Abstract
Most brain tumors oversecrete vascular endothelial growth factor (VEGF), which leads to an abnormally permeable tumor vasculature. This hyperpermeability allows fluid to leak from the intravascular space into the brain parenchyma, which causes vasogenic cerebral edema and increased interstitial fluid pressure. Increased interstitial fluid pressure has an important role in treatment resistance by contributing to tumor hypoxia and preventing adequate tumor penetration of chemotherapy agents. In addition, edema and the corticosteroids needed to control cerebral edema cause significant morbidity and mortality. Agents that block the VEGF pathway are able to decrease vascular permeability and, thus, cerebral edema, by restoring the abnormal tumor vasculature to a more normal state. Decreasing cerebral edema minimizes the adverse effects of corticosteroids and could improve clinical outcomes. Anti-VEGF agents might also be useful in other cancer-related conditions that increase vascular permeability, such as malignant pleural effusions or ascites.
Introduction
Cerebral edema is defined as an increase in brain volume owing to an increase in brain water and sodium content.1,2 It is a significant cause of neurological morbidity and mortality in patients with a variety of central nervous system (CNS) pathologies, including brain tumors, infections, stroke, or trauma. The two main types of cerebral edema are cytotoxic edema and vasogenic edema. Cytotoxic edema is the result of hypoxia, which leads to increased intracellular fluid and cell death caused by failure of the sodium–potassium ion pump. Cytotoxic edema is often caused by ischemic stroke or traumatic brain injury. Vasogenic edema, typically associated with primary and metastatic brain tumors, is caused by increased vascular permeability.
Over 200,000 individuals are diagnosed with primary and metastatic brain tumors in the US each year,3 and vasogenic cerebral edema occurs in the majority of these cases. In patients with high-grade gliomas, extensive vasogenic edema, as measured by MRI, is associated with short survival, which indicates a clear need to better understand and manage this complication.4 Any advance in the management of vasogenic edema, therefore, has broad clinical implications.
Corticosteroids were introduced in 1957 as a treatment for vasogenic cerebral edema caused by metastatic cancers from another primary source;5 however, corticosteroids are usually associated with only temporary clinical benefit and a high incidence of toxicity. Corticotrophin-releasing factor has also been studied in phase I clinical trials of patients with vasogenic cerebral edema because of a putative role in modulating brain blood-vessel permeability.6–8 Corticotrophin-releasing factor has demonstrated limited efficacy, however, and is not currently approved for use in this condition.7,8 Consequently, corticosteroids remain the mainstay of treatment, and the identification of novel, effective, antiedema agents is a high priority.
Antiangiogenic agents are a class of cancer drugs that target tumor vasculature and might reduce vessel permeability through a ‘normalizing’ effect on vessel morphology.9 Normalization of tumor vessels restores vascular integrity, thus alleviating the edema associated with a brain tumor. This review will focus on the pathophysiology of vasogenic edema and the potential use of drugs and biological agents that target angiogenesis, and particularly the vascular endothelial growth factor (VEGF) pathway, in the treatment of vasogenic cerebral edema.
Pathogenesis of vasogenic cerebral edema
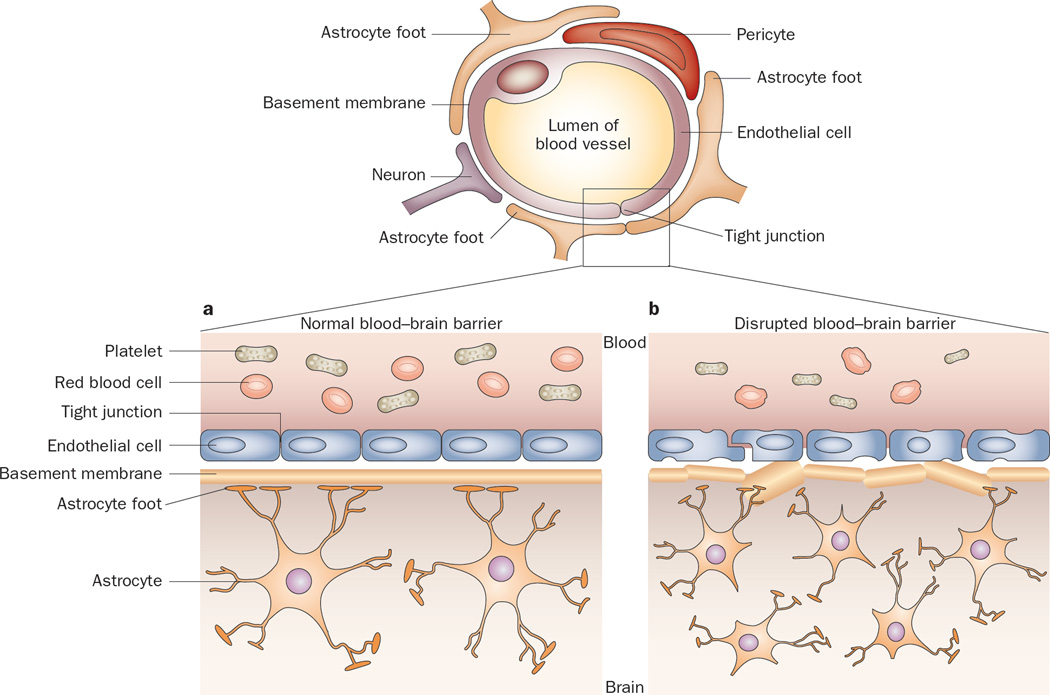
As noted above, the underlying mechanism of vasogenic cerebral edema is increased vascular permeability. One function of the blood–brain barrier (BBB) is to prevent leakage of plasma fluid and proteins into the brain parenchyma. The BBB is composed of a complex network of endothelial cells, pericytes, and astrocyte foot processes that form tight, almost impermeable, junctions (Figure 1). Additionally, minimal pinocytotic activity occurs across the cells. Under normal physiological conditions, the BBB selectively excludes exogenous hydrophilic molecules with molecular weight over 180 Da from passively entering the CNS.10 In conditions associated with BBB disruption (for example, metastatic or primary brain tumors), extravasation of plasma fluid and proteins occurs across the disrupted BBB, which results in vasogenic edema and increased interstitial fluid pressure (IFP) within the tumor.11 Ultimately, these conditions might also result in increased intracranial pressure.
Figure 1.
The permeability of junctions in normal and disrupted blood–brain barrier. a | The normal blood–brain barrier is composed of an intricate network of astrocytes, pericytes, endothelial cells, and neurons that form tight, impermeable junctions, which exclude large cells, marcomolecules, and excess fluid from the central nervous system. b | In the setting of a brain tumor, the tumor astrocytes are more densely packed and irregular, the basement membrane is disrupted and thickened, and the tight junctions are widened, allowing passage of macromolecules and fluid.
In the presence of a brain tumor, the balanced interaction of cells comprising the BBB is disturbed, which leads to failure of this important protective barrier. Histological studies of the BBB in primary and metastatic brain tumors reveal abnormal tight junctions, increased pinocytotic activity, and the presence of fenestrations. Additionally, the basement membrane is thickened and irregular with diminished interactions between pericytes and astrocytes.12–14 The result is a poorly functioning, hyperpermeable BBB with pores up to 550 nm in diameter, which allows the passage of plasma fluid into the CNS.15
The precise mechanism of BBB disruption in the setting of a brain tumor is still being explored. As tumor cells initially proliferate in the CNS, they grow along existing blood vessels (vascular co-option). Once the tumor has grown beyond 1–2 mm in size, however, continued survival of the tumor requires the sprouting of new blood vessels to form new vasculature (that is, angiogenesis).16 The driving force for tumor-related angiogenesis is hypoxia because the existing blood supply is no longer adequate for the enlarging tumor. Angiogenesis in brain tumors is a complicated process and beyond the scope of this review, but has been reviewed in more detail in 2007.17 We will focus on those aspects of tumor angiogenesis that are most directly related to the generation of vasogenic cerebral edema.
VEGF is a major proangiogenic peptide that is partly responsible for the loss of integrity of the BBB in brain tumors. Gliomas, meningiomas, and metastatic tumors all have upregulation of VEGF.4,18–20 In addition to hypoxia, genetic mutations and microenvironmental factors (for example, low pH) commonly found in tumors can lead to overexpression of VEGF.21,22 VEGF is secreted by tumor cells as well as host stromal cells, and binds to its receptors, VEGFR1 and VEGFR2, which are located primarily on the surface of endothelial cells. VEGF stimulates the creation of interendothelial gaps, fragmentations, and fenestrations in the brain endothelium, which are associated with degeneration of the basement membrane.23 These structural changes lead to fluid leakage from the intravascular compartment into the brain parenchyma, which results in vasogenic edema and increased IFP. In gliomas, VEGF messenger RNA levels correlate with capillary permeability and vascular volume.24 As the malignant grade of a glioma increases, VEGF expression increases,25 and high-grade tumors are typically associated with more edema compared with low-grade tumors.26
The mass effect caused by elevated IFP can result in debilitating neurological symptoms—generally more symptoms than the tumor alone would cause. Edema can cause focal neurological symptoms as well as more global problems such as headache, confusion and, in cases of extreme increased intracranial pressure, brain herniation and death. Corticosteroids can achieve an antiedema effect by reducing vascular permeability. This effect is achieved in part by downregulation of VEGF.27,28 Although corticosteroids can reduce the signs and symptoms of vasogenic cerebral edema, these drugs are associated with numerous adverse effects such as insomnia, altered mental status, myopathy, osteopenia, hyperglycemia, and gastrointestinal irritation. These complications might compromise the quality of life of patients with brain tumors. Moreover, increased, and potentially more toxic, doses of corticosteroids are often required for a sustained antiedema effect. Consequently, alternative treatments for cerebral edema are needed.
Vascular normalization
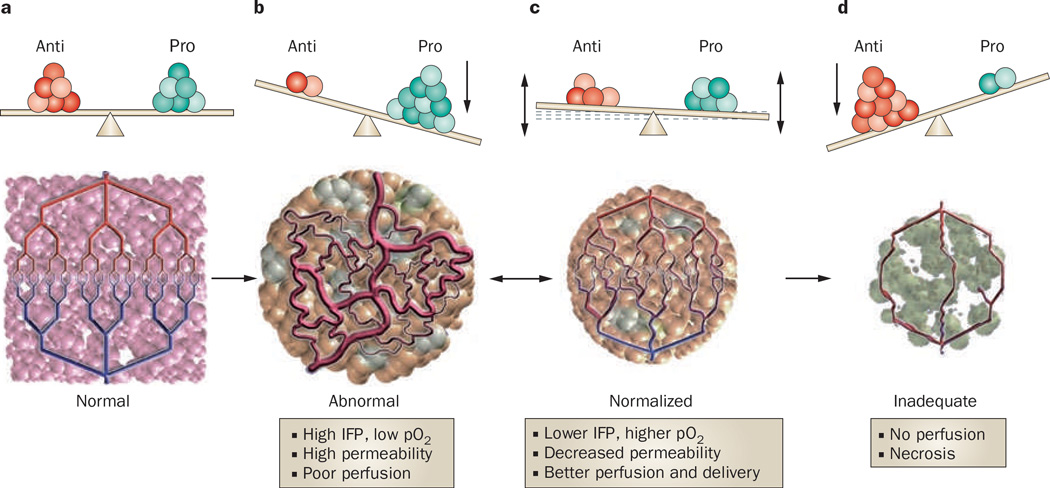
Preclinical and clinical observations that angiogenesis is a prerequisite for brain tumor growth beyond a certain size generated interest in the use of angiogenesis inhibitors as anticancer therapy.29 A possible mechanism by which these agents might achieve an antitumor effect is through vascular normalization of the dilated and tortuous tumor blood vessels (Figure 2).9 The VEGF-dependent abnormal vascular network produced by tumor angiogenesis leads to regional heterogeneity in blood flow with some areas of the tumor receiving increased blood flow while others receive minimal blood flow. A consequence of this disorganization is inefficient delivery of oxygen, which results in a hypoxic tumor microenvironment. These regional differences in blood flow and hypoxia, combined with increased IFP from increased tumor vascular permeability, all compromise the cytotoxic effects of chemotherapy and radiation, and contribute to treatment resistance. Independent of treatment resistance, hypoxia itself can select a more malignant and invasive tumor phenotype.
Figure 2.
Schematic diagram to illustrate the process of vascular normalization a | In health, an exquisite balance of signaling from proangiogenic and antiangiogenic molecules maintains an organized and efficient vascular supply. b | Tumors produce angiogenic factors (various shades of green) that induce an abnormal, inefficient vascular network. c | Judiciously administered antiangiogenic therapy can bring the balance back to a more normal state and normalize the vascular network, improving drug delivery and efficacy. d | If antiangiogenesis is potent and/or persistent it can destroy the network totally, impeding delivery of oxygen and nutrients, and ultimately starving the tumor. In preclinical models, panel c usually progresses towards panel d (single arrow) with currently approved antiangiogenic agents. However, in human tumors, panel c commonly reverts to panel b after a ‘window of normalization’ (double arrow). Abbreviations: Anti, antiangiogenic molecules; IFP, interstitial fluid pressure; pO2, tissue oxygen level; Pro, proangiogenic molecules.
The judicious administration of anti-VEGF agents can temporarily restore tumor vessels to a more normal state and improve the delivery of drugs and oxygen to the tumor. Improved oxygen delivery might enhance the efficacy of cytotoxic therapies like chemotherapy and radiation. Another potential benefit of vascular normalization is restoration of the integrity of the BBB and reduction of vasogenic cerebral edema and tumor IFP. A reduction in tumor hypoxia, improved delivery of therapeutics and enhanced efficacy of cytotoxic radiation after anti-VEGF therapy have all been demonstrated in preclinical tumor models.30–32
Role of antiangiogenic agents
Agents that target the VEGF signaling pathway have the potential to normalize tumor vasculature.31,33,34 In a U87 human glioma xenograft mouse model, the IFP dropped by 74% in mice treated with an anti-VEGF antibody, and in most treated animals IFP was close to normal.32 In a murine renal-cell-carcinoma model, MRI analysis showed that the pan-VEGFR inhibitor vatalanib decreased vascular permeability and blood volume.35 These effects were confirmed in a phase I/II study of patients with recurrent glioblastoma multiforme in which vatalanib decreased permeability and cerebral blood volume, as measured by MRI.36 Finally, in separate studies, bevacizumab, a monoclonal antibody to VEGF, and cediranib, an oral pan-VEGFR tyrosine kinase inhibitor, reduced cerebral edema in patients with malignant glioma, as measured by MRI.37–39 The decrease in edema was noted as early as 18 days after treatment with bevacizumab and a reduction in permeability was noted after 24 h in patients treated with cediranib.
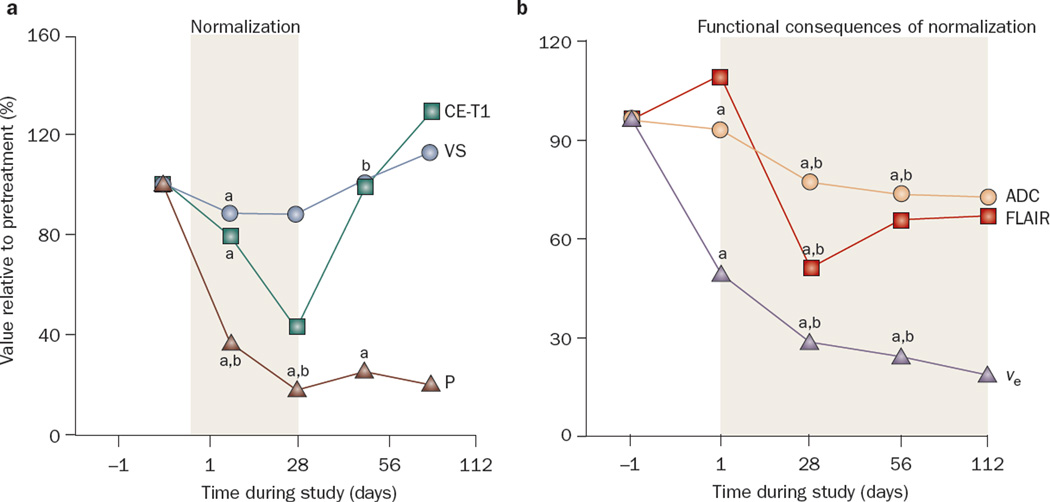
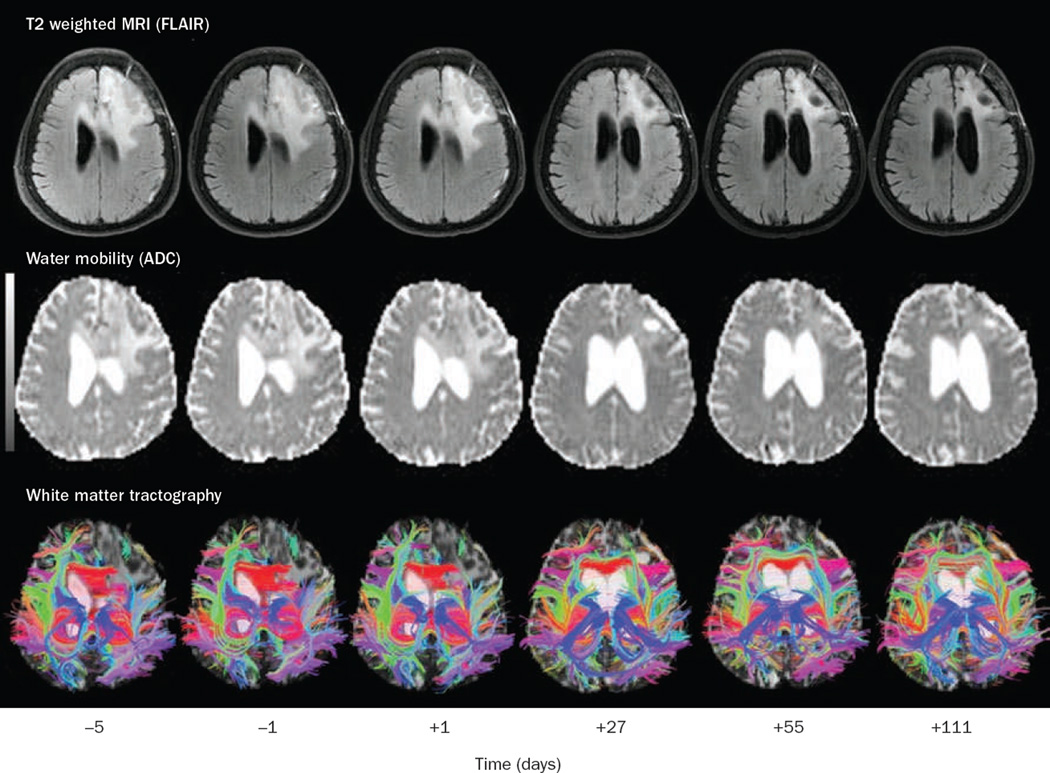
The potential use of anti-VEGF therapy for the alleviation of vasogenic cerebral edema was demonstrated in a phase II study of cediranib in patients with recurrent glioblastoma multiforme.39 Specialized MRI sequences to detect cerebral edema (fluid attenuation inversion recovery [FLAIR], apparent diffusion coefficient maps, and extracellular extravascular space fraction) demonstrated a significant reduction in tumor-associated edema, although these changes were not evident for several weeks (Figures 3 and 4). Of the first 15 consecutive patients enrolled in the trial, 14 had reduction in mass effect as demonstrated by decreased brain midline shift or compression of surrounding normal brain and improvement in visibility of white matter tract anatomy on diffusion tensor imaging. These radiographic changes translated into clinical benefits: 8 of the 11 patients who were taking corticosteroids while on cediranib had their dose reduced, and the other 3 of the 11 patients stopped completely. Once cediranib treatment was stopped, however, most patients required reinitiation of corticosteroids because of the development of rebound edema.
Figure 3.
Effects of cediranib on cerebral edema in patients with recurrent glioblastoma. a | Median values for contrast-enhanced T1-weighted tumor volume (CE-T1), vessel size (VS), and permeability (P) of the tumor over time as measured by an independent expert. Day –1 was set as 100% in all tumors, and changes during cediranib treatment were plotted for all patients. Note the rebound of CE-T1 volume and vessel size after day 28, which indicates a partial closure of the normalization window. By contrast, permeability (P) remains diminished for a longer period of time. b | Median values of potential MRI markers of cerebral edema including T2-weighted abnormality volume measured by fluid attenuated inversion recovery images (FLAIR), apparent diffusion coefficient (ADC), and extracellular–extravascular volume fraction (ve), before and during treatment, showing a sustained decrease of edema while taking cediranib. aP <0.05 for values compared with day –1. bP <0.05 for values compared with day +1. Abbreviations: ADC, apparent diffusion coefficient; CE-T1, contrast-enhanced T1-weighted tumor volume; FLAIR, fluid attenuation inversion recovery; P, permeability; ve, extracellular–extravascular volume fraction; VS, vessel size. Permission obtained from Elsevier © Batchelor, T. T. et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
Figure 4.
serial MRI images from the same patient (as shown in Figure 3) with a left frontal glioblastoma treated with cediranib. Days –5 and –1 are baseline studies before initiation of treatment, followed by images on day +1, +27, +55, +111 after cediranib therapy. Top row: FLAIR images demonstrating resolution of edema with treatment. Second row: Apparent diffusion coefficient maps (measurement of water mobility) demonstrating improved water mobility (that is, less edema causing restricted diffusion) with treatment. Bottom row: White matter tractography demonstrating improvement in the architecture of white matter fiber tracts as edema resolves and mass effect and tissue displacement diminish. Abbreviations: ADC, apparent diffusion coefficient; FLAIR, fluid attenuation inversion recovery. Permission obtained from Elsevier © Batchelor, T. T., et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11, 83–95 (2007).
This study also demonstrated that cediranib was associated with reduction in tumor vessel diameter and permeability within 24 h of treatment, consistent with vascular normalization. The reduction of vessel diameter, as measured by gradient and spin-echo MRI, however, was reversed 28 days after the start of treatment, while the reduction of vessel permeability was sustained for longer than 28 days, even in the setting of tumor progression. These observations imply that anti-VEGF agents can result in sustained reduction in vascular permeability and vasogenic cerebral edema independent of their antitumor effect.
Imaging cerebral edema
A limitation in the interpretation of results from clinical studies of patients with brain tumors treated with anti-VEGF therapies is the inability to distinguish the antitumor effects versus the antipermeability and antiedema effects of this class of agents.40 MRI is the best non invasive tool for the assessment of cerebral edema and its response to therapeutic agents. The most common MRI sequences used to visualize the tumor mass, vascular permeability and cerebral edema are postcontrast T1, T2, diffusion and FLAIR sequences. Postcontrast T1 MRI sequences reflect the amount of contrast dye that leaks from the intravascular compartment across the disrupted BBB because contrast dye is normally excluded from the CNS by an intact BBB. When the BBB is disrupted, gadolinium, the most commonly used contrast agent, leaks into the tumor and surrounding brain tissue. Precontrast T1-weighted image sequences are occasionally helpful as they demonstrate hypointensity in areas of cerebral edema relative to surrounding normal brain.
T2 sequences and FLAIR sequences are hyperintense in areas of cerebral edema and typically reveal more-extensive areas of involvement than indicated by postcontrast T1 images. The hyperintensity observed on T2 images, however, might also reflect infiltrating glioma cells or gliosis.41 Distinguishing between vasogenic edema and infiltrative tumor is important when exploring the potential antiedema versus antitumor effects of anti-VEGF agents. In the cediranib study, the FLAIR signal began to increase after an average of 28 days of treatment, while alternative MRI measures of permeability remained low. One interpretation of these discordant changes is that the antiedema effect of cediranib was long-lasting but that the nonenhancing tumor eventually progressed to infiltrate surrounding brain tissue. In fact, it has been suggested that anti-VEGF agents might increase the invasiveness of tumor cells while restoring the integrity of the BBB, and this effect might confound the interpretation of conventional MRI sequences.42
Other MRI sequences such as diffusion-weighted imaging—particularly apparent diffusion coefficient maps, which detect water diffusion and can reflect cell density—have been disappointing in distinguishing tumor from edema.43,44 New MRI techniques, such as dynamic contrast-enhanced MRI, allow calculation of permeability on the basis of kinetic models of contrast agent diffusion. Variability in the algorithms employed to calculate dynamic contrast-enhanced MRI leads to variable results between different models. Advanced forms of diffusion imaging such as q-space imaging, are under active investigation. Overall, no single approach is optimum and a combination of MRI sequences will probably be required in order to better distinguish infiltrative tumor versus vasogenic edema.45
Toxic effects associated with treatment
As with corticosteroids, antiangiogenic agents are not without risk.46 Major risks include thromboembolic events, hemorrhage, surgical wound dehiscence, gastrointestinal perforation and reversible posterior leukoencephalopathy syndrome. The risk of intracerebral or intratumoral hemorrhage seems to be relatively low; none of the 31 patients with glioblastoma multiforme in the aforementioned cediranib study experienced a CNS hemorrhage, and only 1 of the 35 patients treated with a combination of bevacizumab and irinotecan experienced a hemorrhage after 60 weeks of treatment.39,47 Studies with a large numbers of participants followed for a long duration are needed before the risk of CNS hemorrhage can be definitively assessed in the brain tumor population.
Common adverse effects such as hypertension, fatigue, and diarrhea are generally manageable and the frequency of these adverse effects varies from agent to agent. Long-term adverse effects are unknown, but the cardiovascular and potential thyrotoxic effects of extended VEGF blockade in particular will need to be addressed, especially if prolonged administration of anti-VEGF agents is contemplated for conditions like vasogenic cerebral edema.48
Unanswered questions
Several questions need to be addressed before this class of agent is approved for the treatment of vasogenic cerebral edema, not the least of which is the high financial cost of these drugs. One critical issue is to define the optimum dose and duration of treatment. To date, clinical trials have focused on the antitumor effect of these agents. High doses necessary to treat the tumor might or might not be needed to control vasogenic edema. As demonstrated in the cediranib study, vessel permeability remained low even when the tumor progressed in some patients. A chronic, low-dose schedule might be sufficient to control edema and might reduce the risks of toxicity. Nonetheless, chronic VEGF blockade could lead to upregulation of alternative proangiogenic pathways that involve other signaling molecules such as basic fibroblast growth factor or stromal cell-derived factor 1-α, which could result in new vessel formation, disruption of the BBB and vasogenic cerebral edema.39,49 Thus, combination strategies of antiangiogenic agents that target different factors (for example, VEGF and basic fibroblast growth factor) might be necessary for sustained antiedema effects.
Not all drugs that target angiogenesis will have antiedema properties. The various drugs in this class target many steps in the angiogenesis pathway, not all of which include VEGF. The drugs that directly interfere with VEGF signaling seem to be the agents most effective in controlling edema. Most antiangiogenic agents, however, have not been systematically studied with respect to preventing or controlling cerebral edema. Agents that mimic or upregulate endogenous antiangiogenic agents, for example thrombospondins, could possibly also have antiedema effects via vascular normalization.50
VEGF-independent pathways might also contribute to edemagenesis. Not all tumors that are associated with edema have elevated VEGF secretion. In a microarray analysis of DNA from patients with newly diagnosed malignant gliomas, VEGF expression was variable and some tumors with high edema content did not have elevated VEGF expression.3 Consequently, edema associated with these tumors might not be susceptible to anti-VEGF therapy. Clinically, this finding is supported by the heterogeneous antiedema responses observed with bevacizumab and cediranib.38
Finally, the optimum integration of antiangiogenic therapy for edema management in the glioma population should also take into consideration the possible adverse effect of these therapies. Preclinical studies have shown that anti-VEGF treatment might enhance the migration of cancer cells from the primary tumor along adjacent preexisting blood vessels (vascular co-option).17,51,52 In addition, the combination of vandetanib, an antiangiogenic agent that targets VEGFR2, and temozolomide, resulted in decreased tumor cell apoptosis in a xenograft glioma model. The authors hypothesized that this result could have occurred because of vandetanib-induced restoration of the BBB and reduced tumor penetration of temozolomide.53 Clearly, an increased understanding of the mechanisms of action of individual agents will be critical for the future clinical use of these agents with chemotherapy and radiation.
Conditions associated with hyperpermeability
VEGF inhibitors could also be useful in the treatment of patients with cancer who have other conditions associated with increased vascular permeability. Cerebral radiation necrosis is a complication that occurs in some patients who have received prior brain irradiation, and is associated with vasogenic cerebral edema that is only marginally responsive to corticosteroids. Patients with this condition often require prolonged treatment with corticosteroids to control neurological symptoms. Although the underlying mechanism of cerebral radiation necrosis is unclear, endothelial cell damage and the release of VEGF can cause disruption of the BBB. This disruption results in an influx of fluid into the brain parenchyma and increased cerebral IFP. In one study, eight patients with cerebral radiation necrosis were treated with bevacizumab and subsequent MRI scans demonstrated significant reduction in contrast leakage and edema.54 These patients were also able to decrease their corticosteroid dose. Large, prospective studies are needed to confirm this potential beneficial effect of anti-VEGF therapy in this population.
Malignant effusions such as ascites or pleural effusions are also the result of increased vascular permeability as a consequence of VEGF secretion.55 The concentration of VEGF is considerably higher in malignant pleural or peritoneal effusions than effusions secondary to infection, congestive heart failure, or in healthy individuals.56,57 Consequently, inhibition of VEGF signaling could be effective in the treatment of malignant effusions. In mouse models, vatalanib, an oral pan-VEGFR antagonist, was associated with a reduction in the size of malignant effusions by reducing vascular permeability.58,59 In another study in mice, a neutralizing antibody to VEGF blocked the development of ascites associated with primary effusion lymphoma.60 In humans, case reports suggest that bevacizumab might have use in the treatment of malignant ascites from ovarian cancer, colon cancer, and adenocarcinoma of unknown origin.61,62 Although prospective studies are necessary to confirm these preliminary observations, The role of antiangiogenic agents in patients with cancer might expand beyond targeting the tumor, and achieve wider use in the treatment of conditions associated with VEGF-induced vascular permeability.
Conclusions
Vasogenic cerebral edema is a significant cause of morbidity in patients with brain tumors—both from the direct effects of the edema and from indirect effects related to the requirement for chronic corticosteroids. VEGF is a critical modulator of BBB disruption and edemagenesis in patients with brain tumors. Antiangiogenic agents have the potential to reduce cerebral edema by blocking VEGF signaling and reducing vascular permeability. This blockade is associated with normalization of the tumor vasculature and restoration of the ability of the BBB to exclude plasma proteins and fluid from the CNS. The lack of a reliable test to distinguish infiltrating tumor from tumor-associated edema has limited our ability to study potential antiedema therapies; however, technological advances such as dynamic contrast-enhanced MRI hold promise in this regard. Not all antiangiogenic agents are likely to have antiedema properties, and the optimum dose and schedule of agents that can alleviate edema need to be established. In addition, more studies will be necessary to determine the long-term effects of these agents, and the best combination strategies with cytotoxic therapy. Nevertheless, more than 50 years after the introduction of corticosteroids for the treatment of vasogenic cerebral edema, vascular normalization by anti-VEGF agents holds new promise as an effective therapeutic strategy for this common and serious complication of brain tumors.
Key points.
-
▪
Peritumoral vasogenic cerebral edema is a significant cause of morbidity and mortality in patients with brain tumors
-
▪
VEGF is secreted by brain tumors and has an important role in increasing vascular permeability and, thus, contributing to peritumoral edema
-
▪
Peritumoral edema increases interstitial fluid pressure, which leads to poor penetration of chemotherapeutics and treatment resistance
-
▪
Antiangiogenic agents, particularly those that target the VEGF pathway, have been shown to reduce peritumoral edema in phase II studies of patients with brain tumors, and have been shown to improve progression-free survival and overall survival
-
▪
Anti-VEGF therapy exerts its effects by restoring vascular permeability and normalizing tumor blood vessels
-
▪
Anti-VEGF agents could also be useful in other cancer-related conditions that increase vascular permeability, such as malignant pleural effusions or ascites
Acknowledgments
TT Batchelor has declared associations with the following companies: Acceleron Pharma, AstraZeneca, Enzon, Exelixis, Genentech, Imclone, Millenium Pharmaceuticals, Schering Plough and Vertex Pharmaceuticals. RK Jain has declared associations with the following companies: AstraZeneca, Dyax, Millennium Pharmaceuticals, Takeda and SynDevRx. AG Sorensen has declared associations with the following companies: ACRIN image Matrix, AstraZeneca, Amgen, Epix Pharmaceuticals, Exelixis, GlaxoSmithKline, Genentech, General Electric Healthcare, Millennium Pharmaceuticals, Mitsubishi Pharma, the National Institutes of Health, Novartis Pharmaceuticals, Schering–Plough and Siemens Medical Solutions. See the article online for full details of the relationships.
Footnotes
Competing interests
The other authors declared no competing interests.
References
- 1.Del Maestro RF, Megyesi JF, Farrell CL. Mechanisms of tumour-associated edema: a review. Can. J. Neurol. Sci. 1990;17:177–183. doi: 10.1017/s0317167100030419. [DOI] [PubMed] [Google Scholar]
- 2.Klatzo I. Presidential address. Neuropathological aspects of brain edema. J. Neuropathol. Exp. Neurol. 1967;26:1–14. doi: 10.1097/00005072-196701000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 4.Carlson M, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin. Cancer Res. 2007;13:2592–2598. doi: 10.1158/1078-0432.CCR-06-2772. [DOI] [PubMed] [Google Scholar]
- 5.Kofman S, Garvin JS, Nagamani D, Taylor SG., 3rd Treatment of cerebral metastases from breast carcinoma with prednisolone. J. Am. Med. Assoc. 1957;163:1473–1476. doi: 10.1001/jama.1957.02970510039008. [DOI] [PubMed] [Google Scholar]
- 6.Hendryk S, Jedrzejowska-Szypulka H, Josko J, Jarzab B, Döhler KD. Influence of the corticotropin releasing hormone (CRH) on the brain–blood barrier permeability in cerebral ischemia in rats. J. Physiol. Pharmacol. 2002;53:85–94. [PubMed] [Google Scholar]
- 7.Villalona-Calero MA, et al. A phase I trial of human corticotropin-releasing factor (hCRF) in patients with peritumoral brain edema. Ann. Oncol. 1998;9:71–77. doi: 10.1023/a:1008251426425. [DOI] [PubMed] [Google Scholar]
- 8.Tjuvajev J, et al. Corticotropin-releasing factor decreases vasogenic brain edema. Cancer Res. 1996;56:1352–1360. [PubMed] [Google Scholar]
- 9.Jain RK. Normalizing tumour vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 10.Kroll RA, Neuwelt EA. Outwitting the blood–brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 11.Boucher Y, Salehi H, Witwer B, Harsh GR, 4th, Jain RK. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br. J. Cancer. 1997;75:829–836. doi: 10.1038/bjc.1997.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertossi M, Virgintino D, Maiorano E, Occhiogrosso M, Roncali L. Ultrastructural and morphometric investigation of human brain capillaries in normal and peritumoral tissues. Ultrastruct. Pathol. 1997;21:41–49. doi: 10.3109/01913129709023246. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa H, Ushio Y, Hayakawa T, Yamada K, Mogami H. Changes of the blood–brain barrier in experimental metastatic brain tumours. J. Neurosurg. 1983;59:304–310. doi: 10.3171/jns.1983.59.2.0304. [DOI] [PubMed] [Google Scholar]
- 14.Stewart PA, Hayakawa K, Farrell CL, Del Maestro RF. Quantitative study of microvessel ultrastructure in human peritumoral brain tissue. Evidence for a blood–brain barrier defect. J. Neurosurg. 1987;67:697–705. doi: 10.3171/jns.1987.67.5.0697. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs SK, et al. Regulation of transport pathways in tumour vessels: role of tumor type and microenvironment. Proc. Natl Acad. Sci. USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucano CD. The seed and soil hypothesis: vascularization and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, et al. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 18.Provias J, et al. Meningiomas: role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery. 1997;40:1016–1026. doi: 10.1097/00006123-199705000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Strugar JG, Criscuolo GR, Rothbart D, Harrington WN. Vascular endothelial growth/permeability factor expression in human glioma specimens: correlation with vasogenic brain edema and tumor-associated cysts. J. Neurosurg. 1995;83:682–689. doi: 10.3171/jns.1995.83.4.0682. [DOI] [PubMed] [Google Scholar]
- 20.Yano S, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60:4959–4967. [PubMed] [Google Scholar]
- 21.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas. in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 22.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 23.Dobrogowska DH, Lossinky AS, Tarnawski M, Vorbrodt AW. Increased blood–brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J. Neurocytol. 1998;27:163–173. doi: 10.1023/a:1006907608230. [DOI] [PubMed] [Google Scholar]
- 24.Machein MR, Kullmer J, Fiebich BL, Plate KH, Warnke PC. Vascular endothelial growth factor expression, vascular volume, and, capillary permeability in human brain tumors. Neurosurgery. 1999;44:732–740. doi: 10.1097/00006123-199904000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Chan AS, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am. J. Surg. Pathol. 1998;22:816–826. doi: 10.1097/00000478-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Tervonen O, Forbes G, Scheithauer BW, Dietz MJ. Diffuse “fibrillary” astrocytomas: correlation of MRI features with histopathologic parameters and tumor grade. Neuroradiology. 1992;34:173–178. doi: 10.1007/BF00596330. [DOI] [PubMed] [Google Scholar]
- 27.Heiss JD, et al. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J. Clin. Invest. 1996;98:1400–1408. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machein M, et al. Differential downregulation of vascular endothelial growth factor by dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol. Appl. Neurobiol. 1999;25:104–112. doi: 10.1046/j.1365-2990.1999.00166.x. [DOI] [PubMed] [Google Scholar]
- 29.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 30.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 31.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Lee CG, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 33.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19:7–16. [PubMed] [Google Scholar]
- 34.Jain RK. Taming vessels to treat cancer. Sci. Am. 2008;298:56–63. doi: 10.1038/scientificamerican0108-56. [DOI] [PubMed] [Google Scholar]
- 35.Drevs J, et al. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–4022. [PubMed] [Google Scholar]
- 36.Conrad C, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) [abstract 1512] ASCO Meeting Abstracts. 2004;22:1512. [Google Scholar]
- 37.Vredenburgh JJ, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 38.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 39.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat. Clin. Pract. Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura T, Ohkubo M, Igarashi H, Kwee IL, Nakada T. Increase in glutamate as a sensitive indicator of extracellular matrix integrity in peritumoral edema: a 3.0-tesla proton magnetic resonance spectroscopy study. J. Neurosurg. 2007;106:609–613. doi: 10.3171/jns.2007.106.4.609. [DOI] [PubMed] [Google Scholar]
- 42.Rubenstein JL, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;4:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauleit D, et al. Can the apparent diffusion coefficient be used as a noninvasive parameter to distinguish tumor tissue from peritumoral tissue in cerebral gliomas? J. Magn. Reson. Imaging. 2004;20:758–764. doi: 10.1002/jmri.20177. [DOI] [PubMed] [Google Scholar]
- 44.van Westen D, Lätt J, Englund E, Brockstedt S, Larsson EM. Tumor extension in high-grade gliomas assessed with diffusion magnetic resonance imaging: values and lesion-to-brain ratios of apparent diffusion coefficient and fractional anisotropy. Acta Radiol. 2006;47:311–319. doi: 10.1080/02841850500539058. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen AG. Magnetic resonance as a cancer imaging biomarker. J. Clin. Oncol. 2006;24:3274–3281. doi: 10.1200/JCO.2006.06.6597. [DOI] [PubMed] [Google Scholar]
- 46.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat. Rev. Cancer. 2007;7:475–485. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 47.Vredenburgh JJ, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 48.Jain RK, Finn AV, Kolodie FD, Gold HK, Virmani R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:491–502. doi: 10.1038/ncpcardio0979. [DOI] [PubMed] [Google Scholar]
- 49.Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- 50.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 51.Kunkel P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 52.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claes A, et al. Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization. Mol. Cancer Ther. 2008;7:71–78. doi: 10.1158/1535-7163.MCT-07-0552. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Senger D, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 56.Sack U, et al. Vascular endothelial growth factor in pleural effusions of different origin. Eur. Respir. J. 2005;25:600–604. doi: 10.1183/09031936.05.00037004. [DOI] [PubMed] [Google Scholar]
- 57.Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. Oncologist. 2000;5:45–50. doi: 10.1634/theoncologist.5-suppl_1-45. [DOI] [PubMed] [Google Scholar]
- 58.Yano S, et al. Treatment for malignant pleural effusion of human lung adenocarcinoma by inhibition of vascular endothelial growth factor receptor tyrosine kinase phosphorylation. Clin. Cancer Res. 2000;6:957–965. [PubMed] [Google Scholar]
- 59.Xu L, et al. Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int. J. Oncol. 2000;16:445–454. doi: 10.3892/ijo.16.3.445. [DOI] [PubMed] [Google Scholar]
- 60.Aoki Y, Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi sarcoma associated herpesvirus-infected primary effusion lymphoma. Blood. 1999;94:4247–4254. [PubMed] [Google Scholar]
- 61.Numnum TM, Rocconi RP, Whitworth J, Barnes MN. The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian carcinoma. Gynecol. Oncol. 2006;102:425–428. doi: 10.1016/j.ygyno.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Pichelmayer O, Gruenberger B, Zielinski C, Raderer M. Bevacizumab is active in malignant effusion. Ann. Oncol. 2006;17:1853. doi: 10.1093/annonc/mdl143. [DOI] [PubMed] [Google Scholar]