Abstract
HIV-1 infection typically results from the transmission of a single viral variant, the transmitted/founder (T/F) virus. Studies of these HIV-1 variants provide critical information about the transmission bottlenecks and the selective pressures acting on the virus in the transmission fluid and in the recipient tissues. These studies reveal that T/F virus phenotypes are shaped by stochastic and selective forces that restrict transmission and may be targets for prevention strategies. In this Review, we highlight how studies of T/F viruses contribute to a better understanding of the biology of HIV-1 transmission and discuss how these findings affect HIV-1 prevention strategies.
HIV-1 is the retrovirus (genus Lentivirus) responsible for acquired immunodeficiency syndrome (AIDS). The virus replicates predominantly in CD4+ T cells, which produce virus that is readily and persistently detected in the blood and other bodily fluids. Most HIV-1 transmission events worldwide are a result of heterosexual sex with an infected partner, and approximately 80% of heterosexual transmission events and infections are established from a single HIV-1 variant — termed the transmitted/founder virus (T/F virus) — as based on analyses of the complexity of the virus in the blood during the first several weeks of infection1–4. Shortly after transmission, HIV-1 populations in the blood of the newly infected individuals are largely homogenous and evolve in a manner consistent with exponential viral replication3, which allows for the genetic sequence of a T/F virus to be inferred as the same as the consensus sequence constructed from the viral population present early in infection3. In contrast to the homogeneous viral population observed in the recipients shortly after transmission, there is typically a diverse viral population in the blood of infected donors, which indicates that there are one or more strong bottlenecks that result in the transmission of a single T/F virus (FIG. 1). Therefore, there is continued interest in understanding whether these bottlenecks are stochastic and restrict all viruses (for example, nonspecific barrier functions) or whether there are selective pressures favouring certain phenotypes in the T/F virus. Extensive efforts have been made to find viral phenotypes that correlate with transmission, as exploring these phenotypes may elucidate the biology of HIV-1 transmission and inform novel prevention approaches.
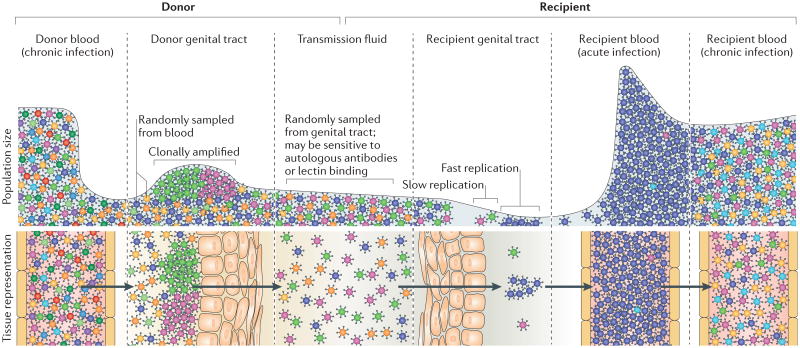
Figure 1. The transmitted/founder virus is shaped by multiple genetic bottlenecks.
Chronically infected individuals have extremely diverse HIV-1 populations in their blood. Some viruses from the blood seed the genital tract of the donor, where the resulting viral population is less diverse than in the blood and is often dominated by a few clonally amplified variants. It is unknown whether replication in the genital tract selects for specific phenotypes. Viruses sampled from the donor genital tract are present in the transmission fluids (cervicovaginal mucus, semen or rectal secretions). These fluids may contain proteins that enhance (for example, semen-derived enhancers of virus infection) or reduce (for example, cytokines, chemokines, antimicrobials, lectins and autologous antibodies) viral infectivity. Differential sensitivity to these proteins could select for specific viral phenotypes. The vast majority of viruses within the transmission fluid do not penetrate the genital or rectal mucosa of the recipient. Damage due to sexually transmitted infections or intercourse can increase the ability of viruses to penetrate the mucosa. Most of the viruses that are able to infect the recipient genital tract have a low reproductive rate (R0<1) owing to low densities of target cells, low viral fitness or susceptibility to host defences (such as phagocytosis or production of interferons) and will not contribute to the systemic infection. Typically, when a systemic infection is established after sexual exposure to HIV-1, the initial viral population in the recipient's blood will be genetically homogeneous because it was established from a single viral genotype (the transmitted/founder virus) that was able to replicate in the recipient genital tract. On progression to the chronic stages of infection, infected individuals display extremely diverse HIV-1 populations in their blood.
The selective pressures that shape the bottlenecks that lead to the transmission of a T/F virus can occur at different stages in the transmission cycle: in the donor variants at the site of transmission; during the transmission process of moving the virus particles from the donor to the site of infection in the recipient; with the infection of the initial cell in the recipient; or in the first few rounds of replication, during which inefficient viral spread might result in the infection being extinguished (FIG. 1). As the stochastic and selective forces that act at these different stages will differ based on the donor and recipient environment, there is unlikely to be a single phenotype or genetic sequence that is shared by all T/F viruses. Rather, phenotypes that increase the probability of transmission will be over-represented in T/F viruses. In this Review, we discuss the different bottlenecks that shape the transmission of T/F viruses, including the conditions that enhance or limit HIV-1 transmission, and the features of the viruses that are selected during transmission, highlighting how these findings have the potential to inform the development of biological interventions directed against HIV-1.
Transmission bottlenecks in the donor
Individuals chronically infected with HIV-1 have diverse viral populations in their blood, but that diversity can be reduced by bottlenecks that take place as viruses seed the genital tract (GT) and enter the transmission fluid (semen, cervicovaginal mucus (CVM) or rectal mucus) (FIG. 1).
Compartmentalization at the donor site of transmission
The migration of HIV-1 from the blood into the transmission fluid is likely to be greatly influenced by the trafficking of infected immune cells and/or free viruses from the blood into the GT and the rectum. This notion is consistent with an analysis of the env sequences of simian immunodeficiency virus (SIV) in male macaques, which showed that SIV populations that are present in the semen and tissues of the male GT are derived from the viral populations that are present in the blood5. However, it is also possible that viruses replicate locally in the GT. Therefore, the composition of viral populations at the site of transmission in the donor is likely to reflect a dynamic relationship between the trafficking of free virus from the blood, HIV-1-infected cells from the blood that release viruses into the GT, and the local replication of viruses in the GT.
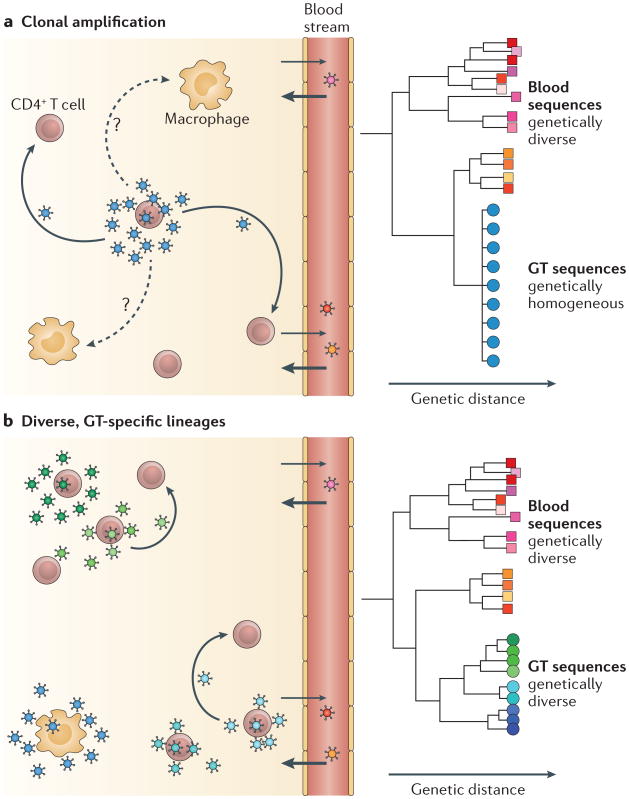
Sustained replication in the GT can create genetically distinct, compartmentalized lineages of viruses (FIG. 2). The existence of compartmentalized lineages in the GT is somewhat surprising given the evidence for frequent, bidirectional trafficking of HIV-1 between the blood and GT6, which is predicted to homogenize the populations in these two compartments. Nonetheless, compartmentalization in the GT of both males7–15 and females8,14–17 infected with HIV-1 has been reported. Notably, viral populations compartmentalized in the GT can include clonally amplified and/or diverse, GT-specific lineages (FIG. 2). The majority of these GT-specific lineages are clonally amplified in both males7,8 and females8,18,19. Furthermore, when sampled longitudinally, most clonally amplified GT-specific lineages are expressed transiently8,18.
Figure 2. Compartmentalization of genital tract-specific viral lineages.
HIV-1 populations in the blood are genetically diverse during chronic infection, but genital tract (GT)-specific lineages can be either homogeneous or diverse. a. Clonally amplified viruses are produced when one or a few cells (most likely T cells) are infected with very similar viruses. Phylogenetic analyses illustrate that clonally amplified viruses that are present in the GT (blue) are much less diverse than viruses that are present in the blood (red, orange and pink). b. Viral replication in the GT for many generations can produce diverse, GT-specific lineages (blue and green) that are phylogenetically distinct from viruses that are present in the blood (red, orange and pink). These diverse lineages may have adapted to replication in different cell types (such as macrophages) and/or evolved independently in different parts of the GT.
These observations are consistent with a model in which inflammation, which is most commonly caused by a change in the viral, bacterial and/or fungal species present in the GT, especially following the acquisition of a sexually transmitted pathogen20, can drive HIV-1 replication in the GT. In this model, GT inflammation, whether clinical or subclinical, may lead to bursts of virus production from a small cluster of T cells infected by a single virus, resulting in clonal viral amplification (FIG. 2). Inflammation may also create clusters of HIV-1-susceptible cells in the GT by recruiting these cells from the periphery, causing their local proliferation and activation in the GT. Furthermore, these newly recruited, HIV-1-susceptible cells may amplify viral variants that exist in the GT and/or viral variants that are trafficked to the GT by infected T cells. In any case, as the inflammation resolves and the number of HIV-1-susceptible cells in the GT returns to pre-inflammation levels, the amplified viral variant is predicted to quickly decay.
In contrast to these clonally amplified viral lineages that are present in the GT, a few GT-specific lineages have been observed to be moderately8 to substantially7 diverse in their genetic complexity (FIG. 2). These diverse lineages are produced through multiple rounds of isolated viral replication and evolution in the GT of males7 and females8. Although our view of clonally amplified GT viruses is that they typically represent transient lineages8,18, a longitudinal analysis of a female patient who had a moderately diverse, compartmentalized lineage showed that this diversity persisted in the GT over a period of 28 days8. The small number of observations of diverse GT compartmentalized lineages may reflect either that these lineages rarely arise or that they frequently arise but persist at lower abundance than clonally amplified variants. Furthermore, it is unknown whether these lineages represent viruses that have adapted to replicate in new target cells in the GT or viruses that are simply replicating independently in an area of the GT that is seldom reached by blood viruses.
Clonally amplified viruses in the GT would be predicted to have a transmission advantage owing to their higher frequency in the transmission fluid. However, a study of transmission pairs found compartmentalized variants in the GT of seven out of eight donors and showed that, at the time of sampling — which was between 2 and 12 weeks after the transmission event — the T/F viruses were minority (not clonally amplified) variants in the GT of each donor8. Without samples taken at the time of transmission (an impossible task), the possibility that the T/F variants were clonally amplified at the time of transmission cannot be ruled out, but these observations suggest that downstream bottlenecks may reduce or obscure any transmission advantage associated with viral frequency in the transmission fluid.
The effect of compartmentalization on transmission is also complicated by the fact that viral populations in the GT can be further divided into free virus and cell-associated virus groups. Little is known about whether these forms are genetically distinct and whether they both contribute to transmission. One study of six men who have sex with men (MSM) transmission pairs suggested that each T/F virus was more similar to free viruses present in the seminal plasma of the donor than to viral DNA from the donor's seminal cells, which is consistent with sexual transmission typically occurring by transfer of cell-free virus21. However, a re-analysis of the viral sequences questioned this conclusion for technical reasons22. An additional study found some evidence of transmission of a cell-associated variant23 in humans, and studies of SIV-infected macaques have revealed that transmission can occur by vaginally inoculating females with either cell-associated24 or cell-free viruses25. Although both routes of infection are possible, infection studies in macaques almost exclusively use cell-free viruses and have proven that this route is highly effective, whereas there is relatively little evidence that cell-associated virus can be transmitted. This discrepancy could be due to technical challenges that make it difficult to illustrate transmission of cell-associated virus or due to cell-associated virus being a highly inefficient mode of transmission. This is an important issue for developing prevention strategies.
Selection in the transmission fluid
Selection in the transmission fluid is another possible bottleneck during the transmission cycle of HIV-1. For example, the transmission fluid may contain neutralizing antibodies or lectins that prevent some HIV-1 variants from being transmitted. Alternatively, some viruses may become trapped in the mucosal secretions.
The Env protein is expressed on the surface of HIV-1 particles and mediates target cell binding; however, its exposure at the surface of the virus also makes the Env protein a target of neutralizing antibodies. A great deal of effort has been put into examining env gene sequences and Env protein phenotypes in T/F viruses, trying to identify genetic or phenotypic evidence of selection during transmission. These studies have attempted to compare the T/F virus to the total donor population in linked transmission events or have compared T/F viruses with a surrogate population meant to approximate the donor population. After identifying a statistical relationship between a specific genotype or phenotype and transmission, researchers try to infer where in the transmission process the selective pressure occurred that skewed the viral population. The search for genetic variation has focused on identifying ‘signature sequences’ that are associated with increased transmission probability26.
One feature that has been observed in T/F viruses is their differential levels of glycosylation. For example, T/F viruses of HIV-1 subtypes A, C and D1,27–29 have fewer N-linked glycosylation sites encoded by their env gene, but this pattern is less obvious for subtype B T/F viruses27,30,31. Interestingly, the role of glycosylation in transmission may differ for men and women and may depend on the mode of viral transmission. A study of heterosexually transmitted subtype C viruses found that the bias towards transmission of low glycosylation viruses was particularly pronounced in female-to-male transmission, suggesting that female-to-male transmission either selects viruses with fewer glycosylation sites or selects viruses lacking specific glycosylation sites29. A potential explanation for these observations is a model in which heavily glycosylated viruses are less likely to be sexually transmitted32,33 because they are more easily trapped in the transmission fluid or inhibited by agents present in the transmission fluid (FIG. 3). As intrapartum transmission requires a virus to interact with a transmission fluid (the CVM), this model would predict that intrapartum transmission also selects for T/F viruses with fewer glycosylation sites. Consistent with this prediction, viruses transmitted intrapartum display fewer glycosylation sites than those transmitted intrauterine, which is a mode of transmission that does not involve interaction of HIV-1 with the CVM34. Notably, although heavier glycosylation reduces the probability of sexual and intrapartum HIV-1 transmission, it may provide the virus with an evolutionary advantage at later stages of infection by increasing viral resistance to neutralizing antibodies29,32,33 through the establishment of a ‘glycan shield’, which is known to facilitate escape from neutralizing antibodies by obscuring viral epitopes35,36.
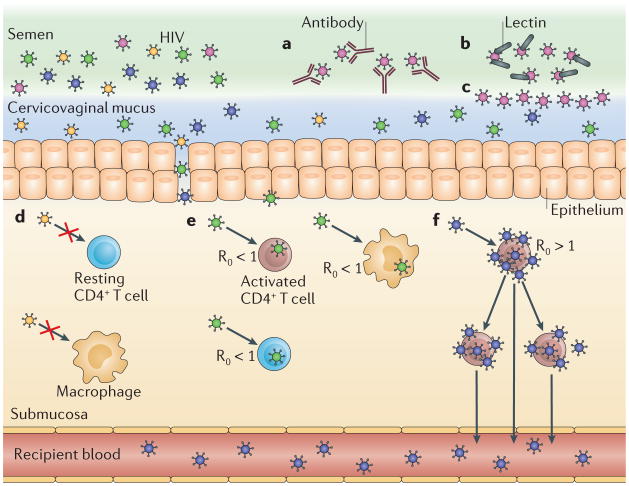
Figure 3. Selection of transmitted/founder virus phenotypes.
Transmitted/founder (T/F) HIV-1 viruses may be enriched for phenotypes that increase the probability of transmission. a. Viruses may have a low probability of being transmitted if they are sensitive to autologous antibodies present in the transmission fluids, such as semen or cervicovaginal mucus. b. Highly glycosylated viruses may also have a lower probability of being transmitted if they are bound by lectins in the transmission fluid. c. Glycans on the surface of viruses may restrict their migration through the transmission fluid. d–f. In the genital submucosa of the recipient, viruses may have a reduced probability of transmission if their Env protein is poorly adapted to entering cells in that tissue (part d). Similarly, a virus may have a reduced probability of transmission if it replicates slowly because it has a low-fitness genotype or it preferentially infects target cells that do not replicate viruses rapidly (for example, macrophages and resting CD4+ T cells). In this case, the basic reproductive rate of the virus (R0, which is an estimate of the number of cells that will be infected from a single infected cell) will be lower than 1 and the virus will be lost (part e). By contrast, the probability of transmission may be increased if a virus replicates rapidly because it has a high-fitness genotype or an ability to preferentially infect target cells that rapidly amplify virus (for example, activated CD4+ T cells). In this case, the R0 of the virus will be higher than 1 and the virus can establish an infection and will eventually be present in the blood of the recipient (part f).
Despite the differences in glycosylation observed in T/F viruses, the examination of neutralization sensitivity to specific antibodies has not revealed consistent differences between T/F viruses and viruses present during chronic infection. This is somewhat surprising given that T/F viruses are generally less glycosylated and would be predicted to be more sensitive to neutralization. Consistent with this prediction, subtype B T/F viruses have been reported to be moderately more sensitive to neutralizing antibodies that target the CD4-binding site of Env37; however, this has not been seen with subtype C T/F viruses29. Similarly, one study observed that the Env proteins of subtype C T/F viruses are more sensitive to autologous donor antibodies1 compared with the average donor virus. However, several studies have shown that Env proteins from T/F viruses and Env proteins from viruses present at chronic stages of infection rarely differ in their sensitivity to heterologous antibodies1,29. If we consider sensitivity to heterologous antibodies as a probe for Env protein conformation, these data seem to indicate that there is no conformational difference between the T/F viruses and the typical viruses that are present in the donor. However, the observation of a difference in sensitivity to autologous antibodies between T/F viruses and the donor viruses that are present in the peripheral blood during chronic infection needs to be confirmed, and at the moment the significance of this difference is unclear.
Glycosylation can also affect viral binding by broadly neutralizing antibodies (BNAbs), as these antibodies can include a glycan as part of their epitope target (FIG. 3). For example, an analysis of sera from nine patients with strong BNAb responses to HIV-1 revealed that eight produced BNAbs with glycan-dependent epitopes38. Notably, loss of a glycosylation site can make a virus resistant to certain BNAbs that bind to glycans on the Env surface, such as 2G12, PG9 and PG16 (REF. 36). Interestingly, the slight overall reduction in glycosylation of subtype C T/F viruses is mostly represented in lost glycosylation sites within the highly variable regions of Env, not the more conserved glycosylation sites that are recognized by these well-characterized BNAbs. This explains how T/F viruses can have fewer glycosylation sites than viruses isolated from subjects during chronic infection (about 7% fewer) yet T/F viruses are not, on average, more resistant to BNAbs that bind epitope targets that include conserved glycans29.
Lectins may also select for under-glycosylated Env proteins during transmission (FIG. 3). Many lectins have been shown to bind glycans on the surface of the envelope glycoprotein gp120 and thus inhibit HIV-1 (or simian–human immunodeficiency virus (SHIV)) infection in cell culture, in ectocervical explants and in macaque models (reviewed in REF. 39). One of these lectins, the mannose-binding lectin (MBL), plays an important part in innate immunity by binding carbohydrates on the surface of many pathogens40 and activating the complement system. For example, lower MBL levels are associated with susceptibility to Streptococcus pneumoniae, Neisseria meningitidis and influenza A virus, as well as with the severity of hepatitis B infection (reviewed in REF. 40). Notably, genetic differences between donors in serum MBL levels have been shown to vary by more than 1,000-fold41. Studies examining the relationship between MBL levels in serum and HIV acquisition have reached different conclusions, with some studies suggesting that MBL is protective and others suggesting that it increases susceptibility to HIV-1 (REF. 40). To understand the role of MBL in HIV-1 transmission, it may be useful to examine whether MBL levels in genital secretions influence HIV-1 susceptibility. Interestingly, MBL is found at low concentrations in semen42, but whether MBL is present in CVM is unknown.
Another donor factor that could influence transmission is a peptide fragment of the semen-derived protein prostatic acid phosphatase (PAP; also known as ACPP), which forms amyloid fibrils that capture HIV-1 and facilitate its attachment to target cells in the recipient43. These fibrils, termed semen-derived enhancers of virus infection (SEVI), have been shown to enhance HIV-1 infection in a dose-dependent manner44 in vitro. Similarly, semenogellin-derived amyloid fibrils in semen are also capable of enhancing virus attachment and entry45. The potential importance of a SEVI-like activity is also demonstrated by infectivity studies in cell culture-based assays, in which the inclusion of semen reduced the activity of several antivirals in blocking infectivity (with maraviroc — an antiretroviral agent developed to prevent HIV-1 infection by blocking the C–C chemokine receptor type 5 (CCR5) co-receptor46 — being a notable exception)47. Although semen-derived amyloid fibrils enhance infection in vitro, less is known about their effects in vivo. For example, an analysis of the effect of SEVI on the transmission of SIVmac in macaques did not provide conclusive evidence for SEVI enhancing infection48, and there are currently no human data indicating that SEVI enhance transmission.
Additional donor effects increasing HIV-1 transmission
In addition to GT compartmentalization and to the selection of viral variants in the transmission fluid, other donor factors, such as the viral load at the time of transmission, may alter HIV-1 transmission rates. It has long been recognized that donors with higher viral loads in their blood49,50 and/or GT51,52 transmit HIV-1 more readily than donors with lower viral loads. This relationship is further supported by a recent study showing that initiation of antiretroviral therapy, which lowers viral loads, results in lower transmission rates49.
The concentration of HIV-1 in blood plasma and genital secretions can vary by several orders of magnitude owing to many factors, including concomitant viral, bacterial or parasitic infections that enhance (reviewed in REF. 53) or suppress54,55 replication of HIV-1. For example, confection with Neisseria gonorrhoeae is associated with elevated concentrations of HIV-1 in the blood plasma of women56 and in semen57. N. gonorrhoeae produces heptose monophosphate, which stimulates an innate immune response that results in increased nuclear factor-κB (NF-κB)-mediated transcription of the HIV-1 long terminal repeats (LTR)58 as one mechanism that could affect the level of virus. Similarly, co-infection with herpes simplex virus 2 (HSV-2) has also been associated with elevated concentrations of HIV-1 in genital secretions (reviewed in REF. 59), but the mechanistic relationship between HSV-2 infection and HIV-1 replication is still being investigated60. Nonetheless, a general inflammatory response and the influx of inflammatory cells into the GT as a result of HSV-2 co-infection are likely to be major factors in determining the HIV-1 viral load. The effect that sexually transmitted infections (STIs) have on HIV-1 replication is further illustrated by observations that treating co-infected men for N. gonorrhoeae significantly reduces HIV-1 viral load in semen57 and treating co-infected women for HSV-2 significantly reduces HIV-1 viral load in genital secretions and blood plasma59.
Transmission bottlenecks in the recipient
The most severe bottleneck that occurs during transmission takes place in the GT of the recipient, where the viral diversity present in the transmission fluid is typically reduced to a single genotype, which initiates the systemic infection (FIG. 1).
Infection of target cells in the recipient genital tract
For an HIV-1 particle to initiate an infection, it must reach and infect subepithelial cells in the recipient GT, particularly CD4+ T cells. The ability to enter these cells is governed by the viral surface Env protein. When translated, the Env precursor protein is cleaved into two subunits, gp120 and gp41, which stay non-covalently associated as heterodimers. Three of these heterodimers are organized as a trimeric spike on the surface of the virion, with gp41 being a transmembrane protein anchoring the trimer and gp 120 being exposed at the apex of the trimer and most distal to the viral surface. Viral entry is initiated when the CD4-binding site of gp120 of one or more trimers binds CD4 molecules on the surface of the host cell, which results in conformational changes that expose the co-receptor-binding site on gp120. This co-receptor-binding site then engages the CCR5 co-receptor or, in some cases, the C-X-C chemokine receptor type 4 (CXCR4) co-receptor, which are expressed on the surface of the host cell. Co-receptor engagement causes the extracellular domain of gp41 to insert its amino-terminal fusion peptide into the host cell membrane, initiating the formation within gp41 of a six-helix bundle, which brings the host and viral membranes together to generate a fusion pore between the viral and host membranes that the viral capsid can use to enter the host cell61. Given the central part played by Env during viral entry, several Env-mediated phenotypes have been proposed to have a role in the infection of subepithelial target cells and to define the nature of the target cell in transmission.
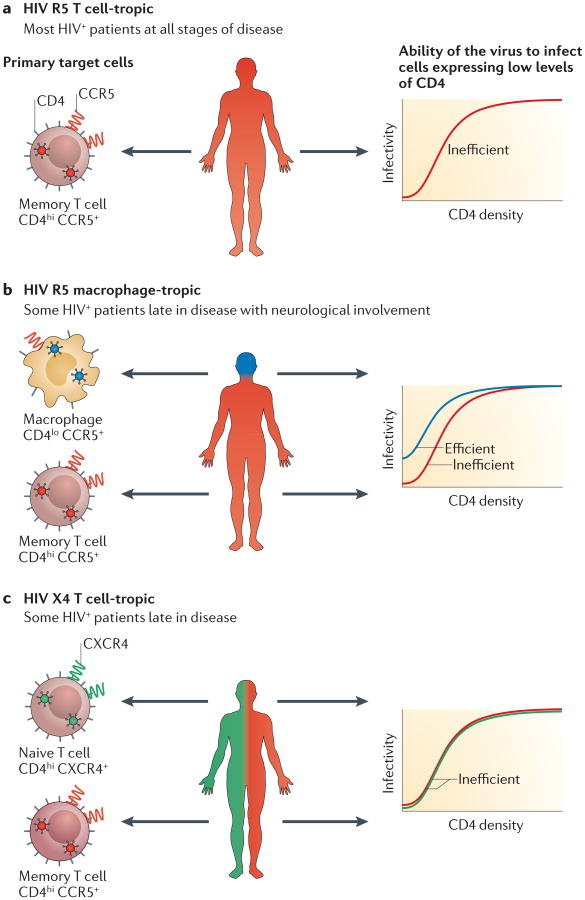
The discovery of co-receptors for HIV-1 entry (REF 62) led to an understanding that the T/F virus typically uses CCR5 for entry, but the role of CD4 in defining the tar‑ get cell has only recently been clarified. In recent years, the ability of HIV-1 to infect cells expressing different levels of the entry receptor CD4 and its co-receptor CCR5 has been assayed using the Affinofile (receptor affinity–profiling) cell line, in which expression of CD4 and CCR5 is under inducible control63. These assays have confirmed that the vast majority of blood-derived viruses use the CCR5 co-receptor but that they poorly infect cells expressing low levels of CD4 (REFS 29,64,65). CD4 is expressed at a high density on CD4+ T cells but at a much lower density on other CD4-expressing cells, such as monocytes and macrophages64. Thus, most blood-derived HIV-1 viruses can be termed ‘R5 T cell-tropic’. Similarly, HIV-1 T/F viruses are nearly always R5 T cell-tropic (FIG. 4). Conversely, several studies have shown that T/F viruses are unable to efficiently infect macrophages64,66–69 or other cells expressing low levels of CD4 (REFS 29,64) (FIG. 4). For example, pseudotyped viruses made with env clones from 33 heterosexually transmitted T/F viruses were inefficient at infecting cells expressing low levels of CD4 (REF. 29). Similarly, these 33 subtype C T/F viruses29 and 55 subtype B T/F viruses3 all used CCR5 as the co-receptor, with only one virus being capable of also using the alternative co-receptor CXCR4 (that is, being ‘dual-tropic’).
Figure 4. Characteristics of transmitted/founder viruses.
The dfferent cell types that are available for infection by HIV-1 transmitted/founder (T/F) viruses in the genital tract of the recipient differ greatly in their surface expression of the CD4 receptor and of the C-C chemokine receptor type 5 (CCR5) or C-X-C chemokine receptor type 4 (CXCR4) co-receptors. For example, CD4+ T cells express much higher densities of CD4 than do macrophages. Importantly, memory CD4+ T cells express both the CCR5 and the CXCR4 co-receptors, but for simplicity reasons, only one of the co-receptors is displayed in each cell. a. Most viral lineages have adapted to replicating in CD4+ T cells. These T cell-tropic viruses are inefficient at entering cells expressing low levels of CD4 (such as macrophages) and require high levels of CD4+ for entry (such as those expressed by CD4+ T cells)64. T cell-tropic viruses can be further divided by whether they use the CCR5 or the CXCR4 co-receptor. Most T/F viruses use the CCR5 co-receptor3,29, making them R5 T cell-tropic. These viruses are frequently transmitted to new individuals. b. A few HIV-1 lineages have been identified, mostly in the central nervous system of people at end-stage disease, that primarily replicate in macrophages. These macrophage‑tropic viruses are able to enter cells expressing low levels of CD4. However, no macrophage-tropic T/F viruses have been reported, suggesting that these viruses are not transmitted to new individuals. c. Some T cell-tropic viruses can use the CXCR4 co-receptor, making them X4 T cell-tropic. These viruses are transmitted occasionally.
T/F viruses have also been shown to have unexpected features associated with the use of CCR5. For example, T/F env clones are less likely to be able to use a maraviroc-resistant form of CCR5 than chronically derived viruses29. This phenotype is clearly observed when cells express high levels of CCR5 (REFS 29,70) but not when cells express lower levels of CCR5 (REFS 29,65). These data are consistent with CCR5 existing in multiple conformations when expressed at high levels, and either resistant viruses using a novel maraviroc-bound form of CCR5 or CCR5 existing in conformations that maraviroc cannot bind but that some viruses can use for entry. Regardless of the specific mechanism, transmission seems to select for viruses that are more restricted in the use of CCR5 co-receptor variants than blood-derived chronic viruses.
T/F viruses also differ from other HIV-1 viruses in their ability to incorporate Env protein in newly formed virions, and one study of infectious molecular clones (IMCs) of T/F viruses revealed that they produce viruses that contain twice as much Env protein as IMCs of chronically derived control viruses71. This is consistent with a study of subtype B env sequences showing that transmitted env genes are enriched for encoding a basic amino acid at position 12 in the Env leader sequence, which increases Env density on pseudoviruses72. Higher Env protein concentration may increase the probability that a virus attaches to and infects a target cell in the genital mucosa or rectum of the recipient.
T/F viruses also display enhanced binding to dendritic cells (DCs) and are subsequently transferred to T cells more efficiently than viruses derived from chronic infections71. This could facilitate efficient transport of T/F viruses from the epithelial surface to the stroma or to lymphoid tissue, where the virus can be presented to, and infect, T cells73. A study exposing human vaginal explants to HIV-1 found that T/F viruses efficiently bound DCs, which then transported the viruses through the mucosa and presented them to T cells74. Similarly, in vitro studies have shown that DCs are able to facilitate infection after being incubated with HIV-1 and then co-cultured with T cells75–78. These results suggest that DCs may facilitate HIV-1 infection of T cells during transmission, but there is little evidence that DCs themselves are productively infected79–81, consistent with their low density of surface CD4.
Finally, studies of SIV transmission indicate that resting CD4+ T cells are the most abundant CD4-expressing cells in the subepithelial tissue before infection and are the earliest cells infected. However, once infected, resting CD4+ T cells produce fewer virus particles than activated T cells82,83. Furthermore, treatment of macaques with the antimicrobial agent glycerol monolaurate can suppress immune signalling in the female GT and prevent transmission of SIV, probably by inhibiting recruitment of T cells and DCs84. These observations suggest that recruiting T cells, and perhaps DCs, to the site of exposure enhances viral transmission and the establishment of systemic infection, which provides at least a partial explanation for why bacterial vaginosis85 and HSV-2 (REF. 86) infections — which result in the recruitment of immune cells to the site of infection — are associated with increased susceptibility to HIV-1.
Viral replication in the recipient genital tract
There is now strong genotypic and phenotypic evidence that transmission selects for viruses with higher replication rates. In a recent study87 of gag, pol and nef sequences from 137 linked transmission pairs, donor sequences that more closely matched the cohort consensus sequence were more likely to be transmitted than were sequences that differed more from the consensus. This advantage is thought to arise because being more similar to the consensus sequence produces higher-fitness viruses with elevated infectiousness or burst size. The higher-fitness nature of the consensus sequence is supported by studies showing that when low-fitness cytotoxic T lymphocyte (CTL)-escape mutants 88–90 are transmitted, they often quickly revert to the consensus sequence at that site87,91–93. The emerging picture is that transmission occurs in a multiple-step process that probably involves stochastic bottlenecks that limit transmission of all viruses and selective bottlenecks that select for phenotypes such as reduced glycosylation and higher fitness (BOX 1; FIG. 3).
Box 1. Relationship between viral fitness and transmission.
The probability that an infection will die out (that is, will not be transmitted to new cells) is predicted by the virus's basic reproductive rate, R0, which is an estimate of the number of cells that will be infected from a single infected cell121. On average, infections will be lost when R0 <1, and will be sustained and spread when R0 >1. Viral lineages may differ in their R0 owing both to stochastic factors that reduce replication of all viral lineages (for example, low target cell density) and to specific viral phenotypes that increase viral replication (for example, resistance to interferon-α (IFNα)). The bias toward transmission of high-fitness viruses87 suggests that most HIV-1 infected cells die out in the early days of the infection and do not contribute to a systemic infection (R0 <1; see the figure, dashed red line). Analyses of T/F viruses also indicate that HIV-1 replicates exponentially early in infection3, and this corresponds to a rapid increase in both R0 (see the figure, solid red line) and viral load (see the figure, solid blue line). This rapid replication reduces the proportion of cells that are susceptible to infection and stimulates a CD8+ T cell-mediated immune response that kills infected cells (cytotoxic T lymphocyte (CTL) response), which are two factors that reduce the R0 and eventually cause it to rapidly decline until it is less than 1. The combination of R0 <1 and death of infected cells generates a precipitous drop in viral load. The subsequent emergence of viral mutants that can escape CD8+ T cell-mediated killing (CTL-escape mutants) allows R0 to rebound to steady-state, at which point the average infected cell will generate one additional infected cell (R0 = 1), and the viral load is maintained at set point (set point viral load (SPVL)). The timing of these events and the SPVL are probably determined by several factors, including the host immune response and the fitness of the transmitted virus.
As noted, R0 at steady state is 1, meaning that each infected cell gives rise to another infected cell. However, the SPVL varies dramatically between people, from <50 copies per ml of viral RNA in elite controllers to >100,000 copies per ml in rapid progressors. These differences can be achieved either by each cell producing different numbers of virus particles in different people, or by different numbers of cells being infected at steady state in different people. The latter explanation seems more likely, given that SPVL is associated with the rate of disease progression122. These data justify the interest in studying the viral genetics of SPVL, especially the idea that each viral lineage includes a genetic component that is, in part, responsible for setting the SPVL in the host. Indeed, recent studies suggest that there is some intermediate SPVL that maximizes transmission, and viruses that help achieve that SPVL will be selected on a population basis123,124. Other studies have analysed the link between phylogenetic relatedness of T/F viruses and SPVL125,126. To date, these approaches have revealed a wide range of values for the contribution of the viral genotype to determining the SPVL and thus this remains an area of active interest.

Bias towards transmission of higher-fitness variants reveals information about the environment in which transmission takes place. Such evolutionary change could be generated only if higher-fitness viruses have a higher probability of generating a systemic infection (that is, there is selection) and viruses replicating at the site of exposure differ in their fitness (that is, there is genetic variation). Higher-fitness viruses could be selected under multiple scenarios, which differ primarily in the number of viruses replicating at the site of transmission. For example, exposure to HIV-1 could result in the initial replication of multiple, heterogeneous viruses at the site of transmission, followed by a second stage in which only one virus establishes systemic infection. In this scenario, the virus with higher fitness would be successful and lower-fitness viruses would be lost. A prediction of this first scenario is that the multiple lineages that replicate initially at the site of transmission would occasionally give rise to viral recombinants, but deep sequencing viral populations during acute infection has not revealed recombinant genomes or rare genomes94,95 (but see REF. 96). Therefore, if multiple viruses are indeed transmitted to new HIV-1 recipients, most are probably lost in the initial rounds of replication. In an alternative scenario, after exposure to HIV-1, no more than one virus replicates at the site of transmission and most replicating viruses do not generate systemic infection. In this case, when a systemic infection is established it is typically from a higher-fitness virus. Under this scenario, the local replication of viruses that are ultimately lost would have to be very limited and not sufficient to induce an adaptive immune response (that is, seroconversion without systemic infection). Both scenarios strongly suggest that T/F viruses are the only variants that have sustained replication at the site of transmission and that all other variants are lost very quickly (FIG. 3).
Understanding when the bias toward transmission of higher-fitness variants occurs can also reveal additional information about the factors limiting transmission. For example, biased transmission of viruses closer to the consensus sequence was more severe in female-to-male transmission than in male-to-female transmission, with infection of females allowing the transmission of lower-fitness genotypes and transmission to males more strongly favouring higher-fitness variants87. Exceptions to this pattern arise when a female donor has a higher viral load or when male recipients recently had a genital ulcer or inflammation. Under these conditions, lower-fitness variants have an increased probability of being transmitted to males. Similarly, selection for reduced glycosylation of the surface Env protein is stronger in female-to-male transmission than in male-to-female transmission29. Together, these studies29,87 suggest that infection of the first cell and subsequent spread to other cells may be more difficult in males than in females, but that amplifying factors can overcome these difficulties to increase the probability of lower-fitness variants being transmitted (BOX 1).
This initial stage of viral replication in the GT of newly infected individuals has also been suggested to induce an antiviral state through interaction with the innate immune system, and that this antiviral state could inhibit viral replication and select for interferon-α (IFNα) resistance. Indeed, recent studies examining T/F IMCs have reported that they have enhanced IFNα resistance71,97. However, at least some of the genetic determinants that define IFNα sensitivity in T/F viruses are CTL-escape mutations97 and may also reduce fitness. Therefore, it is difficult to know whether transmission directly selects for IFNα-resistant viruses or whether IFNα resistance is a by-product of selection for increased viral fitness. Although IFNα is generally thought to limit transmission, this interpretation is complicated by questions about whether these factors inhibit or promote transmission and whether they are expressed early enough to influence transmission at all. In SIV-infected macaques, vaginal transmission results in rapid induction of pro-inflammatory cytokines (for example, tumour necrosis factor (TNF), macrophage inflammatory protein 1α (MIP1α) and interleukin-6 (IL-6)) that may quickly recruit target cells to the mucosal tissue, whereas peak production of IFNα and IFNβ occurs later98. These complications are further illustrated by an additional SIV–macaque experiment indicating that high levels of IFNα initially blocked transmission, whereas continued exposure to high levels of IFNα reduced the expression of antiviral proteins, allowing macaques to become infected and experience increased disease severity99. Thus the timing of the IFN effect determines whether the induced inflammatory response controls or enhances the infection.
Factors involved in the establishment of systemic infection
While many questions persist about the viral factors involved in transmission and replication in the GT, even less is known about whether factors that influence the ability of HIV-1 to replicate in specific cell types and other tissues could influence the establishment of systemic infection and the seeding of infection in other compartments in the body. For example, an analysis of a small number of viruses suggested that transmission selects for T/F viruses with increased affinity for α4β7 integrin, which is expressed on some CD4+ T cells100. This gut-homing integrin can retain T cells in the gut-associated lymphoid tissue (GALT)101, where HIV-1 is known to replicate very rapidly. However, an analysis of a much larger panel of pseudotyped viruses did not detect a difference in the ability of an α4β7-specific antibody to block infection of viruses with the Env protein of T/F viruses compared with viruses expressing the Env protein of viruses taken from chronic infection65, suggesting that transmission does not select for viruses with an increased affinity for α4β7. These observations are supported by a recent study showing that most Env proteins, including those expressed by T/F viruses, are unable to efficiently bind α4β7 (REF. 102). By contrast, the sequence motif that binds α4β7 is over-represented in several South African subtype C lineages of HIV-1 relative to other clades103, therefore making it difficult to determine whether this motif has been selected in these lineages to increase α4β7 binding or is simply an ancestral sequence and has not been selected to increase α4β7 binding. The potential role of this integrin in infection was shown with the ability of an antibody directed against α4β7 to protect macaques from infection104. Furthermore, when animals treated with the α4β7-specific antibody became infected, they maintained CD4+ T cell counts in their blood and GALT that were significantly higher than those of infected, untreated animals. Although it is unclear whether transmission selects for viruses with an increased ability to bind α4β7 integrin, there is encouraging information that blocking this receptor could become a method of pre-exposure prophylaxis (PrEP; see below).
As most transmission events occur with a single HIV-1 variant, it is not possible to use genetic diversity to assess the early seeding of different compartments. However, the simultaneous transmission of multiple viruses provides an opportunity to investigate viral preference for some compartments. Surprisingly, at early stages during acute infection, some transmitted viral lineages can be largely absent in the blood but present in the cerebral spinal fluid or the central nervous system, suggesting that it is possible for multiple viruses to be transmitted and for some of these to be sequestered in at least one compartment and not be readily detected in the blood105,106. Although the extent to which this happens in other compartments and how this affects the establishment of the systemic infection are unknown, these data suggest that compartment-specific factors can influence how the systemic infection unfolds. Molecularly tagged SIV variants that can be used as an artificial swarm107 will be a useful tool to address this question in the macaque model, although it must be kept in mind that even low-dose challenges in the macaque model represent a transmission frequency that is 10–100-fold higher than that seen in humans.
Bottlenecks inform prevention strategies
HIV prevention can be divided into behavioural and biological approaches, although these clearly overlap. Perhaps the greatest attention has been given to keeping HIV-negative people uninfected through safer sex that involves reliable condom usage, monogamy and avoidance of concurrent (that is, overlapping) partnerships, and health-seeking behaviour that leads to detection and treatment of STIs (reviewed by REF. 108). As noted above, untreated STIs cause inflammation that seems to reduce the ‘fitness barrier’ in transmission87 and lead to a larger average number of variants transmitted4. However, attempts to reduce HIV-1 incidence in the general population by mass treatment of STIs have generally been unsuccessful109.
Biological interventions include circumcision and the use of antiretroviral agents and vaccines. Male circumcision leads to large and sustained reduction in HIV-1 acquisition110. This observation demonstrates that either the cells in the foreskin are highly susceptible to HIV-1 infection or the environment created by the foreskin increases susceptibility to infection.
In recent years, antiviral agents have been deployed in two ways to stop HIV-1 transmission. First, antiviral agents have been used as treatment, as effective treatment of an infected person reduces transmission probability in both heterosexual couples49,111 and MSM couples112 to a negligible level. Second, antiviral agents have been used as PrEP for men and women at high risk of HIV-1 infection. Currently, the combination of tenofovir disoproxil fumarate and emtricitabine (Truvada; Gilead) is approved for this purpose in the United States, but access outside of the United States is largely limited to clinical trials or demonstration projects; other drugs are in development for oral, injectable or topical usage as PrEP. As with ‘treatment as prevention’, the anti viral agents must be present in sufficient concentration to eliminate all potential T/F viruses from establishing a sustained infection. Studies of non-human primates suggest that these target cells are at or near the genital mucosal surfaces113, but more distant cells throughout the female reproductive tract (including the ovaries) may be exposed to virus and infected114.
Vaccines offer the most important hope for the constraint of HIV-1, and the success of a vaccine depends entirely on the immunity evoked being able to eliminate potential T/F viruses. Currently, there are three ways in which an HIV-1 vaccine might work alone or in combination: by priming CD8+ T cells to eliminate the initial cells infected by the T/F virus; by eliciting antibodies that use antibody-dependent cytotoxic means to kill infected cells (which is apparently the way in which the RV144 vaccine reduced HIV-1 incidence115); or by eliciting BNAbs that will neutralize the T/F virus. The success of each of these approaches depends on the ability of host defences to interact effectively with the T/F viruses. There are now specific examples of the importance of this interaction in the detection of genetic sieving of founder/breakthrough variants in the context of vaccine trials. In the RV144 trial, there was an association between the generation of antibodies to variable region 1 (V1) and V2 and protection from infection115, and there was a similar correlation in the sieving of specific sequences in V1 and V2 of breakthrough viruses in the vaccine arm116. These are both indications of selective pressure on this region due to prior exposure to the vaccine116. Similarly, selective pressure against T cell epitopes was detected in breakthrough virus in the context of the STEP vaccine trial117. An analysis of breakthrough virus in a vaccine trial using the SIV/macaque system demonstrated selection against neutralization-sensitive viruses in the challenge stock that carried a two amino acid signatures in Env118, again showing that prior immunization is able to apply selective pressure on the challenge virus.
Outlook
Thirty years of research have helped to clarify many of the details about how HIV-1 is transmitted. Of particular importance is the recent realization that transmission involves both stochastic and fitness bottlenecks that act both in the donor and in the recipient (FIG. 1). In the donor, bottlenecks are observed when viruses seed the donor GT (FIG. 2) and in the transmission fluid (FIG. 3). In the recipient, bottlenecks occur as the virus crosses the mucosal membrane or when it replicates in the recipient GT (FIG. 3). These bottlenecks ensure that sexual exposure to HIV-1 only occasionally generates a systemic infection, and when transmission does occur it usually involves a single viral genotype. Furthermore, these studies reveal information about points of transmission vulnerability that may be addressed by prevention strategies.
Future prevention efforts will build on these successes and insights. Antiretrovirals with long half-lives or slow-release formulations are being developed to ensure that antiretroviral drugs are maintained at high enough concentrations to be effective PrEP agents119. The potential role of endogenous lectins could be enhanced with the introduction of potent exogenous lectins, such as components in microbicides120. Furthermore, the induction of BNAbs by vaccination or gene therapy could create more stringent bottlenecks in the exposed person, which could further lower the probability of viral transmission. Finally, large-scale studies that are amenable to the genetic and phenotypic analysis of the T/F virus will continue to offer important evidence of selective pressure short of complete protection that can inform future efforts.
Acknowledgments
This work was funded by awards from the US National Institutes of Health (U01 AI067854 (CHAVI)), and R37 AI44667 to R.S. and R37 DK049381 to M.S.C.).
Glossary
- Retrovirus
A member of the family Retroviridae. Retroviruses use reverse transcriptase to convert their single-stranded RNA genome into double-stranded DNA, which is subsequently integrated into the host genome. The integrated proviral genome may be transcribed, translated and assembled into new virions
- Lentivirus
Genus of the family Retroviridae that includes HIV and simian immunodeficiency virus (SIV)
- Transmitted/founder virus
(T/F virus). The virus that forms a systemic infection after being transferred from an infected individual to an uninfected individual. HIV-1 infections are typically established from a single T/F virus
- env
Gene encoding the HIV-1 Env glycoprotein. This protein is cleaved into two subunits (gp120 and gp41), which are non-covalently bound as heterodimers and organized as trimers on the virion surface. Env glycoproteins facilitate virus binding to and fusion with host cells
- Compartmentalized lineages
Virus variants found outside the blood that are genetically distinct from variants in the blood. Compartmentalized lineages evolve after many generations of independent replication in a tissue or compartment
- Transmission pairs
HIV-1 donors and the recipient that each infected. Studies of transmission pairs are useful for examining many aspects of transmission biology, including factors that influence the probability of transmission and whether transmission bottlenecks select for specific viral phenotypes
- Neutralizing antibodies
Antibodies that inactivate infectious agents after recognizing antigens on their surface
- Lectins
Carbohydrate-binding proteins capable of neutralizing viruses by binding glycans on the viral surface
- Glycosylation
The host process of attaching glycans to proteins. Glycosylation of HIV-1 Env has the effect of shielding epitopes on Env from antibody binding
- HIV subtypes
Group M HIV-1 contains multiple lineages, termed subtypes
- Autologous donor antibodies
Antibodies that are produced by the same individual who produced the viruses that they are neutralizing. May inhibit transmission by neutralizing viruses in the transmission fluid
- Heterologous antibodies
Antibodies that are produced by an individual different from the one who produced the viruses that they are neutralizing
- Pseudotyped viruses
Viruses produced in vitro using a method that allows the researcher to control the specific Env proteins expressed on the surface of the virion
- Infectious molecular clones
(IMCs). Full-length DNA clones of HIV-1 genomes capable of producing replication-competent viruses
- Cytotoxic T lymphocyte (CTL)-escape mutants
Viral variants that carry mutations in viral proteins that allow viruses to avoid detection by CTL. These mutations can reduce viral replicative fitness
- Interferon-α
(IFNα). A cytokine whose expression is rapidly upregulated after virus exposure to signal cells to create an antiviral environment. IFNα can reduce HIV-1 replication
Footnotes
Competing interests statement: The authors declare no competing interests.
References
- 1.Derdeyn CA, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams MR, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. One of the first studies using single genome amplification to amplify env genes from blood samples collected during acute infection and infer the T/F sequence and T/F virus phenotypes. It found that 76% of sexual transmission events were established from a single T/F virus and that 98% were CCR5 tropic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haaland RE, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fieni F, et al. Viral RNA levels and env variants in semen and tissues of mature male rhesus macaques infected with SIV by penile inoculation. PLoS ONE. 2013;8:e76367. doi: 10.1371/journal.pone.0076367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaillon A, et al. HIV migration between blood and cerebrospinal fluid or semen over time. J Infect Dis. 2014;209:1642–1652. doi: 10.1093/infdis/jit678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JA, et al. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog. 2010;6:e1001053. doi: 10.1371/journal.ppat.1001053. This study illustrates that diverse, compartmentalized viral lineages can be detected in some semen samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boeras DI, et al. Role of donor genital tract HIV-1 diversity in the transmission bottleneck. Proc Natl Acad Sci USA. 2011;108:E1156–E1163. doi: 10.1073/pnas.1103764108. A study of transmission pairs suggesting that the transmission bottleneck is not just stochastic and may select for rare variants in the donor GT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrn RA, Kiessling AA. Analysis of human immunodeficiency virus in semen: indications of a genetically distinct virus reservoir. J Reprod Immunol. 1998;41:161–176. doi: 10.1016/s0165-0378(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 10.Delwart EL, et al. Human immunodeficiency virus type 1 populations in blood and semen. J Virol. 1998;72:617–623. doi: 10.1128/jvi.72.1.617-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diem K, et al. Male genital tract compartmentalization of human immunodeficiency virus type 1 (HIV) AIDS Res Hum Retroviruses. 2008;24:561–571. doi: 10.1089/aid.2007.0115. [DOI] [PubMed] [Google Scholar]
- 12.Gupta P, et al. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral quasi species between blood and semen. J Infect Dis. 2000;182:79–87. doi: 10.1086/315644. [DOI] [PubMed] [Google Scholar]
- 13.Kiessling AA, et al. Human immunodeficiency virus in semen arises from a genetically distinct virus reservoir. AIDS Res Hum Retroviruses. 1998;14:S33–S41. [PubMed] [Google Scholar]
- 14.Overbaugh J, Anderson RJ, Ndinya-Achola JO, Kreiss JK. Distinct but related human immunodeficiency virus type 1 variant populations in genital secretions and blood. AIDS Res Hum Retroviruses. 1996;12:107–115. doi: 10.1089/aid.1996.12.107. [DOI] [PubMed] [Google Scholar]
- 15.Zhu TF, et al. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bull M, et al. Compartmentalization of HIV-1 within the female genital tract is due to monotypic and low-diversity variants not distinct viral populations. PLoS ONE. 2009;4:e7122. doi: 10.1371/journal.pone.0007122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poss M, et al. Evolution of envelope sequences from the genital tract and peripheral blood of women infected with clade A human immunodeficiency virus type 1. J Virol. 1998;72:8240–8251. doi: 10.1128/jvi.72.10.8240-8251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bull ME, et al. Human immunodeficiency viruses appear compartmentalized to the female genital tract in cross-sectional analyses but genital lineages do not persist over time. J Infect Dis. 2013;207:1206–1215. doi: 10.1093/infdis/jit016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bull ME, et al. Monotypic human immunodeficiency virus type 1 genotypes across the uterine cervix and in blood suggest proliferation of cells with provirus. J Virol. 2009;83:6020–6028. doi: 10.1128/JVI.02664-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts L, et al. Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J Infect Dis. 2012;205:194–203. doi: 10.1093/infdis/jir715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler DM, et al. The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med. 2010;2:18re1. doi: 10.1126/scitranslmed.3000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heath L, Frenkel LM, Foley BT, Mullins JI. Comment on “The origins of sexually transmitted HIV among men who have sex with men”. Sci Transl Med. 2010;2:50le1. doi: 10.1126/scitranslmed.3001416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianella S, et al. Sexual transmission of predicted CXCR4-tropic HIV-1 likely originating from the source partner's seminal cells. Virology. 2012;434:2–4. doi: 10.1016/j.virol.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salle B, et al. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 25.Miller CJ, et al. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnanakaran S, et al. Recurrent signature patterns in HIV-1 B clade envelope glycoproteins associated with either early or chronic infections. PLoS Pathog. 2011;7:e1002209. doi: 10.1371/journal.ppat.1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chohan B, et al. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol. 2005;79:6528–6531. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagar M, et al. Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis. 2009;199:580–589. doi: 10.1086/596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ping LH, et al. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol. 2013;87:7218–7233. doi: 10.1128/JVI.03577-12. This study of a large number of heterosexually transmitted T/F env genes found that they are exclusively T cell-tropic. This study also observed that, relative to their controls, T/F viruses isolated from males have fewer glycosylation sites than T/F viruses isolated from females. This suggests that female-to-male transmission either selects viruses with fewer glycosylation sites or selects viruses lacking specific glycosylation sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frost SD, et al. Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J Virol. 2005;79:6523–6527. doi: 10.1128/JVI.79.10.6523-6527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, et al. Env length and N-linked glycosylation following transmission of human immunodeficiency virus Type 1 subtype B viruses. Virology. 2008;374:229–233. doi: 10.1016/j.virol.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curlin ME, et al. HIV-1 envelope subregion length variation during disease progression. PLoS Pathog. 2010;6:e1001228. doi: 10.1371/journal.ppat.1001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagar M, Wu X, Lee S, Overbaugh J. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol. 2006;80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell ES, et al. The genetic bottleneck in vertical transmission of subtype C HIV-1 is not driven by selection of especially neutralization-resistant virus from the maternal viral population. J Virol. 2011;85:8253–8262. doi: 10.1128/JVI.00197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 36.West AP, et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilen CB, et al. Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. J Virol. 2011;85:8514–8527. doi: 10.1128/JVI.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavine CL, et al. High-mannose glycan-dependent epitopes are frequently targeted in broad neutralizing antibody responses during human immunodeficiency virus type 1 infection. J Virol. 2012;86:2153–2164. doi: 10.1128/JVI.06201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balzarini J. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol. 2007;5:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballegaard V, Haugaard AK, Garred P, Nielsen SD, Munthe-Fog L. The lectin pathway of complement: advantage or disadvantage in HIV pathogenesis? Clin Immunol. 2014;154:13–25. doi: 10.1016/j.clim.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Adamek M, et al. Characterization of mannose-binding lectin (MBL) variants by allele-specific sequencing of MBL2 and determination of serum MBL protein levels. Tissue Antigens. 2013;82:410–415. doi: 10.1111/tan.12232. [DOI] [PubMed] [Google Scholar]
- 42.Wing JB, et al. Mannose-binding lectin is present in human semen and modulates cellular adhesion of Neisseria gonorrhoeae in vitro. Clin Exp Immunol. 2009;157:408–414. doi: 10.1111/j.1365-2249.2009.03984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munch J, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Kim KA, et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:1–12. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roan NR, et al. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011;10:541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulick RM, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. New Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zirafi O, et al. Semen-mediated enhancement of HIV infection markedly impairs the antiviral efficacy of microbicides. AIDS Res Hum Retroviruses. 2014;30:A183–A183. [Google Scholar]
- 48.Munch J, et al. Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology. 2013;10:1–9. doi: 10.1186/1742-4690-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen MS, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. This study showed that early antiretroviral therapy reduced transmission rates by 96%. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn TC, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 51.Baeten JM, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler DA, et al. Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS. 2008;22:1667–1671. doi: 10.1097/QAD.0b013e32830bfed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351:5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 54.Moss WJ, et al. Suppression of human immunodeficiency virus replication during acute measles. J Infect Dis. 2002;185:1035–1042. doi: 10.1086/340027. [DOI] [PubMed] [Google Scholar]
- 55.Watt G, Kantipong P, Jongsakul K. Decrease in human immunodeficiency virus type 1 load during acute dengue fever. Clin Infect Dis. 2003;36:1067–1069. doi: 10.1086/374600. [DOI] [PubMed] [Google Scholar]
- 56.Anzala AO, et al. Acute sexually transmitted infections increase human immunodeficiency virus type 1 plasma viremia, increase plasma type 2 cytokines, and decrease CD4 cell counts. J Infect Dis. 2000;182:459–466. doi: 10.1086/315733. [DOI] [PubMed] [Google Scholar]
- 57.Cohen MS, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 58.Malott RJ, et al. Neisseria gonorrhoeae-derived heptose elicits an innate immune response and drives HIV-1 expression. Proc Natl Acad Sci USA. 2013;110:10234–10239. doi: 10.1073/pnas.1303738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagot N, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. New Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 60.Nixon B, et al. Genital herpes simplex virus type 2 infection in humanized HIV-transgenic mice triggers HIV shedding and is associated with greater neurological disease. J Infect Dis. 2014;209:510–522. doi: 10.1093/infdis/jit472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilen CB, Tilton JC, Doms RW. In: HIV: From Biology to Prevention and Treatment. Bushman FD, Nabel GJ, Swanstrom R, editors. Cold Spring Harbor Laboratory; 2011. [Google Scholar]
- 62.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 63.Johnston SH, et al. A quantitative affinity-profiling system that reveals distinct CD4/CCR5 usage patterns among human immunodeficiency virus type 1 and simian immunodeficiency virus strains. J Virol. 2009;83:11016–11026. doi: 10.1128/JVI.01242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joseph SB, et al. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J Virol. 2014;88:1858–1869. doi: 10.1128/JVI.02477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parrish NF, et al. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7. PLoS Pathog. 2012;8:e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Isaacman-Beck J, et al. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol. 2009;83:8208–8220. doi: 10.1128/JVI.00296-09. The first study illustrating that T/F viruses are not macrophage-tropic and that transmission does not select for enhanced macrophage-tropism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, et al. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ochsenbauer C, et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salazar-Gonzalez JF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parker ZF, et al. Transmitted/founder and chronic HIV-1 envelope proteins are distinguished by differential utilization of CCR5. J Virol. 2013;87:2401–2411. doi: 10.1128/JVI.02964-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parrish NF, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. A Thorough analysis of T/F virus phenotypes, which observed that subtype B T/F viruses are more resistant to IFNα than controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asmal M, et al. A signature in HIV-1 envelope leader peptide associated with transition from acute to chronic infection impacts envelope processing and infectivity. PLoS ONE. 2011;6:e23673. doi: 10.1371/journal.pone.0023673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 74.Shen R, et al. Vaginal myeloid dendritic cells transmit founder HIV-1. J Virol. 2014;88:7683–7688. doi: 10.1128/JVI.00766-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cameron PU, et al. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–386. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 76.Pope M, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 77.Kawamura T, et al. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J Exp Med. 2000;192:1491–1500. doi: 10.1084/jem.192.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawamura T, et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc Natl Acad Sci USA. 2003;100:8401–8406. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tenner-Racz K, et al. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation, the impact of highly active antiretroviral therapy. J Exp Med. 1998;187:949–959. doi: 10.1084/jem.187.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Ende ME, et al. CD4 T cells remain the major source of HIV-1 during end stage disease. AIDS. 1999;13:1015–1019. doi: 10.1097/00002030-199906180-00002. [DOI] [PubMed] [Google Scholar]
- 81.Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang ZQ, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 83.Zhang ZQ, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci USA. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 87.Carlson JM, et al. HIV transmission. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science. 2014;3451254031 doi: 10.1126/science.1254031. Shows that the severe genetic bottleneck at the time of transmission selects for high-fitness viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boutwell CL, et al. Frequent and variable cytotoxic-T-lymphocyte escape-associated fitness costs in the human immunodeficiency virus type 1 subtype B Gag proteins. J Virol. 2013;87:3952–3965. doi: 10.1128/JVI.03233-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinez-Picado J, et al. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol. 2006;80:3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schneidewind A, et al. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007;81:12382–12393. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allen TM, et al. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J Virol. 2004;78:7069–7078. doi: 10.1128/JVI.78.13.7069-7078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leslie AJ, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 93.Li B, et al. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J Virol. 2007;81:193–201. doi: 10.1128/JVI.01231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fischer W, et al. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS ONE. 2010;5:e12303. doi: 10.1371/journal.pone.0012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henn MR, et al. Whole genome deep sequencing of HIV-1 reveals the impact of early minor variants upon immune recognition during acute infection. PLoS Pathog. 2012;8:e1002529. doi: 10.1371/journal.ppat.1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kijak GH, et al. Cryptic multiple HIV-1 infection revealed by early, frequent, and deep sampling during acute infection. AIDS Res Hum Retroviruses. 2014;30:A58. [Google Scholar]
- 97.Fenton-May AE, et al. Relative resistance of HIV-1 founder viruses to control by interferon-α. Retrovirology. 2013;10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sandler NG, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nawaz F, et al. The genotype of early-transmitting HIV gp120s promotes α4β7-reactivity, revealing α4β7+/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7:e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagner N, et al. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 102.Perez LG, Chen H, Liao HX, Montefiori DC. Envelope glycoprotein binding to the α4β7 integrin complex is not a general property of most HIV-1 variants. J Virol. 2014;88:10767–10777. doi: 10.1128/JVI.03296-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richardson SI, et al. The sequence of the α4β7-binding motif on gp120 of transmitted/founder viruses contributes to the dependence on the integrin for HIV infection. AIDS Res Hum Retroviruses. 2014;30:A56. [Google Scholar]
- 104.Byrareddy SN, et al. Targeting αβ integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20:1397–1400. doi: 10.1038/nm.3715. This study illustrates that an antibody to α4β7 integrin can protect macaques from SIV acquisition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–2407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sturdevant CB, et al. Central nervous system compartmentalization of HIV-1 subtype C variants early and late in infection in young children. PLoS Pathog. 2012;8:e1003094. doi: 10.1371/journal.ppat.1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Del Prete GQ, et al. Molecularly tagged simian immunodeficiency virus SIVmac239 synthetic swarm for tracking independent infection events. J Virol. 2014;88:8077–8090. doi: 10.1128/JVI.01026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wetmore CM, Manhart LE, Wasserheit JN. Randomized controlled trials of interventions to prevent sexually transmitted infections: learning from the past to plan for the future. Epidemiol Rev. 2010;32:121–136. doi: 10.1093/epirev/mxq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gray RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. These results of a randomized clinical trial show that circumcision significantly reduces the rate of HIV acquisition. [DOI] [PubMed] [Google Scholar]
- 111.Muessig KE, Cohen MS. Advances in HIV prevention for serodiscordant couples. Curr HIV/AIDS Rep. 2014;11:434–446. doi: 10.1007/s11904-014-0225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodger A, et al. HIV transmission risk through condomless sex if the HIV+ partner is on suppressive ART: PARTNER study. Top Antivir Med. 2014;22:24–25. [Google Scholar]
- 113.Smedley J, et al. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS ONE. 2014;9:e92830. doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stieh DJ, et al. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog. 2014;10:e1004440. doi: 10.1371/journal.ppat.1004440. This study demonstrates that cells throughout the reproductive tract of female macaques are susceptible to SIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. New Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rolland M, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rolland M, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17:366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roederer M, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dolgin E. Long-acting HIV drugs advanced to overcome adherence challenge. Nat Med. 2014;20:323–324. doi: 10.1038/nm0414-323. [DOI] [PubMed] [Google Scholar]
- 120.Koharudin LMI, Gronenborn AM. Antiviral lectins as potential HIV microbicides. Curr Opin Virol. 2014;7:95–100. doi: 10.1016/j.coviro.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribeiro RM, et al. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol. 2010;84:6096–6102. doi: 10.1128/JVI.00127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mellors JW, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 123.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fraser C, et al. Virulence and pathogenesis of HIV-1 infection: an evolutionary perspective. Science. 2014;343:1243727. doi: 10.1126/science.1243727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hodcroft E, et al. The contribution of viral genotype to plasma viral set-point in HIV infection. PLoS Pathog. 2014;10:e1004112. doi: 10.1371/journal.ppat.1004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang JM, et al. HLA allele sharing and HIV type 1 viremia in seroconverting Zambians with known transmitting partners. AIDS Res Hum Retroviruses. 2004;20:19–25. doi: 10.1089/088922204322749468. [DOI] [PubMed] [Google Scholar]