Abstract
While transfusion of red blood cells (RBCs) is effective at preventing morbidity and mortality in anemic patients, studies have indicated that some RBC components have functional defects (“RBC storage lesions”) that may actually cause adverse events when transfused. For example, in some studies patients transfused with RBCs stored >14 days have had statistically worse outcomes than those receiving “fresher” RBC units. Recipient-specific factors may also contribute to the occurrence of these adverse events. Unfortunately, these events have been difficult to investigate because up to now they have existed primarily as “statistical occurrences” of increased morbidity and mortality in large data sets. There are currently no clinical or laboratory methods to detect or study them in individual transfusion recipients.
Herein, we propose a unifying hypothesis, centered on Insufficient NO Bio-Availability (INOBA), to explain the increased morbidity and mortality observed in some patients following RBC transfusion. In this model, variables associated with RBC units (storage time; 2,3-DPG concentration) and transfusion recipients (endothelial dysfunction) collectively lead to changes in NO levels in vascular beds. Under certain circumstances, these variables are “aligned” such that NO concentrations are markedly reduced, leading to vasoconstriction, decreased local blood flow and insufficient O2 delivery to end organs. Under these circumstances, the likelihood of morbidity and mortality escalates. If the key tenets of the INOBA hypothesis are confirmed, it may lead to improved transfusion methodologies including altered RBC storage/processing conditions, novel transfusion recipient screening methods, and improved RBC/recipient matching.
Keywords: Red blood cells, transfusion, nitric oxide
Background
The last quarter of the 20th century produced dramatic improvements in blood testing methodologies that markedly reduced infectious disease transmission, one of the most obvious complications of blood transfusion. Accordingly, investigators have now begun to study less obvious, but no less important problems associated with transfusion. For example, recent clinical studies have shown that patient outcome is worse following transfusion of aged (“non-fresh”) RBC units compared to “fresh” units, and with higher Hct targets as compared to lower ones [1, 2]. Because patients transfused with RBCs are often quite ill, and further disease-related complications are not unexpected, many post-transfusion complications may not be obvious. Furthermore, there are currently no effective clinical or laboratory assessments to determine whether these patients died from complications of the RBC transfusion or from the underlying disease. Nonetheless, the available epidemiologic data argue that these adverse events following RBC transfusion are relatively common, and are of great significance to patient outcome [1, 2].
The “post-infectious era” in transfusion medicine
Blood transfusion is the most commonly employed procedure among discharge codes recorded for hospital inpatients in the US [3]. Despite its common use, blood transfusion has rarely been subjected to modern assessments of efficacy and safety, though it is well recognized that transfusion is associated with a number of serious adverse events [4]. These events are often categorized as infectious or noninfectious serious hazards of transfusion (NiSHOTs)[5]. Technological advancements in donor screening over the last 20-30 years have markedly decreased the risks of transmitting pathogens (viral, bacterial, and other agents) by transfusion [6], [7]. Thus, there is a real possibility that we are entering a new phase in the evolution of transfusion medicine: the post-infectious era. Despite our successes with infectious agents, NiSHOTs continue to cause significant morbidity and mortality for transfusion recipients and represent the next significant challenge for further improving the safety and efficacy of blood transfusion. The list of NiSHOTs has typically included mistransfusion, transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO) [5, 8-11]. An expanded list may also include transfusion-related immunomodulation (TRIM), alloimmunization in specialized patient populations such as patients with sickle cell disease, hyperkalemia and cardiac arrest in pediatric surgery patients, transfusion-associated graft vs. host disease (TA-GVHD), and under-transfusion [4, 12, 13].
RBC transfusion-associated morbidity and mortality
The role of RBC storage time
Even when the well-recognized NiSHOTs are excluded from analysis, transfusion (particularly of RBCs) remains an independent contributor to (or predictor of) morbidity and mortality [1, 2, 14-29]. Tinmouth et al reviewed 9 studies, mostly retrospective, investigating the occurrence of RBC-associated adverse reactions. Together these studies included over 2800 cardiac surgery, trauma, and ICU patients [14]. While the clinical manifestations were quite heterogeneous, most of the studies showed that the rates of mortality, multiorgan failure, infections or length of hospital stay increased in proportion to the age of the RBC units or the number of units transfused. A more recent very large retrospective study specifically examined adverse events related to the storage age of RBCs in 6002 cardiac surgery patients who received a total of 19,584 transfusions [1]. Patients were divided into those that received fresher blood (≤ 14 days of storage) versus older blood (15-42 days storage). Patients that received older units had higher rates of in-hospital mortality (2.8% vs. 1.7%, P=0.004), as well as higher rates of extended intubation, renal failure, and sepsis. Overall, complications were more common in the patients that received older units (25.9% vs. 22.4%; P=0.001) and 1-year mortality was also significantly greater than for patients receiving fresher units (11.0% vs. 7.4%; P<0.001). While there are a number of concerns with this and other retrospective studies, the results suggest that older RBC transfusions are associated with a diverse array of atypical adverse events in a subset of transfusion recipients. Results from the ongoing NHLBI/TMH-funded prospective RECESS study should help clarify the potential risks of transfusion of stored RBCs.
How does the “RBC storage lesion” affect patient outcome?
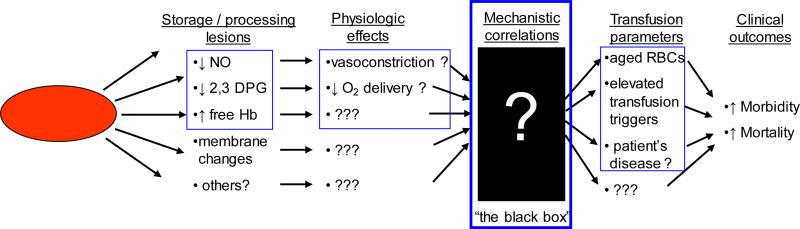
While RBC transfusions are very effective therapies, the clinical studies described above suggest that they are also imperfect, poorly characterized therapies that may be associated with mechanistically-undefined adverse effects. Since RBCs are a biologic therapy, we ideally need to understand: the changes that occur to RBCs during collection, processing, and storage; the physiological and functional effects of these changes on transfusion efficacy in the recipient; appropriate ways to monitor functional as well as adverse effects of transfusion in the recipient; and the mechanistic relationship between these physiological effects and clinical outcomes in transfusion recipients with various disease states. Unfortunately, very few of these issues have been addressed, and are significant “unknowns” that may compromise transfusion safety and efficacy (Figure 1).
Figure 1.
Schematic diagram showing known changes that occur with RBC storage as well as the significant “unknown” links that that may lead from RBC storage lesions to adverse outcomes in transfusion recipients.
While Figure 1 illustrates a number of unknowns that lead from RBC changes to adverse outcomes in the recipient, in our opinion the most significant of the unknowns is represented by the black box. For example, it is unclear whether the reduced 2,3-DPG levels seen after 14 days of RBC storage has an appreciable affect on blood flow and O2 delivery in transfusion recipients. One of the most important goals of our studies is to eliminate the black box and directly investigate in human transfusion recipients the linkage between storage-related lesions in the RBCs and resulting changes in blood flow and peripheral O2 delivery after transfusion. Furthermore, these studies will be aided by bringing together these parameters through a single unifying hypothesis centered on insufficient NO bioavailability (INOBA).
The INOBA hypothesis
Insufficient NO bioavailability due to transfused RBCs and recipient factors may cause post-transfusion morbidity and mortality
The specific RBC storage lesions that lead to adverse events in transfusion recipients are not understood. Because these reactions may be clinically significant and common, dissecting the mechanisms and developing approaches to prevent their occurrence are important for improving patient outcome. In order to understand how the RBC storage lesion may produce morbidity and mortality after transfusion, we first sought to develop an hypothesis that not only accounts for these adverse events but also provides a role for recipient factors and known RBC alterations (reduced 2,3-DPG). This unifying hypothesis is centered on the underlying pathophysiology of insufficient NO bioavailability (INOBA; Figure 2), and can be stated as follows: When the cumulative effects of RBC transfusions and recipient factors reduce local NO bioavailability to levels below a critical threshold, tissue perfusion is insufficient to meet metabolic demands leading to morbidity and mortality in the transfusion recipient.
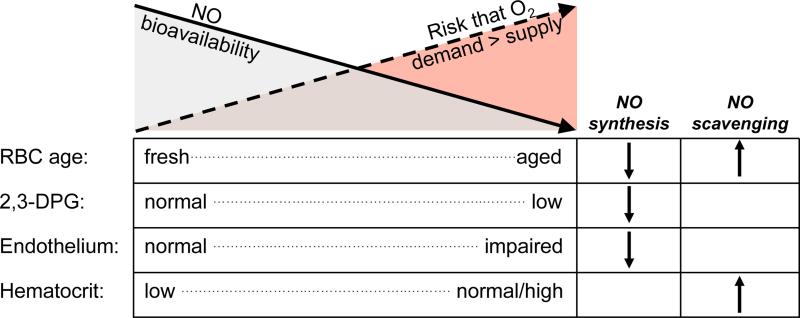
Figure 2.
The INOBA hypothesis. Insufficient NO Bio-Availability (INOBA) is the common pathophysiological underpinning that ties together a number of diverse variables and manifestations associated with post-transfusion morbidity and mortality: RBC storage time (age), 2,3-DPG content, endothelial function, and post-transfusion hematocrit targets.
As shown in Figure 2, both RBC-specific (storage time; 2,3-DPG) and recipient-specific (endothelial function; Hct) factors are postulated to reduce bioavailable NO through decreased synthesis and/or increased scavenging. As more of these factors are shifted toward a state with lower NO levels (eg, transfusion of aged, 2,3-DPG-depleted blood to a patient with endothelial dysfunction; solid arrow), cumulative NO bioavailability becomes limiting. This leads to insufficient vasodilation, reduced blood flow to end organs, and an increased risk that O2 delivery will be inadequate to meet tissue demands (dashed arrow). The end result in ill patients can be an increased incidence of multiorgan failure and death. This hypothesis is based on numerous previous studies, as described in more detail in the following sections.
The INOBA hypothesis leads to a number of readily testable predictions. In order to study these predictions in clinically relevant systems, we are performing in vitro assays of NO bioavailability, biochemical assays for NO reaction products, in vivo models to test for NO- and non-NO-mediated changes in forearm blood flow and O2 delivery in healthy transfused volunteers, and non-invasive in vivo studies to test for the interactions of transfused RBCs and dysfunctional endothelial cells in patients with cardiovascular disease. If the INOBA hypothesis is borne out through these studies, it could lead to specific approaches for remediation (eg, supplementation of RBC units with NO, routine rejuvenation of RBCs to replete 2,3-DPG, selection of fresh units for patients with CVD, novel approaches to match RBC units to recipients) that have important implications for transfusion policies.
The studies we have designed to investigate the INOBA hypothesis, particularly the in vivo human transfusion models, are highly sensitive and specific. Should the results of these studies be negative, contrary to our expectations, we would be able to accordingly conclude that in contrast to abundant literature there is no evidence that stored, processed, or modified RBCs significantly affect NO-mediated changes in blood flow and O2 delivery. Despite being a negative result, this conclusion would be very important as it would redirect efforts to understand the effects of storage-related changes away from the NO-based focus that has recently gained support.
The roles of nitric oxide (NO) in the control of vascular tone
The microcirculation is composed of a continuum of small vessels including small arterioles, capillaries, and post-capillary venules. The microcirculation represents an actively-adjusting vascular circuit that matches blood flow (and O2 delivery) to local tissue oxygen demands [30, 31]. While the endothelium actively regulates many aspects of vascular function, from the perspective of transfusion medicine the most important function is control of vascular tone in the microcirculation [32, 33]. By regulating the tone of the underlying smooth muscle, the endothelium can control the caliber of the blood vessel and thus local blood flow. While the physiologic mechanisms that match O2 delivery to local requirements are incompletely understood, endothelium-derived nitric oxide (NO) clearly plays an important role [30, 31]. Other factors released by the endothelium are also able to either contract (thromboxane and endothelin) or relax (endothelium-derived hyperpolarizing factor [EDHF]) the smooth muscle, reducing or increasing blood flow, respectively [32, 33]. Our studies focus on the vasodilatory effects of NO. However, because each study has a physiologic output (eg, aortic relaxation in vitro or alterations in blood flow and arterial diameter in vivo), the other factors (which may work cooperatively with NO [34]) can also be investigated. For example, EDHF can be up-regulated when the vasodilatory effects of NO are suppressed [35].
NO signaling from the endothelium is controlled by nitric oxide synthase (NOS) activity, and is described as paracrine regulation of vascular tone. There are three isoforms of NOS, neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) [32, 33]. eNOS is thought to be the major source of NO for regulating vasoactivity, but nNOS and possibly iNOS may also play roles. In addition, as described below, there are “endocrine” NO signals involving NO production by blood proteins, especially Hb. NO acts by diffusing into the smooth muscle cells and activating guanylate cyclase resulting in smooth muscle relaxation and vasodilation [32, 33]. Because endothelial NO production is critical for control of vascular tone, the INOBA hypothesis suggests that reduced NO production by dysfunctional endothelium contributes to adverse effects after RBC transfusion. Interestingly, recent work has revealed that in addition to transporting O2 and CO2, the RBC also controls local NO concentrations and thus may also play a surprisingly important role in regulating blood flow in the microcirculation [36, 37].
Hb/RBC-derived (endocrine) NO and the control of hypoxic vasodilation
It is well known that RBCs support cellular metabolism by carrying O2 to, and CO2 from, tissues. However, it is less well appreciated that RBCs may also control tissue oxygenation at another level: intrinsic RBC regulatory mechanisms appear to control local blood flow in order to preferentially perfuse, and thus provide O2 for, the most hypoxic tissues [38]. This process has been termed hypoxic vasodilation, meaning the relaxation of vascular smooth muscle with increases in blood flow in response to low pO2 [38]. These actions of RBCs, working at two distinct levels to control oxygenation, are physiologically attractive since RBCs are uniquely suited to monitor O2 and because these mechanisms can work synergistically to dramatically increase O2 delivery to tissues in oxygen debt [38]. Although not yet fully understood at a biochemical level, NO released by RBCs is the most likely candidate for the RBC-derived hypoxic vasodilatory signal [38]. Based on this important role in tissue perfusion and oxygenation, NO has been described as “nature's third respiratory gas” [39].
Mechanisms of NO production by Hb
In contrast to endothelial production of NO, the exact mechanisms by which Hb/RBCs produce NO are a matter of significant debate. Recent studies have suggested that S-nitrosylation of hemoglobin (SNO-Hb) regulates hypoxic vasodilation by RBCs [40, 41]. In this model, NO can be captured by the cys β-93 on the R (relaxed, oxygenated) conformation of Hb forming SNO-Hb. SNO-Hb can then release NO when pO2 is reduced and the Hb transitions to the T-state (deoxygenated). Thus, RBCs may serve as a storage pool for NO, forming SNO-Hb under oxygenated conditions for subsequent downstream release of NO to promote vasodilation when O2 tension is low (hypoxic conditions) [25, 38]. Because SNO-Hb declines rapidly during RBC storage, it has been postulated that the RBC storage lesion is due, at least in part, to a deficiency of SNO-Hb [42]. However, the known effects of 2,3-DPG raise questions about this model. 2,3-DPG stabilizes deoxyhemoglobin which should promote NO release, while depleted 2,3-DPG levels should promote formation of SNO-Hb [43]. However, both 2,3-DPG and SNO-Hb fall during RBC storage, although with much different kinetics.
Others have argued on the basis of both chemistry and quantitative analyses that SNO-Hb cannot be converted to vasoactive NO, and even if it could the amount of SNO-Hb in RBCs is not physiologically meaningful [44]. As an alternative mechanism, investigators have shown that nitrite (which is present at high levels in plasma) can be converted to NO by Hb, and that SNO-Hb is only formed as a side reaction in this process [45]. In this model, deoxygenated Hb binds nitrite and a proton to generate NO and met-Hb. The NO can then leave the RBC to cause vasodilation or can bind a second deoxyhemoglobin to form iron-nitrosyl-Hb (HbFe2+-NO). By promoting O2 release and stabilizing deoxy-Hb, 2,3-DPG facilitates NO production through this pathway. Thus, stored RBCs with depleted 2,3-DPG may be NO deficient. Interestingly, the time course of 2,3-DPG depletion (after ~14 days of storage) correlates with studies showing that RBCs stored >14 days are more likely to cause morbidity and mortality [1, 26]. With either mechanism, NO must escape from the RBC to act on smooth muscles and produce vasodilation, although the mechanism is unclear [36, 46].
Recent studies have shown that stored blood, which is NO-depleted, perturbs normal vascular tone: arterial vessels (in vitro and in vivo) show reduced dilation in the presence of stored blood [25]. These effects can be partially reversed by NO repletion of blood before infusion [25]. Reduced dilation should result in decreased blood flow and O2 delivery. The resulting hypoxia may lead to infarction and organ failure, and may account for the adverse effects of stored blood seen in clinical studies, as suggested by the INOBA hypothesis.
Scavenging of NO by Hb
As an alternative mechanism for controlling local NO bioavailability, Hb can also scavenge NO produced by endothelial cells. This mechanism is not mutually exclusive of the above mechanisms of NO synthesis; in fact, Hb/RBCs could regulate local NO concentrations through both synthesis and scavenging of NO. In most circumstances, however, scavenging may not play an important role since encapsulation of Hb in RBCs appears to inhibit NO scavenging [36, 46]. In addition, under normal circulatory dynamics, RBCs are most concentrated in the middle of the blood stream, and there is an RBC-free zone adjacent to the endothelium (Fahreaus Effect) [47]. In contrast, under conditions where free Hb is present (eg, with transfusion of stored RBCs that have undergone some hemolysis) or where RBCs may be closer to the vessel walls (eg, at elevated hematocrit levels), scavenging of endothelial-derived NO by Hb may become physiologically important, leading to significant decreases in bioavailable NO [47, 48][48]
Endothelial dysfunction in transfused patients may modify the effects of the RBC storage lesion
The above discussion highlights the importance of NO to the control of vasodilation, which is central to regulating tissue blood flow. Hb/RBCs play an important role in this process, through NO synthesis and/or NO scavenging, and the resulting process of hypoxic vasodilation is an attractive mechanism for matching O2 delivery to local O2 demand [38]. In addition, as described above, endothelial cells also make significant contributions to regulating vascular tone. Endothelial cells synthesize NO as well as other regulators of smooth muscle contraction, and are located closer to the smooth muscle cells than are RBCs [31]. The INOBA hypothesis postulates that the likelihood of adverse events after RBC transfusions is determined by a combination of patient-specific differences in endothelial function together with RBC-specific changes following blood processing and storage. Endothelial dysfunction, which leads to reduced NO bioavailability, is a common finding in many ill patients, including the over 70 million Americans with cardiovascular disease (CVD) [49]. Many of the subjects of the clinical studies showing adverse effects of RBC storage were critically ill, and likely had some degree of endothelial dysfunction. In fact, most transfusions are given to patients who are severely ill and would be expected to have endothelial dysfunction as well. Thus, if stored RBCs also reduce NO availability, this effect would be expected to synergize with preexisting endothelial dysfunction, markedly reducing NO levels and producing relative vasoconstriction.
Reduced NO production in patients with endothelial dysfunction can be quantified by non-invasive ultrasound measurements of the brachial artery. In addition, a variety of CVD risk factors and plasma biomarkers also provide an index of the degree of endothelial dysfunction (described below). These biomarkers should not only be useful for studying the INOBA hypothesis in the research setting, but may also serve as predictive assays to identify patients at-risk of morbidity and mortality from transfusion and thus provide a novel approach for matching blood components to transfusion recipients.
Oxidative stress (OS), systemic inflammation, and circulating progenitor cells
Quantifiable biomarkers associated with endothelial dysfunction
Oxidative stress (OS) is an important component of the pathophysiology of CVD. Ongoing oxidative processes in the body reflect the balance of pro-oxidants and anti-oxidants. Traditional risk factors for CVD (sedentary lifestyle, obesity, hypercholesterolemia, hypertension, diabetes, insulin resistance, smoking, and aging) tip this balance, producing a significant net increase in prooxidative processes [50-52]. The resulting OS produces secondary effects including elevated inflammation and ultimately endothelial dysfunction and reduced NO synthesis [53]. Lending support to this model, the total burden of CVD risk factors (which can be expressed using the Framingham score) correlates with the extent of resulting endothelial dysfunction [54-56]. There are a number of molecular markers of OS that can be readily assayed in peripheral blood and urine samples and used as probes for distinct oxidative pathways. Assaying these biomarkers in combination not only provides more complete data of ongoing OS, but also allows us to ask which marker(s) are most closely related to adverse effects of stored RBC transfusions.
Numerous serum markers of inflammation have also been found to be associated with CVD risk, to be upregulated in response to OS, and to be independently associated with endothelial dysfunction. The most common of these, which are currently used in clinical CVD research projects at Emory, include hsCRP and LpPLA2. hsCRP is a 5-subunit protein that is predominantly produced by the liver and rapidly upregulated by inflammatory cytokines [57]. In the Women's Health Study, baseline levels of hsCRP were shown to provide additional prognostic information in subjects across all Framingham Risk Score strata [58]. LpPLA2 is another circulating inflammatory marker that appears to predict future adverse CVD events, and is independent of hsCRP levels [59]. A recent meta-analysis of 14 eligible studies (N = 20,549 patients) demonstrated that elevated levels of LpPLA2 were associated with an increased risk of CVD events (adjusted OR of 1.60) [60].
Summary and future directions
In summary: 1) RBC transfusion is a very commonly employed therapy that is associated with significant numbers of serious adverse reactions and/or events including increased morbidity and mortality in some recipient populations; 2) the INOBA hypothesis, based on a large amount of experimental data, postulates that some of the adverse events are due to time- and storage condition-dependant changes in NO production (and/or increased scavenging) by RBCs coupled with endothelial dysfunction in transfusion recipients, leading to reductions in blood flow, and thus oxygen delivery, to end organs; 3) the INOBA hypothesis leads to a number of directly testable predictions; 4) if the INOBA hypothesis is confirmed, and a role for NO in RBC transfusion-associated morbidity and mortality is identified, the results may lead to translational research to re-engineer and manufacture preservative/storage solutions that would mitigate this deleterious effect; 5) the results could also lead to an improved approach to “personalized transfusion medicine”, where donors and recipients are matched at physiologic levels as well as by immunologic parameters; and, 6) given recent publications in both the scientific and the lay press, with titles such as “Bad Blood? Old Units Might be Substandard” [61] and with conclusions suggesting it may be time for “a wholesale change in blood-banking practices” regarding the “optimal shelf of stored blood” [61], it is important that the role of NO, and the mechanisms of RBC-associated morbidity and mortality, be determined through detailed experimentation, in order to accurately inform policy decisions regarding RBC storage.
Acknowledgements
The authors would like to acknowledge the early contributions of Drs. Christopher Hillyer, David Harrison, Sergey Dikalov, and Beth Shaz to the development and framing of the INOBA hypothesis, as well as for their contributions to the NHLBI grant application from which this manuscript originated.
This work was supported in part by NIH/NHLBI funding (R01 HL095479).
Footnotes
The authors declare that they have no conflicts of interest relevant to this manuscript submitted to TRANSFUSION
Reprints will not be available from the authors.
Contributor Information
John D. Roback, Center for Transfusion and Cellular Therapies, Department of Pathology and Laboratory Medicine, Emory University School of Medicine
Robert B. Neuman, Division of Cardiology, Department of Medicine, Emory University School of Medicine
Arshed Quyyumi, Division of Cardiology, Department of Medicine, Emory University School of Medicine.
Roy Sutliff, Division of Pulmonology, Department of Medicine, Emory University School of Medicine.
References
- 1.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 2.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 3. [March 24, 2008];Health Care Cost and Utilization Project (HCUP) Facts and Figures, Statistics on hospital-based care in the United States, 2005, Exhibit 3.1. Most Frequent All-listed Procedures. At http://www.hcupus.ahrq.gov/reports.jsp. [cited.
- 4.Hillyer CD, Blumberg N, Glynn SA, Ness PM. Transfusion recipient epidemiology and outcomes research: Possibilities for the future. Transfusion. 2008 doi: 10.1111/j.1537-2995.2008.01807.x. in press. [DOI] [PubMed] [Google Scholar]
- 5.Association Bulletin #01-4: Non-infectious serious hazards of transfusion - NiSHOTs. American Association of Blood Banks; Bethesda: [4/7/08]. Available http://aabb.org/Content/Members_Area/Association_Bulletins/ab01-4.htm. [cited. [Google Scholar]
- 6.Dodd RY, Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population.[comment]. Transfusion. 2002;42(8):975–9. doi: 10.1046/j.1537-2995.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 7.Corash L. Pathogen reduction technology: methods, status of clinical trials, and future prospects. Current Hematology Reports. 2003;2(6):495–502. [PubMed] [Google Scholar]
- 8.Kleinman S. A perspective on transfusion-related acute lung injury two years after the Canadian Consensus Conference. Transfusion. 2006;46(9):1465–8. doi: 10.1111/j.1537-2995.2006.00956.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuehnert MJ, Roth VR, Haley NR, Gregory KR, Elder KV, Schreiber GB, Arduino MJ, Holt SC, Carson LA, Banerjee SN, Jarvis WR. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41(12):1493–9. doi: 10.1046/j.1537-2995.2001.41121493.x. [DOI] [PubMed] [Google Scholar]
- 10.Eder AF, Kennedy JM, Dy BA, Notari EP, Weiss JW, Fang CT, Wagner S, Dodd RY, Benjamin RJ. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004-2006). Transfusion. 2007;47(7):1134–42. doi: 10.1111/j.1537-2995.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 11.Tobian AA, Sokoll LJ, Tisch DJ, Ness PM, Shan H. N-terminal pro-brain natriuretic peptide is a useful diagnostic marker for transfusion-associated circulatory overload. Transfusion. 2008 doi: 10.1111/j.1537-2995.2008.01656.x. [DOI] [PubMed] [Google Scholar]
- 12.Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus Med Rev. 2007;21(2):118–33. doi: 10.1016/j.tmrv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Blajchman MA. Transfusion-associated immunomodulation and universal white cell reduction: are we putting the cart before the horse? Transfusion. 1999;39(7):665–70. doi: 10.1046/j.1537-2995.1999.39070665.x. [DOI] [PubMed] [Google Scholar]
- 14.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 15.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46(10):1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 16.Mou SS, Giroir BP, Molitor-Kirsch EA, Leonard SR, Nikaidoh H, Nizzi F, Town DA, Roy LC, Scott W, Stromberg D. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351(16):1635–44. doi: 10.1056/NEJMoa041065. [DOI] [PubMed] [Google Scholar]
- 17.Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104(5):911–20. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Garcia MG, Duenas E, Sola MC, Hutson AD, Theriaque D, Christensen RD. Epidemiologic and outcome studies of patients who received platelet transfusions in the neonatal intensive care unit. J Perinatol. 2001;21(7):415–20. doi: 10.1038/sj.jp.7210566. [DOI] [PubMed] [Google Scholar]
- 19.Del Vecchio A, Sola MC, Theriaque DW, Hutson AD, Kao KJ, Wright D, Garcia MG, Pollock BH, Christensen RD. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion. 2001;41(6):803–8. doi: 10.1046/j.1537-2995.2001.41060803.x. [DOI] [PubMed] [Google Scholar]
- 20.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132(6):620–4. discussion 624-5. [PubMed] [Google Scholar]
- 21.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–8. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 22.Smith MJ, Le Roux PD, Elliott JP, Winn HR. Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg. 2004;101(1):1–7. doi: 10.3171/jns.2004.101.1.0001. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 24.Kneyber MC, Hersi MI, Twisk JW, Markhorst DG, Plotz FB. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33(8):1414–22. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghio M, Contini P, Mazzei C, Brenci S, Barberis G, Filaci G, Indiveri F, Puppo F. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93(5):1770–7. [PubMed] [Google Scholar]
- 28.Corwin HL, Carson JL. Blood transfusion--when is more really less? N Engl J Med. 2007;356(16):1667–9. doi: 10.1056/NEJMe078019. [DOI] [PubMed] [Google Scholar]
- 29.Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8(4):267–81. doi: 10.1177/108925320400800402. [DOI] [PubMed] [Google Scholar]
- 30.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 31.Suematsu M, Suganuma K, Kashiwagi S. Mechanistic probing of gaseous signal transduction in microcirculation. Antioxid Redox Signal. 2003;5(4):485–92. doi: 10.1089/152308603768295230. [DOI] [PubMed] [Google Scholar]
- 32.Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 2006;70(5):840–53. doi: 10.1038/sj.ki.5001680. [DOI] [PubMed] [Google Scholar]
- 33.Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1(10):2112–8. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 34.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–25. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 35.Bryan RM, Jr., You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology. 2005;102(6):1261–77. doi: 10.1097/00000542-200506000-00028. [DOI] [PubMed] [Google Scholar]
- 36.Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide. 2008;18(1):47–60. doi: 10.1016/j.niox.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr., Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107(2):566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen BW, Piantadosi CA. How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm. Am J Physiol Heart Circ Physiol. 2006;291(4):H1507–12. doi: 10.1152/ajpheart.00310.2006. [DOI] [PubMed] [Google Scholar]
- 39.Dzik S. Nitric oxide: nature's third respiratory gas. Transfusion. 2002;42(12):1532–3. doi: 10.1046/j.1537-2995.2002.00288.x. [DOI] [PubMed] [Google Scholar]
- 40.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218(3):739–49. [PubMed] [Google Scholar]
- 41.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89(1):444–8. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawloski JR, Stamler JS. Nitric oxide in RBCs. Transfusion. 2002;42(12):1603–9. doi: 10.1046/j.1537-2995.2002.00278.x. [DOI] [PubMed] [Google Scholar]
- 43.Winslow RM, Intaglietta M. Red cell age and loss of function: advance or SNO-job? Transfusion. 2008;48(3):411–4. doi: 10.1111/j.1537-2995.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 45.Chen K, Popel AS. Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radic Biol Med. 2006;41(4):668–80. doi: 10.1016/j.freeradbiomed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10(7):1185–98. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lancaster J, Hutchings A, Kerby JD, Patel RP. The hemoglobin-nitric oxide axis: implications for transfusion therapeutics. Transfusion Alternatives in Transfusion Medicine. 2007:273–280. [Google Scholar]
- 48.Lancaster J, Hutchings A, Kerby JD, Patel RP. The hemoglobin-nitric oxide axis: implications for transfusion therapeutics. Transfusion Alternatives in Transfusion Medicine. 2007;9(4):273–280. [Google Scholar]
- 49.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol. 2006;291(3):H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 50.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105(1A):32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 51.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 52.Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37(4):1047–52. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 53.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17(2):48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDermott DH, Halcox JP, Schenke WH, Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA, Murphy PM. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89(5):401–7. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- 55.Zhu J, Quyyumi AA, Rott D, Csako G, Wu H, Halcox J, Epstein SE. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation. 2001;103(8):1071–5. doi: 10.1161/01.cir.103.8.1071. [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Quyyumi AA, Wu H, Csako G, Rott D, Zalles-Ganley A, Ogunmakinwa J, Halcox J, Epstein SE. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23(6):1055–9. doi: 10.1161/01.ATV.0000074899.60898.FD. [DOI] [PubMed] [Google Scholar]
- 57.Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005;46(3):389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 59.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25(5):923–31. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 60.Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82(2):159–65. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 61.Seppa N. Bad blood? Old units might be substandard. 2008 www.ScienceNews.org.