Abstract
Summary
We compared temporal trends in serum 25-hydroxyvitamin D and parathyroid hormone (PTH) in two primary hyperparathyroidism (PHPT) cohorts recruited 20 years apart. The prevalence of 25-hydroxyvitamin D levels <20 and <30 ng/mL declined by 30–50 %, respectively, and was accompanied by lower PTH. In the older cohort, higher PTH may be due to lower 25-hydroxyvitamin D.
Introduction
Vitamin D deficiency may exacerbate PHPT. Whether there have been temporal trends in 25-hydroxyvitamin D (25OHD) levels in PHPT is unclear. The prevalence of low vitamin D levels (25OHD <20 and <30 ng/mL) and associated biochemical and bone mineral density (BMD) profiles were assessed in two PHPT cohorts recruited over 20 years apart.
Methods
This is a cross-sectional comparison of serum 25OHD levels, calciotropic hormones, and BMD between two PHPT cohorts recruited at the same hospital: the “old” (N=103) and “new” (N=100) cohorts were enrolled between 1984 and 1991 and between 2010 and 2014, respectively. Results Mean 25OHD levels were 26 % higher in the new cohort (23±10 vs. 29±10 ng/mL, p<0.0001). Levels of 25OHD <20 and <30 ng/mL declined from 46 and 82 %, respectively, to 19 and 54 % (both p<0.0001). Supplemental vitamin D use was common in the new (64 %) but not the old cohort (0 %). The new cohort demonstrated 33 % lower serum PTH levels (p<0.0001). Neither serum nor urine calcium differed. BMD was higher in the new cohort at all skeletal sites (all p<0.001).
Conclusion
With the rise in vitamin D supplementation over the last two decades, low 25OHD levels are no longer common in PHPT patients in the New York area. Those with 25OHD <20 and <30 ng/mL have declined by over 50 and 30 %, respectively. The lower mean PTH levels in the new cohort are most likely accounted for by higher vitamin D intake. Whether improved vitamin D status also underlies the relatively higher BMD in the more vitamin D replete cohort of PHPT patients is unknown.
Keywords: Prevalence, Primary hyperparathyroidism, Vitamin D deficiency
Introduction
Vitamin D deficiency is common in primary hyperparathyroidism (PHPT) and has been reported to occur more frequently than in those without PHPT [1, 2]. Several pathophysiological mechanisms may contribute to the association between vitamin D deficiency and PHPT. Parathyroid hormone (PTH) may facilitate the conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D by stimulating renal 1-alpha hydroxylase activity [3]. Increased levels of 1,25-dihydroxyvitamin D in PHPT have also been proposed to affect overall vitamin D status, by inhibiting the production of vitamin D in the skin and its subsequent conversion in the liver. The half-life of 25-hydroxyvitamin D, the liver product, may also be shortened in PHPT, with increased metabolic clearance due to enhanced hepatic inactivation [4]. Alternatively, chronic vitamin D deficiency, which causes parathyroid gland stimulation, may lead to subsequent hyperplasia and autonomous adenomatous change. Some studies suggest that PHPT is more severe when vitamin D deficiency is present, a concept that was first proposed over 40 years ago [5]. Lower levels of 25-hydroxyvitamin D in PHPT have been associated with greater adenoma weight; more markedly elevated levels of PTH, calcium, and markers of bone turnover; lower bone mineral density (BMD); as well as the development of frank radiological features of PHPT, such as osteitis fibrosa cystica [1, 6–12].
Use of vitamin D-containing multivitamins and vitamin D supplements is now widespread in the USA [13]. Although data are limited, some reports suggest that the prevalence of vitamin D deficiency may be declining in the general population [14]. Other reports suggest that the prevalence is actually increasing due to the increase in obesity, which is associated with low circulating 25-hydroxyvitamin D levels through a variety of potential mechanisms [15, 16]. Whether the secular trends in vitamin D status in the general population are also apparent in PHPT is unclear.
Given the reported effects of low 25-hydroxyvitamin D on the clinical profile of PHPT, ascertaining comparative changes in vitamin D in patients with PHPT is important. The purpose of this study was to compare the prevalence of low vitamin D levels as well as accompanying differences in serum biochemistries, calciotropic hormones, and BMD in two cohorts of PHPT patients recruited from the same demographic area over two decades apart at the same institution. The definitions of vitamin D insufficiency and deficiency are controversial even in the general population, and data on appropriate cut points in PHPT are lacking. For purposes of this analysis, we utilized commonly employed vitamin D thresholds which are recommended by the Endocrine Society Clinical Practice guidelines and other bone and mineral organizations (<30 and <20 ng/mL) [17].
Methods
Design and subjects
This is a cross-sectional analysis comparing 25-hydroxyvitamin D and other calciotropic hormone levels, biochemistries, and areal BMD in two cohorts of PHPT patients. Both cohorts were recruited primarily by referrals from endocrinologists within the Metabolic Bone Diseases Unit and General Endocrinology Clinic or parathyroid surgeons in the Endocrine Surgery Clinic at Columbia University Medical Center (CUMC). Although CUMC is a tertiary care center for PHPT, our clinic serves the catchment area surrounding our hospital. The only difference in recruitment strategies between the cohorts was that in the new cohort, 3 % of study referrals came from the Internet. No filters were applied to the referral process except for the enrollment criteria below. The first PHPT cohort included the 103 of 139 patients enrolled between 1984 and 1991 in our longitudinal natural history study of PHPT in whom 25-hydroxyvitamin D and BMD values were available [18, 19]. Serum calcium and PTH levels in this subgroup were similar to those of the overall cohort. The second cohort represents participants enrolled between 2010 and 2014 in a cross-sectional study of the effect of vitamin D on skeletal health. Participants in the latter study represent consecutive patients who met enrollment criteria and agreed to participate. Participants in both studies were healthy ambulatory patients with PHPT. All patients gave written, informed consent. Both studies were approved by the Institutional Review Board of CUMC.
In both cohorts, PHPT was defined as the presence of hypercalcemia with a frankly elevated or inappropriately normal PTH level. None had thiazide- or lithium-induced hypercalcemia or familial hypocalciuric hypercalcemia (FHH). FHH was excluded on the basis of family history and a 24-h urine calcium excretion. No patients in the old cohort were taking bisphosphonates. Exclusion criteria for the more recent study included bisphosphonate use within the past 2 years; current use of cinacalcet or denosumab; current or previous use of prednisone >7.5 mg for more than 6 months; current or past use of carbamazepine, phenytoin, or phenobarbital for more than 3 months; malignancy within 5 years other than nonmelanomatous skin cancer; granulomatous diseases, HIV, serum creatinine level ≥1.5 mg/dL; liver disease; gastrointestinal diseases known to affect calcium metabolism or cause secondary hyperparathyroidism such as Crohn's disease, celiac disease, or gastric bypass; and pregnancy. Both symptomatic (i.e., those with nephrolithiasis) and asymptomatic PHPT were enrolled. Likewise, PHPT participants were enrolled whether or not they met 2008 consensus guidelines for parathyroidectomy [20].
Clinical and biochemical evaluation
Demographic data, medical history, and medication use were obtained from participants as previously described [18, 21]. Fasting baseline samples were obtained for measurement of biochemistries. Serum calcium (normal range 8.4–10.2 mg/dL), creatinine, as well as urine calcium were measured by automated analysis in both cohorts. Intact PTH was measured by IRMA in the old and new cohorts. The assay utilized for the old cohort was manufactured by Nichols Institute (normal range 10–65 pg/mL; intra- and inter-assay CV 3.4–4.1 and 1.0–3.5 %, respectively, over the range of the calibration curve; limit of detection 2.8 pg/mL) while the assay used for the new cohort was by Scantibodies Laboratory, Inc., Santee, CA (normal range 14–66 pg/mL; intra- and inter-assay CV 3.2–4.8 and 3.6–6.8 %, respectively; limit of detection 1.2 pg/mL) [22, 23]. These assays have similar performance characteristics with average PTH differences of only 1 pg/mL [24]. In the 1984–1991 cohort, 25-hydroxyvitamin D was quantitated by competitive protein-binding assay (intra- and inter-assay CV 8.3 and 8–16.5 %, respectively) and 1,25-dihydroxyvitamin D was measured by radioreceptor assay (intra- and inter-assay CV 6.8 and 11.8 %, respectively) [25, 26]. In the 2010–2014 cohort, serum 25-hydroxyvitamin D (intra- and inter-assay CV 7.0–7.7 % for D3 and 4.5–8.3 % for D2; limit of detection 4 ng/mL) and 1,25-dihydroxyvitamin D (intra- and inter-assay CV 6–8 and 9–10 %; limit of detection 8 pg/mL) were measured by liquid chromatography/tandem mass spectroscopy.
Bone mineral density
1984–1991 cohort
Before 1991, the BMD of the lumbar spine, femoral neck, and distal one third of the non-dominant radius was measured using single-photon and dual-photon absorptiometry (SP2 and DP3 scanners, respectively; Lunar Radiation, Madison, WI); thereafter, dual-energy X-ray absorptiometry (QDR-1000, Hologic, Waltham, MA) was used. All BMD measurements are presented in terms of converted values using methods previously described [18, 19].
2010–2014 cohort
Areal BMD was measured by DXA at the lumbar spine, femoral neck, and one third distal radius sites (Hologic Inc., Waltham, MA). In vivo precision, determined according to the standard method at this facility, is 1.28 % at the lumbar spine, 1.36 % at the hip, and 0.70 % for the one third distal radius [27]. BMD measurements are presented as Z-scores. The use of T-scores was not widely adopted until 1994 and thus participants in the old cohort do not have T-scores.
Statistical analysis
Between-group differences in demographic and skeletal indices were evaluated by independent two-sided Student's t test, chi-square, or Fisher's exact test as appropriate. Critical test values were adjusted for unequal variances when appropriate. Associations between variables were assessed using Pearson correlation. Generalized linear models were used to assess between-group differences, adjusting for covariates (age and body mass index, BMI). Adjusted values are expressed as mean±SEM. For all analyses, a two-tailed p<0.05 was considered to indicate statistical significance. With the current sample size, the study had 80 % power with a two-tailed alpha of 5 % to detect between-group differences in 25-hydroxyvitamin D and PTH larger than 3.96 ng/mL and 23 pg/mL, respectively. Statistical analysis was performed using SAS, version 9.3 (Cary, NC).
Results
Both cohorts were predominantly female and had evidence of mild primary hyperparathyroidism; none had osteitis fibrosa cystica (Table 1). The old cohort (1984–1991) included participants who were younger on average (p=0.0002), but there were no between-group differences in gender, race, ethnicity, or height. The more recent (2010–2014) cohort tended to weigh more (7 %, p=0.06) and have a higher BMI (6 %, p=0.07) compared to the old cohort (Table 1).
Table 1.
Demographic Profiles of PHPT Cohorts
| Variable | 1984-1991 cohort | 2010-2014 cohort | p value |
|---|---|---|---|
| Age (years) | 55.4±12.1 | 61.9±12.3 | 0.0002 |
| Gender (% female) | 79 % | 79 % | 0.91 |
| Race (% White) | 87.4 % | 89.6 % | 0.63 |
| Ethnicity (% Hispanic) | 17% | 17% | 0.92 |
| Height (in.) | 64.5±3.3 | 64.8±3.7 | 0.57 |
| Weight (lbs) | 156.8±38.2 | 167.8±42.8 | 0.06 |
| BM (kg/m2) | 26.4±5.7 | 28.0±6.0 | 0.07 |
| Vitamin D supplementation (IU/day) | 0 | 800 | N/A |
Values represent means±SD or percentages or median in the case of vitamin D supplementation
BMI body mass index, N/A not applicable
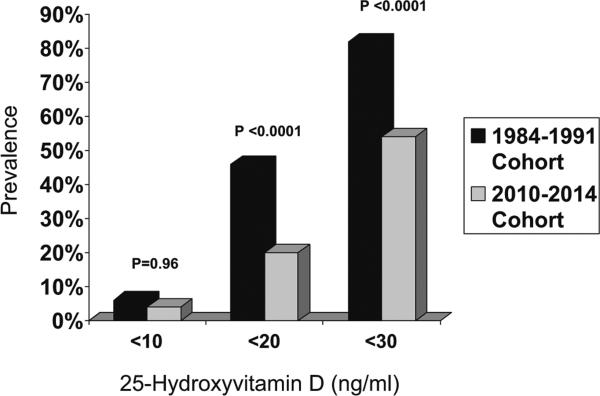
Despite their tendency toward greater weight and BMI, the more recent cohort had 26 % higher mean 25-hydroxyvitamin D levels (p<0.0001) (Table 2). As shown in Fig. 1, the prevalence of 25-hydroxyvitamin D <20 and <30 ng/mL was higher in the old as compared to the new cohort (p<0.0001 for both): 46 % had 25-hydroxyvitamin D levels <20 ng/mL (mean 15±4 ng/dL) and 82 % had a level <30 ng/mL (mean 19±6 ng/mL) in the old cohort as compared to 19 % (mean 14±3 ng/mL) and 54 % (mean 21± 6 ng/mL), respectively, in the new cohort. There were no differences in the prevalence of subjects with extremely low 25-hydroxyvitamin D (<10 ng/mL), which was uncommon in both cohorts (p=0.96).
Table 2.
Comparison of vitamin D and PTH levels by cohort
| Measurement | 1984–1991 cohort | 2010–2014 cohort | p value |
|---|---|---|---|
| Entire cohort | N=103 | N=100 | |
| 25OHD (ng/mL) | 23±10 | 29±10 | <0.0001 |
| PTH (pg/mL) | 127±69 | 85±48 | <0.0001 |
| 25OHD<10 ng/mL | N=10 | N=4 | |
| 25OHD (ng/mL) | 7±3 | 8±2 | 0.28 |
| PTH (pg/mL) | 185±113 | 121±41 | 0.32 |
| 25OHD<20 ng/mL | N=47 | N=19 | |
| 25OHD (ng/mL) | 15±4 | 14±3 | 0.33 |
| PTH (pg/mL) | 149±75 | 126±62 | 0.26 |
| 25OHD<30 ng/mL | N=84 | N=54 | |
| 25OHD (ng/mL) | 19±6 | 21±6 | 0.03 |
| PTH (pg/mL) | 132±72 | 97±54 | 0.002 |
Values represent means±SD or percentages
Fig. 1.
Percentage of patients with 25-hydroxyvitamin D <20 and <30 ng/mL in the two PHPT cohorts. The 1984–1991 cohort is shown in black while the more recent 2010–2014 cohort is shown in gray
No patients in the 1984–1991 cohort were taking vitamin D supplements while 64 % in the new cohort were taking vitamin D supplements. The median daily supplement dosage was 800 IU/day (range 71–7143 IU/day) in the new cohort, and 84 % of those with a 25OHD level >30 ng/mL were consuming vitamin D supplements.
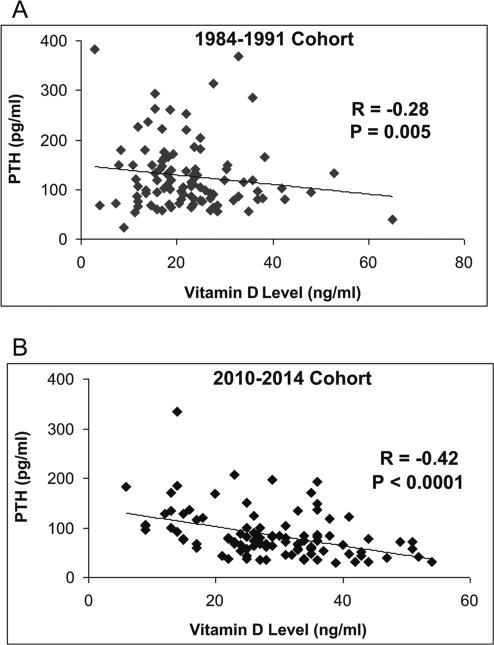
Mean PTH levels were 33 % lower in the new as compared to the older cohort (Table 2; p<0.0001). This pattern was observed in the subset of patients with 25-hydroxyvitamin levels <30 ng/mL, whereas there were no between-group differences in PTH levels for those with 25-hydroxyvitamin D <20 or <10 ng/mL. There was a negative association between 25-hydroxyvitamin D and PTH in both the old (r=−0.28, p=0.005; regression equation: y=−2.016x + 171.81) and newer (r=−0.40, p<0.0001; y=−1.653x+132.59) cohorts (Fig. 2). The slopes of regression lines did not differ (p=0.66).
Fig. 2.
The relationship between 25-hydroxyvitamin D and PTH levels in the two PHPT cohorts. a 1984–1991 cohort, b 2010–2014 cohort
There were no between-group differences in serum calcium concentration or in urinary calcium excretion (Table 3). 1,25-Dihydroxyvitamin D levels were 21 % higher (p=0.0002) in the more recent cohort. In the new cohort, there was a trend toward higher 1,25-dihydroxyvitamin D levels in supplement users versus non-users (75±31 vs. 66±29 pg/mL, p=0.09). BMD as measured by DXA, presented as Z-scores in order to take into account age differences among the cohorts, was higher in the new versus old cohort at the lumbar spine, femoral neck, and one-third radius (Table 4).
Table 3.
Biochemical profiles of two cohorts
| Biochemistries | 1984–1991 cohort | 2010–2014 cohort | p value |
|---|---|---|---|
| Serum calcium (mg/dL) | 10.6±0.6 | 10.7±0.6 | 0.14 |
| Serum 1,25-dihydroxyvitamin D (pg/mL) | 57±20 | 69±24 | 0.0002 |
| Urine calcium excretion (mg/day)a | 229±119 | 250±144 | 0.28 |
Values represent means±SD
Available on 186 of 203 participants
Table 4.
Bone mineral density by DXA in the two cohorts
| Z-scores | 1984–1991 cohort | 2010–2014 cohort | p value |
|---|---|---|---|
| Lumbar spine | –0.4±1.7 | 0.5±1.7 | 0.0008 |
| Femoral neck | –0.9±1.0 | 0.0±0.9 | <0.0001 |
| 1/3 radius | –1.0±1.3 | 0.2±1.4 | <0.0001 |
Values represent means±SD
Of note, 25-hydroxyvitamin D levels remained higher (least squares mean±SEM 29.0±1.1 vs. 22.3±1.1 ng/mL, p<0.0001) and PTH levels lower (least squares mean±SEM 83±6 vs. 128±6 pg/mL, p<0.0001) in the more recent cohort after adjusting for differences in age and BMI. BMD Z-scores at all sites also remained higher after adjusting for BMI (all p<0.01).
Discussion
This first report of temporal trends in vitamin D levels in PHPT assessed the prevalence of low 25-hydroxyvitamin D levels in two cohorts recruited from the same catchment area at the same institution over a period spanning 30 years. Our results demonstrate a significant decline in percentage of patients with low 25-hydroxyvitamin D; 50 % fewer had 25-hydroxyvitamin D <20 ng/mL while 30 % fewer had 25-hydroxyvitamin D <30 ng/mL. Today, approximately half of our PHPT patients have 25-hydroxyvitamin D levels >30 ng/ mL. This increase in 25-hydroxyvitamin D levels occurred despite the trend toward greater weight and higher BMI in the more recent cohort, which should predispose to lower circulating 25-hydroxyvitamin D levels.
The difference in vitamin D status is likely due to increased use of vitamin D supplements, initiated on the part of either physicians or the patients themselves. There are several reasons for the more liberal use of vitamin D supplements in PHPT. First, in 2008, the Third International Workshop on Asymptomatic PHPT recommended measuring 25-hydroxyvitamin D in all patients with PHPT and repleting vitamin D to a 25-hydroxyvitamin D level >20 ng/mL, a recommendation reiterated in the newest guidelines as well [28, 29]. Second, there is increased public awareness regarding vitamin D deficiency and its potential association with health outcomes such as osteoporosis, cardiovascular disease, breast cancer, and other chronic illnesses that has led to widespread self-supplementation. Our results mirror some recent reports of changes in vitamin D supplementation and vitamin D status in the general population without PHPT [14, 30].
In tandem with the increase in mean 25-hydroxyvitamin D level over time, we found lower PTH levels without any differences in serum or urine calcium. Although our data are cross-sectional and causality cannot be definitively imputed, it is possible that the differences in vitamin D status between the cohorts may account for the lower PTH concentration in the more recent, more vitamin D replete group of PHPT patients. Data from longitudinal studies have shown that vitamin D supplementation reduces PTH levels in PHPT [31–35]. In the older cohort, the PTH elevations may have been heightened by coexisting vitamin D deficiency. We cannot completely rule out the converse possibility, namely that other factors may have resulted in a decline in PTH over time leading to higher 25-hydroxyvitamin D levels (due to reduced transcription of the 1-α-hydroxylase). Alternative explanations could include changes in our referral patterns, changes in the disease itself, changes in disease incidence, or earlier detection of PHPT leading to a milder phenotype. Arguing against some of these possibilities, similar recruitment methods were utilized for both cohorts and the cohorts had similar demographics. More importantly, the similar slopes for the regression of PTH upon 25-hydroxyvitamin D in the two cohorts support the conclusion that the disease has not changed, only the prevalence of low vitamin D levels. Furthermore, if the decline in PTH were the primary factor, we would have expected to see lower levels of 1,25-dihydroxyvitamin D in the new cohort. Instead, levels were higher, likely reflecting greater substrate. Supporting this concept, we found a tendency toward higher 1,25-dihydroxyvitamin D levels among supplement users in the new cohort.
In addition to the biochemical differences between the two cohorts, we also found that the newer cohort had higher areal BMD. Whether the higher serum vitamin D levels, lower PTH levels, or both factors contributed to this BMD difference is unclear. A recent RCT found that treatment with 2800 IU/day of vitamin D improved vitamin D levels from 20 to 38 ng/mL and decreased PTH while lumbar spine BMD increased by 2.5 % versus the placebo, though there was no change in proximal femur or forearm BMD [36]. In contrast, uncontrolled studies have shown variable effects of vitamin D repletion upon BMD in PHPT [33, 37]. Caution needs to be exercised, however, in attributing causality to the association between higher vitamin D levels and better BMD in the new cohort. Other factors may also in part be responsible for this finding. There have been changes in BMD technology over time; although, measurements were compared using conversion methods previously validated in our longitudinal studies [18, 19]. It is also likely that changes in the referent databases used to calculate Z-scores occurred over time. Our newer cohort also had higher BMI, which predispose to better BMD, but BMD remained higher even after adjustment for this factor. Finally, the recent study more rigorously excluded those with conditions or medications known to influence bone metabolism other than PHPT.
It is, however, well-documented that the biochemical and skeletal presentation of PHPT has changed over time, so improved BMD over time would not be unexpected. Once a disease of “bones, stones, and abdominal groans,” most patients with PHPT are now asymptomatic. Osteitis fibrosa cystica is rarely seen in the USA and PHPT patients most often have only subclinical evidence of bone loss detectable by DXA [18]. The change in presentation is largely attributed to the routine measurement of calcium, which began in the 1970s. At that time, it became clear that PHPT was more common than initially surmised and that many patients were, in fact, asymptomatic. Some work implicates higher 25-hydroxyvitamin D levels contributing to the decline in osteitis fibrosa cystica [38]. One might anticipate that further improvement in vitamin D status and PTH would be associated with higher BMD.
Our study has several limitations, the most important of which is that the assays utilized to measure calciotropic hormones changed over time. Recent recognition of inaccuracies and inconsistencies among different commercially available 25-hydroxyvitamin D assays has led to vitamin D standardization programs to promote improved accuracy and reliability [39]. The method utilized for the new cohort (liquid chromatography/tandem mass spectrometry) is currently considered to be the gold standard. Cross-validation of vitamin D assays within our lab was not possible as there was no remaining serum from the old cohort and the older competitive protein-binding assay is no longer commercially available. Although we recognize this significant limitation, published data suggest that the competitive binding assay overestimates vitamin D compared to liquid chromatography/tandem mass spectroscopy when the major circulating metabolite is vitamin D3, as would be expected for the old cohort in whom there was no vitamin D supplementation. Since the older method tends to provide higher rather than lower values, any potential bias would have been expected to prejudice against the finding we report in this manuscript [40, 41]. Comparative studies have shown the two PTH assays utilized in the two cohorts provide similar results with average differences of only 1 pg/mL [24]. Therefore, although assay variability remains an important limitation, we believe our inferences are likely to be valid. Our study has several important strengths. The two cohorts are relatively large for studies of PHPT. The subjects come from the same geographic locale (latitude) and are well-characterized. Further, to our knowledge, this is the first study to assess temporal trends in vitamin D in patients with PHPT.
In conclusion, with the rise in vitamin D supplementation over the last two decades, fewer patients with mild PHPT in our catchment area have low 25-hydroxyvitamin D levels. The frequency of levels <20 and <30 ng/mL has declined by over 30 and 50 %, respectively. In the old cohort, PTH elevations due to PHPT may have been further heightened by coexisting vitamin D deficiency. Conversely, differences in vitamin D status may underlie the lower PTH concentration in the more recent, more vitamin D replete cohort of PHPT patients. These differences may be associated with a further subtle shift in the skeletal profile of modern day PHPT, as the negative effect of concomitant vitamin D deficiency becomes less evident.
Acknowledgments
Funding sources This work was supported by NIH grants R01 DK084986, K24 DK074457, R01 32333, as well as the Joseph Weintraub Family Foundation.
Footnotes
Conflicts of interest None.
References
- 1.Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol (Oxf) 2005;63:506–513. doi: 10.1111/j.1365-2265.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- 2.Boudou P, Ibrahim F, Cormier C, Sarfati E, Souberbielle JC. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J Endocrinol Invest. 2006;29:511–515. doi: 10.1007/BF03344140. [DOI] [PubMed] [Google Scholar]
- 3.Clements MR, Davies M, Hayes ME, Hickey CD, Lumb GA, Mawer EB, Adams PH. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol (Oxf) 1992;37:17–27. doi: 10.1111/j.1365-2265.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 4.Clements MR, Davies M, Fraser DR, Lumb GA, Mawer EB, Adams PH. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci (Lond) 1987;73:659–664. doi: 10.1042/cs0730659. [DOI] [PubMed] [Google Scholar]
- 5.Lumb GA, Stanbury SW. Parathyroid function in human vitamin D deficiency and vitamin D deficiency in primary hyper-parathyroidism. Am J Med. 1974;56:833–839. doi: 10.1016/0002-9343(74)90812-2. [DOI] [PubMed] [Google Scholar]
- 6.Rao DS, Honasoge M, Divine GW, Phillips ER, Lee MW, Ansari MR, Talpos GB, Parfitt AM. Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J Clin Endocrinol Metab. 2000;85:1054–1058. doi: 10.1210/jcem.85.3.6440. [DOI] [PubMed] [Google Scholar]
- 7.Rao DS, Agarwal G, Talpos GB, Phillips ER, Bandeira F, Mishra SK, Mithal A. Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyper-parathyroidism: a global perspective. J Bone Miner Res. 2002;17(Suppl 2):N75–N80. [PubMed] [Google Scholar]
- 8.Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. The effects of vitamin D insufficiency in patients with primary hyper-parathyroidism. Am J Med. 1999;107:561–567. doi: 10.1016/s0002-9343(99)00294-6. [DOI] [PubMed] [Google Scholar]
- 9.Ozbey N, Erbil Y, Ademoglu E, Ozarmagan S, Barbaros U, Bozbora A. Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyper-parathyroidism. World J Surg. 2006;30:321–326. doi: 10.1007/s00268-005-0239-y. [DOI] [PubMed] [Google Scholar]
- 10.Beyer TD, Chen EL, Nilubol N, Prinz RA, Solorzano CC. Short-term outcomes of parathyroidectomy in patients with or without 25-hydroxy vitamin D insufficiency. J Surg Res. 2007;143:145–150. doi: 10.1016/j.jss.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Moosgaard B, Christensen SE, Vestergaard P, Heickendorff L, Christiansen P, Mosekilde L. Vitamin D metabolites and skeletal consequences in primary hyperparathyroidism. Clin Endocrinol (Oxf) 2008;68:707–715. doi: 10.1111/j.1365-2265.2007.03109.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H, Noguchi S, Uchino S, Watanabe S, Koike E, Murakami T, Fujihira T, Koga Y, Masatsugu T, Yamashita H. Vitamin D status in Japanese patients with hyperparathyroidism: seasonal changes and effect on clinical presentation. World J Surg. 2002;26:937–941. doi: 10.1007/s00268-002-6622-z. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson A, Blatman J, El-Dash N, Franco JC. Consumer usage and reasons for using dietary supplements: report of a series of surveys. J Am Coll Nutr. 2014;33:176–182. doi: 10.1080/07315724.2013.875423. [DOI] [PubMed] [Google Scholar]
- 14.Berger C, Greene-Finestone LS, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res. 2012;27:1381–1389. doi: 10.1002/jbmr.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–1255. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 19.Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93:3462–3470. doi: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilezikian JP, Khan AA, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–339. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker MD, Cong E, Kepley A, et al. Association between serum 25-hydroxyvitamin D level and subclinical cardiovascular disease in primary hyperparathyroidism. J Clin Endocrinol Metab. 2014;99:671–680. doi: 10.1210/jc.2013-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts JT, Jr, Segre GV. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hyper-calcemia. Clin Chem. 1987;33:1364–1367. [PubMed] [Google Scholar]
- 23.Inaba M, Nakatsuka K, Imanishi Y, Watanabe M, Mamiya Y, Ishimura E, Nishizawa Y. Technical and clinical characterization of the Bio-PTH (1-84) immunochemiluminometric assay and comparison with a second-generation assay for parathyroid hormone. Clin Chem. 2004;50:385–390. doi: 10.1373/clinchem.2003.026831. [DOI] [PubMed] [Google Scholar]
- 24.Cantor T, Yang Z, Caraiani N, Ilamathi E. Lack of comparability of intact parathyroid hormone measurements among commercial assays for end-stage renal disease patients: implication for treatment decisions. Clin Chem. 2006;52:1771–1776. doi: 10.1373/clinchem.2006.071589. [DOI] [PubMed] [Google Scholar]
- 25.Preece MA, O'Riordan JL, Lawson DE, Kodicek E. A competitive protein-binding assay for 25-hydroxycholecalciferol and 25-hydroxyergocalciferol in serum. Clin Chim Acta. 1974;54:235–242. doi: 10.1016/0009-8981(74)90241-1. [DOI] [PubMed] [Google Scholar]
- 26.Reinhardt TA, Horst RL, Orf JW, Hollis BW. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984;58:91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- 27.Bonnick SL, Johnston CC, Jr, Kleerekoper M, Lindsay R, Miller P, Sherwood L, Siris E. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–110. doi: 10.1385/jcd:4:2:105. [DOI] [PubMed] [Google Scholar]
- 28.Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340–350. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 29.Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99:3561–3569. doi: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell D, Lee H, Greendale G, Cauley JA, Burnett-Bowie S, Finkelstein JS. Increasing 25-hydroxyvitamin D levels over time: the Study of Women's Health Across the Nation (SWAN) ASBMR; Houston, TX: 2014. [Google Scholar]
- 31.Shah VN, Shah CS, Bhadada SK, Rao DS. Effect of 25 (OH) D replacements in patients with primary hyperparathyroidism (PHPT) and coexistent vitamin D deficiency on serum 25(OH) D, calcium and PTH levels: a meta-analysis and review of literature. Clin Endocrinol (Oxf) 2014 doi: 10.1111/cen.12398. [DOI] [PubMed] [Google Scholar]
- 32.Rao RR, Randeva HS, Sankaranarayanan S, Narashima M, Mohlig M, Mehanna H, Weickert MO. Prolonged treatment with vitamin D in postmenopausal women with primary hyperparathyroidism. Endocr Connect. 2012;1:13–21. doi: 10.1530/EC-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grey A, Lucas J, Horne A, Gamble G, Davidson JS, Reid IR. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency. J Clin Endocrinol Metab. 2005;90:2122–2126. doi: 10.1210/jc.2004-1772. [DOI] [PubMed] [Google Scholar]
- 34.Grubbs EG, Rafeeq S, Jimenez C, Feng L, Lee JE, Evans DB, Perrier ND. Preoperative vitamin D replacement therapy in primary hyperparathyroidism: safe and beneficial? Surgery. 2008;144:852–858. doi: 10.1016/j.surg.2008.06.032. discussion 858-859. [DOI] [PubMed] [Google Scholar]
- 35.Tucci JR. Vitamin D therapy in patients with primary hyper-parathyroidism and hypovitaminosis D. Eur J Endocrinol. 2009;161:189–193. doi: 10.1530/EJE-08-0901. [DOI] [PubMed] [Google Scholar]
- 36.Rolighed L, Rejnmark L, Sikjaer T, Heickendorff L, Vestergaard P, Mosekilde L, Christiansen P. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J Clin Endocrinol Metab. 2014;99:1072–1080. doi: 10.1210/jc.2013-3978. [DOI] [PubMed] [Google Scholar]
- 37.Kantorovich V, Gacad MA, Seeger LL, Adams JS. Bone mineral density increases with vitamin D repletion in patients with coexistent vitamin D insufficiency and primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:3541–3543. doi: 10.1210/jcem.85.10.6909. [DOI] [PubMed] [Google Scholar]
- 38.Bilezikian JP, Meng X, Shi Y, Silverberg SJ. Primary hyper-parathyroidism in women: a tale of two cities—New York and Beijing. Int J Fertil Womens Med. 2000;45:158–165. [PubMed] [Google Scholar]
- 39.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 40.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006;52:1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 41.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]