Abstract
The scarcity of donor organs has led to the development of devices that provide optimal long-term respiratory or cardiopulmonary support to bridge recipients as they wait for lung and/or heart transplantation. This study was designed to evaluate the 30-day in-vivo performance of the newly developed pediatric pump-lung (PediPL) for cardiopulmonary support using a juvenile sheep model. The PediPL device was placed surgically between the right atrium and descending aorta in eight sheep (25.4 to 31.2kg) and evaluated for 30 days. Anticoagulation was maintained with continuous heparin infusion (ACT 150–200 sec). The flow rate was measured continually and gas transfer was measured daily. Plasma free hemoglobin, platelet activation, hematologic data, and blood biochemistry were assessed twice a week. Sheep were euthanized after 30 days. The explanted devices were examined for gross thrombosis. Six sheep survived for 30 to 32 days. During the study, the oxygen transfer rate of the devices was 54.9 ± 13.2mL/min at a mean flow rate of 1.14 ± 0.46 L/min with blood oxygen saturation of 95.4% ± 1.7%. Plasma free hemoglobin was 8.2 ± 3.7 mg/dL. Platelet activation was 5.35 ± 2.65%. The animals had normal organ chemistries except for surgery-related transient alterations in kidney and liver function. Although we found some scattered thrombi on the membrane surfaces of some explanted devices during the necropsy, the device function and performance did not degrade. The PediPL device was capable of providing cardiopulmonary with long-term reliability and good biocompatibility over the 30 day duration and offering the potential option for bridging to heart and/or lung transplant pediatric patients with end-stage heart or lung disease.
Keywords: pediatric, lung transplantation, Heart transplantation, cardiopulmonary support, artificial pump lung
Introduction
The need for mechanical cardiopulmonary and respiratory support devices for young children with advanced heart or lung disease is well established. It is estimated that a minimum of 32,000 infants are affected with congenital heart disease each year in the United States (1). Aggressive mechanical circulatory interventions may improve the likelihood of survival in pediatric patients who experience heart failure and become refractory to conventional therapies. Acute respiratory failure is often associated with pneumonia. According to the World Health Organization pneumonia was the single largest worldwide cause of death in children age <5 years, accounting for 1.1 million deaths a year in 2013 (2). In spite of the improved mechanical ventilator therapy, the mortality rate remains high for these young patients. For end-stage lung disease, lung transplantation is the preferred option for patients, but remains limited due to scarcity of suitable donor organs.
Extracorporeal membrane oxygenation (ECMO) has been routinely used to support heart and lung failure in the pediatric population. Though their survival to discharge remains at 40% to 50% for the past decades and is fraught with complications that increase dramatically when support exceeds a few days (3). In the recent years ECMO entered a new era of rapid evolution as the progress in understanding of therapeutic principle and complications has been made. Newer equipment and components, including console, pump, oxygenator and cannulae, are emerging. Long term ECMO support has been explored to bridge the sickest patients while waiting for a compatible lung. In some cases the bridging duration was more than 100 days (4). Good short and medium term outcomes haven been demonstrated in the literature (5, 6). However, the existing ECMO systems are often loosely constructed from a number of components: a blood pump, a blood oxygenator with or without a heat exchanger, a set of access cannulae and connecting tubing. These traditional ECMO systems are complex and bulky, which make them a less than optimal choice for long term support.
The lack of chronic pediatric mechanical circulatory/cardiopulmonary support options prompted the National Heart, Lung and Blood Institute (NHLBI) to award contracts to five selected consortiums for the development of novel pediatric circulatory support systems in 2004 (7). In 2010, the NHLBI awarded four contractors the Pumps for Kids, Infants, and Neonates (PumpKIN) Pre-Clinical Program to bring four promising pediatric circulatory/cardiopulmonary assist devices to human clinical trial. The PediPL device is one of the four PumpKIN devices and was specifically designed for use up to 30 days in patients weighing 5–20 kg. The device is indicated to treat: 1) pediatric patients with failure to wean from cardiopulmonary bypass who are not sufficiently stable to be moved from the operating room without mechanical circulatory support; 2) children with severe primary respiratory failure or secondary failure associated with cardiac disease, and 3) children with profound cardiogenic shock who require urgent support and may or may not require transition to long-term ventricular assistance. In this report we present our pre-clinical animal experience with the PediPL in a juvenile ovine model in terms of its biocompatibility and oxygen transfer function over a 30-day duration.
Materials and Methods
Device description
The PediPL is conceptualized to be a fully integrated pump lung for pediatric cardiopulmonary or respiratory support. The pumping function of the PediPL was designed based on the magnetically levitated bearingless impeller/motor technology which was previously implemented in the Thoratec CentriMag® blood pump (Thoratec, Pleasanton, CA), a continuous-flow, and centrifugal-type rotary blood pump. The oxygenation component is made of microporous hollow fiber membranes (HFM). To achieve the most effective use of the fiber membranes, maximum gas exchange and elimination of deleterious flow stagnancy, a cylindrically HFM bundle with a unique circumferential-radial uniform outside-in flow path design is employed. The PediPL device weights about 280 grams and blood contact surface of HFM is about 0.3 m2. The priming volume is about 110mL. Figure 1A shows the disposable pump-oxygenator of the PediPL. The details of the PediPL design can be found in the reference (8)
Figure 1.
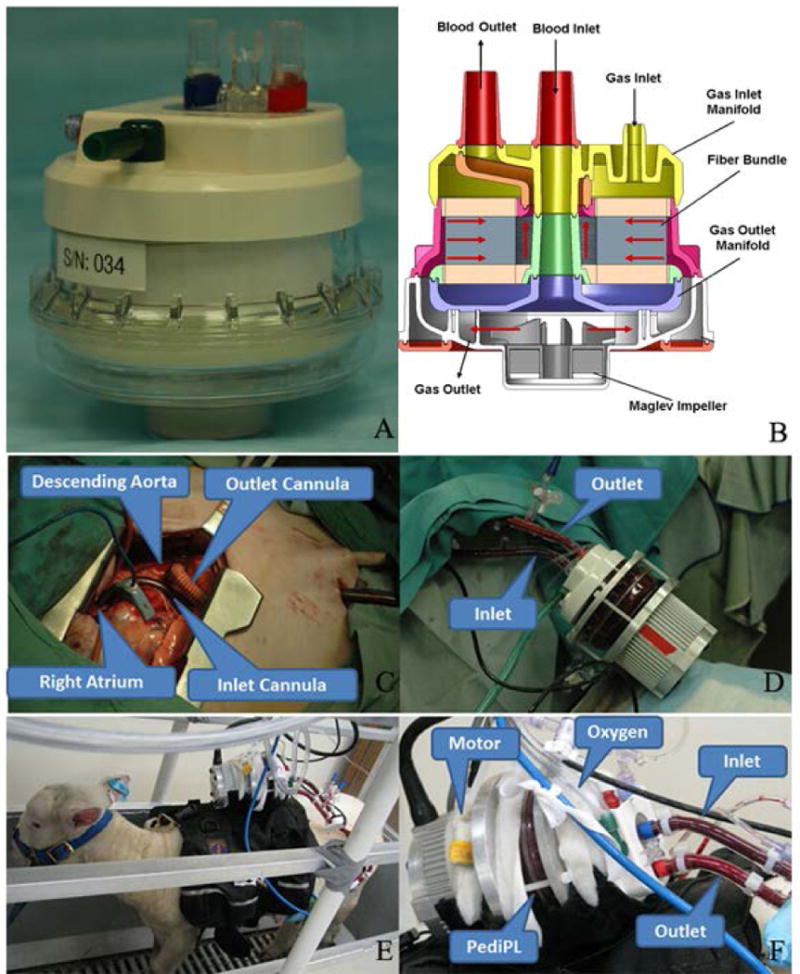
Photographs of the PediPL device and its internal flow path. (A) the disposable PediPL component; (B) internal flow path; (C) Surgical cannulation of the PediPL device in sheep model; (D) circulation of the PediPL after implantation; (E) a sheep with the PediPL after surgery and (F) the PediPL device description in working mode.
Figure 1B shows the cross-sectional view of the flow path inside the PediPL. Venous blood is drawn from the patient into the PediPL pump chamber from a central cylindrical inlet through a drainage cannula. Driven by a magnetically levitated rotating centrifugal pump impeller, the blood is propelled through the diffuser section and flows toward the space between the outer housing and the polymethylpentene HFM bundle. While the blood passes through the HFM bundle, the oxygen is transferred from the fiber lumen to the blood and the carbon dioxide is removed from the blood. The oxygenated blood is collected at the space between the HFM bundle and the center tube and returned back to the patient through the return cannula. The sweep gas enters the lumens of individual hollow fibers of the potted HFM bundle from the top and exits the device at the bottom through the channels imbedded in the diffuser fins. The PediPL is designed to be placed paracorporeally in the bed side and portable. In the animal study, the device was placed on the back of the animal (Figure 1D–F), and the power supply and controller used to operate the device was placed outside of the body and mounted on a cage.
Surgical procedure
Eight Dorset crossbred juvenile sheep (24.2–31.2 kg) were used in the study. All the surgical procedures and post-operative care were carried out according to the approved protocol by the Institutional Animal Care and Use Committee (IACUC) of the University Of Maryland School Of Medicine. During the course of the animal experiments, all the animals received humane care in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86–23, revised 1996).
After the general anesthesia and preoperative preparation, a small left thoracotomy incision was made. An outflow cannula (8 mm custom made polyurethane cannula with Dacron graft tip for first two sheep, and Medtronic DLP One Piece Pediatric Arterial Cannula 16 or 12 Fr. for the other six sheep) was sutured on the descending aorta or inserted into the descending aorta. An inflow cannula (Medtronic DLP Single Stage Venous Cannula: 20 Fr.) was inserted into the right atrial about 3 cm and secured with the purse string sutures (Figure 1C). The two cannulae were tunneled under skin and exited on the lateral wall. A right atrium to descending aorta bypass with the PediPL device was set up (Figure 1D). Within 24 hours after the implant surgery, all the animals recovered and were standing, eating and drinking (Figure 1E). The animals were anticoagulated with continual heparin infusion (IV) for a targeted ACT from 150 to 200 seconds and observed for 30 days.
Blood Chemistry, Hematologic, and Biocompatibility Measurements
Blood samples were collected prior to the implant surgery, after implantation, and twice a week for determination of complete metabolic panel (CMP), complete blood count (CBC), plasma free hemoglobin (PFH), lactic acid dehydrogenase (LDH), and platelet activation (percentage of CD62p positive platelets). Collected blood samples were sent to an outside laboratory (Antech Diagnostics, Lake Success, NY) for CMP, CBC, and LDH determination. PFH was measured using a modified cyanomethemoglobin method and CD62p expression on platelets was quantified with flow cytometry, respectively, as described previously (9–11).
Oxygen transfer
The ratio of the blood flow rate to sweep gas was maintained at 1:1 during the 30 day study period. The 95% oxygen mixed with 5% carbon dioxide was used as the sweep gas to avoid the excessive removal of carbon dioxide by both the animal itself and the device. Blood samples at the inlet and outlet of the PediPL were collected for blood gas analysis using a blood gas analyzer (Stat Profile PhoxPlus L; Nova Biomedical, Waltham, MA). The oxygen transfer rate was calculated according to the published method(12). Because 95% oxygen mixed with 5% carbon dioxide was used as the sweep gas, the carbon dioxide transfer rate could not be reliably evaluated and was not presented.
Necropsy
Necropsy was performed for each animal at the endpoint of the 30-day study or at the time of unanticipated death and early termination. The animals were euthanized by potassium chloride injection (1–2mmol/kg) under general anesthesia using ventilated isoflurane (1–4%) or Ketamine (2.2–7.5 mg/kg, IV). The animal’s organs were excised for tissue sampling. Pathological examination was performed for all the organs. The explanted devices were examined for gross thrombosis.
Statistics
All data were presented as mean ± standard deviation (SD). Statistical analysis was performed using the statistical package for social science (SPSS, 18.0 for Windows, SPSS, Chicago, IL). Data collected daily over the 30-day study period were averaged every 5 days. Comparisons between the measured data at the baseline (pre-implant or initiation of device operation) and at subsequent times were performed using a mixed model with the sheep number as the subject variable and the data collection time as the fixed, repeated measure variable. Post hoc analysis using the least significant difference (LSD) corrected confidence interval was used to compare all the postoperatively measured variables with the baseline data to examine the changes with time.
Results
Study summary
The PediPL was successfully implanted on the eight sheep. All of the animals recovered from the surgery and were able to stand, drink, and eat within 24 hours. Immediately after the implant surgery, anticoagulation treatment with heparin was initiated with a targeted activated clotting time of 150 to 200 seconds. Escalating heparin infusion dosage was required initially to maintain the targeted ACT (Figure 2). However the required dosage became stabilized after the first week. Heparin dosage was 103±29 units/kg/hr after the first week for these animals.
Figure 2.
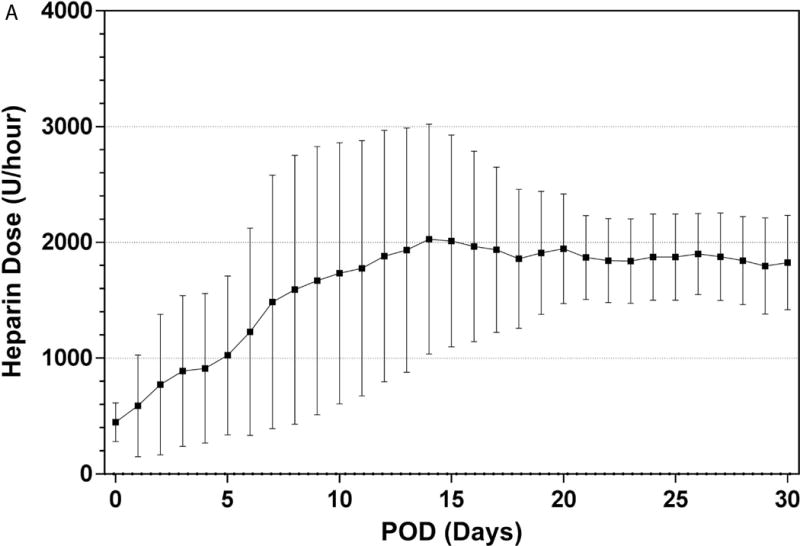
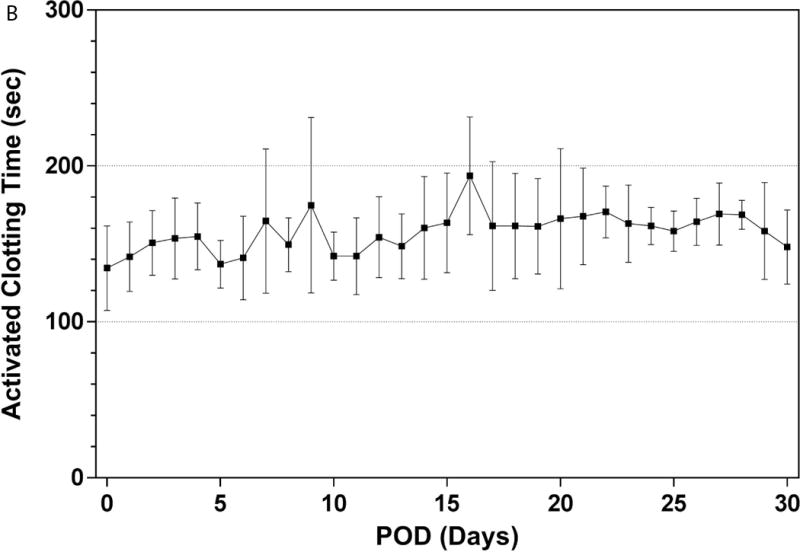
Anticoagulation treatment: (A) Heparin dose for anticoagulation treatment over the 30-day study period; and (B) achieved activated clotting time during the study.
The outcome results of the eight in vivo animal experiments are summarized in Table 1. Six sheep survived for 30 to 32 days and were electively terminated. One sheep was terminated early because of poor prognosis. The lung edema was found during the necropsy and attributed to the excessive fluid infusion during the implant surgery. The other animal expired because of mechanical damage in the device on the post-operative day (POD) 22. The device was found broken and sheep died from bleeding. All the devices in the animals that survived to the study endpoint functioned normally. Only one PediPL device was used for each animal without any device exchanging during the 30-day study period. Thus only the data from the six successful animal experiments are presented in the following sections.
Table 1.
Summary of in vivo animal experiments
| Sheep No. | Weight (kg) | Duration (days) | Flow Rate (L/min) | Cause of termination |
|---|---|---|---|---|
| 1 | 27 | 32 | 0.84–1.12 | Elective termination |
| 2 | 30 | 30 | 0.67–1.16 | Elective termination |
| 3 | 25 | 1 | 0.90–1.12 | Volume overloading |
| 4 | 24.2 | 30 | 0.74–1.04 | Elective termination |
| 5 | 31.2 | 32 | 0.54–1.11 | Elective termination |
| 6 | 25.8 | 22 | 0.57–1.21 | Device failure |
| 7 | 27 | 30 | 0.96–1.21 | Elective termination |
| 8 | 25.4 | 30 | 0.92–1.48 | Elective termination |
Pumping Function
The device flow rate was set at about 1.0 L/min at the rotational speed of about 2500 rpm in the first four animals over the 30-day study period. The initial device flow rate in the other two animals was set at about 1.0 L/min on the first two days and then adjusted to 1.5 L/min. As the animals recovered from the surgery, the flow rate fluctuated between 0.4 and 1.5 L/min, depending on the gesture of the animals (standing vs. sitting) when the data was collected. In some cases, the external tubing might had been bent or kinked. After the appropriate adjustment, the flow rate returned to the normal. In general, the flow rate decreased slightly as the hematocrit increased with time and became stable at the same rotational speed. Figure 3 shows the device flow rates over the 30 day study period at the two rotational speeds for the two groups of the study animals.
Figure 3.
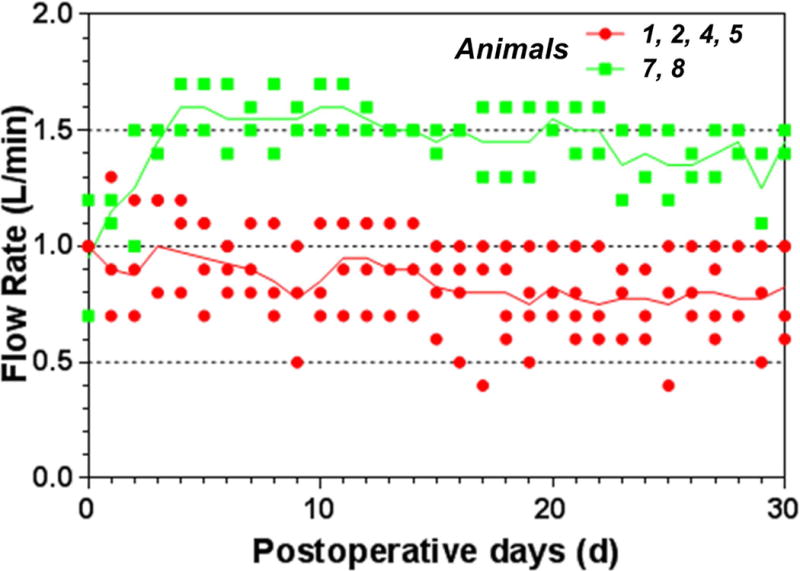
Device flow rate over the 30-day study period.
Oxygen transfer
The oxygen transfer performance of the PediPL device is shown in Figure 4. The oxygenation transfer of the PediPL device was stable during the 30-day study duration. The rate maintained in the range of 49.7 ± 14.1 mL/min to 65.2± 18.0mL/min at a mean flow rate of about 1 L/min. In spite of varying inlet blood conditions, the oxygen saturation of the blood at the device outlet was always above 95%. There was no gradual decrease in oxygen transfer rate or saturation over the 30 day period.
Figure 4.
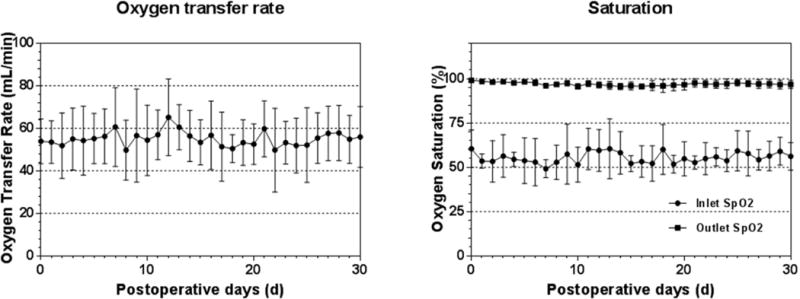
Oxygen transfer rate and saturation over the 30-day study period.
Plasma free hemoglobin and Lactate dehydrogenase
Compared with the preoperative baseline level of 7.9 ± 4.4mg/dL, PFH did not increase after the implant surgery and remained in the normal range (<20 mg/dL) throughout the 30 day duration (Figure 5). LDH significantly elevated immediately after the implant surgery, but returned to the normal range within two weeks, and then remained stable throughout the study duration (Figure 5). The peak value occurred on POD 1 and was significant higher than the baseline level (p<0.05). This trend reflected a surgery-related transient alteration. No hematuresis or other symptom of hemolysis was found in the animals during the 30-day study duration.
Figure 5.
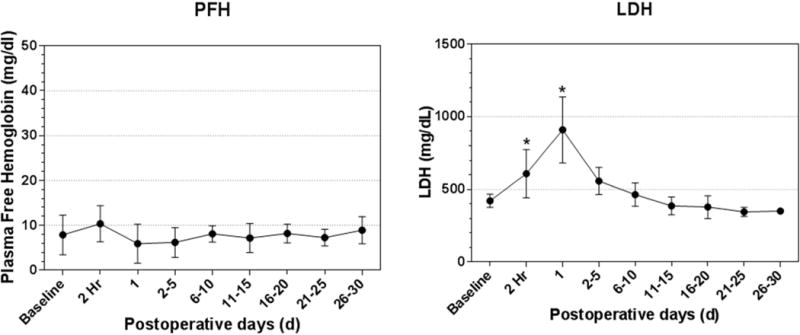
Plasma free hemoglobin and lactate dehydrogenase over the 30-day study period. PFH= plasma free hemoglobin; LDH= lactate dehydrogenase; * P<0.05, compared to the preoperative value.
Platelet activation
Platelet counts dropped significantly during and immediately after the surgery compared with the preoperative baseline value (p< 0.05), and then recovered after the surgery (Figure 6). The platelet activation was minimal under 8% during the study (Figure 6). The platelet activation was comparable to the pre-surgical baseline level. There was no statistical difference between the levels of platelet activation at the baseline and the postoperative time points.
Figure 6.
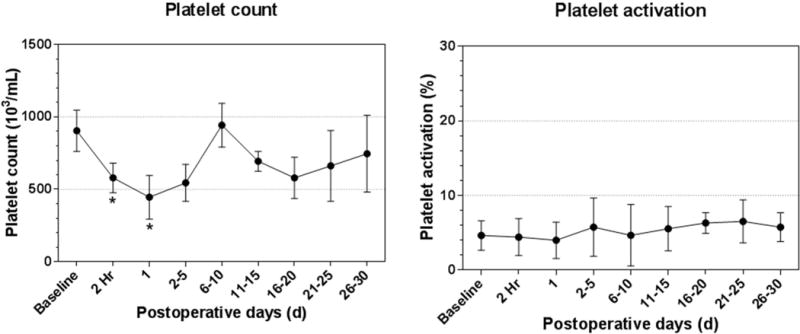
Platelet count and platelet activation over the 30-day study period. * P<0.05, compared to the preoperative value.
Hematology and Blood chemistry
The data of hematology and blood chemistry tests are summarized in Table 2. There was a drop in hematocrit and hemoglobin after the surgery. The postoperative hemodilution could be attributed to blood loss and fluid infusion during the surgery. Two weeks after the surgery, the hematocrit returned to the baseline level and gradually increased until the study endpoint. Blood transfusion was not needed for any animal. White blood cell counts did not increase significantly after the surgery and remained within the normal reference ranges throughout the study period. Selected laboratory tests for kidney function (creatinine, blood urea nitrogen), liver function (alaninetransaminase, aspartate aminotransferase), and cell and tissue injury (creatine phosphokinase) were normal except that some of these tests exhibited surgery-related transient alterations during the first week. They were all significantly elevated acutely after the surgery, returned to the normal ranges at POD 5 to 15, and then remained stable until the endpoint of the study.
Table 2.
Summary of the hematology and blood chemistry data during 30 days experiments
| Measurements | Preoperative | Postoperative 2 hours | POD1 | POD2-5 | POD6-10 | POD11-15 | POD16-20 | POD21-25 | POD26-30 |
|---|---|---|---|---|---|---|---|---|---|
| WBC (103/uL) | 7.77±1.73 | 8.17±2.45 | 7.66±1.62 | 10.23±4.29 | 9.60±2.81 | 9.59±3.28 | 10.89±3.21 | 10.64±3.11 | 10.76±3.77 |
| Hemoglobin (mg/dL) | 9.72±1.25 | 7.62±1.49* | 7.93±2.01 | 8.32±1.54 | 8.81±0.58 | 8.60±1.70 | 9.12±2.41 | 9.48±2.85 | 9.33±2.07 |
| Hematocrit | 30.63±4.07 | 25.50±2.35 | 22.44±5.43 | 24.80±3.11 | 25.60±2.88 | 26.60±5.90 | 25.00±5.79 | 28.60±5.03 | 30.00±6.82 |
| ALT (U/L) | 14.67±3.83 | 19.50±5.47 | 43.33±15.58* | 23.50±7.50 | 17.00±4.10 | 14.17±3.31 | 12.67±3.93 | 13.33±6.09 | 13.17±4.98 |
| AST (U/L) | 97.33±10.67 | 123.83±22.24 | 213.67±49.92* | 94.33±24.99 | 70.33±14.29 | 63.83±14.22 | 67.17±17.36 | 76.83±28.53 | 63.83±19.85 |
| CPK (U/L) | 137.00±52.69 | 4398.33±1988.62* | 1193.83±268.29* | 146.83±59.51 | 81.83±29.30 | 96.67±40.81 | 103.50± 32.15 | 122.17±44.64 | 109.50±36.29 |
| Creatinine (mg/dL) | 0.63±0.10 | 0.70±0.09 | 0.70±0.25 | 0.58±0.15 | 0.67±0.12 | 0.63±0.22 | 0.63±0.17 | 0.75±0.21 | 0.65±0.16 |
| BUN (mg/dL) | 19.83±6.37 | 25.67±6.71 | 22.17±6.55 | 13.83±1.72 | 17.83±4.88 | 15.85±4.26 | 19.88±4.57 | 20.83±6.60 | 17.17±5.67 |
WBC=White blood cells; ALT=alanine aminotransferase; AST=aspartate aminotransferase; CPK=creatine phosphokinase; BUN=blood urea nitrogen; POD=postoperative day;
means P<0.05, compared to the preoperative value.
Necropsy
After 30 days the survival animals underwent necropsy. The internal organs, cannulae and implanted PediPL device were grossly examined for abnormality and thrombosis. No visible thrombus was found in the cannulae or circulation. The right kidney from one animal had a 4×4 mm infarct while the left kidney was free of infarcts. The kidney function of this animal was completely normal over the course of the study. All of the PediPL devices explanted from the six successful 30-day animals were generally clean and without structural failure. There were no massive occlusive clots or deposited materials in the fiber bundle as commonly observed in the explanted oxygenators used in cardiopulmonary bypass or respiratory support. Two of the six explanted devices had isolated clots in the space between the outer housing and the outer layer of the HFM bundle. There were scattered microthrombi (<1 mm) in some areas of the HFM bundle and flow path (Figure 7 (A) (B)). Since only one device was used for the 30-day duration in each animal, it was expected that there would have been some thrombi in the fiber bundle in some devices because of the limitation of the current hollow membrane technology and a slightly variability in anticoagulation. The other four explanted devices were free of gross thrombotic formation in the fiber bundle and flow path (Figure 7 (C) (D)).
Figure 7.
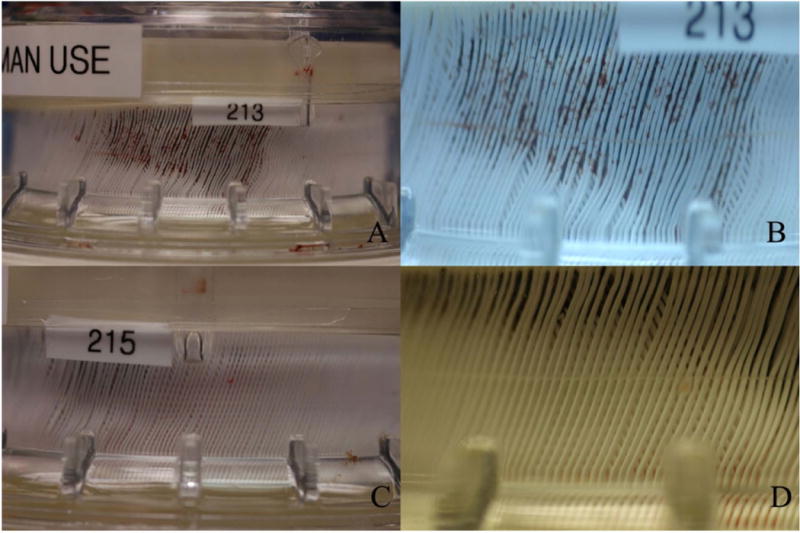
Explanted PediPL devices and Fibers from necropsy. A–B: two device with isolated clots on the fiber bundle; C–D: representative PediPL devices free of visible scattered thrombi.
Discussion
It has been reported the mortality of infants waiting for a heart transplant is 25% to 30%, which is the highest among all pediatric and adult patients (13). With an increasing demand on the supply of organs available for pediatric heart or lung transplant, alternative strategies are being sought to minimize wait-list-associated mortality for pediatric patients with end-stage heart/lung disease. As one of the PumpKIN devices, the PediPL device was designed as a compact pediatric pump-lung for cardiopulmonary or respiratory support for pediatric patients who need cardiopulmonary or respiratory support. The purpose of this study was to evaluate the PediPL device for cardiopulmonary support in an animal model.
In the present study, the experimental focus was placed on the unloading function and oxygenation function. Thus a veno-arterial mode was set up in the animal model. According to the collected data, the device flow rate of 1.14 ± 0.46 L/min was maintained during the 30 day study, suggesting that no occlusive thrombosis in the flow path. It was observed that the cannulation and the circuit might be very important for maintaining the appropriate flow rate during the support. The device flow rate fluctuated more frequently in the first two sheep when the 8 mm custom made polyurethane cannula with Dacron graft tip was used as the outflow cannula. The device flow rate was more stable in the other animals when the Medtronic DLP One Piece Pediatric Arterial Cannulas were used. The oxygen transfer rates were maintained as 54.9 ± 13.2mL/min during the 30 day duration with over 95% oxygen saturation of the blood at the outlet. This demonstrated an appropriate and stable oxygen transfer function. The small blood contact surface area of HFM (0.3 m2) might have improved the biocompatibility of the PediPL. PFH and platelet activation were minimal and within the normal ranges throughout the 30 day period. The hematology and blood chemistry tests were normal except for surgery-related transient alterations. Most importantly, only one device was used for each animal without need for exchange during the 30-day duration. During the necropsy, all the explanted devices were generally clean and without structural failure. There were no massive occlusive clots or deposited materials in the fiber bundle. There were scattered microthrombi (<1 mm) in some areas of the fiber bundle and flow path in two explanted devices. However, the device function and performance did not degrade during the 30 day support period. As a 30 days device, it might be acceptable compared to the oxygenators used in cardiopulmonary bypass or respiratory support in clinics.
One experiment was terminated early due to poor prognosis after the implant surgery. The lung edema was found during the necropsy of this animal. When the anesthesia record and surgical procedures were reviewed and compared to the other animals, it was noticed that the excessive fluid infusion was given during the implant surgery. About 2000 mL Ringer’s solution was infused because of blood lose during the surgery. Oliguria and pink bubble sputum cough were observed after the animal recovered from anesthesia. The pulmonary edema never happened in the rest of the animals with limited fluid infusion. One animal expired early from bleeding because of mechanical damage in the device on POD 22. The animal died in the cage when it was found. It was found that the device was broken between the housing and the fiber bundles. Since the device was designed for human use, it is expected that the device would be handled with great caution. During the animal experiments, the device was placed on the back of the animal which was constrained in a cage. There was unpredicted motion which could result in mechanical impact between the device and the cage wall. After this animal, the blood chamber of the devices was assembled with improved manufacturing processes. All the devices were evaluated for 24 hours with saline before implantation.
Because the sheep used in the study were healthy, we noticed that both the implanted PediPL device and the normally functional lungs of the animal removed excessive carbon dioxide. In some cases, the partial pressure of CO2 in venous blood could drop below 35 mmHg. Thus, 95% oxygen mixed with 5% carbon dioxide was used as the sweep gas during this study to avoid the excessive removal of carbon dioxide. Therefore, the removal performance of carbon dioxide of the PediPL could not be reliably evaluated and was not presented in the present study. However, the function of CO2 removal of the PediPL device can be evaluated acutely by surgically placing the device from the right atrium to the pulmonary artery. The respiratory failure can be emulated by reducing the ventilation rate to 1 ~ 2 pulses/min with a mixture of air and oxygen for ventilation. The experiments for evaluating the CO2 removal performance of the PediPL have been initiated.
Limitation
Only the eight animals were used in this study since the main purpose of this study was to demonstrate the feasibility that the PediPL device could provide both the ventricular unloading and oxygenation functions over the 30 day duration in an animal model. More data will be collected in the upcoming experiments to show its additional functionalities. The juvenile sheep that were used as the animal model did not simulate the exact infant body weight. The lamb might be used to evaluate the lower flow rate mode in the future. This study was performed in healthy animals without cardiopulmonary failure. The cardiac and respiratory dysfunction should be considered if the PediPL device is used to support patients with cardiopulmonary failure in the future. We will implant the PediPL device to these animal models to further understand its performance and limitation.
Conclusion
In the present study, it was demonstrated that the PediPL was capable of providing cardiopulmonary with long-term reliability and good biocompatibility over the 30 day duration. With rigorous manufacturing processes, it is anticipated that the PediPL could potentially provide a bridging option for pediatric patients waiting for lung and/or heart transplantation in the future. It could also be used to support the patients with acute cardiopulmonary/respiratory failure to recovery.
Acknowledgments
This study was supported in part by the National Institutes of Health (Contract Number: HHSN268201000014C and Grant Numbers: R01HL082631, R01 HL 088100).
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Pneumonia Factsheet no. 331. Geneva: World Health Organization; Reviewed November 2013 http://www.who.int/mediacentre/factsheets/fs331/en/accessed March 10, 2014. [Google Scholar]
- 3.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2009;55:111–6. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 4.Iacono A, Groves S, Garcia J, Griffith B. Lung transplantation following 107 days of extracorporeal membrane oxygenation. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2010;37:969–71. doi: 10.1016/j.ejcts.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. The Journal of thoracic and cardiovascular surgery. 2012;144:716–21. doi: 10.1016/j.jtcvs.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Lang G, Taghavi S, Aigner C, et al. Primary lung transplantation after bridge with extracorporeal membrane oxygenation: a plea for a shift in our paradigms for indications. Transplantation. 2012;93:729–36. doi: 10.1097/TP.0b013e318246f8e1. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin JT, Borovetz HS, Duncan BW, et al. The National Heart, Lung, and Blood Institute Pediatric Circulatory Support Program. Circulation. 2006;113:147–55. doi: 10.1161/CIRCULATIONAHA.105.571422. [DOI] [PubMed] [Google Scholar]
- 8.Wu ZJ, Gellman B, Zhang T, Taskin ME, Dasse KA, Griffith BP. Computational Fluid Dynamics and Experimental Characterization of the Pediatric Pump-Lung. Cardiovasc Eng Technol. 2:276–87. doi: 10.1007/s13239-011-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu ZJ, Zhang T, Bianchi G, et al. Thirty-day in-vivo performance of a wearable artificial pump-lung for ambulatory respiratory support. The Annals of thoracic surgery. 2012;93:274–81. doi: 10.1016/j.athoracsur.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu ZJ, Taskin ME, Zhang T, Fraser KH, Griffith BP. Computational model-based design of a wearable artificial pump-lung for cardiopulmonary/respiratory support. Artificial organs. 2012;36:387–99. doi: 10.1111/j.1525-1594.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 11.Kilic A, Nolan TD, Li T, et al. Early in vivo experience with the pediatric Jarvik 2000 heart. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2007;53:374–8. doi: 10.1097/MAT.0b013e318038fc1f. [DOI] [PubMed] [Google Scholar]
- 12.Dierickx PW, De Wachter DS, De Somer F, Van Nooten G, Verdonck PR. Mass transfer characteristics of artificial lungs. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2001;47:628–33. doi: 10.1097/00002480-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Mah D, Singh TP, Thiagarajan RR, et al. Incidence and risk factors for mortality in infants awaiting heart transplantation in the USA. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009;28:1292–8. doi: 10.1016/j.healun.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


