Abstract
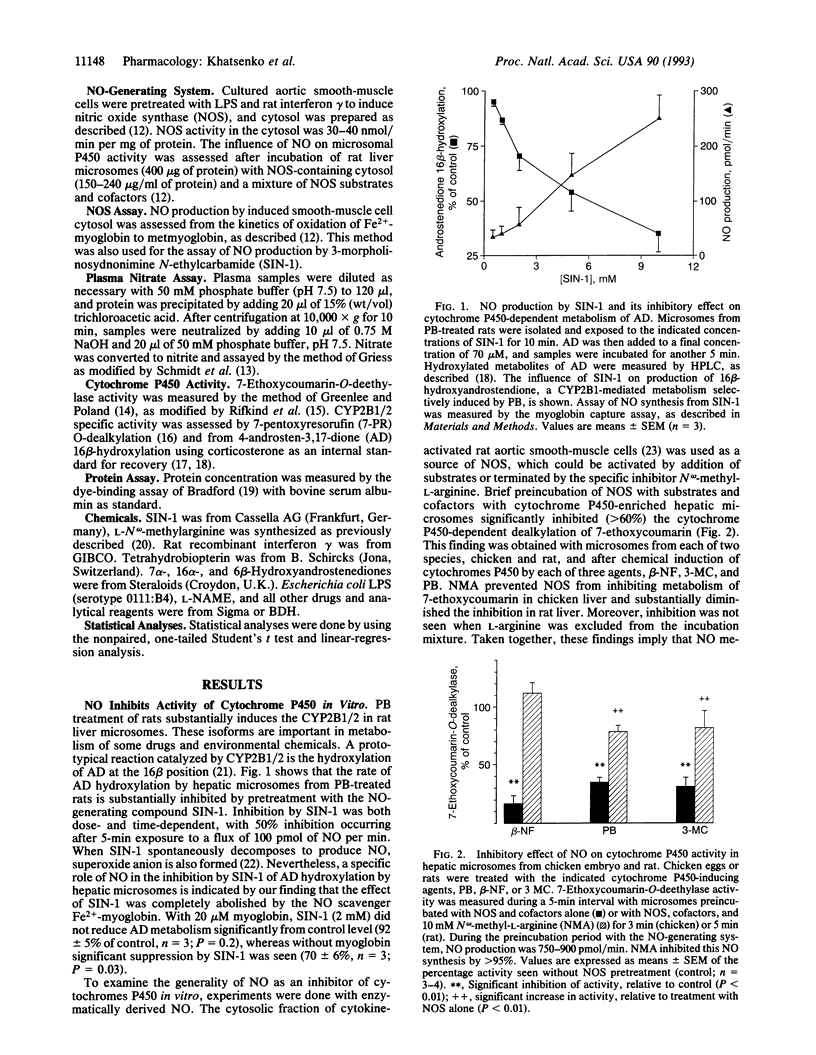
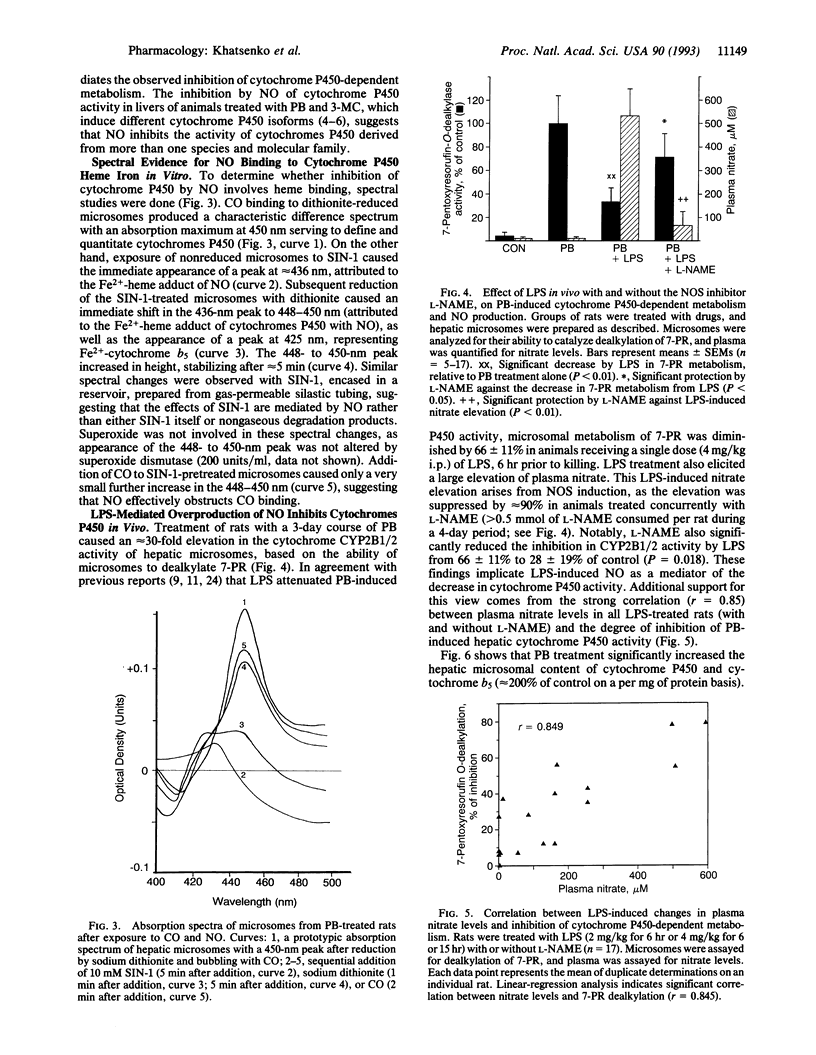
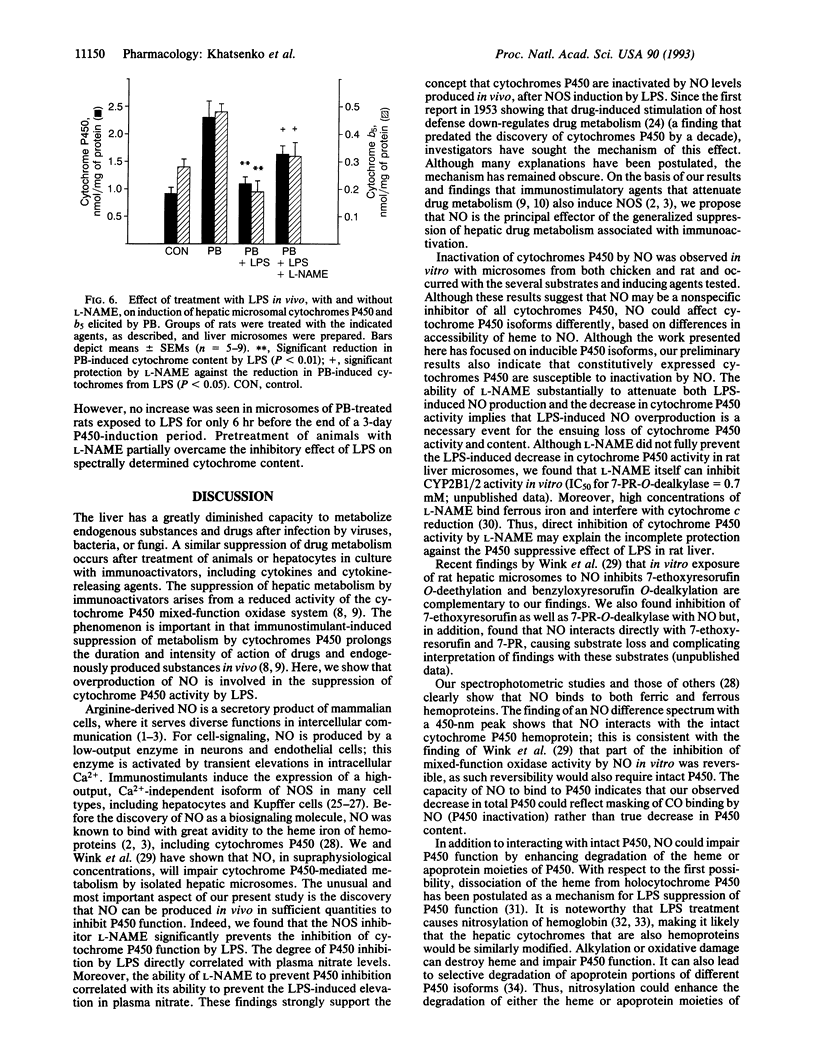
Bacterial lipopolysaccharide (LPS) and a diverse array of other immunostimulants and cytokines suppress the metabolism of endogenous and exogenous substances by reducing activity of the hepatic cytochrome P450 mixed-function oxidase system. Although this effect of immunostimulants was first described almost 40 yr ago, the mechanism is obscure. Immunostimulants are now known to cause NO overproduction by cells via induction of nitric oxide synthase. We have investigated whether NO overproduction is involved in suppressing hepatic metabolism by LPS. In vitro treatment of hepatic microsomes with NO, produced by chemical decomposition of 3-morpholinosydnonimine or by nitric oxide synthase, substantially suppressed cytochrome P450-dependent oxygenation reactions. This effect of NO was seen with hepatic microsomes prepared from two species (rat and chicken) and after exposure to chemicals that induce distinct molecular isoforms of cytochromes P450 (beta-naphthoflavone, 3-methylcholanthrene, and phenobarbital). Spectral studies indicate that NO reacts in vitro with both Fe(2+)- and Fe(3+)-hemes in microsomal cytochromes P450. In vivo, LPS diminished the phenobarbital-induced dealkylation of 7-pentoxyresorufin by rat liver microsomes and reduced the apparent P450 content as measured by CO binding. These LPS effects were associated with induction of NO synthesis; LPS-induced NO synthesis showed a strong positive correlation with the severity of cytochrome P450 inhibition. The decrease in both hepatic microsomal P450 activity and CO binding caused by LPS was largely prevented by the selective NO synthase inhibitor N omega-nitro-L-arginine methyl ester. Our findings implicate NO over-production as a major factor mediating the suppression of hepatic metabolism by immunostimulants such as LPS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. NG-methylarginine, an inhibitor of endothelium-derived nitric oxide synthesis, is a potent pressor agent in the guinea pig: does nitric oxide regulate blood pressure in vivo? Biochem Biophys Res Commun. 1989 Apr 28;160(2):881–886. doi: 10.1016/0006-291x(89)92517-5. [DOI] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Erroi A., Villa P., Ghezzi P. Dexamethasone modulation of in vivo effects of endotoxin, tumor necrosis factor, and interleukin-1 on liver cytochrome P-450, plasma fibrinogen, and serum iron. J Leukoc Biol. 1989 Sep;46(3):254–262. doi: 10.1002/jlb.46.3.254. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Ferrari F. K., Williams D. L., Simmons R. L. Kupffer cell:hepatocyte cocultures release nitric oxide in response to bacterial endotoxin. J Surg Res. 1990 Apr;48(4):349–353. doi: 10.1016/0022-4804(90)90073-b. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome P-450 heme and the regulation of hepatic heme oxygenase activity. Arch Biochem Biophys. 1976 Sep;176(1):91–102. doi: 10.1016/0003-9861(76)90144-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke M. D., Mayer R. T. Differential effects of phenobarbitone and 3-methylcholanthrene induction on the hepatic microsomal metabolism and cytochrome P-450-binding of phenoxazone and a homologous series of its n-alkyl ethers (alkoxyresorufins). Chem Biol Interact. 1983 Jul 15;45(2):243–258. doi: 10.1016/0009-2797(83)90072-8. [DOI] [PubMed] [Google Scholar]
- Coon M. J., Ding X. X., Pernecky S. J., Vaz A. D. Cytochrome P450: progress and predictions. FASEB J. 1992 Jan 6;6(2):669–673. doi: 10.1096/fasebj.6.2.1537454. [DOI] [PubMed] [Google Scholar]
- Correia M. A., Sugiyama K., Yao K. Q. Degradation of rat hepatic cytochrome P-450p. Drug Metab Rev. 1989;20(2-4):615–628. doi: 10.3109/03602538909103565. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel R. E., O'Keefe D. H., Peterson J. A. Nitric oxide complexes of cytochrome P-450. FEBS Lett. 1975 Jul 15;55(1):198–201. doi: 10.1016/0014-5793(75)80991-4. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Ostrowski J., Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S13–S22. [PubMed] [Google Scholar]
- Greenlee W. F., Poland A. An improved assay of 7-ethoxycoumarin O-deethylase activity: induction of hepatic enzyme activity in C57BL/6J and DBA/2J mice by phenobarbital, 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Pharmacol Exp Ther. 1978 Jun;205(3):596–605. [PubMed] [Google Scholar]
- Gross S. S., Jaffe E. A., Levi R., Kilbourn R. G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem Biophys Res Commun. 1991 Aug 15;178(3):823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992 Dec 25;267(36):25722–25729. [PubMed] [Google Scholar]
- Guengerich F. P. Reactions and significance of cytochrome P-450 enzymes. J Biol Chem. 1991 Jun 5;266(16):10019–10022. [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatsenko O. G., Gulyaeva L. F., Mishin V. M., Lyakhovich V. V. Dynamics of androstenedione metabolite formation in rat liver microsomes during temporal phenobarbital induction. Comp Biochem Physiol C. 1991;99(3):257–261. doi: 10.1016/0742-8413(91)90238-o. [DOI] [PubMed] [Google Scholar]
- Kosaka H., Harada N., Watanabe M., Yoshihara H., Katsuki Y., Shiga T. Synergistic stimulation of nitric oxide hemoglobin production in rats by recombinant interleukin 1 and tumor necrosis factor. Biochem Biophys Res Commun. 1992 Nov 30;189(1):392–397. doi: 10.1016/0006-291x(92)91571-7. [DOI] [PubMed] [Google Scholar]
- Mannering G. J., Deloria L. B. The pharmacology and toxicology of the interferons: an overview. Annu Rev Pharmacol Toxicol. 1986;26:455–515. doi: 10.1146/annurev.pa.26.040186.002323. [DOI] [PubMed] [Google Scholar]
- Morgan E. T. Down-regulation of multiple cytochrome P450 gene products by inflammatory mediators in vivo. Independence from the hypothalamo-pituitary axis. Biochem Pharmacol. 1993 Jan 26;45(2):415–419. doi: 10.1016/0006-2952(93)90078-b. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Peterson D. A., Peterson D. C., Archer S., Weir E. K. The non specificity of specific nitric oxide synthase inhibitors. Biochem Biophys Res Commun. 1992 Sep 16;187(2):797–801. doi: 10.1016/0006-291x(92)91266-s. [DOI] [PubMed] [Google Scholar]
- Peterson T. C., Renton K. W. Depression of cytochrome P-450-dependent drug biotransformation in hepatocytes after the activation of the reticuloendothelial system by dextran sulfate. J Pharmacol Exp Ther. 1984 Apr;229(1):299–304. [PubMed] [Google Scholar]
- Pittner R. A., Spitzer J. A. Endotoxin and TNF alpha directly stimulate nitric oxide formation in cultured rat hepatocytes from chronically endotoxemic rats. Biochem Biophys Res Commun. 1992 May 29;185(1):430–435. doi: 10.1016/s0006-291x(05)81003-4. [DOI] [PubMed] [Google Scholar]
- Rifkind A. B., Troeger M., Muschick H. Kinetic evidence for heterogeneous responsiveness of mixed function oxidase isozymes to inhibition and induction by allylisopropylacetamide in chick embryo liver. J Biol Chem. 1982 Oct 10;257(19):11717–11727. [PubMed] [Google Scholar]
- Ryan D. E., Levin W. Purification and characterization of hepatic microsomal cytochrome P-450. Pharmacol Ther. 1990;45(2):153–239. doi: 10.1016/0163-7258(90)90029-2. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Nakane M., Förstermann U., Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992 Apr;41(4):615–624. [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Botting R. M. Secretory functions of the vascular endothelium. J Physiol Pharmacol. 1992 Sep;43(3):195–207. [PubMed] [Google Scholar]
- Waxman D. J., Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992 Feb 1;281(Pt 3):577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Ko A., Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–11947. [PubMed] [Google Scholar]
- Wink D. A., Osawa Y., Darbyshire J. F., Jones C. R., Eshenaur S. C., Nims R. W. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993 Jan;300(1):115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]



