Abstract
Background
The condition known as cachexia presents in most patients with malignant tumours, leading to a poor quality of life and premature death. Although the cancer‐cachexia state primarily affects skeletal muscle, possible damage in the cardiac muscle remains to be better characterized and elucidated. Leucine, which is a branched chain amino acid, is very useful for preserving lean body mass. Thus, this amino acid has been studied as a coadjuvant therapy in cachectic cancer patients, but whether this treatment attenuates the effects of cachexia and improves cardiac function remains poorly understood. Therefore, using an experimental cancer‐cachexia model, we evaluated whether leucine supplementation ameliorates cachexia in the heart.
Methods
Male Wistar rats were fed either a leucine‐rich or a normoprotein diet and implanted or not with subcutaneous Walker‐256 carcinoma. During the cachectic stage (approximately 21 days after tumour implantation), when the tumour mass was greater than 10% of body weight, the rats were subjected to an electrocardiogram analysis to evaluate the heart rate, QT‐c, and T wave amplitude. The myocardial tissues were assayed for proteolytic enzymes (chymotrypsin, alkaline phosphatase, cathepsin, and calpain), cardiomyopathy biomarkers (myeloperoxidase, tissue inhibitor of metalloproteinases, and total plasminogen activator inhibitor 1), and caspase‐8, ‐9, ‐3, and ‐7 activity.
Results
Both groups of tumour‐bearing rats, especially the untreated group, had electrocardiography alterations that were suggestive of ischemia, dilated cardiomyopathy, and sudden death risk. Additionally, the rats in the untreated tumour‐bearing group but not their leucine‐supplemented littermates exhibited remarkable increases in chymotrypsin activity and all three heart failure biomarkers analysed, including an increase in caspase‐3 and ‐7 activity.
Conclusions
Our data suggest that a leucine‐rich diet could modulate heart damage, cardiomyocyte proteolysis, and apoptosis driven by cancer‐cachexia. Further studies must be conducted to elucidate leucine's mechanisms of action, which potentially includes the modulation of the heart's inflammatory process.
Keywords: Cancer, Cachexia, Leucine, Heart, Biomarkers, Electrocardiography, Apoptosis
Background
Cancer‐cachexia is a complex syndrome that is characterized by inflammation, involuntary body weight loss, and adipose and muscle tissue wasting and leads to premature death.1, 2 In this multifactorial state, the development of anorexia, early satiety, asthenia, weakness, and anaemia are commonly noticed, as well as changes in the metabolism of carbohydrates, fats, and proteins.3 Moreover, the cachectic state could be the main determinant of the reduced the quality and lifetime of these cancer patients.4, 5, 6 Anorexia and catabolism, which are promoted by the presence of tumours, are the main factors that lead to the state of cachexia,3 which can also be caused by cytokines produced by tumours or released by the host immune system in response to the effects of cancer, which promote lipolysis and proteolysis in these patients.7
While observations of death in cancer patients related to cardiovascular insufficiency are not new,8 studies have only recently focused on how cachexia is responsible for or contributes to cardiac failure. Thus, the alterations in cardiac muscle structure and metabolism that occur during cancer‐cachexia progression are poorly understood.9, 10
Emerging studies provide suggestions concerning how cancer‐induced cachexia might lead to heart damage. TIAN and colleagues9, 11 noted that cachectic mice presented decreased contractile cardiac function and heart rate, with concomitant increased fibrosis, cardiac atrophy, remodelling, and the presence of pro‐inflammatory cytokines in the heart tissue. Other studies showed the contribution of proteolysis and oxidative stress to cancer‐cachexia‐induced heart damage.10, 12
Several processes, rather than protein spoliation itself, might be involved in the reported cardiac atrophy. Among these processes, programmed cell death, or apoptosis, with a general loss of healthy myocardial tissue, is reported to be increased in several cardiomyopathies.13, 14, 15, 16 This process involves the activation of caspases and the cleavage of apoptosis regulators, which could be related to myocardial failure.17, 18
The use of biomarkers in association with electrocardiographic information generates more accurate information regarding cardiac damage.19, 20 In the cardiac context, myeloperoxidase (MPO) is a marker of oxidative stress and inflammation. In addition, tissue inhibitor of metalloproteinases 1 (TIMP‐1) is linked to pathological tissue remodelling, and the total plasminogen activator inhibitor 1 (Total PAI‐1) level is related to thrombosis risk and fibrosis. All of these biomarkers are involved in cardiomyopathies, cardiac dysfunction, ischemia, and heart failure.21, 22
Protein synthesis requires an appropriate balance of amino acids, and some amino acids, especially branched chain amino acids, are reduced in patients with cancer‐cachexia.23 Studies show the benefits of a semi‐purified diet containing a high content of leucine for biochemical changes related to protein metabolism in the gastrocnemius muscle of young tumour‐bearing rats24, 25; however, to our knowledge, no studies focused on whether leucine could contribute to the attenuation of cardiac spoliation induced by cancer‐cachexia. Therefore, this study aimed to observe the effect of leucine modulation on cardiac damage and failure in a cancer‐cachexia model.
Methods
Animals and diet
Male Wistar rats obtained from the animal facilities at the State University of Campinas, UNICAMP, Brazil were housed in collective cages during the entire experimental period. The animals received food and water ad libitum with controlled light and darkness (12–12 h), temperature (22 ± 2 C), and humidity (50–60%). The semipurified diets were provided in accordance with AIN‐93 M from the American Institute of Nutrition.26 The normoprotein diet (C) included 18% protein. The leucine‐rich diet (L) contained 18% protein plus 3% L‐leucine24, 27 (Table 1).
Table 1.
Semi‐purified diet composition according to AIN‐93 M
| Ingredients* | Control | Leucine |
|---|---|---|
| Cornstarcha | 397.5 g | 385.5 g |
| Casein** | 200 g | 200 g |
| Dextrina | 132 g | 122 g |
| Sugar | 100 g | 90 g |
| Cellulose micro fibre | 50 g | 50 g |
| Salt mix*** | 35 g | 35 g |
| Vitamin mix*** | 10 g | 10 g |
| Cystineb | 3 g | 3 g |
| Choline | 2.5 g | 2.5 g |
| Soy oil | 70 g | 70 g |
| Leucineb | — | 30 g |
Composition and amount of nutrients in 1 kg of diet, based on the American Institute of Nutrition—AIN‐93 (REEVES et al, 199326).
Correction of casein to total protein content equal to 74.4%, casein protein source contains 1.6% leucine.
In accordance with AIN‐93 (REEVES et al, 1993).
Ingredion (São Paulo, Brazil) donation.
Ajinomoto Interamericana Ind. (São Paulo, Brazil) donation.
Tumour implant
The tumour‐bearing rats received a subcutaneous injection of approximately 2.5 × 106 Walker‐256 carcinoma cells in a 0.9% NaCl suspension (0.5 mL) in the right flank.25, 28 The rats without tumours received a unique injection containing a 0.5 mL NaCl solution (0.9% w/v) without anaesthesia.
The general guidelines of the United Kingdom Co‐ordinating Committee on Cancer Research (UKCCCR, 1998)29 for animal welfare were followed, and the experimental protocols were approved by the Institutional Committee for Ethics in Animal Research (CEEA.IB/UNICAMP, protocol No. 2678‐1).
Experimental protocol
The adult rats (90 days old) were distributed into four groups (n = 5 animals/group) according to tumour implant and nutritional scheme, as follows: control (C) rats fed a control diet, tumour‐bearing rats (W), rats fed a leucine‐rich diet (L), and tumour‐bearing rats fed a leucine‐rich diet (WL). Body weights were recorded 3 times/week. Twenty‐one days after tumour implantation (pre‐agonic state), the rats were submitted to electrocardiogram measurement and sacrificed via deep anaesthesia to collect samples from the heart and blood for biochemical analysis.
Electrocardiogram (ECG)
The rats were anaesthetized (100 mg/kg of ketamine + 7 mg/kg body weight of xylazine, i.m., diluted in a vehicle solution of 0.9% sodium chloride (saline)), fixed in the supine position and subjected to ECGs for spontaneous breathing. Recordings were made with electrodes in the form of hypodermic needles using a computerized four‐channel ECG MLS360/7 ECG Analysis Module (AD Instruments—Australia). Wave amplitudes were measured in millivolts (mV), and the durations of the intervals were measured in milliseconds (ms).
Cardiac biomarker analysis
Heart samples
The heart ventricle samples were homogenized with sample buffer (Multiplex MAP Cell Signalling Buffer, Millipore, USA) according to the manufacturer's instructions.
Analyses of cardiac biomarkers (MPO, TIMP 1, and PAI‐1) in the serum and heart tissue were performed using immunoassay kits (Multiplex Map Cell Signalling assay, Millipore) and fluorescent flow cytometry using Luminex equipment (Millipore, USA).
Histological analysis
Hearts from additional experimental groups (n = 5 per group) were rapidly excised, and a portion of the ventricles were fixed in 10% neutral‐buffered formalin and embedded in paraffin. Sections with a thickness of 5 µm were stained with HE trichrome and then analysed using light microscopy.
Apoptotic and proteolytic enzymes
Heart tissue caspase activity was determined using a method described by Koeplinger and colleagues.30 Briefly, heart tissue samples [after homogenization in sample buffer (20 mM Tris, 1 mM DTT, 2 mM ATP, and 5 mM MgCl2)] were incubated for 30 min in the dark with 20 μM fluorogenic substrate, followed by fluorescence measurement in a Hidex ChamaleonTM V plate reader (excitation 355 nM, emission 460 nM). For caspases 3 and 7, we used the Z‐Asp‐Glu‐Val‐Asp‐AMC substrate; for caspase 8 we used the Z‐Ile‐Glu‐Thr‐Asp‐AMC substrate; and for caspase 9 we used the Ac‐Leu‐Glu‐His‐Asp‐AMC substrate. All substrates were purchased from AnaSpec (AnaSpec, San Jose, CA, USA).
The myocardium muscle homogenate was also assessed for chymotrypsin‐like, cathepsin, and calpain enzyme activities. Chymotrypsin‐like activity was evaluated using the substrate N‐Succinyl‐Leu‐Leu‐Val‐Tyr‐7‐Amino‐4‐Methylcoumarin diluted in dimethyl sulfoxide (DMSO) and Tris (pH 8.0) and assessed using the fluorometric method with a 360 nm excitation wavelength and a 460 nm emission wavelength.31 The activity of cathepsin was evaluated using a fluorometric method and the substrate benzyloxycarbonyl‐phenylalanine‐arginine 4‐methyl‐7‐coumarin amide with a 340 nm excitation wavelength and a 460 nm emission wavelength.32 Calpain was measured by incubating the samples in reaction buffer with casein for 5 min, followed by the addition of 5 mM CaCl2 and subsequent reading of the absorbance at 500 nm in a spectrophotometer, in accordance with colorimetric methodology.32
The total protein content of the heart tissue samples, which was used to normalize the enzymatic activities, was determined using the Bradford method with bovine serum albumin (BSA) as a standard.33
Statistical analysis
The data are expressed as the mean ± SEM. The data were analysed statistically using two‐way ANOVA to determine the effects of diet and/or tumour growth on heart parameters. Comparisons among the groups were assessed using a post‐hoc Bonferroni multiple comparison test (Graph Pad Prism software, v3.00 for Windows 98, USA). The results were considered statistically significant when the P value was less than 5%.34
Results
Body and heart parameters
In this study, the final body weight decreased, as did the percentage of weight and weight gain, in both tumour‐bearing rats (W and LW groups) (Table 1). Thus, the effect of tumour development was considered very significant (F = 8.83, P < 0.0001), although diet did not modulate weight loss. No differences in tumour weight or relative tumour weight (Table 1) were observed in groups W and LW. We observed a decrease in heart weight and a higher relative heart rate in tumour‐bearing rats; these results were produced primarily by the effects of the tumour (F = 10.45, P = 0.0019, indicating that this result is very significant), but the effect of diet was not considered to be significant. On the other hand, the leucine‐rich diet had a positive effect on the myocardial protein content in group L and the maintenance of the heart protein content in group LW in comparison to the C groups (Table 1; diet effects correspond to F = 6.93, P = 0.0129 and tumour effects correspond to F = 6.03, P = 0.0197). However, this positive effect did not affect the reduction in left ventricular thickness that was observed in both tumour‐bearing groups (tumour effect corresponds to F = 70.63, P < 0.0001) or the reduction in right ventricular thickness that was observed only in group LW (F = 14.98, P = 0.0005), revealing a very significant interaction between diet and tumour effects (Table 2).
Table 2.
Morphometric parameters of body weight and heart and tumour tissues
| Groups | C | W | L | LW |
|---|---|---|---|---|
| Initial body weight (g) | 401.6 + 9.1 | 413.4 + 5.93 | 417.7 + 8.08 | 402.5 + 7.35 |
| Final body weight (g) a | 454.6 + 10.1 | 397.6 + 17.7* | 476.2.9 + 10.9 | 396.1 + 12.15*# |
| Percentage of final body weight (%) a § | 113.3 + 1.0 | 95.9 + 3.4* | 113.9 + 1.1 | 98.5 + 2.5*# |
| Weight gain (g) per day a § | 2.52 + 0.21 | −0.75 + 0.67* | 2.78 + 0.23 | −0.31 + 0.49*# |
| Heart weight (grams) b | 1.27 + 0.05 | 1.14 + 0.04* | 1.42 + 0.06* | 1.15 + 0.06*# |
| Relative heart weight (%) b §, § | 0.28 + 0.01 | 0.30 + 0.01* | 0.29 + 0.01 | 0.32 + 0.01*# |
| Myocardial protein (µg/mg) c | 88.65 + 6.89 | 80.84 + 3.51* | 112.20 + 6.07* | 89.75 + 7.58 |
| Heart water content §§§ | 73.33 + 2.91 | 67.54 + 4.02 | 71.37 + 3.10 | 71.84 + 4.05 |
| Left ventricular thickness (µm) d | 281.7+ 12.4 | 221.5+ 9.1*# | 323.8+ 16.8* | 186.5+ 5.9*# |
| Right ventricular thickness (µm) e | 83.9+ 6.2 | 88.9+ 4.2 | 101.2+ 5.3* | 66.4+ 4.6*# |
| Tumour weight (g) | — | 55.66 + 5.98 | — | 48.84 + 7.40 |
| Relative tumour weight (%)f | — | 14.86 + 2.08 | — | 13.97 + 2.14 |
Legend: C (Control group), W (untreated tumour‐bearing rats group), L (leucine‐rich diet group), LW (tumour‐bearing rats fed a leucine‐rich diet).
(Final body weight/Initial body weight) × 100%; weight gain = (final body weight − initial body weight) / days.
(Heart weight/body weight) × 100%.
(Fresh heart weight − dry heart weight) / fresh heart weight × 100%. Decreased body weight, and percentage/weight gain produced by tumour effect.
(Two‐way ANOVA, P < 0.0001; followed by the Bonferroni test *P < 0.05 vs. control group, # P < 0.05 vs. leucine group; n = 5). Decreased heart weight and relative heart weigh produced by tumour effect.
(Two‐way ANOVA, F = 10.45 P = 0.0019; followed by the Bonferroni test *P < 0.05 vs. control group, # P < 0.05 vs. leucine group; n = 5).
Myocardial protein content changed mainly by diet effect (two‐way ANOVA, F = 6.93 P = 0.0129; followed the Bonferroni test * P < 0.05 vs. control group, n = 5) and by tumour effect (two‐way ANOVA, F = 6.03 P = 0.0197; followed by the Bonferroni test * P < 0.05 vs. control group, n = 5).
Left ventricle wall reduction as a tumour effect (two‐way ANOVA, F = 70.63 P < 0.0001; followed by the Bonferroni test * P < 0.05 vs. control group, # P < 0.05 vs. leucine group; n = 5).
Right ventricle wall reduction as an interaction effect between tumour and diet (two‐way ANOVA, F = 14.98 P = 0.0005; followed by the Bonferroni test * P < 0.05 vs. control group, # P < 0.05 vs. leucine group; n = 5).
(Tumour weight/final body weight without tumour) × 100%.
Electrocardiography analysis and cardiac biomarkers
Cancer‐cachexia can affect cardiac tissue and function; to address that topic, we initially used some ECG parameters, such as QT interval corrected for heart rate (QT‐c), T‐wave amplitude, and heart rate. Figure 1A shows a significant decrease in heart rate in both tumour‐bearing groups (tumour effects correspond to F = 25.88, P = 0.0005). On the other hand, tumour evolution increased the QT‐c interval (F = 8.92, P = 0.0105), revealing a significant effect. These data suggested a higher risk of death (sudden death), especially in group W, which strictly correlated with an increase in the area under the curve's T wave (Figure 1C and 1D; the interaction effects between tumour and diet are considered significant; F = 21.12, P = 0.0018). While these results are likely associated with cardiac failure in group W, the ECG register could show an increased time of ventricular depolarization (QRS) adding to an increased repolarization time (higher T wave interval; Figure 1C and 1G). Counteracting this point, despite having increased QT‐c and a reduction in heart rate in association with a thin ventricle wall, leucine treatment strongly prevented the T wave increase, reducing the higher QT‐c interval (11% lower than group W and similar to group C). The ECG register did not show an ischemia process in any studied group (T wave inversion), and no change in ST interval was observed (Figure 1C, E, F, G, and H).
Figure 1.
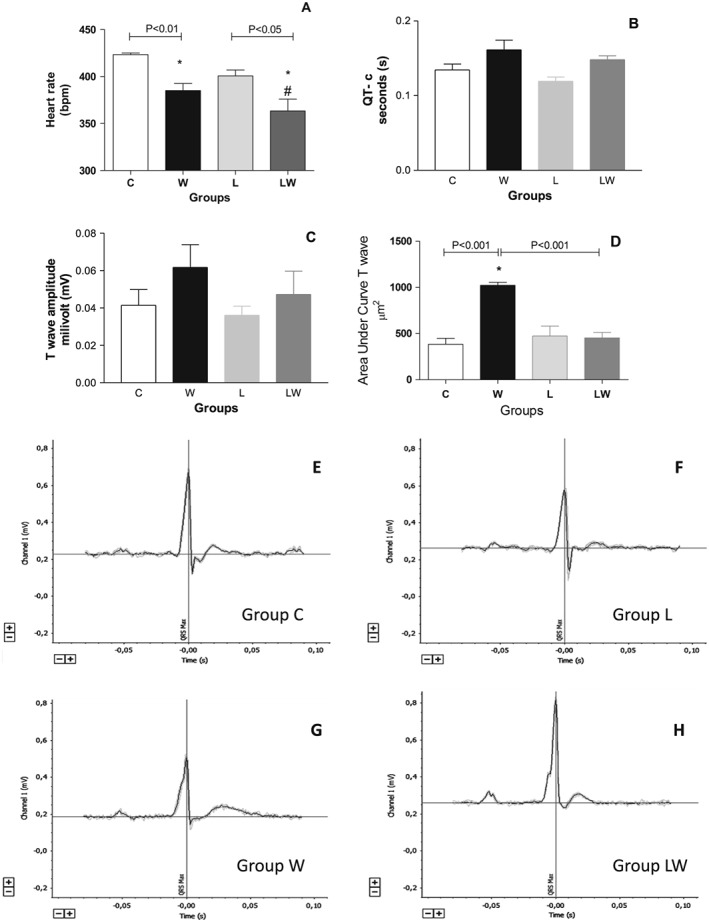
Electrocardiography registers from different experimental groups. A—Heart rate (bpm) (* P < 0.01 vs. control, # P < 0.005 vs. leucine, two‐way ANOVA with Bonferroni test, n = 5); B—QT‐c (seconds—s) (# P < 0.01 vs. leucine, two‐way ANOVA with Bonferroni test, n = 5); C—T‐wave amplitude (millivolt—mV); D—T wave's area under curve (* P < 0.001 vs. C, L, and LW group, two‐way ANOVA with Bonferroni test, n = 5); E—Electrocardiography trace of control group; F—Electrocardiography trace of leucine‐rich diet group; G—Electrocardiography trace of tumour‐bearing group; H—Electrocardiography trace of tumour‐bearing rats fed a leucine‐rich diet. Graphs E, F, G, and H represent the electrocardiography traces in D1 (peripheral derivation one), as the best register representative of each group (n = 5); the grey range along the ECG trace means all registers obtained from all animals per group (n = 4); the black line means the average of the grey range from all of the animals. Legend: C (Control group), W (tumour‐bearing untreated group), L (leucine‐rich diet group), LW (tumour‐bearing group fed a leucine‐rich diet).
To determine whether myocardial tissue can suffer some effects of tumour growth, we also observed that the alkaline phosphatase and chymotrypsin activities were enhanced only in group W (Figure 2A and 2B; the interaction effect of diet and tumour is considered significant, F = 8.35, P = 0.0127). We also found that the leucine‐rich diet led to an increase in chymotrypsin activity, which was verified here in both leucine‐treated groups (Figure 2B; a significant diet/tumour interaction; F = 4.74, P = 0.0501). Furthermore, we found a decrease in cathepsin activity in both tumour‐bearing groups (the tumour effect is considered significant; F = 8.46, P = 0.0108), and calpain activity was similar in all groups (Figure 2C and 2D). Regarding these cardiac biomarkers, in this work, we observed a large increase in all three biomarkers analysed (MPO (interaction effect F = 5.84, P = 0.0288; tumour effect F = 10.32, P = 0.0058), TIMP‐1 (tumour effect F = 6.68, P = 0.0216), and total PAI‐1 (tumour effect F = 8.62, P = 0.0097)) in only untreated tumour‐bearing rats (W) (Figure 3A, 3B, 3C). The leucine‐treated group (LW) also exhibited a slight increase in MPO and TIMP‐1 in comparison to group L (Figure 3A and 3B), with values similar to those of the controls.
Figure 2.
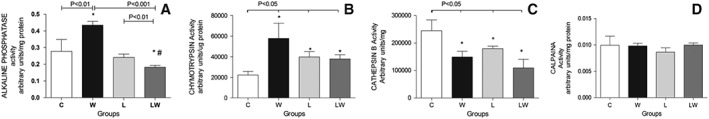
Proteolytic enzyme activity in myocardial muscle from different experimental groups. A—Alkaline phosphatase activity (arbitrary units per protein content per minute) (* P < 0.01 vs. control, # P < 0.001 vs. leucine, two‐way ANOVA with Bonferroni test, n = 5); B—Chymotrypsin activity (* P = 0.05 vs. control, two‐way ANOVA with Bonferroni test, n = 5); C—Cathepsin B activity (* P = 0.01 vs. control, two‐way ANOVA with Bonferroni test, n = 5); D—Calpain activity. For details, see the Material and methods. Legend: C (Control group), W (tumour‐bearing untreated group), L (leucine‐rich diet group), LW (tumour‐bearing group fed a leucine‐rich diet).
Figure 3.
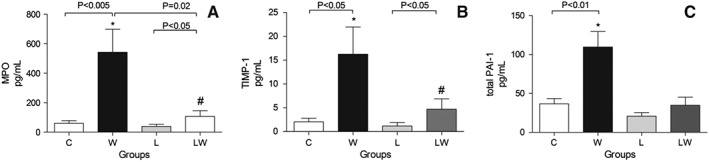
Cardiac biomarkers in myocardial tissue from different experimental groups. A—MPO (Myeloperoxidase—pg/mL) (* P < 0.005 vs. control, # P = 0.02 vs. leucine, two‐way ANOVA with Bonferroni test, n = 5). B—TIMP1 (Tissue Inhibitor Metalloproteinase 1—pg/mL) (* P < 0.05 vs. control, # P < 0.05 vs. leucine, two‐way ANOVA with Bonferroni test, n = 5). C—tPAI‐1 (Total Plasminogen Activator Inhibitor 1—pg/mL) (* P = 0.0097 vs. control, two‐way ANOVA with Bonferroni test, n = 5). For details, see the Material and methods. Legend: C (Control group), W (untreated tumour‐bearing group), L (leucine‐rich diet group), LW (leucine‐treated tumour‐bearing group).
Apoptotic enzymatic activities
In this work, we observed a significant increase in the activity of caspases 3 and 7 in cardiac tissue from untreated tumour‐bearing rats (W) (Figure 4A; tumour effects correspond to F = 17.16, P = 0.0009), suggesting an increase in the apoptotic rate. Regarding the upstream caspase activation pathway, in our model, the observed increase in myocardial apoptosis appears to be linked to the intrinsic pathway rather than the extrinsic pathway, as a trend towards an increase in caspase‐9 activity was observed in untreated tumour‐bearing rats (W; the tumour and diet effect interaction is not significant, F = 4.39, P = 0.0509). In contrast, the caspase‐8 activity did not exhibit any evident changes (Figure 4B and 4C, respectively).
Figure 4.
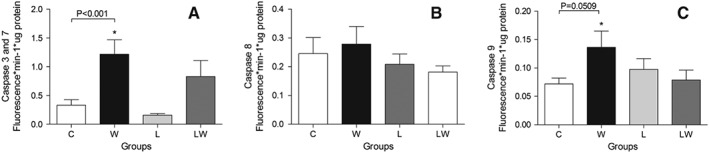
Caspase activity in cardiac tissue from different experimental groups. A—Caspases 3 and 7 (* P < 0.001 vs. control, two‐way ANOVA with Bonferroni test, n = 5). B—Caspase 8. C—Caspase 9 (* P = 0.0509 vs. control, two‐way ANOVA with Bonferroni test, n = 5). For details, see the Material and methods. Legend: C (Control group), W (tumour‐bearing group), L (leucine‐rich diet group), LW (tumour‐bearing rats fed a leucine‐rich diet). * P < 0.05 compared with group C. # P < 0.05 compared with group L.
Discussion
During cancer‐cachexia progression, some metabolic alterations that lead to lean mass and fat spoliation and chronic inflammation and culminate in involuntary weight loss, fatigue, and asthenia are commonly observed.1, 35 Walker tumour growth, as an experimental model of cachexia,36 represents an important effect on lean body mass wasting, as verified here in this work. Additionally, tumour growth exerted some important effects on heart tissue and function, suggesting the presence of cardiac cachexia in these animals. On the other hand, no differences in tumour weight or tumour relative weight were observed, ensuring that the animals from both groups W and LW were potentially exposed to the same effect of tumour growth and cachexia‐inducing factors. However, the leucine treatment could influence the tumour effects and change some host responses, especially heart parameters, which could be related to positive modulation of the effects of tumour damage on cardiac function.
Previous studies demonstrated that the cancer‐cachexia syndrome leads to skeletal muscle protein spoliation1, 9, 24 and that supplementation with branched‐chain amino acids, especially leucine, can restore this alteration24; however, more studies are needed to elucidate whether cancer‐cachexia damages heart tissue and how leucine could modulate this process.
Several studies showed that cancer‐cachexia indeed affects cardiac tissue.9, 10, 12 Furthermore, during years of work on cancer‐cachexia models, we observed several instances of sudden death in animals even before the establishment of a complete cachectic state. Here we show some ECG parameters in tumour‐bearing rats that were compromised by tumour effects (significant decrease in heart rate, alteration in the QT‐c interval, and increased area under the T‐wave curve), suggesting a higher risk of death in the tumour‐bearing group. In association with morphometric parameters, which showed an important reduction in the left ventricle wall, these changes likely suggest cardiac failure.10, 37, 38 Although the electrocardiography yields some interesting and important data, Xu and colleagues demonstrated that the cardiac damage, at the cellular level, that occurs in a cancer‐cachexia model is much more severe than suggested by electrocardiography.20 Meanwhile, in the present work, nutritional supplementation with leucine led to some positive effects on the changes in QT‐c, T‐wave amplitude, and area in group LW, despite the observed reduction in the right and left ventricle walls. The reduction of the ventricular wall might not indicate an impaired contractile force, but when associated with the reduction of cardiac mass (lower weight and protein content, which was present especially in group W, rather than group LW) this change could compromise the contractile strength, reducing the heart rate and indicating cardiac failure.10, 37, 38
To address this issue, we used biomarkers for heart damage (MPO, TIMP‐1, and total PAI‐1) and assessed apoptotic and proteolytic enzyme activities in association with ECG to better understand the extent of the damage that occurs in the heart in the different treatment groups.
Our previous work showed some important impairments in skeletal muscle in tumour‐bearing rats, which had increased chymotrypsin activity, representing enhancements of the ubiquitin–proteasome pathway.24, 27, 39, 40, 41 The present results likely suggest that the higher proteolytic activity (increased chymotrypsin activity) that is associated with higher cell activity (higher AP activity) in heart tissue could be responsible for the ECG failure that was observed only in group W. In our previous research,24, 27, 39, 40, 42 as also found here, the leucine‐rich diet also minimized the observed changes in those enzymes, even in the heart. Despite the presence of tumour, the LW group exhibited some improvements in cardiac tissue, with minimal damaging effects produced by tumour development, as confirmed by the significant diet interaction effect. Indeed, previous studies showed the benefits produced by leucine treatment in skeletal muscle.23, 40, 43, 44, 45 Some studies demonstrated that the main proteolytic pathway that is activated during cancer evolution is the ubiquitin–proteasome pathway; therefore, catabolic processes that involve the lysosomal and calcium‐dependent pathways are less related to muscle mass wasting.1, 46 In this way, we suggest that these pathways are less important in myocardial catabolic process.
The search for and use of cardiac biomarkers have increased considerably for predicting an abnormality before the symptomatic stage of the disease.22 Several studies identified some biomarkers linked to the major heart disorders, such as myocardium infarction, heart failure, coronary disease, fibrosis, and inflammation. One mechanism postulated for cancer‐cachexia involves general chronic inflammation and oxidative stress,1, 35, 47 suggesting that the increased MPO level in cardiac tissue might induce the changes in cardiac tissue parameters that were seen only in tumour‐bearing rats. As neutrophils release MPO, which impairs NO‐induced endothelial relaxation, the myocardial tissue can be subjected to increased coronary arterial disease, ischemia and myocardium infarction, as shown by other researchers.48, 49, 50 In fact, the leucine‐rich diet might have exerted a stronger cell signalling effect, which minimized MPO release, improving some myocardial parameters in the LW group.
Indeed, both tumour and host tissues produce cytokines that act on multiple target sites (from myocytes and endothelial cells to neurons), triggering a complex cascade of biological responses, including oxidative stress, which is responsible for the characteristic wasting associated with cachexia; these changes were also observed in cardiac tissue.1, 9, 12, 35, 47 Additionally, because leucine is essential for protein synthesis, an increasing number of studies suggest that leucine supplementation therapy might attenuate the catabolic and anti‐anabolic effects of the inflammation generated by cancer‐cachexia.45, 51, 52 MPO is a marker for oxidative stress and is also associated with inflammation; in this way, the maintenance of levels of this biomarker that was observed in the leucine‐treated tumour‐bearing group (LW) suggests that leucine likely contributes to both a reduction in the cardiac inflammatory state and oxidative stress.
Similarly, TIMP‐1 can reflect pathological myocardial remodelling processes during heart failure syndromes,22 and this biomarker is also linked to inflammation, as it is synthesized in response to pro‐inflammatory cytokines.53 TIMP‐1 was associated with decreases in cardiac function and acute heart failure and was identified as an independent predictive factor of myocardial infarction, acute heart failure, and death in patients with coronary diseases.53, 54 Thus, once again, leucine treatment seems to reduce the inflammatory process in the myocardial tissue, as untreated tumour‐bearing rats but not rats fed a leucine‐rich diet showed a significant increase in the levels of this biomarker.
Finally, functionally involved in the fibrinolysis system, the increased total PAI‐1 content that was observed only in untreated tumour‐bearing rats is not only an independent prognostic factor marker of heart failure after myocardial infarction but also indicates coronary and thrombolytic diseases and is valuable in predicting subsequent ischemic events.55, 56, 57
In summary, the alterations observed in all three of the analysed biomarkers suggest that the untreated tumour‐bearing group (W) but not the leucine‐treated group (LW) is more likely to experience heart damage that is currently linked to cancer‐cachexia, such as fibrosis and atrophy,9, 11, 12, 58 as well as thrombosis and ischemia, which have not been investigated previously in studies of heart damage induced by cancer‐cachexia.
We believe that the observed alterations in ECG parameters and the expression of biomarkers associated with increased proteolytic pathway activity reinforce the observation that the cardiac damage observed in the untreated tumour‐bearing groups was attenuated by leucine supplementation.
As an example, consider the TIMP‐1 expression and QT‐c data in light of the following facts: (i) cancer‐cachexia might result in heart mass loss, heart dilatation, and a decrease in wall thickness;10, 38 (ii) increases in TIMP‐1 levels were found in heart samples from dogs with cardiac diseases, especially dilated cardiomyopathies;59 and (iii) it is well established that an increase in the QT interval (QT‐c) can be linked to dilated cardiomyopathies or hypertrophy and sudden death risk. Thus, we have two powerful tools that suggest that the untreated tumour‐bearing rats (W) but not their leucine‐supplemented littermates (LW) are more likely to suffer heart failure and/or sudden death.10, 37, 38
Another indicator that leucine might attenuate the processes that occur in the heart in response to cancer‐cachexia lies in the fact that we observed an increase in the area under the T‐wave amplitude, especially in the untreated group, which is typically associated with ischemia. We also observed a concomitant increase in MPO and total PAI‐1 expression in untreated tumour‐bearing rats; these two factors are involved in distinct pathways that can lead to ischemia.50, 55
Taken together, the increase in proteolytic pathway and biomarker expression and the observed alterations in ECG might indicate heart failure and sudden death risk, especially in untreated tumour‐bearing rats (W), suggesting a possible benefit of leucine supplementation for the cardiac function of cancer‐cachectic rats.
Apoptosis is an extremely rare phenomenon in the healthy myocardium.13 Nevertheless, the role of apoptosis in cardiomyopathies and cardiac failure is well documented in animals and humans.14, 15, 16 Thus, the observed increase in effector caspase activity is consistent with the observed alterations in ECG and biomarkers, which suggested the occurrence of ischemia, fibrosis, and heart failure, especially in tumour‐bearing rats (W).
It has been demonstrated that cancer‐cachexia might drive cardiac alterations and heart damage, including atrophy and disruptions in myocardial structure.9, 11 Moreover, although we noticed a tendency towards increased caspase‐3 and ‐7 activity in leucine‐supplemented tumour‐bearing rats (LW) in comparison to the controls (C), this alteration was not significant, providing additional evidence of the possible modulatory effect of the treatment on heart damage. Concurrently, we observed a tendency towards a decrease in caspase‐3 and 7 activity when comparing healthy leucine‐treated rats (L) with the healthy untreated group (C), which supports the hypothesis that leucine reduces the occurrence of apoptosis in the myocardium.
The increased caspase‐3 and ‐7 activity that were observed in group W may also be because of an increase in the expression of these caspases, supplying more substrate to the initiator caspases and thus increasing effector activity. Increased caspase‐3 expression has been reported in animal models of heart failure.60, 61
Given that in addition to cachexia, the chemotherapeutic treatment of malignancies is closely related to future heart damage and failure,62 it is possible that a leucine‐rich diet might improve quality of life and survival in patients undergoing cancer treatment or even in patients in remission.
Conclusions
Our findings support previous achievements regarding the role of cancer‐induced cachexia in the promotion of cardiac failure. This effect is evidenced by the observed increases in MPO, TIMP, and total PAI‐1, which are all biomarkers of heart damage or failure, activated proteasome activity, alterations in ECG, and increased effector caspase activity in untreated tumour‐bearing rats (W). Furthermore, in most cases, a leucine‐rich diet seems to attenuate the deleterious effects on heart tissue that are generated by tumour progression. Thus, in the context of cancer‐cachexia, a leucine‐rich diet might minimize cardiac muscle damage.
Conflict of interest
The authors declare that no conflict of interest relevant to this article exists.
Acknowledgements
The authors are thankful for the financial support of CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico #302863/2013‐3), and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo #2010/00714‐5; #2010/00209‐9; #2009/11993‐5, #2013/16115‐1). The authors gratefully thank Dr. J. Marcondes for statistical and computational support and Dr. M. A. R. Mello for insightful discussion. Carbohydrates and dextrin were donated by Ingredion (Sao Paulo, Brazil), and amino acids were donated by Ajinomoto Brasil (Sao Paulo, Brazil). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle.63 The manuscript was edited by native English‐speaking editors (American Manuscript Editors).
Toneto, A. T. , Ferreira Ramos, L. A. , Salomão, E. M. , Tomasin, R. , Aereas, M. A. , and Gomes‐Marcondes, M. C. C. (2016) Nutritional leucine supplementation attenuates cardiac failure in tumour‐bearing cachectic animals. Journal of Cachexia, Sarcopenia and Muscle, 7: 577–586. doi: 10.1002/jcsm.12100.
References
- 1. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381–410. [DOI] [PubMed] [Google Scholar]
- 2. Argiles JM, Lopez‐Soriano FJ, Busquets S. Mechanisms and treatment of cancer cachexia. Nutr Metab Cardiovasc Dis 2013;23:S19–S24. [DOI] [PubMed] [Google Scholar]
- 3. Mantovani G, Madeddu C. Cancer cachexia: medical management. Support Care Cancer 2010;18:1–9. [DOI] [PubMed] [Google Scholar]
- 4. Tisdale MJ. Cancer cachexia: metabolic alterations and clinical manifestations. Nutrition 1997;13:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Tisdale MJ. Pathogenesis of cancer cachexia. J Support Oncol 2003;1:159–168. [PubMed] [Google Scholar]
- 6. Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiol 2005;20:340–348. [DOI] [PubMed] [Google Scholar]
- 7. Inui A. Cancer anorexia–cachexia syndrome: are neuropeptides the key? Cancer Res 1999;59:4493–4501. [PubMed] [Google Scholar]
- 8. Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975;6:61–64. [PubMed] [Google Scholar]
- 9. Tian M, Nishijima Y, Asp ML, Stout MB, Reiser PJ, Belury MA. Cardiac alterations in cancer‐induced cachexia in mice. Int J Oncol 2010;37:347–353. [DOI] [PubMed] [Google Scholar]
- 10. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, et al Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014;35:932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian M, Asp ML, Nishijima Y, Belury MA. Evidence for cardiac atrophic remodeling in cancer‐induced cachexia in mice. Int J Oncol 2011;39:1321–1326. [DOI] [PubMed] [Google Scholar]
- 12. Hinch ECA, Sullivan‐Gunn MJ, Vaughan VC, McGlynn MA, Lewandowski PA. Disruption of pro‐oxidant and antioxidant systems with elevated expression of the ubiquitin proteosome system in the cachectic heart muscle of nude mice. J Cachexia Sarcopenia Muscle 2013;4:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 1998;83:15–26. [DOI] [PubMed] [Google Scholar]
- 14. Li Z, Bing OH, Long X, Robinson KG, Lakatta EG. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am J Physiol 1997;272:H2313–H2319. [DOI] [PubMed] [Google Scholar]
- 15. Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al Apoptosis in the failing human heart. N Engl J Med 1997;336:1131–1141. [DOI] [PubMed] [Google Scholar]
- 16. Nishida K, Otsu K. Cell death in heart failure. Circ J 2008;72:A17–A21. [DOI] [PubMed] [Google Scholar]
- 17. Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene 2003;22:8543–8567. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S. Caspase function in programmed cell death. Cell Death Differ 2007;14:32–43. [DOI] [PubMed] [Google Scholar]
- 19. Mosleh W, Abdel‐Qadir H, Farkouh M. Biomarkers in the emergency workup of chest pain: uses, limitations, and future. Cleve Clin J Med 2013;80:589–598. [DOI] [PubMed] [Google Scholar]
- 20. Xu H, Crawford D, Hutchinson KR, Youtz DJ, Lucchesi PA, Velten M, et al Myocardial dysfunction in an animal model of cancer cachexia. Life Sci 2011;88:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, et al Increased expression of plasminogen activator inhibitor‐1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol 2004;164:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Florea VG, Anand IS. Biomarkers. Heart Fail Clin 2012;8:207–224. [DOI] [PubMed] [Google Scholar]
- 23. Eley HL, Russell ST, Tisdale MJ. Effect of branched‐chain amino acids on muscle atrophy in cancer cachexia. Biochem J 2007;407:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salomão EM, Toneto AT, Silva GO, Gomes‐Marcondes MCC. Physical exercise and a leucine‐rich diet modulate the muscle protein metabolism in Walker tumor‐bearing rats. Nutr Cancer 2010;62:1095–1104. [DOI] [PubMed] [Google Scholar]
- 25. Gomes‐Marcondes MCC, Ventrucci G, Toledo MT, Cury L, Cooper JC. A leucine‐supplemented diet improved protein content of skeletal muscle in young tumor‐bearing rats. Brazilian J Med Biol Res 2003;36:1589–1594. [DOI] [PubMed] [Google Scholar]
- 26. Reeves PG, Nielsen FH, Fahey GC Jr. AIN‐93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN‐76A rodent diet. J Nutr 1993;123:1939–1951. [DOI] [PubMed] [Google Scholar]
- 27. Salomão EM, Gomes‐Marcondes MCC, Salomao EM. Light aerobic physical exercise in combination with leucine and/or glutamine‐rich diet can improve the body composition and muscle protein metabolism in young tumor‐bearing rats. J Physiol Biochem 2012;68:493–501. [DOI] [PubMed] [Google Scholar]
- 28. Gomes‐Marcondes MC, Honma HN, Areas MA, Cury L. Effect of Walker 256 tumor growth on intestinal absorption of leucine, methionine and glucose in newly weaned and mature rats. Brazilian J Med Biol Res 1998;31:1345–1348. [DOI] [PubMed] [Google Scholar]
- 29. Vale C, Stewart L, Tierney J. Trends in UK cancer trials: results from the UK Coordinating Committee for Cancer Research National Register of Cancer Trials. Br J Cancer 2005;92:811–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koeplinger KA, Mildner AM, Leone JW, Wheeler JS, Heinrikson RL, Tomasselli AG. Caspase 8: an efficient method for large‐scale autoactivation of recombinant procaspase 8 by matrix adsorption and characterization of the active enzyme. Protein Expr Purif 2000;18:378–387. [DOI] [PubMed] [Google Scholar]
- 31. Barrett AJ. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J 1980;187:909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang ST, Wang JH, Chang T, Chen CS. A continuous method for measuring calpain activity. Anal Biochem. [DOI] [PubMed] [Google Scholar]
- 33. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 34. Gad SC. Statistics for toxicologists In Hayes AW, ed. Principles and Methods of Toxicology. 5th ed. New York: CRC Press; 2005. p369–452. [Google Scholar]
- 35. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 36. Emery PW. Cachexia in experimental models. Nutrition 1999;15:600–603. [DOI] [PubMed] [Google Scholar]
- 37. Groarke JD, Cheng S, Jones LW, Moslehi J. Cancer cachexia: getting to the heart of the matter. Eur Heart J 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borges FH, Marinello PC, Cecchini AL, Blegniski FP, Guarnier FA, Cecchini R. Oxidative and proteolytic profiles of the right and left heart in a model of cancer‐induced cardiac cachexia. Pathophysiology 2014;21:257–265. [DOI] [PubMed] [Google Scholar]
- 39. Ventrucci G, Mello MAR, Gomes‐Marcondes MCC. Proteasome activity is altered in skeletal muscle tissue of tumour‐bearing rats a leucine‐rich diet. Endocr Relat Cancer 2004;11:887–895. [DOI] [PubMed] [Google Scholar]
- 40. Ventrucci G, Mello MAR, Gomes‐Marcondes MCC. Leucine‐rich diet alters the eukaryotic translation initiation factors expression in skeletal muscle of tumour‐bearing rats. BMC Cancer 2007;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cruz B, Gomes‐Marcondes MCC. Leucine‐rich diet supplementation modulates foetal muscle protein metabolism impaired by Walker‐256 tumour. Reprod Biol Endocrinol 2014;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gonçalves EM, Salomão EM, Gomes‐Marcondes MCC. Leucine modulates the effect of Walker factor, a proteolysis‐inducing factor‐like protein from Walker tumours, on gene expression and cellular activity in C2C12 myotubes. Cytokine 2013;64:343–350. [DOI] [PubMed] [Google Scholar]
- 43. Mirza KA, Pereira SL, Voss AC, Tisdale MJ. Comparison of the anticatabolic effects of leucine and Ca‐beta‐hydroxy‐beta‐methylbutyrate in experimental models of cancer cachexia. Nutrition 2014;30:807–813. [DOI] [PubMed] [Google Scholar]
- 44. Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604–3609. [PubMed] [Google Scholar]
- 45. Baracos VE, Mackenzie ML. Investigations of branched‐chain amino acids and their metabolites in animal models of cancer. J Nutr 2006;136:237S–242S. [DOI] [PubMed] [Google Scholar]
- 46. Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol 2013;45:2215–2229. [DOI] [PubMed] [Google Scholar]
- 47. Argiles JM, Lopez‐Soriano FJ, Busquets S. Counteracting inflammation: a promising therapy in cachexia. Crit Rev Oncog 2012;17:253–262. [DOI] [PubMed] [Google Scholar]
- 48. Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm 2008;2008:135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ho E, Karimi Galougahi K, Liu C‐C, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 2013;1:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anatoliotakis N, Deftereos S, Bouras G, Giannopoulos G, Tsounis D, Angelidis C, et al Myeloperoxidase: expressing inflammation and oxidative stress in cardiovascular disease. Curr Top Med Chem 2013;13:115–138. [DOI] [PubMed] [Google Scholar]
- 51. Peters SJ, van Helvoort A, Kegler D, Argiles JM, Luiking YC, Laviano A, et al Dose‐dependent effects of leucine supplementation on preservation of muscle mass in cancer cachectic mice. Oncol Rep 2011;26:247–254. [DOI] [PubMed] [Google Scholar]
- 52. Chevalier S, Winter A. Do patients with advanced cancer have any potential for protein anabolism in response to amino acid therapy? Curr Opin Clin Nutr Metab Care 2014;17:213–218. [DOI] [PubMed] [Google Scholar]
- 53. Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, et al Tissue inhibitor of metalloproteinase‐1 (TIMP‐1) is an independent predictor of all‐cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 2006;151:1101.e1–1101.e8. [DOI] [PubMed] [Google Scholar]
- 54. Goldbergova MP, Parenica J, Jarkovsky J, Kala P, Poloczek M, Manousek J, et al The association between levels of tissue inhibitor of metalloproteinase‐1 with acute heart failure and left ventricular dysfunction in patients with ST elevation myocardial infarction treated by primary percutaneous coronary intervention. Genet Test Mol Biomarkers 2012;16:1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gurfinkel E, Altman R, Scazziota A, Rouvier J, Mautner B. Importance of thrombosis and thrombolysis in silent ischaemia: comparison of patients with acute myocardial infarction and unstable angina. Br Heart J 1994;71:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Collet JP, Montalescot G, Vicaut E, Ankri A, Walylo F, Lesty C, et al Acute release of plasminogen activator inhibitor‐1 in ST‐segment elevation myocardial infarction predicts mortality. Circulation 2003;108:391–394. [DOI] [PubMed] [Google Scholar]
- 57. Akkus MN, Polat G, Yurtdas M, Akcay B, Ercetin N, Cicek D, et al Admission levels of C‐reactive protein and plasminogen activator inhibitor‐1 in patients with acute myocardial infarction with and without cardiogenic shock or heart failure on admission. Int Heart J 2009;50:33–45. [DOI] [PubMed] [Google Scholar]
- 58. Borges L d S, Dermargos A, da Silva Junior EP, Weimann E, Lambertucci RH, Hatanaka E. Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J Pineal Res 2014;58:166–172. [DOI] [PubMed] [Google Scholar]
- 59. Zhang W, Zhong M, Yang G, Li J, Guo C, Wang Z, et al Matrix metalloproteinase‐9/tissue inhibitors of metalloproteinase‐1 expression and atrial structural remodeling in a dog model of atrial fibrillation: inhibition with angiotensin‐converting enzyme. Cardiovasc Pathol 2002;17:399–409. [DOI] [PubMed] [Google Scholar]
- 60. Gürtl B, Kratky D, Guelly C, Zhang L, Gorkiewicz G, Das SK, et al Apoptosis and fibrosis are early features of heart failure in an animal model of metabolic cardiomyopathy. Int J Exp Pathol 2009;90:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sepúlveda M, Gonano LA, Back TG, Chen SRW, Vila Petroff M. Role of CaMKII and ROS in rapid pacing‐induced apoptosis. J Mol Cell Cardiol 2013;63:135–145. [DOI] [PubMed] [Google Scholar]
- 62. Yu AF, Steingart RM, Fuster V. Cardiomyopathy associated with cancer therapy. J Card Fail 2014;20:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


