Asp83Asn | 0,41 | 0 | 0 | 0 | 0 | 0,26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| VIII | M | p.Asn228Ser | 0,30 | 0 | 0 | 0 | 0 | 0,24 | 0,21 | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| IX-P | M | c.−10C>T | 0,36 | 0 | 0 | 0 | 0 | 0,32 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| IX-Si | F | c.−l0>T | 0,53 | 0 | 0 | 0 | 0 | 0,30 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 22 |
|
Reference Values (Patients >18 years) |
Normal ranges | Normal ranges | ||||||||||||||||
| 0–2.4 | 0 | 0–0.9 | 0 | 0 | 0–0.3 | 0 | 0–25 | 0 | 0 | 0 | 0 | 0 | 0–17 | 0–14 | 0–55 | |||
Case identifiers: roman numerals refer to individual pedigrees, P = proband; Fa = proband’s father; Mo = proband’s mother; Si = proband’s sister. Gender identifiers: M = male / F = female. Cr = creatinine. Missense exonic GLA gene sequence variants are described according to the amino acid single letter code:
Arg = arginine; Phe = phenylalanine; Leu = leucine; Asn = asparagine; Ser = serine; Cys = cysteine; Asp = aspartate; Ter = premature stop codon. C>T = cytosine to thymine transition.
Abnormal biomarker levels are marked as shaded cells.
In plasma, lyso-Gb3 (mass/charge (m/z) ratio at 786) and its analogs at m/z 802 and m/z 804 were found at increased levels exclusively in patients with pathogenic GLA mutations (p.Arg220Ter, p.Phe113Leu and p.Asn215Ser); the lyso-Gb3 analog at m/z 758 showed a similar pattern but it was additionally detectable in one of the p.Arg118Cys heterozygote (case IV-P); and the lyso-Gb3 analog at m/z 784 was detectable only in patients with pathogenic mutations, but was found above the normal reference range exclusively in the p.Asn215Ser hemizygote. The lyso-Gb3 analog at m/z 820 was detectable at levels slightly above the normal range in one of the individuals carrying non-pathogenic GLA variants; at >2-fold above the upper limit of the normal range (ULN) in the p.Arg220Ter heterozygote; and at >8-fold the ULN in the two patients with the late-onset cardiac variants. The lyso-Gb3 analog at m/z 836 was detectable only in the three patients manifesting clinically severe heart disease, either cardiac variants of FD (cases II, III) or dilated cardiomyopathy of uncertain aetiology (case VIII).
In urine, lyso-Gb3 analogs at m/z 758, 774, 784, 800, 802, and lyso-Gb3 (m/z = 786) itself were found at increased levels only in patients with pathogenic GLA mutations, while Gb3 was within normal range in the p.Asn215Ser hemizygote, but >4.5-fold above the ULN in the p.Phe113Leu hemizygote and modestly increased in the p.Arg220Ter heterozygote. In addition to the patients with pathogenic GLA mutations, the urinary excretion of lyso-Gb3 analog at m/z 820 was detectable at a slightly increased level in one of the p.Arg118Cys heterozygotes (case IV-P). Only the two males with clinically severe heart disease carrying pathogenic GLA mutations showed increased urinary excretion of lyso-Gb3 analog at m/z 836, at >1.5-fold the ULN.
4. DISCUSSION
Overall, the plasma and urine biomarker panels broadly differentiated patients with known pathogenic GLA mutations from individuals carrying VUPS, including exonic variants that either moderately affect (p.Arg118Cys) or do not affect (p.Asp83Asn, p.Asn228Ser) α -Gal enzyme activity, as well as from individuals carrying a functional GLA 5’UTR SNP (c.−10C>T).
The plasma and urinary biomarker profiles observed in the female with the GLA p.Asp83Asn variant was entirely normal. Although the p.Asp83Asn variant has been regarded as a pathogenic mutation by the investigators of large-scale screening studies of FD among European stroke patients [52] and Spanish HD patients [53], that assumption has not been supported by intrafamilial segregation analyses and/or kidney pathology data. In agreement with the in silico predictions, the results of our clinically unbiased family study strongly suggest that GLA p.Asp83Asn is instead a non-pathogenic exonic variant. In the structural analysis of α-Gal, the p.Asp83Asn appears to be a small perturbation in the protein, suggesting that the enzyme could have nearly wild-type activity.
The only abnormality identified in the panel of plasma biomarkers in the male with the GLA p.Asn228Ser variant was the presence of the lyso-Gb3 analog at m/z 836, which is undetectable in healthy controls. The normal lyso-Gb3 level and of all its other analogs is in agreement with the result of a confirmatory α-Gal enzyme assay in plasma and does not corroborate the bioinformatic and structural modelling predictions of pathogenicity. It is of note that lyso-Gb3 analog at m/z 836 was also detected in the plasma of two patients diagnosed with cardiac variants of FD, but was not found neither in the p.Arg118Cys hemizygote male, who showed minimal LVH, probably not related to α-Gal deficiency [46], nor in the p.Arg220Ter heterozygote female, who presented left ventricular noncompaction, probably as an early manifestation of cardiac involvement by FD [43]. This finding is particularly interesting in view of the results of a recent study showing that elevated urinary Gb3 excretion is associated with near-term mortality in non-FD patients with advanced stages of heart disease [54]. Further studies with an increased number of patients will be needed to elucidate whether an increased plasma level of lyso-Gb3 analog at m/z 836 might be an earlier biomarker of progressive heart disease.
A brother and sister carried the GLA 5’UTR SNP c.−10C>T. Hemizygous and heterozygous carriers of the minor c.−10T allele have an average ≈25% lower leukocyte α -Gal activity, as compared to males and females with the wild-type c.−10C or c.−10CC genotypes [40, 50]. The pattern of plasma and urinary biomarkers exhibited by the two siblings was similar to the p.Asp83Asn heterozygote. The marginal increase above normal range upper limit of the plasma lyso-Gb3 analog at m/z 820 observed in patient IX-P most probably is clinically meaningless.
Four females and one male carried the GLA p.Arg118Cys variant, whose minor allele is associated with average residual leukocyte α-Gal activities of ≈30% and ≈60% of the normal mean, respectively in hemizygous males and heterozygous females [46]. The male (case IV-Fa) and two of the females (cases V-P, VI) had normal patterns of plasma and urinary biomarkers. In patient IV-P, the detection of minimal amounts of lyso-Gb3 analog at m/z 758 in plasma and of lyso-Gb3 analog at m/z 820 in urine is probably clinically insignificant. The normal concentrations of lyso-Gb3 in plasma and urine, and of Gb3 in the urine, observed in case IV-P, are in agreement with the results of a kidney biopsy obtained when she was 29 years old, which did not show any signs of FD nephropathy or of abnormal accumulation of Gb3 [55]. Further evidence that the GLA p.Arg118Cys variant does not cause FD nephropathy was the lack of GSL deposits observed by the EM examination of a kidney biopsy from a 42-year-old hemizygous male [46].
The p.Phe113Leu and p.Asn215Ser hemizygotes typically presented with FD cardiac variants. Both patients exhibited rather similar biomarker patterns in plasma and urine, with the exceptions of the plasma concentrations of lyso-Gb3 analog at m/z 784 and of urinary Gb3 excretion levels. The lyso-Gb3 plasma levels were, respectively, ≈40% and ≈20% of the lowest levels observed in Dutch [56, 57] or Japanese [58] males with classic FD, and roughly of the same order of magnitude than those reported in Chinese patients with a late-onset cardiac variant of FD, which is particularly common in Taiwan [59]. Like in the Dutch [56] and the Chinese [59] cohorts, the plasma concentrations of lyso-Gb3 observed in our patients with the p.Phe113Leu or the p.Asn215Ser mutation reflected the severity of LVH; conversely, none of the lyso-Gb3 analogs detected in plasma correlated better with the severity of LVH than the plasma levels of lyso-Gb3.
Remarkably, the urinary Gb3 excretion was >4.5-fold above the ULN in the p.Phe113Leu hemizygote, who showed only minimal proteinuria, but was within the normal reference range in the p.Asn215Ser hemizygote, who presented with CKD stage 3. As this patient also had T2DM and diabetic retinopathy, diabetic nephropathy might be the underlying cause of CKD. A kidney biopsy would be critical to clarify the differential diagnosis but unfortunately could not be obtained.
It has been previously argued that the urine levels of Gb3 cannot be used as a marker of FD in patients with the p.Asn215Ser mutation [24]. This was based on the observation that only three of seven males and none of five females carrying that mutation had elevated urinary Gb3 levels, but no data were reported regarding the kidney disease phenotype of those patients.
The GLA p.Asn215Ser mutation was originally identified in unrelated asymptomatic individuals or mildly affected patients with late-onset manifestations primarily confined to the heart [60]. A recent study has shown that a paediatric Fabry patient cohort with the p.Asn215Ser mutation presents normal urinary Gb3 and lyso-Gb3 levels but show abnormal levels of the urinary analogs [33]. In the United Kingdom, the GLA p.Asn215Ser mutation was identified in three of the five unrelated men diagnosed with FD on the prospective screening of patients presenting with unexplained LVH at ³40 years of age (n = 79), but in none of those who were diagnosed at <40 years of age (n = 74) [61]. Males who are hemizygous for this GLA p.Asn215Ser may present with renal dysfunction at advanced age, with the kidney biopsy showing predominant involvement of podocytes and minimal or absence of GSL deposits in other cell types [62], but only exceptionally [63] has the p.Asn215Ser mutation been identified in FD case-finding studies among HD patients. Indeed, the GLA p.Asn215Ser mutation was not detected in any of 13 large-scale studies screening for FD among patients on RRT that were carried out before 2010 (summarized at [64, 65]), nor in any of those that have been published subsequently [53, 66–70], adding up to more than 20,000 RRT patients screened and more than 30 newly diagnosed carriers of pathogenic GLA mutations. Mutation p.Asn215Ser was also not identified in any of the large stroke patient cohorts of the multinational European “Stroke in Young Fabry Patients study” (n =5023) [52], the “Belgian Fabry study” (n = 1000) [71], the “Stroke Prevention in Young Men Study” (n = 558) [72] and the “PORTYSTROKE study – Screening Genetic Conditions in Portuguese Young Stroke Patients” (n = 493) [45]. Overall, these data suggest that GLA mutation p.Asn215Ser might not be associated with severe, renal or cerebrovascular complications of FD. The same holds true for the epidemiology of GLA mutation p.Phe113Leu, which has never been identified in FD case-finding studies among stroke or RRT patients, except in one of the three newly diagnosed FD patients, among the 2012 males enrolled in the Portuguese screening of HD patients (Ferreira, S. & Oliveira, J. P., personal observation).
Theoretically, relatively hydrophilic biomarkers of systemic GSL metabolic load, like lyso-Gb3 [25], might be filtered and reabsorbed in the kidney [57] and would be expected to be found at increased concentrations in plasma and urine of FD patients. On the other hand, lipophilic biomarkers, like Gb3 [25], reach the urine mainly in desquamated tubular cells and possibly also in podocytes [20, 22], and may better reflect the GSL kidney tissue load [22]. These postulates are in agreement with our present findings and deserve further investigation, particularly in correlation with kidney biopsy data.
In the heterozygous female for the p.Arg220Ter mutation, the plasma levels of lyso-Gb3 and of most of its analogs were abnormally high, while the lyso-Gb3 analogs at m/z 784 and 836 were respectively within the normal range or undetectable. The plasma lyso-Gb3 concentration was of the same order of magnitude of the levels previously reported for Dutch [56, 57], Japanese [58] and Chinese [59] females carrying GLA mutations associated with the classical phenotype of FD. The urinary biomarker profile of the p.Arg220Ter female in this study showed an increased level of excretion for all analogs, except for the analog at m/z 836, which was within the normal values. This urinary lyso-Gb3 profile was similar to that of the p.Phe113Leu hemizygote but with significantly lower amounts of all the analytes except for lyso-Gb3, which was detected at 4- to 5-fold higher concentration. The patient’s lyso-Gb3 urine levels were within the range observed in Dutch males with classic FD [57].
In conclusion, the plasma and urine lyso-Gb3 profiles closely correlated with the phenotypic data for each study subject, supporting the contention that they may constitute clinically useful biomarkers of FD. Our findings are in line with those of Smid et al. [73], who showed that plasma lyso-Gb3 is a reliable diagnostic tool to discern classical FD from subjects without FD, and first describe a similar relationship between lyso-Gb3 analogs and the phenotypic expression of GLA mutations and variants.
The hypothesis that increased levels of plasma and (possibly) urinary lyso-Gb3 analog at m/z 836 are an earlier biomarker of progressive heart disease, and that urinary excretion of Gb3 is a specific biomarker of FD nephropathy, warrants further research studies [74] with larger, carefully selected cardiac and renal patient cohorts.
HIGHLIGHTS.
Individual profiles of Gb3 and lyso-Gb3 and analogs correlate with phenotypic data.
Diagnostic tool to discern classical FD, cardiac variants and patients without FD.
Lyso-Gb3 analog at m/z 836 might be an earlier biomarker of progressive heart disease.
The plasma and urine lyso-Gb3 constitute clinically useful biomarkers of FD.
Acknowledgments
The alphα-galactosidase A assays reported in this manuscript were performed at one of the following laboratories, under the supervision of the acknowledged person: Jan-Eric Månsson, PhD, Laboratory of Neurochemistry, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden; Maria Clara Sá Miranda, PhD, Unidade de Biologia do Lisossoma e do Peroxissoma, Instituto de Biologia Molecular e Celular, Porto, Portugal; Lúcia Lacerda, Unidade de Enzimologia, Centro de Genética Médica Jacinto Magalhães, Centro Hospitalar do Porto, Porto, Portugal.
Portions of this work were supported by grant R01 DK76877 from the NIH to S.C.G.
This manuscript is part of Susana Ferreira’s doctorate thesis research plan supervised by João Paulo Oliveira MD, PhD; some of these data have been presented as a poster at the European Fabry Expert Lounge 2014, Rome, March 21–22, 2014. The research project was reviewed and approved by the Scientific Council of the Faculty of Medicine of the University of Porto.
This study was partly supported by an unrestricted grant from Sanofi – Genzyme (Portugal) and by a Grant-in-Aid of research from Genzyme, A Sanofi Division (Canada) and the Canadian Institutes of Health Research (CIHR). We are grateful to Waters Corporation for their continued scientific support and partnership in Christiane Auray-Blais’ laboratory in Sherbrooke, Quebec, Canada.
No Genzyme staff had any role in the study design, collection, analysis and interpretation of data, writing of the manuscript, or the decision to submit the paper for publication.
We thank the patients and their relatives for their invaluable collaboration in this study. We thank Dr. António Caldeira Gomes (Service of Nephrology, São João Hospital Centre, Porto, Portugal), Dr. Elsa Azevedo (Service of Neurology, São João Hospital Centre, Porto, Portugal), Dr. Inês Ferreira (Haemodialysis Unit, NephroCare, Fafe, Portugal), Dr. Ludovina Paredes (Service of Internal Medicine, Vila Nova de Gaia Hospital Centre, Portugal) and Dr. Pedro Ferreira (Service of Internal Medicine, Matosinhos Hospital Centre, Portugal) for having referred patients to us for comprehensive diagnostic assessment.
Footnotes
CONFLICT OF INTEREST DECLARATION
Susana Ferreira has received unrestricted research grants and funding for research projects from Genzyme, A Sanofi Division; conference registration fees and travel grants from Genzyme, A Sanofi Division and Shire Human Genetic Therapies.
João Paulo Oliveira is member of the European Advisory Board of the Fabry Registry, a global observational registry of patients with Fabry disease sponsored by Genzyme Corporation. He has received unrestricted research grants and funding for research projects from Genzyme Corporation; consulting honoraria and speaker’s fees from Genzyme Corporation; conference registration fees and travel grants from Genzyme Corporation, Shire Human Genetic Therapies and Amicus Therapeutics.
Christiane Auray-Blais has received research grants from Genzyme, A Sanofi Division, Shire and BioMarin Pharmaceuticals Inc. She has received honoraria & funds for travel from Genzyme, A Sanofi Division, Shire and BioMarin Pharmaceuticals Inc. for lectures given. She has received honoraria for consultant services and traveling funds from Amicus Therapeutics Inc. She has received traveling funds from Waters Corporation.
Michel Boutin and Pamela Lavoie have received support for salaries and traveling funds from Genzyme, A Sanofi Division. Michel Boutin has also received salary support from the Michael J Fox Foundation for Parkinson’s research. Pamela Lavoie has also received salary support from Shire and BioMarin Pharmaceuticals Inc.
Contributor Information
Susana Ferreira, Email: susanadg@med.up.pt.
Christiane Auray-Blais, Email: christiane.auray-blais@usherbrooke.ca.
Michel Boutin, Email: michel.boutin2@usherbrooke.ca.
Pamela Lavoie, Email: pamela.lavoie@usherbrooke.ca.
José Pedro Nunes, Email: jplnunes@med.up.pt.
Elisabete Martins, Email: elismartins@med.up.pt.
Scott Garman, Email: garman@biochem.umass.edu.
João Paulo Oliveira, Email: jpo@med.up.pt.
REFERENCES
- 1.Desnick RJ, Ioannou YA, Eng CM. α -Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 2.Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodary PF, Shayman JA, Eitzman DT. Alphα-Galactosidase A in vascular disease. Trends Cardiovasc Med. 2007;17:129–133. doi: 10.1016/j.tcm.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Schiffmann R. Fabry disease. Pharmacol Ther. 2009;122:65–77. doi: 10.1016/j.pharmthera.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab. 2010;99:99–108. doi: 10.1016/j.ymgme.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Weidemann F, Sanchez-Nino MD, Politei J, et al. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis. 2013;8:116. doi: 10.1186/1750-1172-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desnick RJ, Banikazemi M, Wasserstein M. Enzyme replacement therapy for Fabry disease, an inherited nephropathy. Clin Nephrol. 2002;57:1–8. doi: 10.5414/cnp57001. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Linthorst GE, Poorthuis BJ, Hollak CE. Enzyme activity for determination of presence of Fabry disease in women results in 40% false-negative results. J Am Coll Cardiol. 2008;51:2082. doi: 10.1016/j.jacc.2008.02.050. author reply 2082–2083. [DOI] [PubMed] [Google Scholar]
- 10.Migeon BR. X inactivation, female mosaicism, and sex differences in renal diseases. J Am Soc Nephrol. 2008;19:2052–2059. doi: 10.1681/ASN.2008020198. [DOI] [PubMed] [Google Scholar]
- 11.Desnick RJ, Brady R, Barranger J, et al. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003;138:338–346. doi: 10.7326/0003-4819-138-4-200302180-00014. [DOI] [PubMed] [Google Scholar]
- 12.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alphα-Galactosidase A--replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 13.Schiffmann R, Kopp JB, Austin HA, 3rd, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 14.Moore DF, Ries M, Forget EL, Schiffmann R. Enzyme replacement therapy in orphan and ultra-orphan diseases: the limitations of standard economic metrics as exemplified by Fabry-Anderson disease. Pharmacoeconomics. 2007;25:201–208. doi: 10.2165/00019053-200725030-00003. [DOI] [PubMed] [Google Scholar]
- 15.Rombach SM, Hollak CE, Linthorst GE, Dijkgraaf MG. Cost-effectiveness of enzyme replacement therapy for Fabry disease. Orphanet J Rare Dis. 2013;8:29. doi: 10.1186/1750-1172-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desnick RJ. Enzyme replacement therapy for Fabry disease: lessons from two alphα-Galactosidase A orphan products and one FDA approval. Expert Opin Biol Ther. 2004;4:1167–1176. doi: 10.1517/14712598.4.7.1167. [DOI] [PubMed] [Google Scholar]
- 17.Vedder AC, Linthorst GE, Houge G, et al. Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One. 2007;2:e598. doi: 10.1371/journal.pone.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rombach SM, Aerts JM, Poorthuis BJ, et al. Long-term effect of antibodies against infused alphα-Galactosidase A in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS One. 2012;7:e47805. doi: 10.1371/journal.pone.0047805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekri S, Lidove O, Jaussaud R, Knebelmann B, Barbey F. The role of ceramide trihexoside (globotriaosylceramide) in the diagnosis and follow-up of the efficacy of treatment of Fabry disease: a review of the literature. Cardiovasc Hematol Agents Med Chem. 2006;4:289–297. doi: 10.2174/187152506778520718. [DOI] [PubMed] [Google Scholar]
- 20.Schiffmann R, Waldek S, Benigni A, Auray-Blais C. Biomarkers of Fabry disease nephropathy. Clin J Am Soc Nephrol. 2010;5:360–364. doi: 10.2215/CJN.06090809. [DOI] [PubMed] [Google Scholar]
- 21.Auray-Blais C, Cyr D, Ntwari A, et al. Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol Genet Metab. 2008;93:331–340. doi: 10.1016/j.ymgme.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield PD, Calvin J, Hogg S, et al. Monitoring enzyme replacement therapy in Fabry disease--role of urine globotriaosylceramide. J Inherit Metab Dis. 2005;28:21–33. doi: 10.1007/s10545-005-4415-x. [DOI] [PubMed] [Google Scholar]
- 23.Michaud L, Auray-Blais C. Improved ways to screen for patients with Fabry disease, involving optometry in a multidisciplinary approach. Can J Optometry. 2012;74:25–32. [Google Scholar]
- 24.Young E, Mills K, Morris P, et al. Is globotriaosylceramide a useful biomarker in Fabry disease? Acta Paediatr Suppl. 2005;94:51–54. doi: 10.1111/j.1651-2227.2005.tb02112.x. discussion 37–58. [DOI] [PubMed] [Google Scholar]
- 25.Aerts JM, Groener JE, Kuiper S, et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A. 2008;105:2812–2817. doi: 10.1073/pnas.0712309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbey F, Brakch N, Linhart A, et al. Cardiac and vascular hypertrophy in Fabry disease: evidence for a new mechanism independent of blood pressure and glycosphingolipid deposition. Arterioscler Thromb Vasc Biol. 2006;26:839–844. doi: 10.1161/01.ATV.0000209649.60409.38. [DOI] [PubMed] [Google Scholar]
- 27.Auray-Blais C, Ntwari A, Clarke JT, et al. How well does urinary lyso-Gb3 function as a biomarker in Fabry disease? Clin Chim Acta. 2010;411:1906–1914. doi: 10.1016/j.cca.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 28.van der Tol L, Smid BE, Poorthuis BJ, et al. A systematic review on screening for Fabry disease: prevalence of individuals with genetic variants of unknown significance. J Med Genet. 2014;51:1–9. doi: 10.1136/jmedgenet-2013-101857. [DOI] [PubMed] [Google Scholar]
- 29.van der Tol L, Svarstad E, Ortiz A, et al. Chronic kidney disease and an uncertain diagnosis of Fabry disease: Approach to a correct diagnosis. Mol Genet Metab. 2014 doi: 10.1016/j.ymgme.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Auray-Blais C, Boutin M, Gagnon R, Dupont FO, Lavoie P, Clarke JTR. Urinary globotriaosylsphingosine-related biomarkers for Fabry disease targeted by metabolomics. Anal Chem. 2012;84:2745–2753. doi: 10.1021/ac203433e. [DOI] [PubMed] [Google Scholar]
- 31.Auray-Blais C, Boutin M. Novel gb(3) isoforms detected in urine of fabry disease patients: a metabolomic study. Curr Med Chem. 2012;19:3241–3252. doi: 10.2174/092986712800784739. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie P, Boutin M, Auray-Blais C. Multiplex analysis of novel urinary lyso-Gb3-related biomarkers for Fabry disease by tandem mass spectrometry. Anal Chem. 2013;85:1743–1752. doi: 10.1021/ac303033v. [DOI] [PubMed] [Google Scholar]
- 33.Auray-Blais C, Blais C-M, Ramaswami U, et al. Urinary biomarker investigation in children with Fabry disease using tandem mass spectrometry. Clin Chim Acta. 2015;438:195–204. doi: 10.1016/j.cca.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Boutin M, Gagnon R, Lavoie P, Auray-Blais C. LC-MS/MS analysis of plasma lyso-Gb3 in Fabry disease. Clin Chim Acta. 2012;414:273–280. doi: 10.1016/j.cca.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Manwaring V, Boutin M, Auray-Blais C. A metabolomic study to identify new globotriaosylceramide-related biomarkers in the plasma of Fabry disease patients. Anal Chem. 2013;85:9039–9048. doi: 10.1021/ac401542k. [DOI] [PubMed] [Google Scholar]
- 36.Dupont FO, Gagnon R, Boutin M, Auray-Blais C. A metabolomic study reveals novel plasma lyso-Gb3 analogs as Fabry disease biomarkers. Curr Med Chem. 2013;20:280–288. doi: 10.2174/092986713804806685. [DOI] [PubMed] [Google Scholar]
- 37.Boutin M, Auray-Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb3-related analogues in Fabry disease. Anal Chem. 2014;86:3476–3483. doi: 10.1021/ac404000d. [DOI] [PubMed] [Google Scholar]
- 38.Group KDIGOKCW. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira JP, Ferreira S, Reguenga C, Carvalho F, Mansson JE. The g.1170C>T polymorphism of the 5’ untranslated region of the human alphα-Galactosidase gene is associated with decreased enzyme expression--evidence from a family study. J Inherit Metab Dis. 2008;31(Suppl 2):S405–S413. doi: 10.1007/s10545-008-0972-0. [DOI] [PubMed] [Google Scholar]
- 41.Mayes JS, Scheerer JB, Sifers RN, Donaldson ML. Differential assay for lysosomal alphα-Galactosidases in human tissues and its application to Fabry’s disease. Clin Chim Acta. 1981;112:247–251. doi: 10.1016/0009-8981(81)90384-3. [DOI] [PubMed] [Google Scholar]
- 42.Garman SC, Garboczi DN. The molecular defect leading to Fabry disease: structure of human alphα-Galactosidase. J Mol Biol. 2004;337:319–335. doi: 10.1016/j.jmb.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 43.Martins E, Pinho T, Carpenter S, et al. Histopathological evidence of Fabry disease in a female patient with left ventricular noncompaction. Rev Port Cardiol. 2014;33:565. doi: 10.1016/j.repc.2014.02.021. e561–566. [DOI] [PubMed] [Google Scholar]
- 44.Morais P, Santos AL, Baudrier T, Mota AV, Oliveira JP, Azevedo F. Angiokeratomas of Fabry successfully treated with intense pulsed light. J Cosmet Laser Ther. 2008;10:218–222. doi: 10.1080/14764170802275832. [DOI] [PubMed] [Google Scholar]
- 45.Baptista MV, Ferreira S, Pinho EMT, et al. Mutations of the GLA gene in young patients with stroke: the PORTYSTROKE study--screening genetic conditions in Portuguese young stroke patients. Stroke. 2010;41:431–436. doi: 10.1161/STROKEAHA.109.570499. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira S, Ortiz A, Germain DP, et al. The alphα-Galactosidase A p.Arg118Cys variant does not cause a Fabry disease phenotype: Data from individual patients and family studies. Mol Genet Metab. 2015;114:248–258. doi: 10.1016/j.ymgme.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies JP, Winchester BG, Malcolm S. Sequence variations in the first exon of alphα-Galactosidase A. J Med Genet. 1993;30:658–663. doi: 10.1136/jmg.30.8.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu G, Pang S, Feng X, et al. Genetic analysis of lysosomal alphα-Galactosidase A gene in sporadic Parkinson’s disease. Neurosci Lett. 2011;500:31–35. doi: 10.1016/j.neulet.2011.05.238. [DOI] [PubMed] [Google Scholar]
- 49.Ferri L, Guido C, la Marca G, et al. Fabry disease: polymorphic haplotypes and a novel missense mutation in the GLA gene. Clin Genet. 2012;81:224–233. doi: 10.1111/j.1399-0004.2011.01689.x. [DOI] [PubMed] [Google Scholar]
- 50.Oliveira JP, Ferreira S, Barcelo J, et al. Effect of single-nucleotide polymorphisms of the 5’ untranslated region of the human alphα-Galactosidase gene on enzyme activity, and their frequencies in Portuguese caucasians. J Inherit Metab Dis. 2008;31(Suppl 2):S247–S253. doi: 10.1007/s10545-008-0818-9. [DOI] [PubMed] [Google Scholar]
- 51.Auray-Blais C, Millington DS, Barr C, Young SP, Mills K, Clarke JT. Gb(3)/creatinine biomarkers for Fabry disease: issues to consider. Mol Genet Metab. 2009;97:237. doi: 10.1016/j.ymgme.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Rolfs A, Fazekas F, Grittner U, et al. Acute cerebrovascular disease in the young: the Stroke in Young Fabry Patients study. Stroke. 2013;44:340–349. doi: 10.1161/STROKEAHA.112.663708. [DOI] [PubMed] [Google Scholar]
- 53.Herrera J, Miranda CS. Prevalence of Fabry’s disease within hemodialysis patients in Spain. Clinical nephrology. 2014;81:112–120. doi: 10.5414/CN108053. [DOI] [PubMed] [Google Scholar]
- 54.Schiffmann R, Forni S, Swift C, et al. Risk of death in heart disease is associated with elevated urinary globotriaosylceramide. J Am Heart Assoc. 2014;3:e000394. doi: 10.1161/JAHA.113.000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valbuena C, Leitao D, Carneiro F, Oliveira JP. Immunohistochemical diagnosis of Fabry nephropathy and localisation of globotriaosylceramide deposits in paraffin-embedded kidney tissue sections. Virchows Arch. 2012;460:211–221. doi: 10.1007/s00428-011-1182-y. [DOI] [PubMed] [Google Scholar]
- 56.Rombach SM, Dekker N, Bouwman MG, et al. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta. 2010;1802:741–748. doi: 10.1016/j.bbadis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Gold H, Mirzaian M, Dekker N, et al. Quantification of globotriaosylsphingosine in plasma and urine of fabry patients by stable isotope ultraperformance liquid chromatography-tandem mass spectrometry. Clin Chem. 2013;59:547–556. doi: 10.1373/clinchem.2012.192138. [DOI] [PubMed] [Google Scholar]
- 58.Togawa T, Kodama T, Suzuki T, et al. Plasma globotriaosylsphingosine as a biomarker of Fabry disease. Mol Genet Metab. 2010;100:257–261. doi: 10.1016/j.ymgme.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 59.Liao HC, Huang YH, Chen YJ, et al. Plasma globotriaosylsphingosine (lysoGb3) could be a biomarker for Fabry disease with a Chinese hotspot late-onset mutation (IVS4+919G>A) Clin Chim Acta. 2013;426:114–120. doi: 10.1016/j.cca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Eng CM, Resnick-Silverman LA, Niehaus DJ, Astrin KH, Desnick RJ. Nature and frequency of mutations in the alphα-Galactosidase A gene that cause Fabry disease. Am J Hum Genet. 1993;53:1186–1197. [PMC free article] [PubMed] [Google Scholar]
- 61.Sachdev B, Takenaka T, Teraguchi H, et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–1411. doi: 10.1161/01.cir.0000012626.81324.38. [DOI] [PubMed] [Google Scholar]
- 62.Meehan SM, Junsanto T, Rydel JJ, Desnick RJ. Fabry disease: renal involvement limited to podocyte pathology and proteinuria in a septuagenarian cardiac variant Pathologic and therapeutic implications. Am J Kidney Dis. 2004;43:164–171. doi: 10.1053/j.ajkd.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 63.Bekri S, Enica A, Ghafari T, et al. Fabry disease in patients with end-stage renal failure: the potential benefits of screening. Nephron Clin Pract. 2005;101:c33–c38. doi: 10.1159/000085709. [DOI] [PubMed] [Google Scholar]
- 64.Mignani R, Feriozzi S, Schaefer RM, et al. Dialysis and transplantation in Fabry disease: indications for enzyme replacement therapy. Clin J Am Soc Nephrol. 2010;5:379–385. doi: 10.2215/CJN.05570809. [DOI] [PubMed] [Google Scholar]
- 65.Linthorst GE, Bouwman MG, Wijburg FA, Aerts JM, Poorthuis BJ, Hollak CE. Screening for Fabry disease in high-risk populations: a systematic review. J Med Genet. 2010;47:217–222. doi: 10.1136/jmg.2009.072116. [DOI] [PubMed] [Google Scholar]
- 66.Kim J-Y, Hyun Y-Y, Lee J-E, et al. Serum globotriaosylceramide assay as a screening test for fabry disease in patients with ESRD on maintenance dialysis in Korea. Korean J Intern Med. 2010;25:415–421. doi: 10.3904/kjim.2010.25.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishino T, Obata Y, Furusu A, et al. Identification of a novel mutation and prevalence study for fabry disease in Japanese dialysis patients. Ren Fail. 2012;34:566–570. doi: 10.3109/0886022X.2012.669300. [DOI] [PubMed] [Google Scholar]
- 68.Doi K, Noiri E, Ishizu T, et al. High-throughput screening identified disease-causing mutants and functional variants of alphα-Galactosidase A gene in Japanese male hemodialysis patients. J Hum Genet. 2012;57:575–579. doi: 10.1038/jhg.2012.68. [DOI] [PubMed] [Google Scholar]
- 69.Maruyama H, Takata T, Tsubata Y, et al. Screening of male dialysis patients for fabry disease by plasma globotriaosylsphingosine. Clin J Am Soc Nephrol. 2013;8:629–636. doi: 10.2215/CJN.08780812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okur I, Ezgu F, Biberoglu G, et al. Screening for Fabry disease in patients undergoing dialysis for chronic renal failure in Turkey: identification of new case with novel mutation. Gene. 2013;527:42–47. doi: 10.1016/j.gene.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 71.Brouns R, Thijs V, Eyskens F, et al. Belgian Fabry study: prevalence of Fabry disease in a cohort of 1000 young patients with cerebrovascular disease. Stroke. 2010;41:863–868. doi: 10.1161/STROKEAHA.110.579409. [DOI] [PubMed] [Google Scholar]
- 72.Wozniak MA, Kittner SJ, Tuhrim S, et al. Frequency of unrecognized Fabry disease among young European-American and African-American men with first ischemic stroke. Stroke. 2010;41:78–81. doi: 10.1161/STROKEAHA.109.558320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smid BE, van der Tol L, Biegstraaten M, Linthorst GE, Hollak CE, Poorthuis BJ. Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J Med Genet. 2015;52:262–268. doi: 10.1136/jmedgenet-2014-102872. [DOI] [PubMed] [Google Scholar]
- 74.Boutin M, Auray-Blais C. Metabolomic Discovery of Novel Urinary Galabiosylceramide Analogs as Fabry Disease Biomarkers. J Am Soc Mass Spectr. 2015;26:499–510. doi: 10.1007/s13361-014-1060-3. [DOI] [PubMed] [Google Scholar]