Abstract
Nitric oxide (NO) is an inhibitory signalling molecule in the gastrointestinal (GI) tract that is released from neurons and from leucocytes during inflammation. NO stimulates soluble guanylate cyclase (sGC), elevates cyclic guanosine 3′,5′-monophospate (cGMP), and subsequently activates cGMP-dependent protein kinase (PKG). Targets for NO in the guinea pig caecum were investigated by characterizing the cellular distribution of sGC, cGMP and PKG. Immunoreactivity for both isoforms of sGC, sGCα1 and sGCβ1, was observed in the interstitial cells of Cajal (ICC) and enteric neurons in the tunica muscularis. Double labelling with anti-Kit and anti-sGC antibodies showed sGCα1 and sGCβ1-like immunoreactivity (LI) in almost all intramuscular (IM) and myenteric ICC. Neuronal processes with neuronal NO synthase were closely apposed to ICC expressing sGC-LI. Cells with sGC-LI possessed ultrastructural features of ICC-IM: caveolae, close association with nerve bundles and contacts with smooth muscle cells (SMC). Sodium nitroprusside, added with the phosphodiesterase inhibitors (3-isobutyl-1-methylxanthine and zaprinast), enhanced cGMP-LI in almost all ICC and in some enteric neurons. Nerve stimulation also increased cGMP-LI in ICC and enteric neurons. In contrast, no resolvable increase in cGMP-LI was observed in any cells when the sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one was present. ICC and SMC also expressed PKG type I-LI. These data show that ICC express the downstream signalling molecules necessary to transduce nitrergic signals and activate inhibitory pathways and thus are primary targets for NO released from neurons and other cells in the GI tract.
Keywords: cyclic guanosine 3′, 5′-monophospate; cyclic guanosine 3′, 5′-monophospate-dependent protein kinase; enteric nervous system; Kit receptor tyrosine kinase; nitric oxide; soluble guanylate cyclase
INTRODUCTION
Nitric oxide (NO) is a free radical produced from L-arginine by multiple nitric oxide synthase (NOS) enzymes which diffuses freely across biological membranes to reach intracellular targets.1 The main target for NO appears to be a cytosolic heterodimer of α and β subunits known as soluble guanylate cyclase (sGC).2,3 Upon NO binding, sGC catalyzes the formation of cyclic guanosine 3′,5′-monophospate (cGMP) from guanosine 5′-triphosphate. cGMP activates a variety of effector molecules, including cGMP-dependent protein kinases (PKG), phosphodiesterases and cyclic nucleotide-gated ion channels.4,5 NO signalling is an important inhibitory pathway in the gastrointestinal (GI) tract. When NO is released, elevated levels of cGMP activate PKG,6–8 and effector proteins, such as 2-pore K+ channels, that reduce electrical excitability and cause relaxation of GI muscles, are phosphorylated and activated.9,10
Kit receptor tyrosine kinase expressing cells known as interstitial cells of Cajal (ICC) play an important role inNO-dependent signal transduction in theGItract.11–13 One population of ICC lies within muscle bundles [ICC-intramuscular (IM)] in close synaptic-like contact with enteric motor neurons that express neuronal NOS (nNOS).14–17 Physiological experiments have demonstrated that ICC-IM are an important postjunctional mediator of nitrergic neurotransmission, because muscles lacking ICC-IM have greatly reduced responses to inhibitory nerve stimulation.18–21 The concept developed from these studies is that a significant portion of inhibitory motor neurotransmission occurs via ICC-IM. The hypothesis is that receptors for NO are expressed in ICC-IM and signal transduction occurs in ICC-IM causing hyperpolarization and stabilization of membrane potential. Electrical coupling between ICC-IM and smooth muscle cells (SMC) conveys inhibitory signals to the smooth muscle syncytium at large.
Previous studies have shown expression of signalling proteins for nitrergic responses in ICC that are closely associated with enteric motor neurons expressing nNOS.22–25 These proteins were unresolvable in SMC near nerve terminals, suggesting that ICC play an important role in transducing nitrergic inhibitory neural signals. Others have questioned the importance of ICC in enteric nitrergic neurotransmission and reported evidence of relaxations that are sensitive to inhibitors of NOS in animals with reduced populations of ICC.26 These data may indicate that ICC are not the singular postjunctional target for nitrergic neurotransmission. Almost all of the evidence of involvement of ICC in enteric motor neurotransmission comes from studies of rodents. To determine whether ICC are targets for enteric motor neurotransmission in additional species, techniques are needed to directly monitor postjunctional responses of ICC, because ICC-deficient Kit mutants are not readily available in most animal models or humans.
In the present study, we have investigated the signalling cascade for nitrergic neurotransmission in a classic model of enteric inhibitory neurotransmission, the guinea pig caecum.27,28 We used immunohistochemical techniques to localize the functional subunits of NO receptors, sGCα1 and sGCβ1, and the primary effector molecule, PKG. We used functional immunohistochemistry to monitor the responsiveness of cells within the tunica muscularis to nitrergic stimulation. Double immunohistochemical labelling for Kit identified ICC,12,29 and demonstrated that these are the major postjunctional cells in which cGMP responses can be resolved in response to inhibitory nerve stimulation.
MATERIALS AND METHODS
Animals
Female Hartley guinea pigs (200–400 g) and female BALB/c mice (15–25 g) were purchased from Japan SLC (Hamamatus, Japan). All animals were anaesthetized by diethyl ether inhalation and exsanguinated following cervical dislocation. The use and treatment of animals followed the Guidelines for Animal Experiments, University of Fukui Faculty of Medical Sciences.
Immunohistochemical studies
For cryostat studies,25,30 caeca from five guinea pigs were flushed with Krebs’ Ringers buffer (KRB, pH 7.3–7.4) before being pinned to the Sylgard elastomer (Dow Corning Corp., Walnut, CA, USA) floor of a dissecting dish and fixed with Zamboni’s fixative (2% paraformaldehyde and 1.5% saturated picric acid solution in 0.1 mol L−1 phosphate buffer, pH 7.3). Following fixation, tissues were washed with phosphate-buffered saline (PBS) (0.01 mol L−1, PBS, pH 7.4) and embedded in Tissue-Tek (Miles, Elkhart, IN, USA). Sections (12 μm) were cut using a cryostat and collected on glass slides. Sections were preincubated with 5% normal donkey serum before being incubated with rabbit antisGCα1 (G4280, 1 : 1000; Sigma-Aldrich, St Louis, MO, USA), rabbit anti-sGCβ1 (G4405, 1 : 1000; Sigma- Aldrich), sheep anti-cGMP (1 : 4000; Dr J. de Vente, Maastricht University, The Netherlands),31 goat anti- Kit (SC-168, 1 : 1000; Santa Cruz Biotechnology, Santa Cruz, AC, USA), rabbit anti-Kit (PC34, 1 : 500; Oncogene Research Products, San Diego, CA, USA), rabbit anti-PKG type I (PKGI, KAS-PK005, 1 : 2000; Stressgen Biotechnologies, Victoria, Canada), sheep anti-nNOS (1 : 3000; Dr P.C. Emson, The Babraham Institute, UK),32 rabbit anti-PGP9.5 (RA95101, 1 : 8000; Ultra- Clone Ltd, Cambridge, UK) antibodies. Tissues were incubated in secondary antibodies (Alexa Fluor-488 or 555-coupled donkey anti-IgG; Molecular Probes, Eugene, OR, USA, 1 : 400). Specimens were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). The specificity of the antibodies was checked by immunoblot analysis as previously described.25
For whole-mount preparations, stretched caeca were fixed with Zamboni’s fixative, washed with PBS and the mucosa removed by sharp dissection. The tunica muscularis was subsequently washed with PBS containing 0.3% Triton X-100 and incubated with normal donkey serum and antibodies for at least 48 h at 4 °C.
A tyramide signal amplification (TSA) method was used to enable allow us to double-label tissues with rabbit anti-sGCα1 and rabbit anti-sGCβ1. Fluorescent staining with the first primary antibody was enhanced with TSA, and conventional fluorescent staining was then performed with the second primary antibody.33 The first primary antibodies were used at a dilution so low (1 : 50 000) that antigen consistently failed to be recognized by conventional fluorescence secondary antibodies, but was still clearly detectable with the TSA method. After incubation in primary antibody, sections were reacted with biotinylated goat anti-rabbit IgG (Vector; 1 : 200). Biotin was localized by FITC conjugated to TSA (NEN Life Science Products, Boston, MA, USA) according to the manufacturer’s recommendations. The second primary antibody (1 : 1000) was then applied. Immunoreactivity was visualized by Alexa Fluor-555 anti-rabbit IgG. We obtained identical results when the order of the two primary antibodies was reversed.
Tissues were examined with a TCS-SP2 confocal microscope (Leica, Wetzlar, Germany) and images collected and measured using Leica Confocal Software. Immunofluorescence intensity of cGMP was quantified on cryostat specimens from three animals using Leica Software. Sixty cells from each specimen with or without Kit immunoreactivity were sampled randomly. Fluorescence intensities were converted into grey values ranging from zero (black) to 265 (white). Data were expressed as mean ± SEM. A multiple comparison test and t-test were used to investigate the statistical significance.
Immunoelectron microscopic studies
The caeca from five guinea pigs were fixed with Zamboni’s fixative plus 0.1% glutaraldehyde (immunoelectron microscopy25,30) or with a solution containing 3% glutaraldehyde and 4% paraformaldehyde (conventional electron microscopy16). The cryostat sections of full-thickness specimens were reacted with anti-sGCβ1 (1 : 3000), then with biotinylated anti-rabbit IgG and with avidin-biotin-peroxidase complex (Vector). Sections were incubated with a solution containing diaminobenzidine in 0.1 mol L−1 Tris–HCl. The specimens were postfixed in OsO4 and en-block stained with uranyl acetate solution, dehydrated in ethanol and embedded in Epon 812 (Oken, Tokyo, Japan). Ultrathin sections were examined using an H-7000 transmission electron microscope (Hitachi, Tokyo, Japan).
Functional immunohistochemical studies
For functional studies,34 caeca from 12 animals were removed and placed in KRB. The tunica muscularis of each caecum was cut into four pieces (25 × 15 mm) and pinned to the base of small dishes. Tissues were allowed to equilibrate in oxygenated KRB (97% O2–3% CO2) at 37.5 ± 0.5 °C for 60 min. To examine responses to the exogenous application of sodium nitroprusside (SNP; Sigma, 1 mmol L−1), Nω-nitro-L-arginine (L-NNA; Sigma, 100 μmol L−1) and tetrodotoxin (TTX; Sigma, 1 μmol L−1) were added to the KRB for 15 min before experiments were initiated. Tissues were exposed to 3-isobutyl-1-methylxanthine (IBMX; Sigma, 1 mmol L−1) and zaprinast (Sigma; 100 μmol L−1) for 5 min, and then to SNP for 15 min. Some tissues were exposed to 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; Sigma, 10 μmol L−1). After SNP, the tissues were rapidly transferred to Zamboni’s fixative.
To examine cellular responses to enteric nerve stimulation, tissues were cut into strips (5 × 10 mm) and pinned to Sylgard panels. Tissues were immersed in an organ bath and allowed to equilibrate in oxygenated KRB. Five minutes before experiments, IBMX (1 mmol L−1) and zaprinast (100 μmol L−1) were added to the KRB. Neural responses were initiated by electrical field stimulation (EFS; 0.5 ms duration, 5 Hz @ 50 V, 5 min train durations) delivered via platinum electrodes placed on either side of the muscle strips and connected to a square wave pulse generator (SEN-3301, Nihon Kohden, Tokyo, Japan). After stimulation, tissues were rapidly transferred to Zamboni’s fixative.
Immunoblot analysis
The tunica muscularis of the small intestine from guinea pigs and mice were homogenized in 20 mmol L−1 Tris–HCl (pH 7.5), 1 mmol L−1 EDTA as previously described.25 Proteins separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis were transferred to a polyvinylidene difluoride membrane. Membranes were blocked with 5% fat-free dry milk and incubated in anti-PKGI (1 : 2000) antibody. Immune complexes were visualized by using an ECL chemiluminescence system (Amersham Pharmacia, Buckinghamshire, UK).
RESULTS
In a previous study, we briefly described the expression of sGCβ1-like immunoreactivity (sGCβ1-LI) and the proximity of cells with sGCβ1-LI to nerve fibres containing nNOS in the caecum.25 In the present study, we further detailed this expression by quantifying the co-localization of sGCβ1-LI with Kit and investigated whether sGCβ1-LI co-localizes with sGCα1, downstream signalling molecules and production of cGMP in response to nitrergic stimulation. Cryostat sections revealed intense sGCβ1-LI in cells within the tunica muscularis and in the plane of the myenteric plexus (Fig. 1A). In whole-mount preparations, sGCβ1-LI was observed in cells running parallel to the long axis of circular and longitudinal SMC (Fig. 1C). Cells with sGCβ1-LI were spindle shaped and had a prominent nuclear region and long slender processes. Double labelling with sGCβ1 and Kit antibodies revealed that the cells expressing sGCβ1- LI also displayed Kit-LI (Fig. 1A and C). The morphological features of the cells demonstrate that cells with sGCβ1-LI were ICC-IM. Immunohistochemical techniques failed to resolve sGCβ1-LI in SMC. sGCβ1-LI was also expressed in a subpopulation of myenteric neurons (Fig. 1A).
Figure 1.
Immunohistochemical localization of sGC isoforms and nNOS in the guinea pig caecum. Panel (A) Distribution of sGCβ1 (green) and Kit (red) in the circular muscle layer and the myenteric plexus region. Intramuscular ICC (ICC-IM) within the circular muscle layer (arrows) and ICC-MY in the myenteric plexus region (arrowheads) showed intense sGCβ1-LI. Myenteric ganglia (G) showed diffuse sGCβ1-LI. Panel (B) A cross-section through the circular muscle layer showing sGCα1-LI (green) and sGCβ1-LI (red) using the TSA method. sGCα1-LI and sGCβ1-LI were detected in cells within the circular layer (arrows) and in the myenteric plexus (G) region (arrowheads). Panels (C) and (D) Whole-mount preparations of the circular muscle layer showing co-localization of sGCβ1 (Panel C, green), sGCα1 (Panel D, green) and Kit in ICC-IM (red). ICC-IM (arrows) ran parallel to the circular muscle fibres and expressed intense sGC-LI. Panel (E) sGCα1-LI (green) and sGCβ1-LI (red) in a whole-mount preparation of the circular muscle layer revealed using the TSA method. sGCα1-LI was co-localized with sGCβ1-LI. Panel (F) A cross-section through the circular muscle layer revealing nNOS-immunopositive nerve fibres (red) and sGCβ1-immunopositive cells (green). Most nNOS nerve fibres were in close apposition to sGCβ1-immunopositive cells (arrows). Myenteric ganglia (G) contained sGCβ1-immunopositive neurons. Panel (G) nNOS-immunopositive nerve terminals (arrowheads) ran in close apposition to sGCβ1-immunopositive cells for more than 100 μm. Scale bars represent 50 μm for panels (A, B and F) and 20 μm for panels (C–E and G).
The relative number of ICC-IM in the circular muscle layer that were sGCβ1-immunopositive were counted in whole-mounts of four animals double labelled with sGCβ1 and Kit antibodies. Of 1696 ICC-IM (i.e. Kit-LI), 99.1% (1681 cells) showed immunoreactivity for sGCβ1. We found only a few Kit-positive ICC-IM where sGCβ1-LI was undetectable and only a few cells with sGCβ1-LI that were not labelled by Kit antibody.
We also examined the expression of sGCα1-LI, which is a functional partner of sGCβ1.3 The expression patterns of sGCα1-LI were the same as the distribution of sGCβ1 (Fig. 1B and D). Because dimerization of sGCα1 and sGCβ1 subunits is required for enzymatic activity of sGC, we performed TSA double immunolabelling to examine the co-localization of sGCα1-LI and sGCβ1-LI (Fig. 1B and E). Cellular co-localization of the two subunits was common in most cells within the tunica muscularis. Using the TSA method, 99.3% of ICC-IM with amplified sGCα1-LI (i.e. of 1038 cells from two animals) also showed sGCβ1-LI. Only seven cells showed sGCβ1-LI but lacked sGCα1-LI. Conversely, 99.1% of ICC-IM with amplified sGCβ1-LI (995 cells from two animals) showed sGCα1-LI, and only nine cells with sGCα1-LI, lacked sGCβ1-LI.
For ICC-IM to be functionally innervated by enteric inhibitory neurons, ICC must be associated with nerve fibres containing nNOS. Thus, we examined the morphological relationship between nNOS-immunopositive nerve fibres and ICC-IM. We found that varicose nerve fibres with nNOS-LI were closely associated with sGCβ1-immunopositive ICC-IM (Fig. 1F and G). Punctate labelling, indicative of nerve varicosities with nNOS-LI, ran along the surfaces of ICC-IM.
Electron microscopic studies were also performed to verify the cellular identities and to study the ultrastructural features of sGCβ1-immunopositive cells in the circular muscle layer of caecum (Fig. 2A and B). Cells with sGCβ1-LI were located within muscle bundles and in the connective tissue septa that separated circular muscle bundles. These cells were closely associated with nerve terminals and made close contacts with neighbouring SMC. Cells with sGCβ1-LI were rich in mitochondria and rough and smooth endoplasmic reticulum, possessed well-developed Golgi apparatus and caveolae, and lacked thick myofilaments. These features corresponded to the structural features of ICC-IM as determined by conventional electron microscopy (Fig. 2A).
Figure 2.
Ultrastructure of ICC and sGCβ1-immunopositive cells within the circular muscle layer of the guinea pig caecum. Panel (A) is a conventional electron micrograph showing ICC-IM making close contact (arrow) with an adjacent smooth muscle cell. The ICCIM is also in close association with a nerve bundle (N). Caveolae (arrowheads) were distinct along the cell membrane and distinguish these cells from fibroblasts. Panel (B) A sGCβ1-immunopositive cell making close contacts (arrows) with a neighbouring smooth muscle cell. sGCβ1-immunopositive cell was also closely associated with nerve bundles (N). Scale bars represent 1 μm.
As sGC is the major receptor of NO in GI muscles, we hypothesized that the major response to nitrergic stimulation would be elevation of cGMP in ICC. This was tested using immunohistochemical techniques to localize cGMP in specific cells. Under control conditions, cGMP-LI was low in ICC-IM and unresolvable in SMC (Fig. 3A). The muscles were exposed to solutions containing SNP for 15 min. The test solution also contained phosphodiesterase inhibitors IBMX and zaprinast to inhibit the breakdown of cGMP. After this period the muscles were rapidly fixed and examined for cGMP-LI. Double-labelling experiments revealed that cells with elevated cGMP-LI after stimulation with SNP also contained Kit-LI (Fig. 3B) and sGCβ1-LI (Fig. 3C). Exposure to SNP resulted in elevation of cGMP-LI in some myenteric neurons and nerve fibres having PGP9.5-LI (Fig. 3D). Using wholemount preparations, we counted 300 Kit-immunopositive cells (ICC-IM) in the circular muscle layers from four animals, and examined ICC-IM with cGMP-LI. After SNP stimulation in the presence of IBMX and zaprinast, 84.17% of ICC-IM revealed intense cGMP-LI. Before SNP stimulation, 2.75% of ICC-IM displayed detectable cGMP-LI. After SNP stimulation in the presence of sGC antagonist ODQ, 2.58% of ICC-IM showed cGMP-LI.
Figure 3.
Changes in cGMP-LI after stimulation with the NO donor SNP or activation of enteric nerves. Panels (A) and (B) show double-labelled images of cGMP-LI (panels A1 and B1, green) and Kit-LI (panels A2 and B2, red) before (A) and after (B) SNP (1 mmol L−1) in the presence of IBMX and zaprinast. The third image in each series of images (i.e. A3–J3) is a merged image of A1-J1 and A2–J2. ICC (arrows) within the circular muscle layer showed increased cGMP-LI after SNP (panel B). Other cell processes, likely to be nerve fibres (panels B1 and B3, arrowheads) also showed intense cGMP-LI. Panel (C) shows cGMP-LI (green) and sGCβ1-LI (red) in response to SNP. cGMP-immunopositive cells (arrows) that were bipolar in shape had sGCβ1-LI (arrows). Panel (D) shows cGMP-LI and PGP9.5-LI within the circular muscle. cGMP-LI was observed in PGP9.5-immunopositive nerve fibres (arrowheads) and ICC (arrows). Panel (E) shows cells in which cGMP-LI increased in response to electrical field stimulation of intrinsic nerve fibres (panel E1, green; arrows) in the presence of IBMX and zaprinast. Double labelling showed that the cells that responded to nerve stimulation with an increase in cGMP-LI were ICC-IM (panel E3; arrows). L-NNA (panel F) blocked the increase in cGMP-LI in ICC-IM in response to nerve stimulation. Panels (G–J) are cryostat cross-sections of the circular muscle layer showing cGMP-LI (panel G1–J1, green) and Kit-LI (panel G2–J2, red). Panels (G and H) show muscles before (G) and in the presence of SNP (H). IBMX and zaprinast were also present. cGMP-LI was not resolved under control conditions. After SNP, cGMP-LI (panel H1) co-localized with Kit-LI (panels H2 and H3, arrows). SMC also displayed weak cGMP-LI after whole-muscle exposure to SNP. Panel (I) show muscles stimulated with SNP in the presence of IBMX, zaprinast and the sGC inhibitor ODQ. ODQ blocked the increase in cGMP-LI in ICC (arrows) and SMC in response to SNP. Panel (J) shows cGMP-LI levels in tissues that were stimulated with SNP in the absence of IBMX and zaprinast. In the absence of the phosphodisterase inhibitors, ICC-IM (arrows) showed only weak cGMP-LI, demonstrating that cGMP is rapidly metabolized after nitrergic responses. Scale bars represent 20 μm in all images.
We also tested whether ICC would display enhanced levels of cGMP in response to enteric nerve stimulation (EFS). EFS, in the presence of IBMX and zaprinast, caused an increase in cGMP in ICC-IM (Fig. 3E). Elevation of cGMP in ICC-IM in response to EFS was inhibited by L-NNA (Fig. 4F), TTX (not shown) or ODQ (not shown). Similar experiments to localize cGMP-LI were performed using cryostat cross sections (Fig. 3G–J). In these experiments, weak labelling of SMC could be seen after stimulation of the muscles with SNP (compare panels Fig. 3G and H). ODQ blocked the elevation of cGMP-LI in response to SNP (Fig. 3I). In the absence of phosphodiesterase inhibitors, IBMX and zaprinast, weak cGMP-LI increased only in ICC-IM (Fig. 3J).
Figure 4.
Quantification of cGMP-like immunofluorescence in ICC-IM and SMC. (A) and (B) are a summary of cGMP-LI fluorescence intensity per pixel in ICC-IM and SMC in the absence and presence of SNP (corresponding to images in Fig. 3G and H). C and D show cGMP-LI fluorescence intensity in response to SNP in the absence or presence of IBMX, zaprinast, and the sGC inhibitor, ODQ (as indicated; corresponding to the image in Fig. 3I and J). Fluorescence intensity of cGMP-LI was measured in pixels within Kit-immunopositive cell areas (ICC-IM, black) and SMC areas (Kit-immunonegative, SM, grey) using Leica Confocal Software. *P < 0.05 shows significant difference in pixel intensity obtained after SNP addition in the presence of IBMX and zaprinast. There was no significant difference in any cell type in response to SNP in the presence of ODQ.
Quantification of the intensity of cGMP-LI demonstrated significant increases in ICC-IM after SNP stimulation in the presence of IBMX and zaprinast (Fig. 4B, P < 0.05). In these tissues, SMC had a tendency to show cGMP-LI compared with non-stimulated tissues (P > 0.05). The specificity of involvement of NO in SNP induced production was tested by treating tissues with ODQ prior to SNP (Fig. 4C). In these experiments cGMP-LI did not rise significantly (P > 0.05) above control conditions. Experiments without phosphodiesterase inhibitors, IBMX and zaprinast, were also performed (Fig. 4D), and we observed no significant increase in cGMP-LI in both ICC and SMC (P > 0.05).
Elevation of cGMP is transduced into functional responses by activation of PKG. Thus, PKG is also an essential signalling protein in postjunctional effector cells. Immunoblots were performed using an anti-PKGI antibody raised against human proteins (i.e. amino acid residues 657–671 identical to murine protein) and homogenates of guinea pig and murine GI muscles (Fig. 5A). The immunoblots of guinea pig tissues displayed a single band with the same molecular weight as mouse.8 Immunohistochemical observations revealed PKGI-LI in ICC, SMC and neurons (Fig. 5B). Most SMC and ICC, which were co-labelled with Kit antibody, showed intense PKGI-LI around the edges of cells. In whole-mount preparations, all ICC-IM showed intense PKGI-LI (Fig. 5C).
Figure 5.
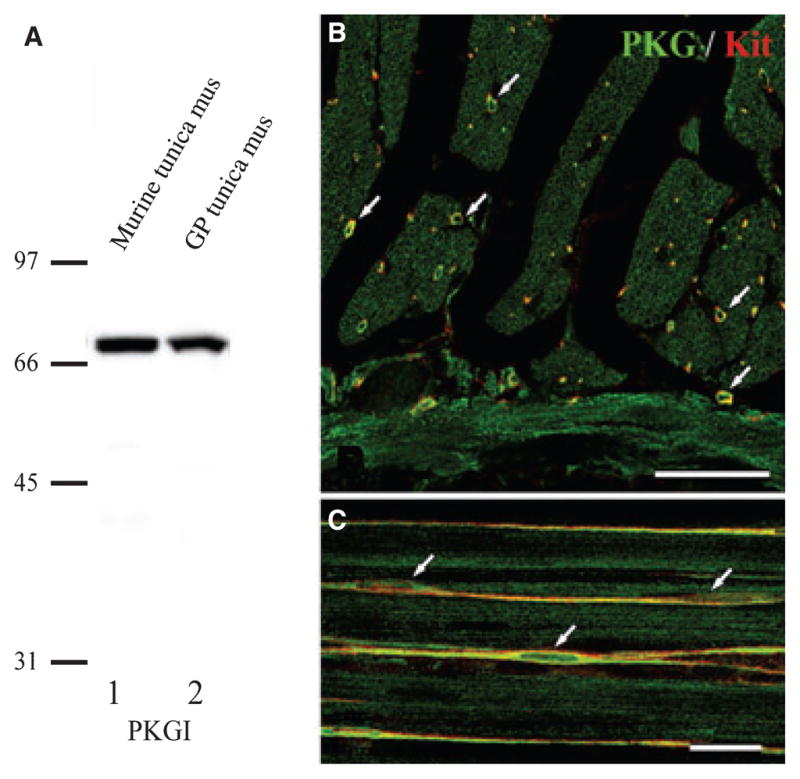
Distribution of PKG type I (PKGI) in the guinea pig caecum. Panel (A) shows immunoblot analysis examining specificity of the PKGI antibody. Lanes 1 and 2 show immunoreactivity to mouse and guinea pig muscles respectively. Molecular markers are shown on the left (in kDa). Panel (B) shows an image of PKGI-LI (green) and Kit-LI (red) in a cross-section through the circular muscle layer. ICC-IM, shown with Kit-LI (arrows), displayed intense PKGI-LI. Weak PKGI-LI was also present in SMC. Panel (C) shows a wholemount preparation of the circular muscle revealing PKGI-LI (green) and Kit-LI (red) in ICC-IM (arrows). Note the faint labelling of PKGI in SMC running parallel to the ICC-IM (green only). Scale bars in panels (B) and (C) represent 50 and 20 μm respectively.
DISCUSSION
There is considerable data in the literature suggesting that a major site of motor innervation in the GI tract is via ICC [ICC-IM or ICC-deep muscular plexus (DMP)] within smooth muscle bundles.11–13 ICC-IM and ICC-DMP lie in close apposition with varicose nerve terminals of nNOS-containing motor neurons and make gap-junctional connections with neighbouring SMC. Nitrergic neurotransmission is largely mediated through signalling molecules such as sGC, cGMP and PKG. It has previously been shown that ICC-DMP express target proteins (sGC and PKG-I) in the small intestine24 and these studies have been expanded to show that ICC in many regions of the guinea pig GI tract express sGCβ1 and sGCα1, the target proteins for NO signalling.25 Here, we have extended these findings by showing that the cGMP signalling pathway is also expressed by ICC-IM in the guinea pig caecum, and we have provided functional immunohistochemical evidence that release of NO from enteric inhibitory nerves triggers production of cGMP in ICC-IM. We could not resolve an increase in cGMP in SMC when NO was released from enteric motor neurons, however faint signals were resolved in SMC in response to whole-tissue exposure to an NO donor.
Most functional evidence for the role of ICC in motor innervation comes from studies of c-kit mutant mice that lack specific types of ICC.15,18–20,35 Such mutants are not readily available for most mammalian species, so in order to generalize this hypothesis, additional techniques are required to evaluate responses of ICC to motor neurotransmission. The present study suggests an important role for ICC in nitrergic enteric inhibitory motor neurotransmission in the guinea pig caecum, a classic preparation for studies of inhibitory neurotransmission.27,28 We found that ICC-IM express functional receptors for NO (i.e. sGCα1 and sGCβ1 isoforms) and the signal transduction molecule, PKGI. Most importantly, however, ICC-IM responded to the application of an NO donor or to inhibitory nerve stimulation with an increase in cGMP. Thus, the signalling pathway that mediates postjunctional nitrergic responses and motor responses is dominantly expressed and functional in ICC. As sGC was abundantly expressed in ICC-IM, that were associated with nerve terminals containing nNOS-LI suggests that ICC are major targets for nitrergic responses. The fact that cGMP increased in ICC-IM after nerve stimulation but not resolved in SMC suggests that NO released from neurons elicited postjunctional effects mainly in ICC-IM. This is possibly due to the fact that inhibitory motor neurons make synaptic-like contacts with ICC-IM,11,13,17 and NO may only reach high enough concentrations to activate sGC appreciably in postjunctional cells close to these synaptic-like contacts. 1 Previous studies have reported synaptic-like associations between nerves and ICC in a variety of species,12 suggesting that innervation via synaptic-like contacts is a functional feature of motor neural control in the GI musculature.
In addition to several studies suggesting that ICC mediate responses to enteric motor neurotransmission, there have also been studies questioning the role of ICC in neurotransmission. For example, we showed in isolated smooth muscle strips of the lower oesophageal sphincter (LOS) that nitrergic neurotransmission was impaired in W/WV mice that lacked most ICC-IM.19 However, a subsequent in vivo study, examined LOS pressures with manometric techniques and concluded that nitrergic regulation of sphincter pressure was unimpaired in W/WV mice.26 A number of criticisms were raised regarding the experimental design and morphological and functional conclusions of this study.36 Others have investigated nitrergic regulation of the internal anal sphincter (IAS) and concluded that relaxation responses to nitrergic nerve stimulation were not substantially impaired in W/WV mice.37 However, in W/WV mice, the IAS displays only a partial reduction in ICC (Ward and Sanders, unpublished observations; Fig. 2 of de Lorijn et al. 37). Thus, it is difficult to deduce the role of ICC in this region using these mutants. The role of ICC in W rat mutants (Ws) have also recently been examined.38 In the LOS, Kit-positive ICC-IM were found to be missing and this was verified by electron microscopy. Cells referred to as ‘fibroblast-like ICC’ were observed in place of Kit-positive cells and were suggested to represent immature ICC.39 It is possible, that while morphologically immature, these cells might provide functional integrity. Another study of nitrergic neurotransmission in the colons of Ws/Ws rats found significant reduction in nitrergic junction potentials, but retention of responses in the remaining cells.40 These authors concluded that nitrergic responses were not dependent upon ICC. Here again, however, there is an incomplete loss of ICC in Ws/Ws rats, so firm conclusions about the significance of ICC in nitrergic responses from studies of these animals is risky. Data from the present study suggest that even if nitrergic neurotransmission occurs ‘in parallel’ in GI muscles (i.e. NO signals are effectively transduced by ICC or SMC), then the transduction mechanisms required for nitrergic effects in GI muscles appear to be heavily weighted toward ICC. Due to this weighting of signalling molecules, partial retention of ICC, as occurs in many regions of the GI tract in mutant animals, might be sufficient to permit transduction of nitrergic responses. It also remains possible that nitrergic responses, while unresolved by the techniques used in the present study, are sufficient to activate effectors directly in SMC. Our findings also suggest that it might also be important to compare the relative expression of nitrergic signalling molecules in wildtype and W mutant SMC to determine whether there has been remodelling of this pathway in mutant animals.
In the caecum nerve varicosities containing nNOS-LI were closely associated with ICC-IM, as in many GI muscles.14,16–18 The major receptor for postjunctional nitrergic responses (sGC) was expressed predominantly in ICC rather than in SMC. Immunoreactivity for sGC in SMC was below the level of resolution using immunohistochemical techniques. However, functional studies suggest that low levels of sGC must also be expressed in SMC, because smooth muscle tissues and cells display cGMP-dependent responses to exogenous NO when ICC are absent.10,18 Part of the response of SMC to exogenous NO appears to be due to direct effects of NO on ion channels.41,42 Although the electrical and mechanical effects of NO released from enteric inhibitory neurons and exogenously applied NO are largely due to cGMP-dependent mechanisms, it should also be noted that a few studies have reported cGMP-independent nitrergic responses in intact muscles, 27 particularly when high concentrations of NO donors were used.43
The present findings, in conjunction with morphological and functional evidence, support primarily a serial arrangement for nitrergic transmitter production and release by enteric motor neurons and postjunctional transduction mechanisms. This concept can be summarized by the following steps: nNOS is expressed in enteric motor neurons; NO is released from nerve terminals; NO may only reach effective concentrations at the synaptic-like contacts between nerve varicosities and ICC-IM; sGC isoforms are expressed predominantly by ICC-IM; NO activates sGC in ICC-IM and causes formation of cGMP; cGMP activates PKGI to elicit cellular responses; electrical responses in ICC-IM are transmitted to SMC via gap junctions; hyperpolarization and stabilization of membrane potential in response to NO reduce contractile responses or produce relaxation. This concept also suggests that additional or different mechanisms mediate responses to NO released from neurons and responses to exogenous NO or endogenous NO released in a paracrine fashion from inflammatory cells, and physiological experiments have shown this to be the case in mutant animals lacking ICC-IM in the gastric fundus.18 It should also be stated that experiments in the present study do not eliminate the possibility of postjunctional responses that are mediated by direct activation of effectors in SMC, but we resolved no evidence for such a transduction pathway activated by NO released from enteric motor neurons.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research from Japan Society for Promotion of Science (SI, KH and YN), the Mishima-Kaiun Memorial Foundation (SI), the Kazato Research Foundation (SI) and P01 DK41315 from the National Institutes of Health, USA (SMW and KMS). We thank Dr J. de Vente (Maastricht University, The Netherlands) for the gift of the anti-cGMP antibody and Dr P. C. Emson (The Babraham Institute, UK) for the gift of the anti-nNOS antibody.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Zabel U, Kleinschnitz C, Oh P, et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol. 2002;4:307–11. doi: 10.1038/ncb775. [DOI] [PubMed] [Google Scholar]
- 2.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–7. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulos TL. Soluble guanylate cyclase. Curr Opin Struct Biol. 2006;16:736–43. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113:1671–6. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 5.Mergia E, Friebe A, Dangel O, Russwurm M, Koesling D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest. 2006;116:1731–7. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber A, Trudrung P, Storr M, et al. Protein kinase G expression in the small intestine and functional importance for smooth muscle relaxation. Am J Physiol Gastrointest Liver Physiol. 1998;275:G629–37. doi: 10.1152/ajpgi.1998.275.4.G629. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer A, Klatt P, Massberg S, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–51. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geiselhoringer A, Werner M, Sigl K, et al. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J. 2004;23:4222–31. doi: 10.1038/sj.emboj.7600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh SD, Monaghan K, Sergeant GP, et al. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–46. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- 10.Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol (Lond) 2001;533:155–63. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–35. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Rumessen JJ, Vanderwinden JM. Interstitial cells in the musculature of the gastrointestinal tract: Cajal and beyond. Int Rev Cytol. 2003;229:115–208. doi: 10.1016/s0074-7696(03)29004-5. [DOI] [PubMed] [Google Scholar]
- 13.Ward SM, Sanders KM, Hirst GDS. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16 (Suppl 1):112–7. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–56. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- 15.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003;311:299–313. doi: 10.1007/s00441-002-0657-1. [DOI] [PubMed] [Google Scholar]
- 17.Beckett EA, Takeda Y, Yanase H, Sanders KM, Ward SM. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J Comp Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- 18.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–29. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 20.Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J Physiol (Lond) 2002;543:871–87. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol (Lond) 2006;573:147–59. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuttleworth CW, Xue C, Ward SM, de Vente J, Sanders KM. Immunohistochemical localization of 3′,5′-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993;56:513–22. doi: 10.1016/0306-4522(93)90350-o. [DOI] [PubMed] [Google Scholar]
- 23.Young HM, McConalogue K, Furness JB, De Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993;55:583–96. doi: 10.1016/0306-4522(93)90526-l. [DOI] [PubMed] [Google Scholar]
- 24.Salmhofer H, Neuhuber WL, Ruth P, Huber A, Russwurm M, Allescher HD. Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell Tissue Res. 2001;305:331–40. doi: 10.1007/s004410100410. [DOI] [PubMed] [Google Scholar]
- 25.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008;152:437–48. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 26.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/Wv mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 27.Young HM, Ciampoli D, Johnson PJ, Stebbing MJ. Inhibitory transmission to the longitudinal muscle of the mouse caecum is mediated largely by nitric oxide acting via soluble guanylyl cyclase. J Auton Nerv Syst. 1996;61:103–8. doi: 10.1016/s0165-1838(96)00064-1. [DOI] [PubMed] [Google Scholar]
- 28.Shuttleworth CW, Sweeney KM, Sanders KM. Evidence that nitric oxide acts as an inhibitory neurotransmitter supplying taenia from the guinea-pig caecum. Br J Pharmacol. 1999;127:1495–501. doi: 10.1038/sj.bjp.0702674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- 30.Iino S, Nojyo Y. Muscarinic M2 acetylcholine receptor distribution in the guinea-pig gastrointestinal tract. Neuroscience. 2006;138:549–59. doi: 10.1016/j.neuroscience.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka J, Markerink-van Ittersum M, Steinbusch HW, De Vente J. Nitric oxide-mediated cGMP synthesis in oligodendrocytes in the developing rat brain. Glia. 1997;19:286–97. [PubMed] [Google Scholar]
- 32.Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 33.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44:1353–62. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 34.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol (Lond) 2004;556:521–30. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol (Lond) 2003;546:751–63. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders KM, Ward SM, Daniel EE. ICC in neurotransmission: hard to swallow a lack of involvement. Gastroenterology. 2002;122:1185–6. doi: 10.1053/gast.2002.32780. (Author Reply 1) [DOI] [PubMed] [Google Scholar]
- 37.de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005;54:1107–13. doi: 10.1136/gut.2004.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsujimura T, Hirota S, Nomura S, et al. Characterization of Ws mutant allele of rats: a 12-base deletion in tyrosine kinase domain of c-kit gene. Blood. 1991;78:1942–6. [PubMed] [Google Scholar]
- 39.Farre R, Wang XY, Vidal E, et al. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil. 2007;19:484–96. doi: 10.1111/j.1365-2982.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 40.Alberti E, Mikkelsen HB, Wang XY, et al. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 41.Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J Physiol (Lond) 1995;489:735–43. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang RJ, Harvey JR, McPhee GJ, Klemm MF. Nitric oxide and thiol reagent modulation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeci. J Physiol (Lond) 2000;525:363–76. doi: 10.1111/j.1469-7793.2000.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanovic A, Jimenez M, Fernandez E. Actions of NO donors and endogenous nitrergic transmitter on the longitudinal muscle of rat ileum in vitro: mechanisms involved. Life Sci. 2001;69:1143–54. doi: 10.1016/s0024-3205(01)01198-5. [DOI] [PubMed] [Google Scholar]






