Abstract
We studied the effects on chemoreception of bilateral focal inhibition of the caudal Nucleus tractus solitarii (cNTS) by microdialysis of muscimol (0.5 mM) in rats during wakefulness and NREM sleep at two temperatures, 24 °C and 30 °C, just below and within the thermoneutral zone, respectively. Body temperature and V̇O2. did not differ at these two temperatures. The CO2 response (% increase in V̇E/V̇O2) did not differ at 24 °C vs. 30 °C and muscimol inhibited the CO2 response equally at both temperatures. In contrast, the hypoxic response (% increase in V̇E/V̇O2) was greater at 30 °C than at 24 °C and muscimol inhibited it only at 30 °C. These effects were similar in wakefulness and NREM sleep. We conclude that: 1) ambient temperature can affect the V̇E/V̇O2 response to hypoxia but not hypercapnia and 2) at 24 °C muscimol in the cNTS affects the CO2 response but not the hypoxic response providing indirect support for the presence of chemoreception within the NTS.
1. Introduction
Our working hypothesis is that central chemoreception, the process by which CO2/pH is detected within the central nervous system and stimulates breathing, involves many sites that are widely distributed in the hindbrain (Coates et al. 1993; Nattie 2000; Feldman et al. 2003; Nattie et al. 2006; Nattie et al. 2008a; Nattie et al. 2008b) including the retrotrapezoid nucleus-RTN (Nattie et al. 1991; Akilesh et al. 1997; Li et al. 1999; Guyenet et al. 2005; Guyenet et al. 2008), the medullary raphe (Nattie et al. 2001b; Nattie et al. 2004; Richerson 2004; Richerson et al. 2005; Hodges et al. 2008), the locus ceruleus (Biancardi et al. 2008), portions of the ventral respiratory group (Nattie et al. 1996), the fastigial nucleus of the cerebellum (Martino et al. 2006), orexinergic neurons of the hypothalamus (Deng et al. 2007; Williams et al. 2007) and the caudal aspect of the Nucleus tractus solitarii (cNTS) (Dean et al. 1989; Teppema et al. 1997; Nattie et al. 2002), the focus of this study. An alternative view is that the RTN “…contains the most important central respiratory chemoreceptors…” (Guyenet et al. 2008), a view not entirely consonant with our own (Nattie et al. 2008a).
The cNTS is well accepted as a site for initial synapse of afferents from the carotid body (Chitravanshi & Sapru 1995; Tabata et al. 2001; Chung et al. 2006; Braga et al., 2007). In addition, there is evidence to suggest that some cNTS neurons are themselves direct participants in chemoreception. Neurons from the cNTS are CO2 sensitive in vitro (Dean et al. 1989) and focal acidification of the cNTS by dialysis of a high CO2 solution stimulates breathing in vivo (Nattie et al. 2002). Exposure of animals to increased CO2 results in the expression of c-fos in cNTS whether the animals are intact (Teppema et al. 1997) or carotid body denervated (Jansen et al. 1996).
In previous experiments we have utilized both focal acidic stimulation by dialysis of high CO2 solutions and focal inhibition by dialysis with the GABAA receptor agonist muscimol to study the role of specific putative chemoreceptor sites in the intact, unanesthetized rat. In the RTN, focal acidification increased breathing (Li et al. 1999) while muscimol decreased the response to systemic administration of CO2 (Nattie et al. 2000). In the medullary raphe, focal acidification in vivo stimulated breathing (Nattie et al. 2001b) and, paradoxically, muscimol increased the response to systemic CO2 (Taylor et al. 2006). In this latter study we found that the effect of muscimol on the ventilatory responses to 7% CO2 or 10% O2 differed when the experiment was carried out at 24 °C (just below the thermoneutral zone) vs. 30 °C (within the thermoneutral zone) (Taylor et al. 2006).
The NTS is rich in GABAergic interneurons (Fong et al. 2005; Bailey et al. 2008) and receives GABAergic innervation from other sites (Fong et al. 2005; Chung et al. 2006). We hypothesize that focal inhibition of the cNTS by bilateral dialysis of muscimol will inhibit both the hypoxic response and the CO2 response, i.e., the cNTS will behave like the RTN not the medullary raphe. We also hypothesize that the effects of muscimol inhibition will vary when studied at 24 °C vs. 30 °C.
2. Methods
2.1. General
All animal experimentation and surgical protocols were within the guidelines of the National Institutes of Health for animal use and care and the American Physiological Society’s Guiding Principles in the Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee at the Dartmouth College Animal Resource Center. The methods are those in common use in our lab (Li et al. 2006; Taylor et al. 2006; Dias et al. 2008). Below they are described in brief.
2.2. Animal surgery
We anesthetized adult male Sprague-Dawley rats weighing 280 to 350g by intramuscular ketamine (100 mg/kg) and xylazine (20 mg/kg). The top of the head and a portion of the abdomen were shaved and the skin sterilized with betadine solution and alcohol. Rats were fixed in a Kopf stereotaxic frame, and two dialysis guide cannulas (0.38mm OD) with a dummy were implanted one in each cNTS. The coordinates for probe placement in cNTS were 0.8 mm lateral and 12.5 mm caudal to bregma. The insertion was at an angle of 10° rostral from vertical such that the probe entered the brain in a caudal direction. The tip was placed 8.5 mm from the skull surface. The guide cannulae were secured with cranioplastic cement. Three EEG electrodes were the screwed into the skull and the wound sutured. A sterile telemetry temperature probe (TA-F20, Data Sciences, St. Paul, MN) was placed in the abdominal cavity. The incision was closed, and the animal was allowed to recover for 7 days.
2.3. Dialysis solution
The artificial cerebrospinal fluid (aCSF) was equilibrated with 5% CO2. The composition of the aCSF was (in mM) 152 sodium, 3.0 potassium, 2.1 magnesium, 2.2 calcium, 131 chloride, and 26 bicarbonate. The calcium was added after the aCSF was equilibrated with CO2. Muscimol was added at a final concentration of 0.5 mM. The dialysis pump was run at a speed of 4 ul/min.
2.4. Ventilation measurement
The plethysmograph chamber used in these experiments is as previously described (Nattie et al. 2002; Li et al. 2006; Dias et al. 2008). It operates at atmospheric pressure, with the inflow and outflow of inspired gases balanced to prevent pressure changes in the box. The inflow gas is humidified, and the flow rate controlled by a flowmeter. The outflow port is connected to a vacuum system via a flowmeter. A high-resistance “bleed” of the outflow line provided ~100 mL/min of outflow gas to the O2 and CO2 analyzers (Applied Electrochemistry). The flow rate through the plethysmograph was maintained at or above 1.4 L/min to prevent CO2 rebreathing. The plethysmograph was calibrated with 0.3-mL injections. The analog output of the pressure transducer was converted to a digital signal and directly sampled at 150 Hz by computer using the DataPac 2000 system. Breath-by-breath analysis was performed with the pressure deflections and the respiratory cycle time for each breath determined over a 20- to 30-s time period. Tidal volume (VT) is calculated and breathing frequency (fR) per breath to estimate ventilation (V̇E) per breath.
2.5. Determination of Temperatures
The chamber temperature was measured by a thermometer inside the chamber. Rat body temperature was measured by using the analog output via telemetry from the temperature probe in the peritoneal cavity.
2.6. EEG and EMG signals
The signals from the EEG and EMG electrodes were sampled at 150 Hz, filtered at 0.3– 50 and 0.1–100 Hz, respectively, and recorded directly on the computer. Arousal state was determined by analysis of EEG and EMG records as previously described (Nattie et al. 2002; Nattie et al. 2004).
2.7. Anatomic analysis
At the end of the experiment, the rats were euthanized, the brain stems were frozen and then sectioned at 50-μm thickness with a Reichert-Jung cryostat. The sections were counterstained with cresyl violet. We identified anatomic landmarks and the site of dialysis probe placement by using a rat brain atlas for reference (Paxinos G. 1998). The guide tubes were removed postmortem but before brain stem removal and sectioning. The manipulation required increased the volume of tissue disruption compared with that produced simply by insertion.
2.8. Experimental Protocol
After insertion of the dialysis probe into the guide tube, the animals were placed into the plethysmograph chamber and allowed 30–40 min to acclimate. Experiments were performed at room temperature (24°C) or at an ambient temperature within the rat thermoneutral zone (30°C) maintained by a heat lamp with a thermostat controller. Once the animals had acclimated, the experimental recording began. Under room air conditions, ventilation and oxygen consumption were measured every 10 min for 40 min, then the inspired air was switched to 7% CO2 balanced with air or 10% O2 balanced with nitrogen. Once the gas analyzer connected to the plethysmograph outflow reached 7% CO2 or 10% O2, which took ~15 min, measurements were made each 10 min for a further 30 min. For presentation of air breathing results we used the last and/or next to last measurement; for CO2 and hypoxia results we used the average of the three data sets. Microdialysis of aCSF was continued throughout the entire experiment. Following the initial control measurements, the same rats were allowed to rest in the chamber for at least one hour under room air conditions with the plethysmograph top open before drug treatment dialysis started. The dialysate was then changed to one with 0.5 mM muscimol and the protocol repeated. There were two experimental groups; one, for the CO2 responses (N=6), the second for the hypoxic responses (N=7). Each rat received only one dialysis probe insertion and one day of experimentation.
2.9. Statistics
There are two steps in the data analysis. First the ventilatory equivalent, V̇E/V̇O2, while breathing air and the test gas (7% CO2 or 10% O2) was evaluated as the repeated measure in an ANOVA with treatment (control, muscimol) and state (awake, NREM sleep) as factors. This was performed separately for the 24 °C and 30 °C results. Post hoc test when appropriate was carried out using the Bonferroni modification. Second, we defined the ‘CO2 response” and the “hypoxic response” as the % increase in the ventilatory equivalent, V̇E/V̇O2, comparing the value while breathing the test gas to that while breathing air. The effect of treatment was then evaluated as the repeated measure in an ANOVA with ambient temperature (24 °C, 30 °C) and state (awake, NREM sleep) as factors. Post hoc test when appropriate was carried out using the Bonferroni modification.
3. Results
3.1. Location of dialysis
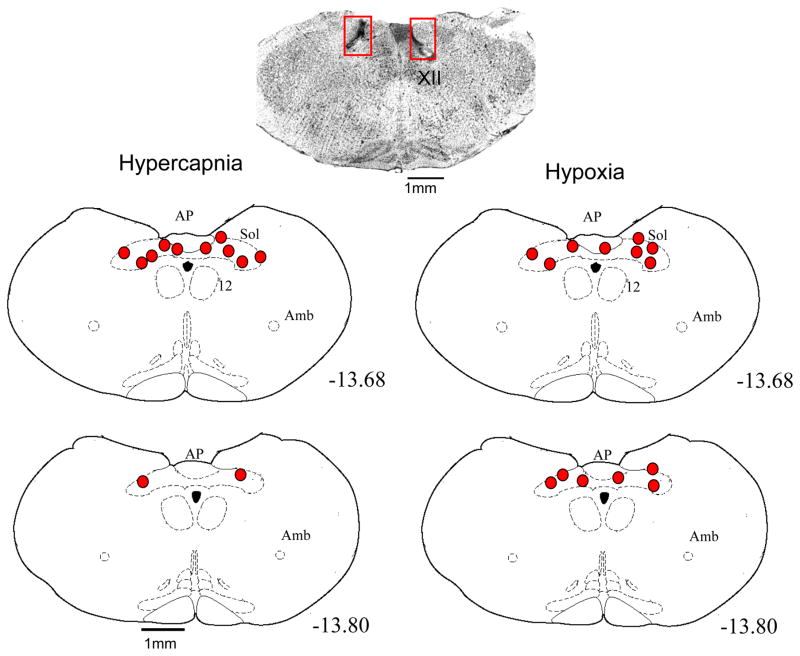
The anatomical location of the microdialysis probe tips are shown on Fig. 1. The cross section at the top is an actual tissue section fixed and stained with cresyl violet. The rectangles outline the areas within which the tissue damage made by the probe tip can be seen, The schematic cross sections show as solid ovals the locations of the probe tips from the 6 rats of the hypercapnic exposure group on the left and the 7 rats of the hypoxic exposure group on the right. All probe tips were within the cNTS.
Fig. 1.
The anatomical location of the dialysis probe tips for the 6 rats used in the hypercapnia protocol (left panels) and the 7 rats used in the hypoxia protocol (right panels). At the top is a cresyl violet stained cross section of the medulla of one rat at the level −13.8 from bregma to show the tissue disruption produced by the chronic guide tube and acute microdialysis probe insertion. The numbers refer to mm caudal to bregma. The schematic sections were drawn from the atlas of Paxinos (1998).
3.2. Body temperature and V̇O2 and sleep-wakefulness
Table 1 shows the values for oxygen consumption and body temperature for the rats of the CO2 and hypoxic exposure groups at the two temperatures in the control and muscimol conditions. Exposure to CO2 increased V̇O2 while exposure to hypoxia decreased it (in either case; P< 0.01; repeated measures ANOVA with no effect of treatment or temperature). Body temperature decreased significantly in both 7% CO2 and 10% O2 conditions (P<0.01; repeated measures ANOVA with no effect of treatment or temperature). We will present and analyze all ventilatory data as the ventilatory equivalent, V̇E/V̇O2, normalizing for any variations in V̇O2 and providing an indirect index of the effective alveolar ventilation.
Table 1.
Oxygen consumption (V̇O2) and body temperature (Tb) of rats breathing air or 7% CO2 (N=6) and rats breathing air or 10% O2 (N=7) before and after bilateral inhibition of the caudal NTS by microdialysis of muscimol. Mean +/− SEM values are shown. The experiments were performed at two different ambient temperatures (TA), 24°C and 30°C. V̇O2 was significantly increased in 7% CO2 and decreased in 10% O2 (P< 0.01; repeated measures ANOVA with no effect of treatment or temperature). Tb was significantly decreased in 7% CO2 and in 10% O2 (P< 0.01; repeated measures ANOVA with no effect of treatment or temperature.
| V̇E/V̇O2 (ml min−1 100g−1) | ||||
|---|---|---|---|---|
| Control | Muscimol | |||
| Air | 7% CO2 | Air | 7% CO2 | |
| 30°C | 1.75 | 2.12 | 1.85 | 2.24 |
| .12 | .08 | .22 | .08 | |
| 24°C | 1.70 | 1.94 | 1.72 | 2.10 |
| .13 | .12 | .07 | .25 | |
| Air | 10% O2 | Air | 10% O2 | |
| 30°C | 2.15 | 1.92 | 2.29 | 1.69 |
| .07 | .27 | .15 | .15 | |
| 24°C | 2.25 | 1.74 | 2.22 | 1.99 |
| .13 | .10 | .12 | .22 | |
| Tb (°C) | ||||
|---|---|---|---|---|
| Control | Muscimol | |||
| Air | 7% CO2 | Air | 7% CO2 | |
| 30°C | 38.4 | 38.0 | 37.9 | 37.4 |
| .3 | .3 | .4 | .5 | |
| 24°C | 38.1 | 37.8 | 37.8 | 37.0 |
| .3 | .4 | .3 | .6 | |
| Air | 10% O2 | Air | 10% O2 | |
| 30°C | 38.3 | 37.4 | 38.2 | 37.5 |
| .1 | .2 | .1 | .3 | |
| 24°C | 38.3 | 37.6 | 37.9 | 37.7 |
| .1 | .1 | .2 | .2 | |
In the 40 min air breathing periods during dialysis of control or muscimol solutions, the rats averaged 43 to 65% of the time awake and 35 to 55% of the time in NREM sleep. During the 30 min exposure to 7% CO2 the rats averaged 67% to 82% of the time awake and 18 to 31% of the time in NREM sleep. During the 30 min hypoxic exposure the rats averaged 36% to 72% of the time awake and 28% to 63% of the time in NREM sleep.
3.2. CO2 response
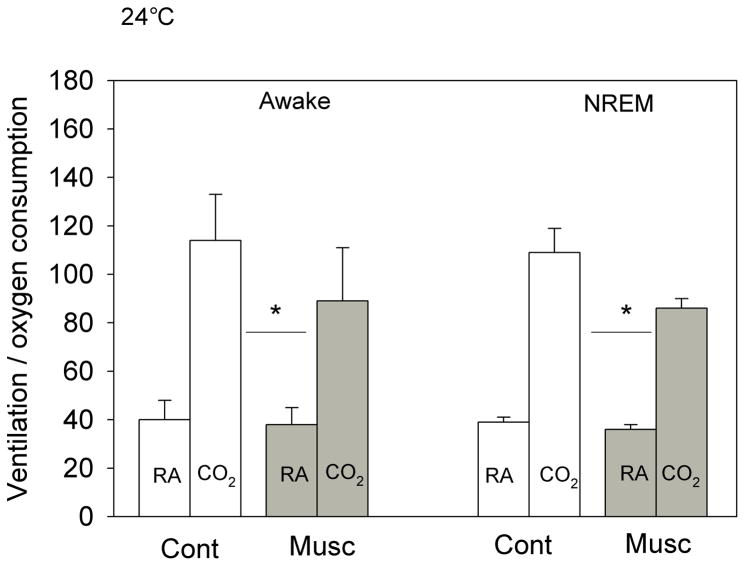
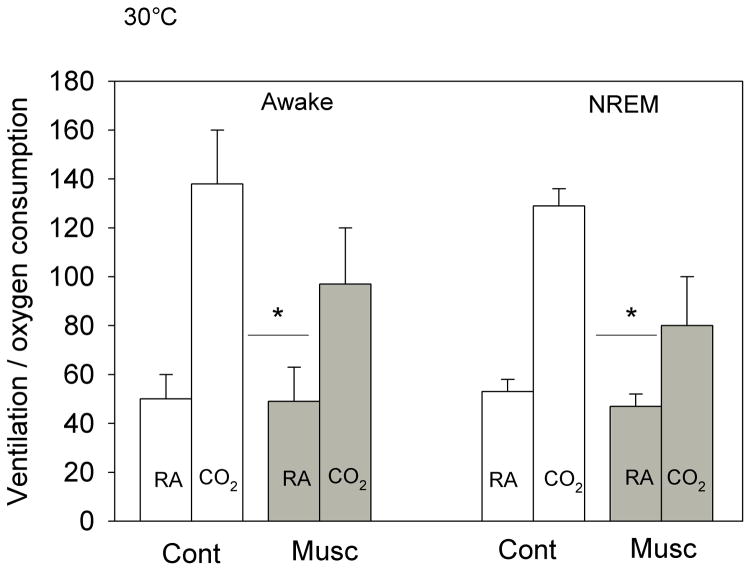
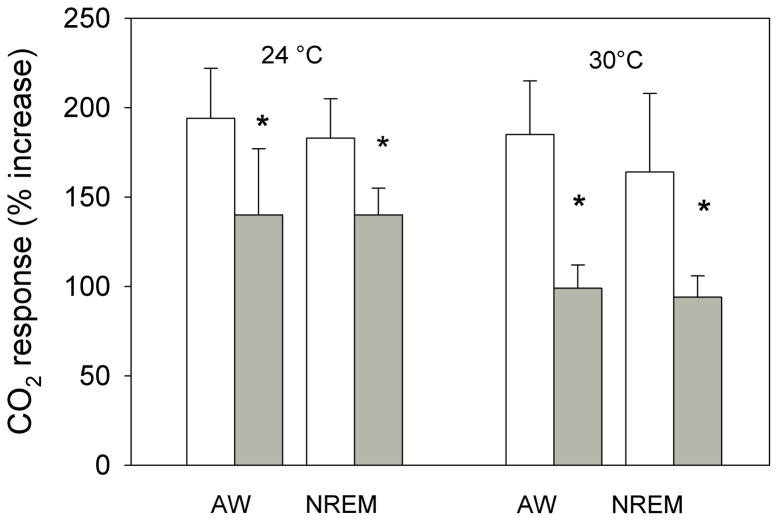
The effects of bilateral dialysis of 0.5 mM muscimol into the NTS on the ventilatory response to 7% CO2 are shown in Fig. 2 for the experiment performed at 24 °C and on Fig. 3 for the experiment performed at 30 °C. At both ambient temperatures and during both wakefulness and NREM sleep muscimol dialysis decreased the repeated measure, V̇E/V̇O2, in air and 7% CO2 (P< 0.001; interaction with treatment, P< 0.01; no interaction with state). Post hoc analysis showed this treatment effect to be with 7% CO2 but not air breathing. We defined the “CO2 response” as the % increase in the ventilatory equivalent, V̇E/V̇O2, and evaluated this variable as shown in Fig. 4. Muscimol treatment decreased the “CO2 response” in wakefulness and NREM sleep at both 24 °C and 30 °C (P< 0.001, repeated measures ANOVA) with no significant interaction with temperature or state (awake, NREM sleep). The muscimol effect on the “CO2 response” was statistically the same at both ambient temperatures and in wakefulness and NREM sleep although there is a trend for a greater effect at 30 °C. The effect was entirely due to changes in breathing frequency.
Fig. 2.
The effects of bilateral dialysis of 0.5 mM muscimol into the NTS on the ventilatory equivalent, the V̇E/V̇O2 ratio, while the rat was breathing air (RA) and 7% CO2 (CO2) during wakefulness (left) and NREM sleep (right) are shown for the experiment performed at 24 °C. Mean +/− SEM values are shown. The line with the * shows that the V̇E/V̇O2 values during 7% CO2 breathing are significantly different during muscimol treatment in both wakefulness and NREM sleep (P< 0.01, repeated measures ANOVA interaction with Treatment). N=5 for awake; N=4 for NREM sleep.
Fig. 3.
The effects of bilateral dialysis of 0.5 mM muscimol into the NTS on the ventilatory equivalent, the V̇E/V̇O2 ratio, while the rat was breathing air (RA) and 7% CO2 (CO2) during wakefulness (left) and NREM sleep (right) are shown for the experiment performed at 30 °C. Mean +/− SEM values are shown. The line with the * shows that the V̇E/V̇O2 values during 7% CO2 breathing are significantly different during muscimol treatment in both wakefulness and NREM sleep (P< 0.01, repeated measures ANOVA interaction with Treatment). N=5 for each group.
Fig. 4.
The “CO2 response” defined as the % increase in the ventilatory equivalent, V̇E/V̇O2, is shown for the data of Figs. 2 and 3. Mean +/− SEM values are plotted. Muscimol treatment decreased the “CO2 response” in wakefulness and NREM sleep at both 24 °C and 30 °C (P< 0.001, repeated measures ANOVA) with no significant interaction with temperature or state (awake, NREM sleep). N=5 for each group.
3.3. Hypoxic response
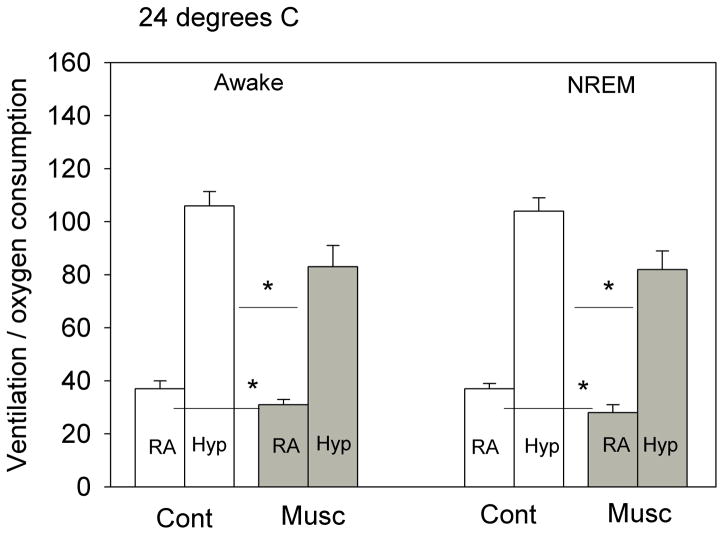
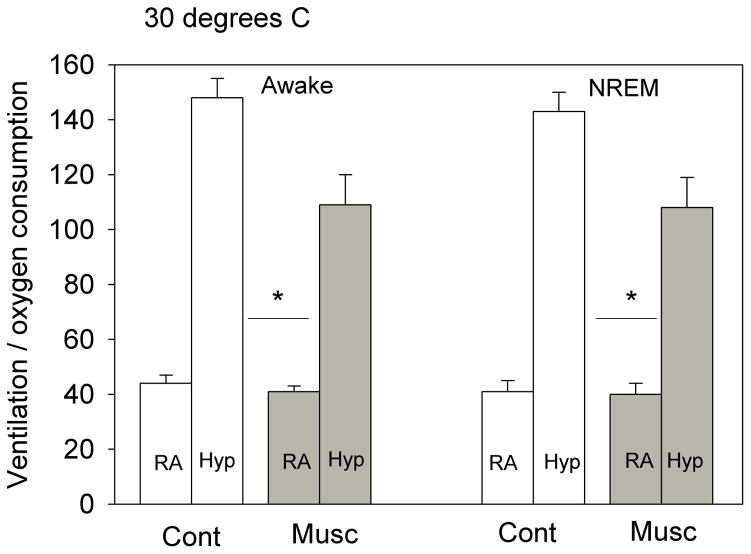
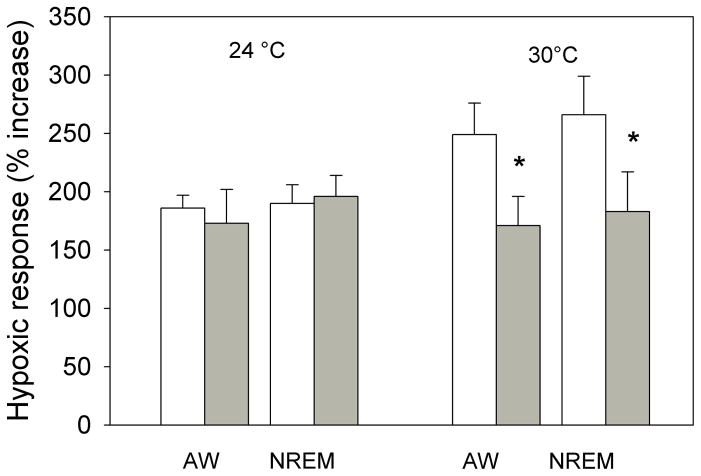
The effects of bilateral dialysis of 0.5 mM muscimol into the cNTS on the ventilatory response to 10% O2 are shown in Fig. 5 for the experiment performed at 24 °C and on Fig. 6 for the experiment performed at 30 °C. At both ambient temperatures and during both wakefulness and NREM sleep muscimol dialysis decreased the repeated measure, V̇E/V̇O2, in air and 10% O2 (P< 0.001; interaction with treatment, P< 0.01; no interaction with state). Post hoc analysis showed this treatment effect to be with 10% O2 but not air breathing at 30 °C but with both 10% O2 and air breathing at 24 °C. We defined the “hypoxic response” as the % increase in the ventilatory equivalent, V̇E/V̇O2, and evaluated this variable as shown in Fig. 7. Muscimol treatment decreased the “hypoxic response” in wakefulness and NREM sleep at 30 °C (P< 0.03, repeated measures ANOVA) with no significant interaction with state (awake, NREM sleep) but did not have a significant effect on the “hypoxic response” at 24 °C. The effects at 30 °C were entirely due to changes in breathing frequency.
Fig. 5.
The effects of bilateral dialysis of 0.5 mM muscimol into the NTS on the ventilatory equivalent (V̇E/V̇O2) while the rat was breathing air (RA) and 10% O2 (Hyp) during wakefulness (left) and NREM sleep (right) are shown for the experiment performed at 24 °C. Mean +/− SEM values are shown. The line with the * shows that the V̇E/V̇O2 values during both air and 10% O2 breathing are significantly different during muscimol treatment in both wakefulness and NREM sleep (P< 0.05, post-hoc test, repeated measures ANOVA P< 0.001; P< 0.02 interaction with treatment). N=7 for each group.
Fig. 6.
The effects of bilateral dialysis of 0.5 mM muscimol into the NTS on the ventilatory equivalent (V̇E/V̇O2) while the rat was breathing air (RA) and 10% O2 (Hyp) during wakefulness (left) and NREM sleep (right) are shown for the experiment performed at 30 °C. Mean +/− SEM values are shown. The line with the * shows that the V̇E/V̇O2 values during 10% O2 breathing are significantly different during muscimol treatment in both wakefulness and NREM sleep (P< 0.05, post-hoc test, repeated measures ANOVA P< 0.001; P< 0.02 interaction with treatment). N=6 for each group.
Fig. 7.
The “hypoxic response” defined as the % increase in the ventilatory equivalent, V̇E/V̇O2, is shown for the data of Figs. 5 and 6. Mean +/− SEM values are plotted. Muscimol treatment decreased the “hypoxic response” in wakefulness and NREM sleep only at 30 °C (P< 0.03, repeated measures ANOVA; P< 0.04 interaction with temperature) with no significant interaction with state (awake, NREM sleep). N=7 for 24 °C; N=6 for 30 °C.
4. Discussion
4.1. General
We used 0.5 mM muscimol in the dialysate for these experiments, which is lower than the 1.0 mM muscimol used in our prior central chemoreceptor site experiments at the RTN (Nattie et al. 2000) and medullary raphe (Taylor et al. 2006). In preliminary experiments, dialysis of 1 mM muscimol in the cNTS produced a drop in body temperature to 35 °C within minutes as well as behavioral agitation. These effects were not observed with dialysis of the 0.5 mM concentration. We report data obtained from 6 rats in the CO2 exposure group and 7 rats in the hypoxic exposure group. These 13 successful experiments with both dialysis guide tubes located in the cNTS and with all data obtained represent a ~50% success rate. To normalize for differences in V̇O2 among animals and to take into account the propensity for muscimol dialysis to inhibit the V̇E/V̇O2 in air breathing we have chosen to express all the ventilatory data only as the V̇E/V̇O2 ratio or the % change of this ratio. Our protocol is not well suited to evaluate the time course of the responses to CO2 or hypoxia because of the slow onset of the stimulus and the relatively slow measure of oxygen consumption due to the time it takes for wash-out of the plethysmograph as compared to ventilation, which is valid in a breath-by-breath manner. Hence we report averaged values over the final 10 or 20 min of the air breathing period and over the 30 min of CO2 or hypoxic exposure. We did not measure blood pressure. Changes in blood pressure within the carotid sinus can affect breathing: increased pressure decreases breathing and vice versa in conscious dogs (Saupe et al., 1995). Hypoxia (10% O2 breathing) in conscious rats increases blood pressure (Sugimura et al., 2008) as does CO2 (Oikawa et al., 2005). GABA applied to the cNTS in conscious rats reduces the baroreflex gain (Callera et al., 2000). Thus one might expect that reduced baroreflex gain would minimize any secondary effects of hypoxia or hypercapnia on breathing via the baroreflex.
4.2. Anatomical location of cNTS guide tubes
As shown on Fig. 1, the dialysis probe tips were located within the cNTS. These sites agree well with the location of NTS neurons found to be chemosensitive in slice experiments (Dean et al. 1989) and with the locations of dialysis probes that when dialyzed with high CO2 solutions resulted in a stimulation of breathing (Nattie et al. 2002). While these sites for the most part are lateral to the more midline location in the commissural nucleus at which the carotid body afferents (Chitravanshi & Sapru, 1995; Braga et al., 2007) and vagal C-fibers synapse (Moreira et al., 2007), we cannot be certain that the spread of muscimol did not affect them. Similarly, our muscimol dialysis could affect neurons in the interstitial nucleus of the cNTS that carry pulmonary afferent information (Kubin et al., 2006) as well as nearby neurons involved in the baroreflex (Loewy, 1990).
4.3. Ambient temperature effect on V̇O2 and rat body temperature
The difference in the two ambient temperatures chosen for study is small, 24 °C vs. 30 °C. The lower temperature is room temperature; it is below the thermoneutral zone of the rat and is the usual ambient temperature for most studies of rat physiology. The higher temperature is within the rat thermoneutral zone. These two ambient temperatures had no significant effect on V̇O2 or body temperature but did affect the absolute values of the V̇E/V̇O2 ratio in air and 7% CO2 or 10% O2 breathing, and the hypoxic response.
4.4. Muscimol effects on air breathing
The V̇E/V̇O2 tended to be lower during air breathing in wakefulness and in NREM sleep after muscimol dialysis in the cNTS in each group although this reached statistical significance only in the hypoxic exposure group at 24 °C. If we pool all V̇E/V̇O2 air breathing data we also find statistical significance. We conclude that dialysis of 0.5 mM muscimol in the cNTS can result in hypoventilation during air breathing.
4.5. The CO2 and hypoxic responses
We have used focal dialysis of muscimol to inhibit neurons within putative central chemoreceptor regions and then examined the ventilatory response to CO2. The RTN contains chemosensitive neurons that express Phox2b (Guyenet et al. 2008) and other neurons including GABAergic processes (Rosin et al. 2006) some of which arise in the NTS (Takakura et al. 2007). Focal application of the GABAA receptor antagonist bicuculline increases ventilation indicating the presence of a tonic GABAergic inhibitory tone in the RTN (Nattie et al. 2001a). When we focally apply muscimol to the RTN we observed hypoventilation during air breathing and a ~ 20% decrease in the response to 7% CO2 (Nattie et al. 2000). From these observations and the fact that focal stimulation of the RTN by dialysis of a high CO2 solution increases ventilation (Li et al. 1999) we concluded that the RTN is a central chemoreceptor site that provides a tonic drive to breathe in the conscious rat in vivo.
The medullary raphe contains serotonergic neurons that are CO2 sensitive in vitro (Richerson 2004; Richerson et al. 2005) and focal stimulation by dialysis with high CO2 solutions in vivo stimulate breathing (Nattie et al. 2001b; Hodges et al. 2004). Specific lesions (Nattie et al. 2004) or inhibition (Taylor et al. 2005) of medullary serotonergic neurons decreases the CO2 response and a mouse with conditional loss of serotonergic neurons has a substantially reduced CO2 response (Hodges et al. 2008). The response to hypoxia was unaffected in these studies. To further understand the role of the medullary raphe in chemoreception we focally applied muscimol by dialysis expecting a decrease in the CO2 response (Taylor et al. 2006). These studies were performed at 24 °C and 30 °C because the muscimol decreased body temperature when dialyzed at 24 °C but not when dialyzed at 30 °C. To our surprise muscimol increased the CO2 response, whether expressed as ventilation or as the V̇E/V̇O2 ratio, at both temperatures and the hypoxic response, expressed as the V̇E/V̇O2 ratio, at the warmer temperature. We suggested that this effect reflected a disinhibition of the serotonergic neurons, e.g., the muscimol in this case may be inhibiting local GABAergic neurons.
The present study was aimed at repeating this protocol but in the cNTS, another putative central chemoreceptor site based on the response of cNTS neurons in vitro to CO2 application (Dean et al. 1989), the expression of c-fos upon CO2 stimulation (Teppema et al. 1997), and the response of the intact animal to focal CO2 dialysis in vivo (Nattie et al. 2002). Since the cNTS receives information from the carotid body we also studied the effect of muscimol on the hypoxic response. We performed the experiment at two ambient temperatures, 24 °C (below the thermoneutral zone) and 30 °C (within the thermoneutral zone). The goal was to minimize any muscimol induced changes in body temperature and discern if the baseline responses and effects of muscimol varied comparing a very mild cold stress to an ambient temperature within the thermoneutral zone. While preliminary experiments at 24 °C using a 1 mM concentration of muscimol in the dialysate (the concentration used in the RTN and medullary raphe studies) demonstrated a marked fall in body temperature, this did not occur when the muscimol concentration was decreased to 0.5 mM. All studies were then performed at this dose.
Muscimol dialysis bilaterally into the cNTS decreased the V̇E/V̇O2 ratio significantly during 7% CO2 breathing in both wakefulness and NREM sleep and decreased the % increase in V̇E/V̇O2, the CO2 response) in wakefulness and NREM sleep. This decrease in the CO2 response did not differ significantly at 24 °C vs. 30 °C. The magnitude of the CO2 response inhibition varied from −24 to −28% at 24 °C to −43 to −47% at 30 °C. These effects in general are larger than the effects observed with dialysis of 1.0 mM muscimol in the RTN, ~ −20% (Nattie et al. 2000) and opposite in direction from those observed with dialysis of muscimol in the medullary raphe (Taylor et al. 2006). The cNTS is involved in the CO2 response.
During 10% O2 breathing muscimol dialysis into the cNTS decreased V̇E/V̇O2 significantly in both wakefulness and NREM sleep and decreased the % increase in V̇E/V̇O2 in both states but only at 30 °C. The magnitude of the hypoxic response inhibition was −31% at 30 °C. GABAergic input in the cNTS inhibits the hypoxic response but at 30 °C not at 24 °C. In the medullary raphe, focal application of muscimol had an opposite effect, an increased response, but again only at 30 °C (Taylor et al. 2006). We do not know how such a small ambient temperature change, without detectable effect on V̇O2 and body temperature, can produce these changes in the hypoxic response. It may be that at room temperature the rats are making subtle adjustments in temperature regulation that also affect the chemical control of breathing. Larger changes in rat body temperature are known to affect chemosensitivity (Maskrey 1990). It is of interest to note that in both this study and our prior study applying muscimol focally in the medullary raphe (Taylor et al. 2005) we observed effects only on the hypoxic response and only at 30 °C, within the thermoneutral zone. This indicates that the response of V̇E/V̇O2 and V̇E/V̇O2 to hypoxia differs in mechanism at 24 °C vs. 30 °C.
4.6. The role of the cNTS in chemoreception
The cNTS is clearly involved as an initial synapse site for carotid body afferent activity related to stimulation by CO2 or hypoxia. Like the RTN, the cNTS is also a putative central chemoreceptor site (see above). Like the cNTS, the RTN also receives afferent carotid body information (Takakura et al. 2006) as well as being a putative central chemoreceptor site (see above). Inhibition of neurons at either site-here by muscimol- could then affect both processes, central and peripheral chemoreception. Our present studies cannot help to discern the relative importance of potential modulation of carotid body afferents vs. direct effects of CO2. The fact that muscimol dialysis in the cNTS at 24 °C inhibits the CO2 response but has no effect on the hypoxic response suggests that the effect on the CO2 response at this temperature is on cNTS neurons that are themselves CO2 sensitive. It is of interest that inhibition of the cNTS with a 0.5 mM muscimol dialysate had a more substantial effect on the CO2 response, especially at 30 °C, than did inhibition of the RTN with a 1.0 mM muscimol dialysate. We suggest that the cNTS may be a much more potent central chemoreceptor site than the RTN. This hypothesis, which requires more specific testing, is at odds with the recent revival of the idea that the ventral medulla, now represented by Phox2b positive neurons of the RTN, is the primary central chemoreceptor site (Guyenet et al. 2008). In addition, the idea that chemoreceptor sites interact to produce an output that is enhanced is supported by our recent experiments in which focal acidification of both the RTN and the medullary raphe produced an enhanced response (Dias et al. 2008). It may be that in both the RTN and the cNTS, CO2 can 1) directly affect chemoresponsive neurons, and 2) enhance their responsiveness to stimulation of afferent input from the carotid body.
4.7. Conclusions
In the absence of arterial blood gases or measurements of brain pH, the CO2 response is effectively expressed as the % increase in the V̇E/V̇O2 ratio, as is the hypoxic response.
The CO2 response is unaffected by ambient temperatures just below vs. within the thermoneutral zone; the hypoxic response is greater at the warmer temperature suggesting that even mild thermal stress alters the way the rat responds to hypoxia. 17
The cNTS is quite important in the CO2 response likely due to a) the inherent CO2 sensitivity of cNTS neurons, and b) its status as a primary synaptic site for carotid body afferents.
Acknowledgments
This work was supported by an NIH grant R37 HL 28066 from the Heart, Lung and Blood Institute to EN. The authors thank Jia-Zhen Cai for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–479. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Braga VA, Soriano RN, Braccialli AL, de Paula PM, Bonagamba LGH, Paton JFR, Machado BH. Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol. 2007;581(3):1129–1145. doi: 10.1113/jphysiol.2007.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callera JC, Bonagamba LGH, Nosjean A, Laguzzi R, Machado BH. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci. 2000;84:58–67. doi: 10.1016/S1566-0702(00)00184-3. [DOI] [PubMed] [Google Scholar]
- Chung S, Ivy GO, Reid SG. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1449–1456. doi: 10.1152/ajpregu.00645.2005. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol. 1995;268:R851–R858. doi: 10.1152/ajpregu.1995.268.4.R851. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772– 1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus (ROb) does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus (RTN) J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AY, Stornetta RL, Foley CM, Potts JT. Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol. 2005;493:274–290. doi: 10.1002/cne.20758. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005;90:247–253. doi: 10.1113/expphysiol.2004.029637. discussion 253–247. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol. 2004;97:2303–2309. doi: 10.1152/japplphysiol.00645.2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AH, Liu P, Weisman H, Chernick V, Nance DM. Effect of sinus denervation and vagotomy on c-fos expression in the nucleus tractus solitarius after exposure to CO2. Pflugers Arch. 1996;431:876–881. doi: 10.1007/s004240050080. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Invited Review. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. pp. 88–103. [Google Scholar]
- Martino PF, Hodges MR, Davis S, Opansky C, Pan LG, Krause K, Qian B, Forster HV. CO2/H+ chemoreceptors in the cerebellar fastigial nucleus do not uniformly affect breathing of awake goats. J Appl Physiol. 2006;101:241–248. doi: 10.1152/japplphysiol.00968.2005. [DOI] [PubMed] [Google Scholar]
- Maskrey M. Body temperature effects on hypoxic and hypercapnic responses in awake rats. Am J Physiol. 1990;259:R492–498. doi: 10.1152/ajpregu.1990.259.3.R492. [DOI] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, Guyenet PG. Activation of 5- Hydroxytryptamine Type 3 Receptor-Expressing C-Fiber Vagal Afferents Inhibits Retrotrapezoid Nucleus Chemoreceptors in Rats. J Neurophysiol. 2007;98:3627–3637. doi: 10.1152/jn.00675.2007. [DOI] [PubMed] [Google Scholar]
- Nattie E. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–235. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci. 2006;126–127:332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nattie E, Shi J, Li A. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat. Respir Physiol. 2001a;124:179–193. doi: 10.1016/s0034-5687(00)00212-7. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001b;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception is a complex system function that involves multiple brainstem sites. J Appl Physiol. 2008a doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A, Richerson G, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol. 2004;556:235–253. doi: 10.1113/jphysiol.2003.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li AH, St John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- Nattie G, Li A. Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol. 2008b;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y. Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo- and baroreceptors. Auton Neurosci. 2005;117:105–114. doi: 10.1016/j.autneu.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Paxino G, WC . The Rat Brain in Stereotaxic Coordinates. Academic; San Diego, CA: 1998. [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. discussion 266–259. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Saupe KW, Smith CA, Henderson KS, Dempsey JA. Respiratory and cardiovascular responses to increased and decreased carotid sinus pressure in sleeping dogs. J Appl Physiol. 1995;78:1688–1698. doi: 10.1152/jappl.1995.78.5.1688. [DOI] [PubMed] [Google Scholar]
- Sugimura M, Hirose Y, Hanamoto H, Okada K, Boku A, Morimoto Y, Taki K, Niwa H. Influence of acute progressive hypoxia on cardiovascular variability in conscious spontaneously hypertensive rats. Auton Neurosci. 2008;141:94–103. doi: 10.1016/j.autneu.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata M, Kurosawa H, Kikuchi Y, Hida W, Ogawa H, Okabe S, Tun Y, Hattori T, Shirato K. Role of GABA within the nucleus tractus solitarii in the hypoxic ventilatory decline of awake rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1411– 1419. doi: 10.1152/ajpregu.2001.281.5.R1411. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543– 557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol. 2006;153:203–216. doi: 10.1016/j.resp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]