Abstract
The transfusion-transmitted cytomegalovirus (TT-CMV) can cause serious morbidity and mortality in low–birth weight infants (LBWIs). Transfusion-transmitted cytomegalovirus can be minimized in LBWIs born to cytomegalovirus (CMV)–seronegative mothers with the use of CMV-seronegative blood components. Despite evidence that has independently shown that either leukoreduction or the use of CMV-seronegative components mitigates TT-CMV, the potential efficacy of combining these 2 strategies has not been substantiated in very LBWIs (<1500 g) born to either CMV-seronegative or CMV-seropositive mothers. Nonetheless, the serious risks of CMV infection posed by allogeneic transfusions and the broad implementation of universal leukoreduction have made this combination strategy the de facto clinical standard for transfusion of LBWIs. Although preferred, this combined approach has not been validated in clinical trials and, thus, warrants a large prospective study to determine whether this is the optimal transfusion tactic or if additional safety measures are necessary to prevent TT-CMV in LBWIs born to both CMV- seronegative and CMV-seropositive mothers. The aim of this prospective birth cohort study, therefore, is to estimate the incidence of TT-CMV in 1300 LBWIs (≤1500 g) who receive CMV-seronegative plus leukoreduced blood products to evaluate the effectiveness of this coupled strategy. Conducted in Atlanta, GA, this study has been registered at the US National Institutes of Health (ClinicalTrials.gov no. NCT00907686).
Primary cytomegalovirus (CMV) infection in immunologically immature low–birth weight infants (LBWIs) is frequently symptomatic, with clinical deterioration including sepsis-like syndromes, respiratory compromise, hepatopathy, neutropenia, and thrombocytopenia.1 Cytomegalovirus-naive neonates of CMV-positive mothers recently infected with CMV often contract viral infection either in utero or from breast milk, and both are generally regarded as being due to vertical transmission. Another important route of transmission is blood transfusion. Low–birth weight infants born to either a CMV-positive or CMV-negative mother have been shown to develop transfusion-transmitted CMV (TT-CMV), resulting in serious morbidity and mortality in half of infected infants.2 Although transfusion medicine practitioners have endeavored to develop improved approaches to prevent TT-CMV in LBWIs, clinical trials to validate these approaches have typically been small and with limited statistical power. Moreover, these studies have been complicated by several factors such as controlling for vertical transmission, leukoreduction filter technology, and availability of CMV-seronegative blood products.
Strategies to prevent TT-CMV in other special immune-compromised populations have been rigorously studied. Nearly 15 years ago, a large multicenter, prospectively randomized controlled trial (RCT) was performed to address the relative safety of CMV-seronegative or leukoreduced blood components in preventing TT-CMV in seronegative bone marrow transplant (BMT) patients.3 Despite findings that showed seronegative and leukoreduced components were not significantly different for TT-CMV risk,3 considerable controversy ensued4 that included the publication of a follow-up study suggesting that leukoreduced blood products may actually lead to a higher incidence of TT-CMV in transplant patients compared to the use of CMV-seronegative products.5 Swiss and Canadian consensus conferences debated these findings and concluded that seronegative components remain the “criterion standard” for CMV safety and that filtered units cannot yet be considered equivalent in this population.6,7
Transfusion of CMV-seronegative components has long been established as the criterion standard for transfusion of at-risk LBWIs based on the pioneering work of Yeager et al2 in the early 1980s. However, in contrast to BMT recipients, RCTs comparing seronegative products directly to leukoreduced transfusions have never been conducted in preterm neonates. Furthermore, the window of opportunity for such a study has passed. First, from an ethical perspective, the previous work by Yeager et al with seronegative units and the unanswered questions of safety with leukoreduced units for adult transplant recipients makes it difficult to justify given the degree of morbidity and potential for mortality in LBWIs, much less the use of a study arm in which blood components are leukoreduced but not screened for CMV antibodies.6 Second, from a practical standpoint, it is increasingly difficult to include a study arm where blood is not leukoreduced and only CMV seronegative because greater than 80% of blood components transfused to children in the United States are leukoreduced.8 As an example, in Atlanta, GA, where this study is being conducted, virtually 100% of units provided by the American Red Cross Blood Services to the participating hospitals are leukoreduced. Furthermore, in Canada, all blood products are leukoreduced, and in certain European countries, most of these are leukoreduced.6,9 Specifically, the Canadian Consensus Conference Statement, published in 2001, did not recommend the combined use of leukoreduction plus CMV-seronegative allogeneic blood for LBWIs. The combined approach for prevention of TT-CMV was recommended for the following groups: transfusions to CMV-seronegative pregnant women, intrauterine fetal transfusions, and CMV-seronegative allogeneic BMT recipients.9 Nonetheless, the scientific question of whether the combined strategy reduces the risk of TT-CMV in any vulnerable population is yet to be answered.6
Given the proven efficacy of seronegative units and the broad implementation of leukoreduction, it is clear that the de facto clinical standard for many neonatologists to prevent TT-CMV in LBWIs has become (or is inevitably becoming) the transfusion of components that are CMV seronegative plus leukoreduced.2,10 Based on these considerations, we believe that, for LBWIs at risk for TT-CMV, the most significant clinical question that remains to be addressed is whether CMV-seronegative plus leukoreduced transfusions actually provide a “zero CMV-risk” blood supply or whether further safety measures (CMV nucleic acid test [NAT] and 100% leukoreduction with unit-by-unit quality control) must necessarily be implemented to protect this extremely vulnerable patient population from CMV infection. This remains an open question because the efficacy and safety of combining serology and leukoreduction to prevent TT-CMV have not been tested in LBWIs or in any other immune-compromised populations.6
METHODS AND DESIGN OF THIS STUDY
Because a randomized controlled clinical trial is not feasible, we chose to address these questions through a combined approach in which we will investigate TT-CMV using LBWIs born to both CMV-seronegative and CMV-seropositive mothers while limiting the risk of vertical transmission by freezing, when possible, the breast milk before orogastric feeding.11 The overall goals of the study are to estimate and compare the incidence of (a) TT-CMV occurrence in LBWIs who receive CMV-seronegative plus leukoreduced blood components for transfusion by maternal CMV status and (b) TT-CMV disease and mortality in LBWIs who receive CMV-seronegative plus leukoreduced blood components for transfusion and to describe the course and outcome of TT-CMV in LBWIs who develop TT-CMV. The following covariates will be tested and analyzed with respect to TT-CMV in LBWIs: CMV infection status of the mother, method of feeding, maternal anti-CMV antibodies transmitted prepartum or via breast milk postpartum, residual white blood cell (WBC) counting to assess leukoreduction filter failures of blood components, and viremic window period detection of CMV in seronegative blood products. In addition to the formal commitment from attending neonatologists, the following factors established the feasibility of conducting this birth cohort study to address these important questions. First, sufficient numbers of LBWIs are born in Atlanta, GA, at the participating hospitals necessary to conduct this study with the required statistical power. Second, although LBWIs receive extensive transfusion support, their volume requirements are low and can be supplied with CMV-seronegative plus leukoreduced units during the proposed clinical study. A third aspect of this study’s feasibility is that most LBWIs in the neonatal intensive care unit (NICU) receive transfusions. A fourth point of study feasibility is the validation of the methods for (a) CMV NAT,12,13 (b) counting of residual WBCs in filtered units14 of transfused blood components to identify episodes of “filter failure,” and (c) a real-time polymerase chain reaction assay for testing of samples from these components to detect transfusions containing CMV DNA, that is, window-phase donations from seroconverting donors.15 To this end, a sufficiently powered, prospective observational cohort study was designed to validate practical testing and processing methodologies to produce optimally CMV-safe blood for transfusion to high-risk LBWIs.
This birth cohort study will provide a sufficiently large study population to adequately assess the incidence of TT-CMV in the 2 proposed study arms: infants born to CMV-negative mothers and those born to CMV-positive mothers. The participating hospitals have approximately 595 LBWIs admitted to the NICU per year (approximately 3000 will be born over the course of this study period). We anticipate that approximately 40% to 50% of our study population will be CMV negative. Thus, we anticipate enrolling 1300 LBWIs (650 born to CMV-negative mothers and 650 born to CMV-positive mothers) born at all hospitals over the course of 5 years. To accrue 1300 patients, it will be necessary to enroll approximately 43% of the eligible LBWIs.
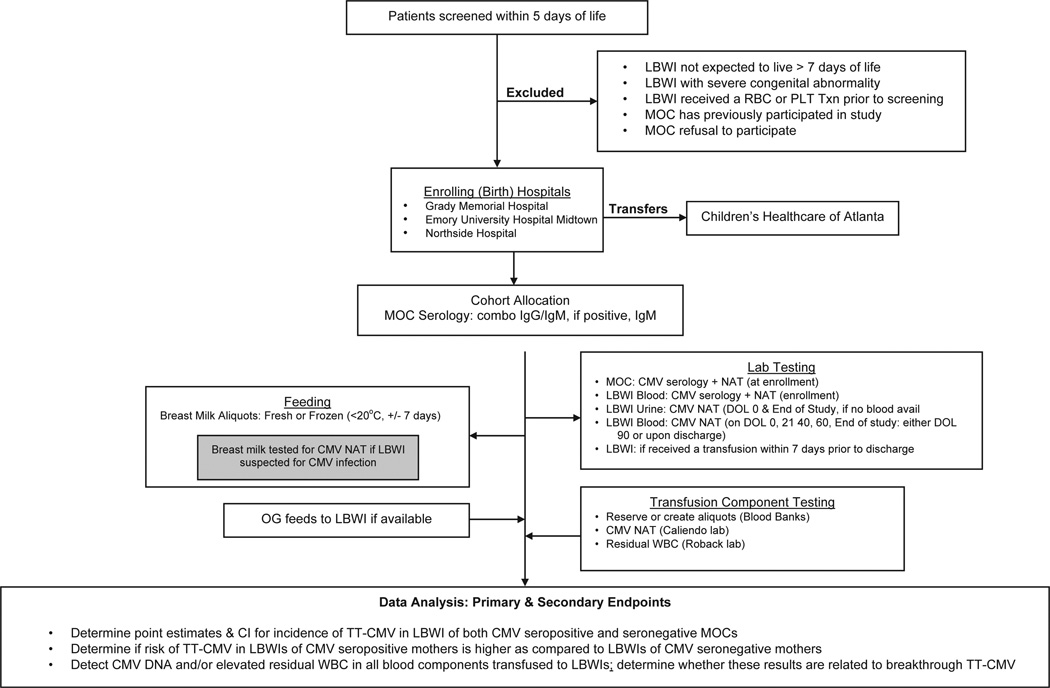
Study Schema
The study schema (see Figure 1) illustrates the recruitment/enrollment of patients, inclusion/exclusion criteria, testing control for CMV transmission other than from blood products, patient and blood component testing schema, and data analysis statements of primary and secondary end points of the study. The schema specifically provides an overview of the testing of mother, infant, breast milk, and blood components. An important and novel benefit of the proposed study design, which was not a component of previous TT-CMV trials, even large RCTs,3 is the association of transfusion recipients with aliquots from the blood components they receive. A secondary objective of this study will allow a detailed retrospective analysis of the association between CMV DNA and elevated WBC counts in transfused units and the development of TT-CMV in the recipient. In positive associations, the occurrences of TT-CMV can be correlated with prior receipt of blood components containing CMV DNA and elevated WBCs (>5 × 106/U). In addition, because segments archived from transfused units will be tested when CMV is suspected, we anticipate that circumstances will be identified in which units containing CMV DNA and/or elevated WBC counts will not produce TT-CMV in the LBWI. This will represent novel data that previously had not been obtained, which should lead to new and more detailed insights into the factors affecting the likelihood that blood components can transmit CMV infection to susceptible transfusion recipients.
Fig 1.
Study schema.
Abbreviations: LBWI, low birth weight infant; CMV, cytomegalovirus; TT-CMV, transfusion transmitted CMV; RBC, red blood cell; PLT, platelet; Txn, transfusion; °C, Celsius; MOC, mother of child; NAT, nucleic acid test; OG, orogastric; DOL, day of life; WBC, white blood cell; CI, confidence interval.
Study Population
We anticipate that the mother of the child (MOC) will be recruited in proportion to the representation of women, Anglos, Black (African Americans), Asians, Latinos (Hispanics), and Native Americans or other demographics within the participating NICUs. Inclusion/exclusion criteria detailed below will not exclude patients, either the MOC or LBWIs, by ethnicity. An analysis of patients (MOC and LBWIs) admitted during 2003 at all participating institutions showed that, on average, they were more than 50% women and more than 25% minorities (Black [African American], Latino [Hispanic], and Asian). Because these demographic data are consistent with our long-term observations, we anticipate that adequate representation of minority LBWIs, hence, will not pursue “outreach” activities to increase these groups’ enrollment.
Potentially eligible LBWIs will be identified by their neonatologist, the mother’s obstetrician, or medical record review by a research staff member. Accrual of LBWIs will not be limited to a certain number of infants per year; rather, all eligible LBWIs will be included at time of eligibility assessment. All MOCs and their LBWIs in any of the 3 birthing hospitals will be screened using defined inclusion/exclusion criteria. Study participants will be LBWIs who are expected to receive allogeneic blood transfusions. The mothers of LBWIs who agree to participate in the study and meet the criteria for inclusion will have the study presented to them during the informed consent process.
Eligibility/Consent
This observational clinical study will be conducted at the 2 Emory University–affiliated hospitals and a community-based hospital. The protocol, informed consent, and study brochure have been reviewed and approved by the institutional review board or research oversight committees of each institution. The protection of participants who meet the eligibility criteria for this study will be implemented for both the mother and her LBWI in a single informed consent document. When possible, the parent(s) of the premature infant shall be approached before their infant’s birth so as to allot adequate time to contemplate their own (mother) and their infant’s participation in the study.
The parent(s) will be given the opportunity to participate in this birth cohort study by prospectively observing mothers and LBWIs for CMV transmission, infection, and disease during the infant’s neonatal intensive care hospital course. In each case, the attending neonatologist or clinical research nurse will verbally explain the study and provide a study brochure that outlines the study’s rationale, the inclusion and exclusion criteria, length of study duration, schedule of study measurements and/or observations, potential risks and benefits, and alternatives to study participation. The study staff will address any questions, and after the verbal acknowledgment to volunteer herself and her newborn for the study, the research nurse will proceed to obtain written consent.
Allocation of infants to either cohort will occur only after consent is obtained and the infant meets the inclusion criteria (birth weight is ≤1500 g at birth, and the LBWI is within first 5 days of life). Assurance of the infant’s exclusion criteria (viz, that the LBWI is not expected to live past its first 7 days of life, has a severe congenital abnormality, has received a red blood cell [RBC] or platelet transfusion before enrollment, or has active CMV infection) will be attained by rigorous initial examination and close evaluation by the infants’ neonatologists. However, if for any reason and at any time during the screening process and after consent is obtained, the infant is determined to be ineligible for the study, both the mother and infant will be withdrawn from the study.
Cohort Allocation
To the extent that eligibility has been established and subjects have been enrolled, the CMV status of the mother will be evaluated by a combination serology assay to detect both IgG and IgM anti-CMV antibodies and CMV NAT results. All LBWIs will be eligible by weight, irrespective of maternal CMV status, and each LBWI will be assigned to 1 of 2 cohorts based on maternal CMV serology results. If the maternal CMV serology is positive, the same sample will be retested with an IgM-specific assay to determine whether active and/or recent infection is present. Cytomegalovirus-seronegative mothers will have repeat serum CMV NAT and serology testing at the time their infant completes the study (on day 90 or upon discharge from hospital, whichever occurs first) to rule out community-acquired CMV.
Vigilance/Observation
The occurrence of CMV infection and disease will be prospectively tracked in all LBWIs, regardless of their mother’s CMV status, through CMV NAT of serum and urine. Low–birth weight infants will be serologically assessed (blood samples previously drawn for standard laboratory tests) upon enrollment (day 1, days 21, 40, 60, and end of study [discharge day or day 90, whichever occurs first]) for evidence of CMV infection. Blood and urine will be evaluated for asymptomatic viremia (by CMV NAT) as well as clinical examination of the LBWI for CMV disease (fever or fever of unknown origin, pneumonitis, hepatitis, and abnormal hematologic indices). In the event of clinical or laboratory suspicion of CMV, clinicians will order standard CMV-related tests on blood, urine, and/or breast milk, and these result will be recorded for the study.
Previous studies have shown that CMV DNA and infectious virus are not usually detected in breast milk until about 3 weeks postparturition,1 although it has been seen earlier in some studies,1 and viral loads of at least 103 CMV genome equivalent per milliliter appear to be needed for transmission.1 In the event that an infant being fed breast milk is found to be CMV NAT positive during the study, the breast milk will be tested for CMV DNA using a real-time polymerase chain reaction assay, which may determine CMV transmission by this route.
All RBC and platelet transfusion products administered will be CMV seronegative and leukoreduced, and records of all transfusions will be maintained. Before issuance, preformed segments from RBC parent units will be removed, or segments will be made from parent units of other products (platelets and cryoprecipitate) ordered for transfusion. These segments will be reserved for CMV NAT and quantitation of residual WBCs. To detect CMV DNA and/or elevated residual WBC counts in blood components transfused to LBWIs and to correlate positive results with episodes of breakthrough TT-CMV, quantitation of residual WBCs in leukoreduced components will be performed by a completely automated flow cytometric method. To measure the association between CMV DNA and elevated WBC counts in transfused units and the development of TT-CMV in the recipient, all peripheral blood samples from LBWIs as well as segments from transfused units will be tested by CMV NAT. Failures will be defined as breakthroughs of TT-CMV and/or failures of WBC leukoreduction (quality control). If a LBWI tests positive for CMV NAT during the study, the breast milk and blood product administered will be tested subsequently outside the routine batching and testing regimen. Lastly, infants of potentially infected mothers will be followed up in their cohort despite confirmation of vertical transmission yet will be excluded from the analysis of TT-CMV transmission (CMV NAT + residual WBCs).
Primary Outcome
According to reports in the literature, breakthrough TT-CMV infection will occur at low rates (<2.5% incidence) in LBWIs of CMV-negative mothers transfused with seronegative plus leukoreduced blood components. After excluding cases of in utero transmission, the risk of TT-CMV in LBWIs of CMV-positive mothers may be higher than that in LBWIs of CMV-negative mothers. All cases of TT-CMV will be analyzed (in the secondary aim) to determine whether they could have been prevented by CMV NAT plus 100% leukoreduction quality control. Thus, the primary aim of this birth cohort study is to estimate the incidence of TT-CMV in LBWIs who receive a combination of CMV-seronegative plus leukoreduced blood products. That is to say, the effectiveness of the 2 strategies coupled together will be assessed for the prevention of TT-CMV in at-risk LBWI born to transmission (CMV NAT + residual WBCs).
Secondary Outcomes
The secondary aim of this study is to detect CMV DNA and/or elevated residual WBC counts in blood components transfused to LBWIs and to determine whether these results are related to episodes of breakthrough TT-CMV in this study population. The following 2 hypotheses relate to this second aim: the risk of TT-CMV will be increased (a) in the population of LBWIs who receive leukoreduced units with WBC filter failures and (b) in those who receive CMV NAT– positive transfusions. Breakthrough episodes of TT-CMV will be identified, and for each case of breakthrough, testing will help examine the temporal relationship between transfusion of RBC and platelet units containing CMV DNA and/or elevated WBC and the development of TT-CMV in the recipient. Further testing will identify multiple episodes, where units that contained CMV DNA or failed leukoreduction did not produce TT-CMV in the recipient. This will allow an analysis of the transmission (CMV NAT + residual WBCs).
Sample Size Considerations
The primary end point of this birth cohort is to estimate the incidence of CMV infection and disease in LBWIs after receiving CMV-seronegative plus leukoreduced blood components for transfusion during their NICU hospital course. To estimate the incidence of CMV infection, confidence intervals (CI) can be used. If the CMV infection rate is as low as 2.5% (95% CI, 1.5%-4.0%) in CMV-seropositive mothers (16 LBWIs with CMV) and 0.5% (95% CI, 0.2%-1.3%) in CMV-seronegative mothers (3 LBWIs with CMV), which we believe are reasonable a priori estimates, then with a sample size of 650 per group, the 95% CI for the percentage of difference (2%) is 0.7% to 3.5%.16 The participating hospitals have approximately 595 NICU admissions of LBWIs per year or 3000 that will have been born over the course of this study. To accrue 1300 patients for this study, it will be necessary to enroll approximately 43% of the eligible LBWIs.
The analysis of primary end points will be summarized using proportions or from Kaplan-Meier analyses of cumulative incidence. Cumulative rates of CMV infection, CMV disease, and mortality will be estimated among LBWIs who were free of congenital CMV infection. The 20-day, 40-day, and 60-day cumulative incidence rates of CMV infection, CMV disease, and mortality will be calculated by maternal CMV status for the birth cohort of LBWIs. Log-rank tests will be used to compare incidence rates according to maternal CMV status. Relative risks will be calculated to measure the degree of association between baseline factors (maternal CMV status, hospital site, method of feeding, maternal anti-CMV antibodies, and leukoreduction filter failures) and outcome (CMV infection, CMV disease, and mortality) by fitting the Cox proportional hazards regression model separately for each baseline factor. Most infants will receive multiple blood product transfusions. To examine the temporal relation between blood product transfusion and CMV infection, we will include blood product transfusion as a time-dependent covariate (CMV negative or CMV positive) in a Cox regression model of time to CMV infection.
Data Management/Monitoring
The data coordinating center for the study will be responsible for (a) monitoring the progress of screening, recruitment, enrollment, and follow-up; (b) providing data management and instituting quality control for all data to ensure the accuracy and reliability of the data including data integrity; and (c) performing statistical analyses for the interim and final analyses that will be communicated to the National Heart, Lung, and Blood Institute.
All LBWIs will be transfused at the discretion of their neonatologist using what has become the standard of care, that is, CMV-seronegative plus leukoreduced units. Adverse events (AEs) are defined as any unfavorable or unintended change in structure, function, signs, and symptoms temporally associated with transfusion of blood component in either study group (CMV-seronegative mothers or CMV-seropositive mothers), whether a causal relationship with the study has been established. Adverse events and serious AEs, related and unrelated to transfusion strategies to prevent TT-CMV and other unexpected outcomes, will be tabulated. Lastly, because the LBWIs will not be specifically phlebotomized for study purposes (CMV NAT), potential protocol-specific risks are not anticipated in this observational birth cohort study.
Enrollment to Date
To date, the 3 (birth) enrolling hospitals have screened 168 infants, and 73 have been enrolled in the study. Of those enrolled, 40 LBWIs have completed study participation, with 2 subjects having been withdrawn (1 infant’s post–birth weight estimate exceeded the pre–birth weight estimate and the other due to the inability to collect the mother’s blood for initial testing), and 5 LBWIs were transferred to the other participating site for surgical intervention and/or follow-up of secondary comorbidities such as necrotizing enterocolitis, retinopathy of prematurity, repair of patent ductus arteriosus, and bronchopulmonary dysplasia.
SUMMARY
Despite concerns regarding transfusion-transmitted viruses, transfusions clearly save the lives of patients in NICUs. Premature LBWIs (≤1500 g) provide an ideal population to study the clinical consequences of TT-CMV as they possess both a high risk of transfusion and significant morbidity and mortality, owing to this disease. Moreover, and of significance, clinical equipoise currently exists among neonatologists and transfusion medicine experts about the reasons for the residual risk of breakthrough infections with filtered, leukocyte-reduced transfusions and/or CMV-seronegative transfusions. The highest quality RCT to date on the risks of TT-CMV in immune-compromised seronegative transfusion recipients compared filtered, leukocyte-reduced blood products and CMV-seronegative blood products for the prevention of transfusion-associated CMV infection (after marrow transplant).3 However, this study did not shed light on the reasons for the residual risk of breakthrough infections with either type of transfusion.
The failure to prevent TT-CMV with seronegative units is usually attributed to donors in the window phase (infectious yet seronegative) of an infection,17 whereas leukoreduced units may transmit CMV if the leukoreduction filters fail to remove a sufficient fraction of CMV-infected WBCs whether because of mechanical problems or high viral loads.18,19 Typically, the motivation to transfuse units that are both CMV seronegative plus leukoreduced is based on the concept that these overlapping safety measures would essentially eliminate TT-CMV. However, there is also support for the alternative hypothesis that the efficacy of these 2 methods to prevent TT-CMV may not be additive. For example, window-phase and seroconverting subjects show high levels of free CMV virus in the plasma as well as WBC-associated virus.20,21 However, although filtration would capture many of the CMV-infected leukocytes, it does not significantly remove plasma-free virus, which is highly infectious.22 On the one hand, if this observational study can demonstrate that the combined strategies alone are sufficient to decrease harmful sequalae in this immune-compromised patient group, such data will provide for a more definitive answer to this previously untested hypothesis. On the other hand, because TT-CMV has been associated with an increased risk of mortality and morbidity in critically ill neonates, this study could reveal the need for additional prevention strategies (eg, leukoreduction quality control and/or CMV NAT) that would result in long-term clinically beneficial consequences in this extremely vulnerable population.
Acknowledgments
Support. Supported in part by NIH awards HL088922, HL086773 (C.J.), HD059142, D043397, HL086773 (J.R.), and P30 AI050409 (A.C.).
REFERENCES
- 1.Forsgren M. Cytomegalovirus in breast milk: Reassessment of pasteurization and freeze-thawing. Pediatr Res. 2004;56:526–528. doi: 10.1203/01.PDR.0000143155.84802.A3. [DOI] [PubMed] [Google Scholar]
- 2.Yeager A, Grumet F, Hafleigh E, et al. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98:281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- 3.Bowden R, Slichter S, Sayers M, et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86:3598–3603. [PubMed] [Google Scholar]
- 4.Landaw E, Kanter M, Petz L. Safety of filtered leukocyte-reduced blood products for prevention of transfusion-associated cytomegalovirus infection. Blood. 1996;87:4910. (letter) [PubMed] [Google Scholar]
- 5.Nichols W, Price T, Gooley T, et al. Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood. 2003;101:4195–4200. doi: 10.1182/blood-2002-10-3143. [DOI] [PubMed] [Google Scholar]
- 6.Blajchman M, Goldman M, Freedman J, et al. Proceedings of a consensus conference: Prevention of post-transfusion CMV in the era of universal leukoreduction. Transfus Med Rev. 2001;15:1–20. doi: 10.1053/tmrv.2001.19946. [DOI] [PubMed] [Google Scholar]
- 7.Zwicky C, Tissot J, Mazouni Z, et al. Prevention of post-transfusion cytomegalovirus infection: Recommendations for clinical practice. Schweiz Med Wochenschr. 1999;129:29–30. [PubMed] [Google Scholar]
- 8.Spinella PC, Dressler A, Tucci M, et al. Survey of transfusion policies at US and Canadian children’s hospitals in 2008 and 2009. Transfusion. 2010;50:2328–2335. doi: 10.1111/j.1537-2995.2010.02708.x. [DOI] [PubMed] [Google Scholar]
- 9.Laupacis A, Brown J, Costello B, et al. Prevention of posttransfusion CMV in the era of universal WBC reduction: A consensus statement. Transfusion. 2001;41:560–569. doi: 10.1046/j.1537-2995.2001.41040560.x. [DOI] [PubMed] [Google Scholar]
- 10.American Association of Blood Banks: Association Bulletin #97-2. Leukocyte reduction for the prevention of transfusion-transmitted cytomegalovirus (TT-CMV) 1997 [Google Scholar]
- 11.Kerrey B, Morrow A, Braunwart J, et al. Pediatric Academic Societies’ Annual Meeting. San Francisco, CA: 2004. A cell culture system for examination of the effect of freezing on the recovery of cytomegalovirus from human breast milk. (abstract) [Google Scholar]
- 12.Roback JD, Hillyer CD, Drew W, et al. Multicenter evaluation of PCR methods for detecting CMV DNA in blood donors. Transfusion. 2001;41:1249–1257. doi: 10.1046/j.1537-2995.2001.41101249.x. [DOI] [PubMed] [Google Scholar]
- 13.Roback JD, Drew WL, Laycock ME, et al. CMV DNA is rarely detected in healthy blood donors using validated PCR assays. Transfusion. 2003;43:314–321. doi: 10.1046/j.1537-2995.2003.00312.x. [comment] [DOI] [PubMed] [Google Scholar]
- 14.Roback JD, Barclay S, Hillyer CD. Automated quantitation of residual WBCs in filtered components by capillary cytometry; American Association of Blood Banks Annual Meeting; 2004. (abstract) [Google Scholar]
- 15.Drew WL, Tegtmeier G, Alter HJ, et al. Frequency and duration of plasma CMV viremia in seroconverting blood donors and recipients. Transfusion. 2003;43:309–313. doi: 10.1046/j.1537-2995.2003.00337.x. [comment] [DOI] [PubMed] [Google Scholar]
- 16.Hamprecht KJ, Maschmann M, Vochem K, et al. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 17.Newcombe RG. Interval estimation for the difference between independent proportions: Comparison of eleven methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Roback JD. CMV and blood transfusions. Rev Med Virol. 2002;12:211–219. doi: 10.1002/rmv.353. [DOI] [PubMed] [Google Scholar]
- 19.Dumont LJ, Luka J, VandenBroeke T, et al. The effect of leukocyte-reduction method on the amount of human cytomegalovirus in blood products: A comparison of apheresis and filtration methods. Blood. 2001;97:3640–3647. doi: 10.1182/blood.v97.11.3640. [DOI] [PubMed] [Google Scholar]
- 20.Visconti MR, Pennington J, Garner SFL, et al. Assessment of removal of human cytomegalovirus from blood components by leukocyte depletion filters using real-time quantitative PCR. Blood. 2004;103:1137–1139. doi: 10.1182/blood-2003-03-0762. [DOI] [PubMed] [Google Scholar]
- 21.Zanghellini F, Boppana SB, Emery VC, et al. Asymptomatic primary cytomegalovirus infection: Virologic and immunologic features. J Infect Dis. 1999;180:702–707. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]
- 22.Roback JD, Buccolo L, Hillyer CD. Effects of filtration and gamma-irradiation on cytomegalovirus (CMV) infectivity and replication: Development of a rhesus macaque transfusion model. Blood. 1997;90:3330. (abstract) [Google Scholar]