Highlights
-
•
Ferret brain architecture, composition, and development are similar to humans.
-
•
Postnatal ferret brain development is comparable to that of premature infants.
-
•
Ferrets have potential to model preterm and term neonatal brain injury.
-
•
Ferrets may fulfill the need for an intermediate model species of neurodevelopment.
-
•
Many opportunities exist to expand the use of ferrets as research subjects.
Keywords: Neurodevelopment, Perinatal brain injury, Ferrets, Animal model, Neurogenesis
Abstract
Complications of prematurity often disrupt normal brain development and/or cause direct damage to the developing brain, resulting in poor neurodevelopmental outcomes. Physiologically relevant animal models of perinatal brain injury can advance our understanding of these influences and thereby provide opportunities to develop therapies and improve long-term outcomes. While there are advantages to currently available small animal models, there are also significant drawbacks that have limited translation of research findings to humans. Large animal models such as newborn pig, sheep and nonhuman primates have complex brain development more similar to humans, but these animals are expensive, and developmental testing of sheep and piglets is limited. Ferrets (Mustela putorius furo) are born lissencephalic and undergo postnatal cortical folding to form complex gyrencephalic brains. This review examines whether ferrets might provide a novel intermediate animal model of neonatal brain disease that has the benefit of a gyrified, altricial brain in a small animal. It summarizes attributes of ferret brain growth and development that make it an appealing animal in which to model perinatal brain injury. We postulate that because of their innate characteristics, ferrets have great potential in neonatal neurodevelopmental studies.
1. Introduction
Survival of preterm infants born at or before 28 weeks of gestation continues to improve, and currently, the majority of infants born as early as 24 weeks of gestation survive (Jarjour, 2015, Stoll et al., 2010). However, nearly 50% of survivors develop moderate to severe neurodevelopmental impairment (Jarjour, 2015, Gargus et al., 2009, Hintz et al., 2011, Ancel et al., 2011). Intraventricular hemorrhage (IVH), hydrocephalus, periventricular white matter (WM) injury and encephalopathy of prematurity are common complications of prematurity and contribute to these poor outcomes (Stoll et al., 2010, Volpe, 2009a). Neonatal inflammation due to maternal chorioamnionitis and postnatal infection can markedly exacerbate neonatal brain injury at all ages (Hagberg et al., 2012, Thornton et al., 2012, Volpe, 2011). While current treatments including antenatal steroids (Friedman and Shinwell, 2004), magnesium sulfate (Crowther et al., 2003), and postnatal caffeine (Schmidt et al., 2012) improve long-term outcomes, there is still great need for additional, more efficacious neuroprotective therapies. Relevant animal models are needed to not only delineate the pathophysiology of neonatal brain injury, but also to discover new therapeutic interventions to halt and/or reverse these processes.
Small and large animal models of preterm brain injury, hypoxic ischemic brain injury and stroke are currently used to understand mechanisms of injury. These models have several advantages and disadvantages, such as similarity to human brain development, cost of husbandry and technical applications. Ferrets (Mustela putorius furo) are domesticated animals, and they have been increasingly used in biomedical research as models of respiratory viral diseases (Ball, 2006, Belser et al., 2011) and gastrointestinal diseases (Alder et al., 1996). More recently they have been used to understand the pathophysiology of immature brain injury. Ferrets lend themselves well to the study of neurodevelopment and brain injury because they are born lissencephalic with a prominent ganglionic eminence, and later undergo postnatal cortical folding to form complex gyrencephalic brain (Reillo and Borrell, 2012), comparable to brain growth as seen in premature human infants (Barnette et al., 2009). Domestic ferrets have social communication skills comparable to dogs (Hernadi et al., 2012) suggesting that sophisticated neurodevelopmental assessments should be plausible in these species. This review describes the suitability of ferrets as species to study perinatal brain injury in humans.
2. Animal models of neonatal brain injury
Historically, the most common animals used to study brain development have been rodents, rabbits and cats. Similar to humans, these animals undergo substantial postnatal brain development. Notable neonatal rodent models are the so-called Vannucci model of hypoxic-ischemic brain injury (Rice et al., 1981), the middle cerebral artery occlusion (MCAO) model of stroke (Ashwal et al., 1995), and the chronic hypoxia model (Schwartz et al., 2004, Salmaso et al., 2012). The classic Vannucci model induces unilateral brain injury in a postnatal day (P) 7 rat by unilateral carotid artery ligation followed by hypoxia (8% oxygen) for 90 min. Since its description, this injury model has been adapted to younger and older rat pups and to mice of various strains, demonstrating the variable vulnerabilities of rodent pups at different ages, and in different species (Northington, 2006). Both the P3 mouse (Dribben et al., 2009) and P3 rat brains (Lodygensky et al., 2014) are considered to have similar brain development as seen in 25-week premature human infant. The MCAO stroke model was initially used in P14–18 rats, but has since been adapted for use in P7 rats (Wen et al., 2004). The Vannucci and MCAO models create large unilateral injuries to both gray and white matter, but the MCAO model includes reperfusion as part of the injury. The chronic hypoxia model exposes animals to 10% oxygen from P3 to P11 and results in white matter injury (Back et al., 2006) and diminished neuronal growth (Schwartz et al., 2004). While similar changes are seen in some preterm infants, the mechanisms of injury are likely quite different since preterm infants do not experience chronic hypoxia. All of these models are limited in their ability to translate effectively to humans because of differences in brain complexity, white to gray matter ratios (Zhang and Sejnowski, 2000), and mechanisms of brain injury.
Rabbits exhibit slightly more complex brain organization compared to rodents, and several rabbit models of brain injury have been developed. They are altricial animals with immature brain at birth (Nicolas et al., 2011). They share the same hemochorial placenta to rodents, sheep and primates (Enders and Carter, 2004). A neonatal rabbit model of global hypoxia-ischemia created by sustained uterine artery occlusion at 70% and 75% of gestation in utero provides a reproducible model of cerebral palsy (Tan et al., 2005, Buser et al., 2010). There is also a rabbit model of IVH created by intraperitoneal glycerol injection of premature rabbits (Georgiadis et al., 2008). These models have moderate to high postnatal mortality rates. Furthermore, glycerol used to induce IVH may have direct cytotoxic effects unrelated to prematurity (Traudt et al., 2014). In comparison to rodents, rabbits do not have an extensive array of antibodies or other standard testing materials available for research. They also have limited behavioral assessments including motor tests (Tan et al., 2005, Yu et al., 2009, Georgiadis et al., 2008), and tests for short term memory and attention (Illa et al., 2013). They also have limited vascular access for monitoring and regular blood sampling (McArdle et al., 2010).
New animal models are being developed to reflect current understanding of the pathophysiology of preterm brain injury that includes varying combinations of inflammation (Kuban et al., 2014, Dammann and Leviton, 2014), hypoxia (Groner, 1997), hypoxia-ischemia (Stridh et al., 2013) and/or hyperoxia (Ritter et al., 2013). Models that demonstrate these effects have been created in immature rabbits (Balakrishnan et al., 2013), newborn kittens (Balasubramaniam and Del Bigio, 2006), dogs (Ment et al., 1982) and rodents (Burd et al., 2012, Dean et al., 2015). Small animal models have several advantages, especially rodents. Rodents have a relatively low cost of care and maintenance accompanied by an accelerated life cycle. Commercial dietary products and species-specific antibodies are readily available and methods of behavioral and electrophysiological testing are well established. Many genetically modified strains exist, and availability of a fully sequenced genome allows for development of therapeutic strategies with specific targeted pathways. Behavioral testing in rodents can include evaluation of motor control, coordination, learning, memory and sensorimotor gating as well as tests of braveness and social interaction (Kim et al., 2015, Ramani et al., 2013, Ten et al., 2004). There are also drawbacks to the use of rodent models. The rodent brain is lissencephalic with a much smaller proportion of white matter than is present in humans (Zhang and Sejnowski, 2000). The foci of neurogenesis and timing of myelination are different. These factors may be important when studying the effect of an early insult on later brain development. The rate of rodent maturation is quite accelerated relative to humans with each day of rat development corresponding to more than a week of human development (Clancy et al., 2001). Despite this, the timing of the cellular response to injury appears to be similar in both species. Thus as brain injury unfolds over hours to days, the developmental context changes differentially in rodents compared to humans; for example, as injury evolves from P7 to P10 in a rat, this time frame would roughly span 32 weeks to term in a human infant. Since the cellular and regional vulnerability of brain varies by developmental stage, the effect of brain injury and its repair may be quite different in rodents than humans (Balasubramaniam and Del Bigio, 2006). These important differences have limited the translation of therapeutic interventions to humans. Despite these shortcomings, these small animal models have formed the cornerstone of our current understanding of mechanisms of brain injury and the basics of neurodevelopmental pathways used to develop new therapies.
Larger mammals such as newborn pig (Iwata et al., 2007, Ezzati et al., 2014), fetal sheep (Castillo-Melendez et al., 2013, Gisslen et al., 2014, Bennet et al., 2007), and nonhuman primates (Inder et al., 2004, Beckstrom et al., 2011) are popular as models of neonatal brain injury because of their more complex brain structure. All of these species have long pregnancies and varying gestational ages when the fetal brain growth is similar to that of a human preterm infant (Clancy et al., 2001, Kim et al., 2014, Wassink et al., 2015, Griffith et al., 2012). Both sheep and piglets are precocious at birth (Butler et al., 2009, Karlsson et al., 2011). They can be chronically instrumented for physiologic measurements (Dalitz et al., 2003, Lemery et al., 1995) and can tolerate repeated blood sampling (Ferrara et al., 1995). However there are very few motor (Duberstein et al., 2014), cognitive and behavioral tests (Sullivan et al., 2013, Friess et al., 2007, Greiveldinger et al., 2011) available to monitor long-term effects of brain injury. Nonhuman primates, in particular, are useful to understand neurobehavioral outcomes of diseases (Mack et al., 2003, Jacobson Misbe et al., 2011) and effects of therapeutic interventions. However, disadvantages such as their higher cost, complicated husbandry (Alonso-Alconada et al., 2015, Nitsos et al., 2006, Inder and Neil, 2005) and large size make them more suitable for late preclinical trials. The use of these valuable animals requires conscientious resource management. Ferrets pose an attractive alternative with less cost but similar cortical development and complex behavior to large animal models (Sawada and Watanabe, 2012). They have great potential as neurodevelopmental research models. The pros and cons of ferrets in comparison to small and large brain injury models are shown in Table 1 .
Table 1.
Comparing ferret to other animals currently used as models of preterm brain injury. G is gestational age, P is postnatal day.
| Mouse | Rat | Rabbit | Ferret | Dog | Pig | Sheep | Non-Human Primate | ||
|---|---|---|---|---|---|---|---|---|---|
| Developmental Biology | Placentation | Hemochorial placenta | Hemochorial placenta | Hemochorial placenta | Endothelio-chorial placenta | Endothelio-chorial placenta | Epithelio-chorial placenta | Epithelio-chorial placenta | Hemochorial placenta |
| Complete gestation (days) | G18.5 | G21.5 | G31 | G41 | G59-63 | G110 | G147 | G185 in baboons | |
| Age comparable to 23–25 week gestation human infant | P3 | P3 | G25 | P10 | P0 | G91 | G91 | G125 in baboons | |
| Altricial | Yes | Yes | Yes | Yes | Yes | No | No | Yes | |
| Financial Consideration | Cost of husbandry | + | + | ++ | ++ | +++ | +++ | +++ | ++++ |
| Artificial feeding and need for other advanced care following brain injury | No | No | Yes | No | No | Yes | No, fetus returned to ewe after procedure with intact uterus | Yes | |
| Brain Development | White matter volume (%) | 9.6 | 12.2 | 16.1 | unknown | unknown | 33 | 32 | 37 |
| Time when eyes open | P16-20 | P15-18 | P9-10 | P35 | P 9-14 | P0 | P0 | G126 | |
| Technical Considerations | Availability of antibodies | ++++ | ++++ | +++ | + | ++ | +++ | +++ | +++ |
| Indwelling catheters for fetal monitoring | No | No | No | No | No | No | Yes | Yes | |
| Cardiovascular monitoring/blood sampling | Non- invasive neonatal test | Non-invasive neonatal tests | Invasive catheters only in adults | Invasive catheters only in adults | Yes | Yes | Yes | Yes | |
| Outcome measures | Long-term outcome measures of brain injury | Yes | Yes | Yes | Yes | No | No | No | Yes |
| Use of complex behavioral studies to assess outcomes of brain injury | Battery of complex motor, behavior and cognitive tests | Battery of complex motor, cognitive and behavioral tests | Mainly motor tests | No | No | No | No | Cognitive and behavioral tests available | |
| Ethical Considerations | + | + | + | ++ | ++ | +++ | +++ | ++++ |
3. Ferrets (Mustela putorius furo)
Ferrets are carnivores belonging to the family Mustelidae, which includes weasels, otters and minks. The European ferret (Mustela putorius furo) has been domesticated for many years and is generally considered a hardy animal with its tolerance of anesthesia and surgical procedures being well described in the veterinary literature (Cantwell, 2001, Brown, 2006, Siperstein, 2008). It is different from the black-footed ferret (Mustela nigripes), which is an endangered species. Ferrets are considered important models for studying effects of respiratory viruses (Maher and DeStefano, 2004) and pathogenesis of respiratory diseases such as cystic fibrosis (Yan et al., 2015). Like humans, they are equally susceptible to respiratory viruses and their lungs have similar physiology and developmental anatomy to humans. They also have similar immune function to humans (Huang et al., 2012) and so are used to develop treatment and vaccines for viral infections, including H5N1 (Zitzow et al., 2002, Kobinger et al., 2007) and severe acute respiratory syndrome from corona virus (See et al., 2008). A draft genome sequence of the ferret has been produced and will enhance the utilization of ferrets as models of respiratory disease, among other pathologies (Peng et al., 2014).
The animals are used in neuro-scientific research due to their complex gyrification and long development period. In the 1950s, ferrets were used in studies to describe the convolutional pattern of the brain through endocranial casts (Darlington, 1957). Studies in the 1980s used the animals in the study of teratology and toxicology, specifically in the context of fetal alcohol syndrome (Rabe et al., 1985). More recent studies have described their use in understanding the effects of traumatic brain injury (Feng et al., 2013, Lighthall, 1988), post hemorrhagic hydrocephalus (Di Curzio et al., 2013), neurotoxins (Johnston et al., 1982), viruses (Margolis and Kilham, 1969), and chronic hypoxia (Tao et al., 2012) on neuronal development. They are also used to study diseases involving visual pathways (Stitt et al., 2015) and have been identified as models for studies of sleep function and effects of sleep aids in early life (Thurber et al., 2008, Hsu et al., 2009). Since their use in neonatal research is constrained by their 6 week long gestation and weaning cycles (Ball, 2002), some of the nuances around whelping and neonatal care are described below.
3.1. Ferret whelping and neonatal care
Like most carnivores, ferrets have endotheliochorial placentation (Lindeberg, 2008, Carter, 2007). Normal ferret gestation is 41–42 days (Moody et al., 1985) and they require surgical intervention to deliver kits after 43 days of gestation. This narrow range of gestation is less variable than that of other model species, such as rats, and has been suggested to yield more consistent levels of central nervous system development at birth when comparing animals from different litters (Christensson and Garwicz, 2005). During parturition, they require close monitoring to prevent shoulder dystocia and cord entanglement. Litter size varies from 1 to 18 kits, with an average litter size of 8 kits, and an average weight of 6–12 gm (Lindeberg, 2008). Weight increases to approximately 150 gm at 28 days of life and up to 250 gm at 6 weeks of life. A significant weight difference between male hobs and female jills develops as early as 30 days of life. Their eyes open around 30 days of life and weaning is completed by 6 weeks of age (Ball, 2006).
Ferrets are bred in limited locations, and they are often shipped long distances with exposure to many intermediate handlers. It is important to minimize stress and limit transportation in weanling and pregnant animals, especially between 28 days of gestation and 8 days postnatal. Transportation related stress can impact research findings significantly and can result in jills cannibalizing their young. Ferret jills have strong maternal instincts and they cross-foster well. They can also be quite protective of their young, and care is required when handling their kits (Ball, 2006).
3.2. Ferret brain development
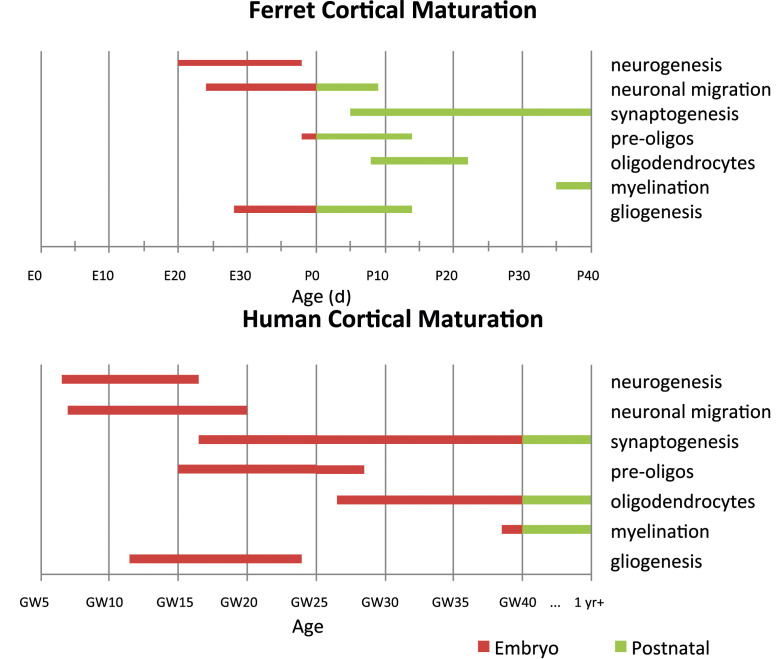
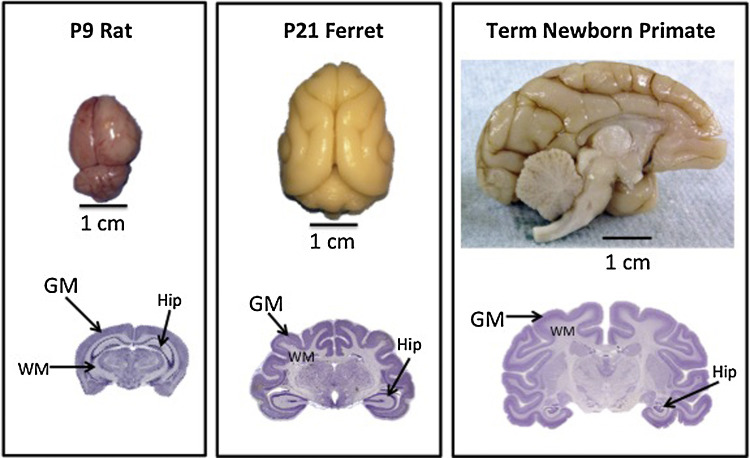
Ferret kits experience significant white matter maturation and complex cortical folding during the first month of life. Neurogenesis is three times longer in ferrets and ten times longer in macaques compared to rodents (Poluch and Juliano, 2015). Onset of neurogenesis occurs earlier in altricial animals such as ferrets when compared to rodents; however, rodents continue neurogenesis, synaptogenesis and axon extension at a faster rate than ferrets. At the time of opening the eyes, maturational points are approximately equivalent across species (Workman et al., 2013). The pattern of ferret cerebral cellular differentiation and morphological changes is similar to those observed in the human brain during the second half of gestation (Workman et al., 2013). Important neurodevelopmental events including synaptogenesis, oligodendrocyte maturation, and gliogenesis largely occur postnatally. Similar events occur in human development during the third trimester, making the ferret an excellent candidate for the study of preterm brain injury. Fig. 1 provides a comparison of events in ferret and human cortical development. Fig. 2 compares a P9 rat brain, a P21 ferret brain, and a term nonhuman primate brain. There is increasing brain complexity from rat to ferret and nonhuman primate. Indeed, ferret brain growth has been compared to growth seen in non-human primates (Sawada and Watanabe, 2012, Fukunishi et al., 2006) and humans (Chi et al., 1977).
Fig. 1.
Comparison of ferret and human cortical maturation timelines for several critical events in corticogenesis. Abbreviations: E-embryonic day; P-postnatal day; GW-gestational week.
Fig. 2.
Comparison of rat, ferret and Macaque nemestrina brains at term equivalence, demonstrating the presence of gyri in ferrets and nonhuman primates, as well as an increase in white to gray matter ratios in ferrets and primates compared to rodent brain. The horizontal line below each brain shows a 1 cm marker for scale. GM denotes gray matter, WM denotes white matter, and hip indicates the location of the hippocampus in each coronal brain section.
Ferret brain neurogenesis begins on embryonic day (E) 24 from cells in the ventricular layer (Martinez-Cerdeno et al., 2012). By E28 the cortical plate is formed (McSherry, 1984, McSherry and Smart, 1986) and neuronal production is completed by E38 (Smart and McSherry, 1986, Noctor et al., 1997). Neurons in the visual cortex begin neurogenesis as early as E20 and continue through P14 (Jackson et al., 1989). At birth, the ferret brain is lissencephalic, and sulci emerge in the rostral cerebrum at P4-10 and in the caudal region at P10–21, with no sexual differences seen between male and female kits (Sawada and Watanabe, 2012). The radial glial cells in the outer subventricular zone play a key role in the pattern of cerebral cortical expansion (Reillo and Borrell, 2012). Maturation of the ferret neocortex is radial in nature (Jespersen et al., 2012) and proceeds in rostral/lateral to caudal/medial direction (Kroenke et al., 2009) due to the transverse neurogenetic gradient (McSherry, 1984, Knutsen et al., 2013). Basilar dendritogenesis of the Layer V neurons begins just after birth, peaking at P21, while layer II/III neurons undergo arborization from P14 to P28 (Zervas and Walkley, 1999). Layer V neurons are also the first to develop corticothalamic tracks, while layer VI neurons take over as the major neocortical track over a protracted period (Clasca et al., 1995). The cortical architecture appears adult-like at P28 (Neal et al., 2007).
The complex gyral folding and progression of myelination have been documented by magnetic resonance imaging (MRI) (Barnette et al., 2009). Diffusion tensor imaging (DTI) on MRI shows a temporal decrease in fractional anisotropy in gray matter due to maturing neurons. An approximate 5-day difference in maturity exists between the rostral/caudal neocortex at the gradient source and the less mature neocortex at the occipital pole (Kroenke et al., 2009). The mean curvature of the brain increases rapidly from P10 to P17 and has a lower rate of growth after P17. The surface area of the neocortex grows at a high rate at approximately P13. This corresponds to cellular transition from proliferation to morphological differentiation at the same time point (Knutsen et al., 2013). The absolute rate of brain growth is significantly lower than that seen in baboons and human infants, but after correcting for species–specific developmental time scales, it appears that ferrets undergo expansion of three to five times compared to that seen in baboons and humans. Ferret cortical sulcal depth also increases steadily postnatally at a high rate until P21 with a decline in rate thereafter.
The developmental transformation of the ferret brain over the first three weeks of life has been correlated to human fetal brain development by using MRI (Barnette et al., 2009). At P4, the ferret brain was characterized by a simple, smooth, thin cortical plate, large ventricles and a prominent subventricular zone. By P10 the ferret cortex had primary sulci that correspond to those seen in human brains at 24 weeks of gestation. The subventricular zone remained prominent. By P17, the cortex appeared thicker, more complex, and the ventricles and the subventricular zone were smaller. At P21 cortical gray matter anisotropy decreased with an increase in WM anisotropy that continued to P35. These findings allowed us to compare development of a P10 ferret with a 25 week gestation human, and a P21 ferret with a term or near term gestation human (Sawada and Watanabe, 2012, Barnette et al., 2009).
Little is known about the development of the glial cells in ferrets. Oligodendrocytes, astrocytes and microglia are identified at E28 in the developing intermediate zone of the cerebral cortex at the same time as neurons are generated in the ventricular zone. The number of glial cells peak in the first week of life. Oligodendrocytes continue dividing after birth and begin differentiation at P8 (Berman et al., 1997). There is initial increase in WM anisotropy on DTI between P10 and P28 (Barnette et al., 2009) corresponding to these cellular changes, and it later declines during postnatal weeks 5–6 with increasing myelination (Barnette et al., 2009). The radial glial cells disappear by P21, having fully transformed into mature astrocytes by postnatal week 6 (Voigt, 1989). The microglial cells are thought to enter the central nervous system through the ventricular zone, and the ramified microglia then continue dividing in situ during development. They do not arise from the neural stem cells in the germinal zones (Berman et al., 1997).
Ferret cerebellar growth is slow and it corresponds to the developmental stages of motor function. The external granular layer generates cerebellar granule cells and influences morphogenesis. This layer is present for 7–8 weeks in ferrets, and is thickest around P22 and disappears by P56 (Christensson et al., 2007). Cerebellar organization and connectivity is important for understanding the effects of brain injury on ferret behavior in various models, however additional research on cerebellar growth and development is needed.
3.3. Ferret as brain injury animal model
In the last three decades, ferrets have been used to understand the pathophysiology of different mechanisms of brain injury and its effect on behavior. Lighthall JW et al. (Lighthall, 1988) used ferrets to study effects of controlled cortical impact on severity of brain injury by producing graded cortical contusions and subcortical injury using precisely controlled contact velocities and range of deformations. They are also used to study effects of blast exposure (Shridharani et al., 2012), neurotoxins such as organophosphorus-induced delayed neurotoxicity (Stumpf et al., 1989) and as models of chronic wasting disease, which belongs to the family of transmissible spongiform encephalopathy (Bartz et al., 1998). Antimitotic methylazoxymethanol given to pregnant jills at E24 induces cortical dysplasia in neonatal ferrets as a result of abnormal neuronal migration and disordered radial glial cells (Poluch and Juliano, 2010).
Tao et al., 2012 evaluated brain injury in neonatal kits after exposure to 10% oxygen from P10 to P20 and P20–30 using a combination of histology and ex vivo DTI on MRI. Vulnerability to hypoxia changed with maturation. Hypoxia exposure at preterm equivalent age (P10–P20) resulted in reduced white matter diffusivity throughout the white matter including the internal capsule, and corpus callosum, astrocytosis and decreased myelination at P20. These changes in DTI resolved by P30 despite persistence of histologic changes. When hypoxia was induced from P20 to P30, DTI showed increased apparent diffusion coefficient and relative anisotropy values in some white matter areas but not others, with no evidence of neuroinflammation or myelin changes.
Di Curzio et al., 2013 injected kaolin (aluminium silicate) into the cisterna magna of P14 ferrets to create a model of hydrocephalus. Histological changes consistent with neuroinflammation, white matter injury, effects on subventricular zone proliferation and associated behavioral changes corresponded with MRI findings of hydrocephalus.
These studies demonstrate the potential use of ferrets as laboratory animals to study effects of injury on brain development and growth in a small, gyrencephalic mammal. However, more physiologically relevant approaches are needed to create a reproducible brain injury pattern that mimics mechanisms of injury seen in premature infants to ultimately determine translatable neuroprotective strategies.
4. Future directions
Ferret cardiovascular anatomical studies suggest opportunities to study cerebrovascular disease at different postnatal ages. These animals have a long cervical arterial trunk with easy access to carotid arteries (Atkinson et al., 1989, Andrews et al., 1979). There are no anastomotic channels between the internal and external carotid arteries, other than the circle of Willis. The anatomy of the cranial arteries is similar to that of rabbits and they have absent carotid rete mirabile (Atkinson et al., 1989). This anatomy provides the opportunity to develop predictable regional cerebral ischemic lesions and create a new neonatal hypoxic-ischemia injury model, possibly through adaptation of the already defined Vanucci (Rice et al., 1981) or MCAO (Ashwal et al., 1995) models. As white matter maturation occurs postnatally in the ferret there is potential opportunity in the P0–P20 timeframe to model varying degrees of prematurity and associated brain injury.
Similar to carotid artery ligation, systemic inflammation has been shown to disrupt white matter development in both animals (Favrais et al., 2011) and humans (Leviton et al., 2011, Zhao et al., 2013). Systemic toll-like receptors play an inherent role in triggering the innate immune system and produce cerebral inflammation. They can either sensitize the brain to injury or induce tolerance (Mallard, 2012) depending on the dose and timing of the inflammatory stimulus (Mallard et al., 2014). When ferrets were exposed to lipopolysaccharide or Poly I:C, which are toll like receptor 4 (TLR4) ligands (Nakata et al., 2009) and TLR 3 ligands (Berendam et al., 2015) respectively, they show a robust systemic cytokine response. However, their effects on neuroinflammation, glial cell activation, cerebral growth and development are unknown. Studies show that the response to endotoxins varies with developmental expression of TLR4 in animals (Harju et al., 2005) and humans (Dowling and Levy, 2014), and the effects may not be comparable between species. Furthermore, the effect of inflammation on white matter depends on the vulnerability of oligodendrocytes at the time of treatment, since preoligodendrocytes are exquisitely sensitive in comparison to mature oligodendrocytes (Back, 2014). Thus it may be prudent to evaluate the ontogeny of toll-like receptors in ferret brains, as well as oligodendrocyte maturation (Tomassy and Fossati, 2014) to optimize the development of a model of preterm brain injury using established methodology.
Cerebellar injury is increasingly recognized as a significant contributor to neuropathology in preterm infants (Volpe, 2009b). Neonatal ferrets have a protracted period of cerebellar development and are ideal models to study the pathophysiological effects of brain injury on cerebellar growth (Hutton et al., 2014). Cerebellar growth and development can be evaluated by MRI as well as by histologic and gross measurements, and these findings correlated to changes in motor function (Christensson et al., 2007). Although a few studies have examined normal (Christensson and Garwicz, 2005) and abnormal (Rabe et al., 1985, Di Curzio et al., 2013, Van Cleave and Shall, 2006) ferret motor behavior at early postnatal time points, more studies are needed to correlate achievement of myelination milestones with motor and cognition tests. Also, better cognitive tests are needed to evaluate the subtle effects of brain injury on development (Sullivan et al., 2013, Lan et al., 2015) such as learning and memory (Barnette et al., 2009). Methods for measuring ferret locomotor activity (Christensson and Garwicz, 2005) and socio-cognitive abilities (Hernadi et al., 2012) have already been described. Ferrets have been shown to exhibit instrumental and visual discrimination learning and perform on tests emphasizing spatial relations, schedule maintained behavior and spontaneously occurring behaviors (Rabe et al., 1985). Finally, recent advances in in utero transgene electroporation in ferrets offers great opportunity to not only understand brain development at the molecular level but also affords potential for genetic intervention in the future (Kawasaki et al., 2012).
5. Limitations
While the ferret has been used for many research purposes already described, there are admittedly gaps in knowledge surrounding aspects of the ferret as a neurodevelopmental model. At this time complex behavioral testing of ferrets has not been extensively established as in rodents and some large mammals. They have, however, been shown to require little pre-training for learning tasks and available data does suggest they will prove desirable candidates for neurobehavioral studies once their behavioral profile is complete and more experimental tasks are devised (Rabe et al., 1985). Neurophysiological tests on in vitro prefrontal cortical slices of ferret brain have increased understanding of schizophrenia and the effects of clozapine on neurons (Gao, 2007).
Neonatal ferrets’ relatively small size, vascular anatomy and availability of phlebotomy sites precludes continuous cardiovascular monitoring and frequent blood sampling (Siperstein, 2008). The larger adult female ferret may allow opportunity for both in utero manipulation (Li et al., 2006) and continuous cardiovascular monitoring. Our own experience at this institution demonstrated that the neonates require minimum intensive care after manipulation and return to nursing without external support (Unpublished data). There are no known ethical issues regarding use of ferrets in locally approved animal research. However, these protocols are closely regulated through the United States Department of Agriculture.
Other technical issues exist such as the limited to non-existent commercial availability of ferret-specific mRNA probes and antibodies for immunohistochemistry. However, some success has been achieved with the use of mouse, rat and rabbit primary antibodies for this purpose (Reillo and Borrell, 2012; Martinez-Cerdeno et al., 2012; Voigt, 1989). Primary antibodies specific to brain injury and neuroinflammation, such as myelin basic protein for myelin, olig2 for oligodendrocytes, glial fibrillary acidic protein for astrocytes, ionized calcium-binding adaptor molecule 1 (Iba1) for microglia, NeuN for neuronal nuclei, activated caspase 3 for apoptotic cells and Ki67 for dividing cells have been successfully tested in ferret tissue and are commercially available (Di Curzio et al., 2013, Tao et al., 2012). In addition, cDNA sequences for inflammatory cytokines like interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL6 and IL8 in ferrets are known and comparable to animals in the Carnivora order such as dogs and cats (Nakata et al., 2008). Gender-related differences in vulnerability to brain injury exist in humans (Smith et al., 2014) but these differences have not yet been specifically investigated in ferrets. Brain sexual dimorphisms are noted in adult ferrets (Tobet et al., 1993), but their impact on neurodevelopmental outcomes following brain injury is yet unknown. Ferret genomic studies are limited (Cavagna et al., 2000) and further studies are needed to expand our current understanding of ferret brain development and later, genomic basis of human preterm brain injury.
6. Conclusion
The domestic ferret presents an excellent opportunity to develop a pathophysiologically relevant model of neonatal brain injury, which will permit future research in neuropathologic, neuroprotective, and regenerative mechanisms relevant to the morbidities associated with premature birth. They have significant advantages over the use of large animal models such as pig and sheep with a comparable gyrencephalic brain and a relatively small size. They are limited by the availability of antibodies and complex behavioral tests similar to those present in established rodent models. However, their long gestation and weaning cycle, along with protracted neurodevelopmental processes and small size make it a relatively cost-effective animal to study long term effects of injury and therapeutic interventions, with the goal to successfully translate these interventions to pre-clinical and clinical human trials.
References
- Alder J.D., Ewing P.J., Mitten M.J., Oleksijew A., Tanaka S.K. Relevance of the ferret model of Helicobacter-induced gastritis to evaluation of antibacterial therapies. Am. J. Gastroenterol. 1996;91(November):2347. [PubMed] [Google Scholar]
- Alonso-Alconada D. Brain cell death is reduced with cooling by 3.5 °C–5 °C but increased with cooling by 8.5 °C in a piglet asphyxia model. Stroke: J. Cerebral Circulation. 2015;46(January):275. doi: 10.1161/STROKEAHA.114.007330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancel P.Y. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: results of the EPIPAGE-2cohort study. JAMA Pediatr. 2011;169(March):230. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- Andrews P.L., Bower A.J., Illman O. Some aspects of the physiology and anatomy of the cardiovascular system of the ferret, Mustela putorius furo. Lab. Anim. 1979;13(July):215. doi: 10.1258/002367779780937771. [DOI] [PubMed] [Google Scholar]
- Ashwal S., Cole D.J., Osborne S., Osborne T.N., Pearce W.J. A new model of neonatal stroke: reversible middle cerebral artery occlusion in the rat pup. Pediatric Neurol. 1995;12(April):191. doi: 10.1016/0887-8994(95)00006-2. [DOI] [PubMed] [Google Scholar]
- Atkinson C.S., Press G.A., Lyden P., Katz B. The ferret as an animal model in cerebrovascular research. Stroke: J. Cereb. Circul. 1989;20(August):1085. doi: 10.1161/01.str.20.8.1085. [DOI] [PubMed] [Google Scholar]
- Back S.A. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 2006;60(December):696. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- Back S.A. Cerebral white and gray matter injury in newborns: new insights into pathophysiology and management. Clin. Perinatol. 2014;41(March):1. doi: 10.1016/j.clp.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B., Dai H., Janisse J., Romero R., Kannan S. Maternal endotoxin exposure results in abnormal neuronal architecture in the newborn rabbit. Dev. Neurosci. 2013;35:396. doi: 10.1159/000353156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam J., Del Bigio M.R. Animal models of germinal matrix hemorrhage. J. Child Neurol. 2006;21(May):365. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- Ball R.S. Husbandry and management of the domestic ferret. Lab Anim. 2002;31(May):37. doi: 10.1038/5000157. [DOI] [PubMed] [Google Scholar]
- Ball R.S. Issues to consider for preparing ferrets as research subjects in the laboratory. ILAR J. 2006;47:348. doi: 10.1093/ilar.47.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnette A.R. Characterization of brain development in the ferret via MRI. Pediatr. Res. 2009;66(July):80. doi: 10.1203/PDR.0b013e3181a291d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J.C., Marsh R.F., McKenzie D.I., Aiken J.M. The host range of chronic wasting disease is altered on passage in ferrets. Virology. 1998;251(November 25):297. doi: 10.1006/viro.1998.9427. [DOI] [PubMed] [Google Scholar]
- Beckstrom A.C., Humston E.M., Snyder L.R., Synovec R.E., Juul S.E. Application of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry method to identify potential biomarkers of perinatal asphyxia in a non-human primate model. J. Chromatogr. A. 2011;1218(April 8):1899. doi: 10.1016/j.chroma.2011.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser J.A., Katz J.M., Tumpey T.M. The ferret as a model organism to study influenza A virus infection. Dis. Mod. Mech. 2011;4(September):575. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet L. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J. Physiol. 2007;578(January):491. doi: 10.1113/jphysiol.2006.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendam S.J., Fallert Junecko B.A., Murphey-Corb M.A., Fuller D.H., Reinhart T.A. Isolation, characterization, and functional analysis of ferret lymphatic endothelial cells. Vet. Immunol. Immunopathol. 2015;163(February 15):134. doi: 10.1016/j.vetimm.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman N.E., Johnson J.K., Klein R.M. Early generation of glia in the intermediate zone of the developing cerebral cortex. Dev. Brain Res. 1997;101(July):149. doi: 10.1016/s0165-3806(97)00060-6. [DOI] [PubMed] [Google Scholar]
- Brown C. Blood collection from the cranial vena cava of the ferret. Lab Anim. 2006;35(October):23. doi: 10.1038/laban1006-23. [DOI] [PubMed] [Google Scholar]
- Burd I., Balakrishnan B., Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am. J. Reprod. Immunol. 2012;67(April):287. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- Buser J.R. Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia. J. Cerebral Blood Flow Metabol.: Off. J. Int. Soc. Cerebral Blood Flow Metabol. 2010;30(May):1053. doi: 10.1038/jcbfm.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.E. The piglet as a model for B cell and immune system development. Vet. Immunol. Immunopathol. 2009;128(March 15):147. doi: 10.1016/j.vetimm.2008.10.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell S.L. Ferret, rabbit, and rodent anesthesia. Vet. Clin. North America. Exo. Anim. Prac. 2001;4(January):169. doi: 10.1016/s1094-9194(17)30056-7. [DOI] [PubMed] [Google Scholar]
- Carter A.M. Animal models of Human Placentation–a review. Placenta. 2007;28(Suppl. A, April):S 41. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Castillo-Melendez M. Experimental modelling of the consequences of brief late gestation asphyxia on newborn lamb behaviour and brain structure. PLoS ONE. 2013;8:e77377. doi: 10.1371/journal.pone.0077377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna P., Menotti A., Stanyon R. Genomic homology of the domestic ferret with cats and humans. Mammalian Genome: Off. J. Int. Soc. 2000;11(October):866. doi: 10.1007/s003350010172. [DOI] [PubMed] [Google Scholar]
- Chi J.G., Dooling E.C., Gilles F.H. Gyral development of the human brain. Ann. Neurol. 1977;1(January):86. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Christensson M., Garwicz M. Time course of postnatal motor development in ferrets: ontogenetic and comparative perspectives. Behav. Brain Res. 2005;158(March):231. doi: 10.1016/j.bbr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Christensson M., Broman J., Garwicz M. Time course of cerebellar morphological development in postnatal ferrets: ontogenetic and comparative perspectives. J. Comp. Neurol. 2007;501(April):916. doi: 10.1002/cne.21291. [DOI] [PubMed] [Google Scholar]
- Clancy B., Darlington R.B., Finlay B.L. Translating developmental time across mammalian species. Neuroscience. 2001;105:7. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clasca F., Angelucci A., Sur M. Layer-specific programs of development in neocortical projection neurons. Proc. Nat. Acad. Sci. U. S. A. 1995;92(November):11145. doi: 10.1073/pnas.92.24.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther C.A., Hiller J.E., Doyle L.W., Haslam R.R. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA: J. Am. Med. Assoc. 2003;290(November 26):2669. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- Dalitz P., Harding R., Rees S.M., Cock M.L. Prolonged reductions in placental blood flow and cerebral oxygen delivery in preterm fetal sheep exposed to endotoxin: possible factors in white matter injury after acute infection. J. Soc. Gynecol. Invest. 2003;10(July):283. doi: 10.1016/s1071-5576(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Dammann O., Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr. Res. 2014;75(March):376. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington D. The convolutional pattern of the brain and endocranial cast in the ferret (Mustela furo L.) J. Anatomy. 1957;91(January):52. [PMC free article] [PubMed] [Google Scholar]
- Dean J.M. A critical review of models of perinatal infection. Dev. Neurosci. 2015;(February 19) doi: 10.1159/000370309. [DOI] [PubMed] [Google Scholar]
- Di Curzio D.L., Buist R.J., Del Bigio M.R. Reduced subventricular zone proliferation and white matter damage in juvenile ferrets with kaolin-induced hydrocephalus. Exp. Neurol. 2013;248(October):112. doi: 10.1016/j.expneurol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Dowling D.J., Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35(July):299. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dribben W.H. High dose magnesium sulfate exposure induces apoptotic cell death in the developing neonatal mouse brain. Neonatology. 2009;96:23. doi: 10.1159/000201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duberstein K.J. Gait analysis in a pre- and post-ischemic stroke biomedical pig model. Physiol. Behav. 2014;125(February 10):8. doi: 10.1016/j.physbeh.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Enders A.C., Carter A.M. What can comparative studies of placental structure tell us-a review. Placenta. 2004;25(Suppl. A, April):S3. doi: 10.1016/j.placenta.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Ezzati M. Pharmacokinetics of dexmedetomidine combined with therapeutic hypothermia in a piglet asphyxia model. Acta Anaesthesiol. Scand. 2014;58(July):733. doi: 10.1111/aas.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrais G. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011;70(October):550. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- Feng Y., Clayton E.H., Chang Y., Okamoto R.J., Bayly P.V. Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography. J. Biomech. 2013;46(March 15):863. doi: 10.1016/j.jbiomech.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara J.J. Effects of dopamine and dobutamine on regional blood flow distribution in the neonatal piglet. Ann. Surg. 1995;221(May):531. doi: 10.1097/00000658-199505000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Shinwell E.S. Prenatal and postnatal steroid therapy and child neurodevelopment. Clin. Perinatol. 2004;31(September):529. doi: 10.1016/j.clp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Friess S.H. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 2007;204(March):234. doi: 10.1016/j.expneurol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunishi K. Development of cerebral sulci and gyri in fetuses of cynomolgus monkeys (Macaca fascicularis) Anatomy Embryol. 2006;211(November):757. doi: 10.1007/s00429-006-0136-7. [DOI] [PubMed] [Google Scholar]
- Gao W.J. Acute clozapine suppresses synchronized pyramidal synaptic network activity by increasing inhibition in the ferret prefrontal cortex. J. Neurophysiol. 2007;97(February):1196. doi: 10.1152/jn.00400.2006. [DOI] [PubMed] [Google Scholar]
- Gargus R.A. Unimpaired outcomes for extremely low birth weight infants at 18 to 22 months. Pediatrics. 2009;124(July):112. doi: 10.1542/peds.2008-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke: J. Cerebral Circulation. 2008;39(December):3378. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- Gisslen T. Repeated exposure to intra-amniotic LPS partially protects against adverse effects of intravenous LPS in preterm lambs. Innate Immunity. 2014;20(February):214. doi: 10.1177/1753425913488430. [DOI] [PubMed] [Google Scholar]
- Greiveldinger L., Veissier I., Boissy A. The ability of lambs to form expectations and the emotional consequences of a discrepancy from their expectations. Psychoneuroendocrinology. 2011;36(July):806. doi: 10.1016/j.psyneuen.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Griffith J.L. MR imaging correlates of white-matter pathology in a preterm baboon model. Pediatr. Res. 2012;71(February):185. doi: 10.1038/pr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner J.I. Endotoxin and transient hypoxia cause severe acidosis in the piglet. J. Pediatr. Surg. 1997;32(July):1123. doi: 10.1016/s0022-3468(97)90413-9. [DOI] [PubMed] [Google Scholar]
- Hagberg H., Gressens P., Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012;71(April):444. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- Harju K. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr. Res. 2005;57(May):644. doi: 10.1203/01.PDR.0000156212.03459.A9. [DOI] [PubMed] [Google Scholar]
- Hernadi A., Kis A., Turcsan B., Topal J. Man's underground best friend: domestic ferrets, unlike the wild forms, show evidence of dog-like social-cognitive skills. PLoS ONE. 2012;7:e43267. doi: 10.1371/journal.pone.0043267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz S.R. Early-childhood neurodevelopmental outcomes are not improving for infants born at <25 weeks’ gestational age. Pediatrics. 2011;127(Jan):62. doi: 10.1542/peds.2010-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu N., Jha S.K., Coleman T., Frank M.G. Paradoxical effects of the hypnotic Zolpidem in the neonatal ferret. Behav. Brain Res. 2009;201(July 19):233. doi: 10.1016/j.bbr.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Huang S.S. Differential pathological and immune responses in newly weaned ferrets are associated with a mild clinical outcome of pandemic 2009 H1N1 infection. J. Virol. 2012;86(December):13187. doi: 10.1128/JVI.01456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton L.C. Injury of the developing cerebellum: a brief review of the effects of endotoxin and asphyxial challenges in the late gestation sheep fetus. Cerebellum (London, England) 2014;13(December):777. doi: 10.1007/s12311-014-0602-3. [DOI] [PubMed] [Google Scholar]
- Illa M. Long-term functional outcomes and correlation with regional brain connectivity by MRI diffusion tractography metrics in a near-term rabbit model of intrauterine growth restriction. PloS one. 2013;8:e76453. doi: 10.1371/journal.pone.0076453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder J., Neil B. Patterns of cerebral injury in a primate model of preterm birth and neonatal intensive care. J. Child Neurol. 2005;20(December):965. doi: 10.1177/08830738050200120601. [DOI] [PubMed] [Google Scholar]
- Inder T., Neil J., Yoder B., Rees S. Non-human primate models of neonatal brain injury. Seminars in Perinatology. 2004;28(December):396. doi: 10.1053/j.semperi.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Iwata O. Therapeutic time window duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;1154(June 18):173. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Jackson C.A., Peduzzi J.D., Hickey T.L. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J. Neurosci. 1989;9(April):1242. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson Misbe E.N., Richards T.L., McPherson R.J., Burbacher T.M., Juul S.E. Perinatal asphyxia in a nonhuman primate model. Dev. Neurosci. 2011;33:210. doi: 10.1159/000327246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour I.T. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr. Neurol. 2015;52(February):143. doi: 10.1016/j.pediatrneurol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Jespersen S.N., Leigland L.A., Cornea A., Kroenke C.D. Determination of axonal and dendritic orientation distributions within the developing cerebral cortex by diffusion tensor imaging. IEEE Trans. Med. Imag. 2012;31(January):16. doi: 10.1109/TMI.2011.2162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M.V., Haddad R., Carman-Young A., Coyle J.T. Neurotransmitter chemistry of lissencephalic cortex induced in ferrets by fetal treatment with methylazoxymethanol acetate. Brain Res. 1982;256(July):285. doi: 10.1016/0165-3806(82)90140-7. [DOI] [PubMed] [Google Scholar]
- Karlsson K.A., Arnardottir H., Robinson S.R., Blumberg M.S. Dynamics of sleep-wake cyclicity across the fetal period in sheep (Ovis aries) Dev. Psychobiol. 2011;53(January):89. doi: 10.1002/dev.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Iwai L., Tanno K. Rapid and efficient genetic manipulation of gyrencephalic carnivores using in utero electroporation. Mol. Brain. 2012;5:24. doi: 10.1186/1756-6606-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.Y. Expression of adrenoceptor subtypes in preterm piglet heart is different to term heart. PLoS ONE. 2014;9:e92167. doi: 10.1371/journal.pone.0092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.R. Studies on the animal model of post-stroke depression and application of antipsychotic aripiprazole. Behav. Brain Res. 2015;287(April 3):294. doi: 10.1016/j.bbr.2015.03.062. [DOI] [PubMed] [Google Scholar]
- Knutsen A.K., Kroenke C.D., Chang Y.V., Taber L.A., Bayly P.V. Spatial and temporal variations of cortical growth during gyrogenesis in the developing ferret brain. Cerebral Cortex (New York, N.Y. 1991) 2013;23(February):488. doi: 10.1093/cercor/bhs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger G.P. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine. 2007;25(July 9):5220. doi: 10.1016/j.vaccine.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke C.D., Taber E.N., Leigland L.A., Knutsen A.K., Bayly P.V. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cerebral Cortex (New York, N.Y. 1991) 2009;19(December):2916. doi: 10.1093/cercor/bhp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban K.C. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol. 2014;(October 16) doi: 10.1016/j.pediatrneurol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K.M., Tien L.T., Pang Y., Bhatt A.J., Fan L.W. IL-1 receptor antagonist attenuates neonatal lipopolysaccharide-induced long-lasting learning impairment and hippocampal injury in adult rats. Toxicol. Lett. 2015;234(April 2):30. doi: 10.1016/j.toxlet.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery D.J., Santolaya-Forgas J., Wilson Bieniarz L.A., Jr., Warsof S.L. A non-human primate model for the in utero chronic catheterization of the umbilical vein. A preliminary report. Fetal Diagnosis Therapy. 1995;10(September–October):326. doi: 10.1159/000264253. [DOI] [PubMed] [Google Scholar]
- Leviton A. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine. 2011;53(January):66. doi: 10.1016/j.cyto.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Sun X., Chen J., Leno G.H., Engelhardt J.F. Factors affecting the efficiency of embryo transfer in the domestic ferret (Mustela putorius furo) Theriogenology. 2006;66(July):183. doi: 10.1016/j.theriogenology.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall J.W. Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma. 1988;5:1. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- Lindeberg H. Reproduction of the female ferret (Mustela putorius furo) Reproduction in Domestic Animals = Zuchthygiene. 2008;43(Suppl. 2, July):150. doi: 10.1111/j.1439-0531.2008.01155.x. [DOI] [PubMed] [Google Scholar]
- Lodygensky G.A. Definition and quantification of acute inflammatory white matter injury in the immature brain by MRI/MRS at high magnetic field. Pediatr. Res. 2014;75(March):415. doi: 10.1038/pr.2013.242. [DOI] [PubMed] [Google Scholar]
- Mack W.J. An improved functional neurological examination for use in nonhuman primate studies of focal reperfused cerebral ischemia. Neurol. Res. 2003;25(April):280. doi: 10.1179/016164103101201346. [DOI] [PubMed] [Google Scholar]
- Maher J.A., DeStefano J. The ferret: an animal model to study influenza virus. Lab. Anim. 2004;33(October):50. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- Mallard C. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr. Res. 2014;75(January):234. doi: 10.1038/pr.2013.188. [DOI] [PubMed] [Google Scholar]
- Mallard C. Innate immune regulation by toll-like receptors in the brain. ISRN Neurol. 2012;2012:701950. doi: 10.5402/2012/701950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. II. Pathologic studies. Lab. Invest.: J. Techn. Methods Pathol. 1969;21(September):189. [PubMed] [Google Scholar]
- Martinez-Cerdeno V. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS ONE. 2012;7:e30178. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A.M., Roberts C.T., Maduwegedera D., Flower R.L., Denton K.M. Chronic maternal hypertension characterized by renal dysfunction is associated with reduced placental blood flow during late gestation in rabbits. Am. J. Physiol. Regul. Integrat. Comp. Physiol. 2010;298(April):R1043. doi: 10.1152/ajpregu.00202.2009. [DOI] [PubMed] [Google Scholar]
- McSherry G.M., Smart I.H. Cell production gradients in the developing ferret isocortex. J. Anatomy. 1986;144(February):1. [PMC free article] [PubMed] [Google Scholar]
- McSherry G.M. Mapping of cortical histogenesis in the ferret. J. Embryol. Exp. Morphol. 1984;81(June):239. [PubMed] [Google Scholar]
- Ment L.R., Stewart W.B., Duncan C.C., Lambrecht R. Beagle puppy model of intraventricular hemorrhage. J. Neurosurg. 1982;57(August):219. doi: 10.3171/jns.1982.57.2.0219. [DOI] [PubMed] [Google Scholar]
- Moody K.D., Bowman T.A., Lang C.M. Laboratory management of the ferret for biomedical research. Lab. Anim. Sci. 1985;35(June):272. [PubMed] [Google Scholar]
- Nakata M., Itou T., Sakai T. Molecular cloning and phylogenetic analysis of inflammatory cytokines of the ferret (Mustela putorius furo) J. Vet. Med. Sci./Japanese Soc. Vet. Sci. 2008;70(June):543. doi: 10.1292/jvms.70.543. [DOI] [PubMed] [Google Scholar]
- Nakata M., Itou T., Sakai T. Quantitative analysis of inflammatory cytokines expression in peripheral blood mononuclear cells of the ferret (Mustela putorius furo) using real-time PCR. Vet. Immunol. Immunopathol. 2009;130(July 15):88. doi: 10.1016/j.vetimm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Neal J. Insights into the gyrification of developing ferret brain by magnetic resonance imaging. J. Anatomy. 2007;210(January):66. doi: 10.1111/j.1469-7580.2006.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas L., Martinez-Gomez M., Hudson R., Bautista A. Littermate presence enhances motor development, weight gain and competitive ability in newborn and juvenile domestic rabbits. Dev. Psychobiol. 2011;53(January):37. doi: 10.1002/dev.20485. [DOI] [PubMed] [Google Scholar]
- Nitsos I. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J. Soc. Gynecol. Invest. 2006;13(May):239. doi: 10.1016/j.jsgi.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Noctor S.C., Scholnicoff N.J., Juliano S.L. Histogenesis of ferret somatosensory cortex. J. Comp. Neurol. 1997;387(October 20):179. [PubMed] [Google Scholar]
- Northington F.J. Brief update on animal models of hypoxic-ischemic encephalopathy and neonatal stroke. ILAR J./Nat. Res. Coun. Inst. Lab. Anim. Res. 2006;47:32. doi: 10.1093/ilar.47.1.32. [DOI] [PubMed] [Google Scholar]
- Peng X. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nat. Biotechnol. 2014;32(December):1250. doi: 10.1038/nbt.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluch S., Juliano S.L. Populations of radial glial cells respond differently to reelin and neuregulin1 in a ferret model of cortical dysplasia. PLoS ONE. 2010;5:e13709. doi: 10.1371/journal.pone.0013709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluch S., Juliano S.L. Fine-tuning of neurogenesis is essential for the evolutionary expansion of the cerebral cortex. Cerebral Cortex (New York, N.Y. 1991) 2015;25(February):346. doi: 10.1093/cercor/bht232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe A., Haddad R., Dumas R. Behavior and neurobehavioral teratology using the ferret. Lab. Anim. Sci. 1985;35(June):256. [PubMed] [Google Scholar]
- Ramani M., van Groen T., Kadish I., Bulger A., Ambalavanan N. Neurodevelopmental impairment following neonatal hyperoxia in the mouse. Neurobiol. Dis. 2013;50(February):69. doi: 10.1016/j.nbd.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reillo I., Borrell V. Germinal zones in the developing cerebral cortex of ferret: ontogeny cell cycle kinetics and diversity of progenitors. Cerebral Cortex (New York, N.Y. 1991) 2012;22(September):2012. doi: 10.1093/cercor/bhr284. [DOI] [PubMed] [Google Scholar]
- Rice 3rd J.E., Vannucci R.C., Brierley J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981;9(February):131. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Ritter J. Neonatal hyperoxia exposure disrupts axon-oligodendrocyte integrity in the subcortical white matter. J. Neurosci. 2013;33(May 22):8990. doi: 10.1523/JNEUROSCI.5528-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso N. Environmental enrichment increases the GFAP+ stem cell pool and reverses hypoxia-induced cognitive deficits in juvenile mice. J. Neurosci. 2012;32(June 27):8930. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Watanabe M. Development of cerebral sulci and gyri in ferrets (Mustela putorius) Congenit Anom (Kyoto) 2012;52(September):168. doi: 10.1111/j.1741-4520.2012.00372.x. [DOI] [PubMed] [Google Scholar]
- Schmidt B. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA: J. Am. Med. Assoc. 2012;307(January 18):275. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- Schwartz M.L. Chronic neonatal hypoxia leads to long term decreases in the volume and cell number of the rat cerebral cortex. Seminars in Perinatology. 2004;28(December):379. doi: 10.1053/j.semperi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- See R.H. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J. Gen. Virol. 2008;89(September):2136. doi: 10.1099/vir.0.2008/001891-0. [DOI] [PubMed] [Google Scholar]
- Shridharani J.K. Porcine head response to blast. Frontiers Neurol. 2012;3:70. doi: 10.3389/fneur.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein L.J. Ferret hematology and related disorders. Vet. Clin. North America. Exo. Anim. Prac. 2008;11(September):535. doi: 10.1016/j.cvex.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Smart I.H., McSherry G.M. Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J. Anatomy. 1986;144(August):27. [PMC free article] [PubMed] [Google Scholar]
- Smith A.L., Alexander M., Rosenkrantz T.S., Sadek M.L., Fitch R.H. Sex differences in behavioral outcome following neonatal hypoxia ischemia: insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp. Neurol. 2014;254(April):54. doi: 10.1016/j.expneurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Stitt I. Auditory and visual interactions between the superior and inferior colliculi in the ferret. Eur. J. Neurosci. 2015;(February 3) doi: 10.1111/ejn.12847. [DOI] [PubMed] [Google Scholar]
- Stoll B.J. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. Sep 2010;126(September):443. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridh L. Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia-ischemia. J. Neurosci. 2013;33(July 17):12041. doi: 10.1523/JNEUROSCI.0673-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf A.M., Tanaka D., Jr., Aulerich R.J., Bursian S.J. Delayed neurotoxic effects of tri-o-tolyl phosphate in the European ferret. J. Toxicol. Environ. Health. 1989;26(61) doi: 10.1080/15287398909531233. [DOI] [PubMed] [Google Scholar]
- Sullivan S. Improved behavior, motor, and cognition assessments in neonatal piglets. J. Neurotrauma. 2013;30(October 15):1770. doi: 10.1089/neu.2013.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. Model of cerebral palsy in the perinatal rabbit. J. Child Neurol. 2005;20(December):972. doi: 10.1177/08830738050200120801. [DOI] [PubMed] [Google Scholar]
- Tao J.D., Barnette A.R., Griffith J.L., Neil J.J., Inder T.E. Histopathologic correlation with diffusion tensor imaging after chronic hypoxia in the immature ferret. Pediatr. Res. 2012;71(February):192. doi: 10.1038/pr.2011.32. [DOI] [PubMed] [Google Scholar]
- Ten V.S. Late measures of brain injury after neonatal hypoxia-ischemia in mice. Stroke: J. Cerebral Circulation. 2004;35(September):2183. doi: 10.1161/01.STR.0000137768.25203.df. [DOI] [PubMed] [Google Scholar]
- Thornton C. Molecular mechanisms of neonatal brain injury. Neurol. Res. Int. 2012;2012:506320. doi: 10.1155/2012/506320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber A., Jha S.K., Coleman T., Frank M.G. A preliminary study of sleep ontogenesis in the ferret (Mustela putorius furo) Behav. Brain Res. 2008;189(May 16):41. doi: 10.1016/j.bbr.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobet S.A., Chickering T.W., Fox T.O., Baum M.J. Sex and regional differences in intracellular localization of estrogen receptor immunoreactivity in adult ferret forebrain. Neuroendocrinology. 1993;58(September):316. doi: 10.1159/000126556. [DOI] [PubMed] [Google Scholar]
- Tomassy G.S., Fossati V. How big is the myelinating orchestra? Cellular diversity within the oligodendrocyte lineage: facts and hypotheses. Front. Cell. Neurosci. 2014;8:201. doi: 10.3389/fncel.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traudt C.M., McPherson R.J., Studholme C., Millen K.J., Juul S.E. Systemic glycerol decreases neonatal rabbit brain and cerebellar growth independent of intraventricular hemorrhage. Pediatr. Res. 2014;75(March):389. doi: 10.1038/pr.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cleave S., Shall M.S. A critical period for the impact of vestibular sensation on ferret motor development. J. Vestibular Res.: Equil. Orient. 2006;16:179. [PMC free article] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J. Comp. Neurol. 1989;289(November):74. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. The encephalopathy of prematurity-brain injury and impaired brain development inextricably intertwined. Seminars in Pediatric Neurology. 2009;16(December):167. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009;24(September):1085. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann. Neurol. 2011;70(October):525. doi: 10.1002/ana.22533. [DOI] [PubMed] [Google Scholar]
- Wassink G. Hypothermic neuroprotection is associated with recovery of spectral edge frequency after asphyxia in preterm fetal sheep. Stroke: J. Cerebral Circulation. 2015;46(February):585. doi: 10.1161/STROKEAHA.114.008484. [DOI] [PubMed] [Google Scholar]
- Wen T.C., Rogido M., Gressens P., Sola A. A reproducible experimental model of focal cerebral ischemia in the neonatal rat. Brain Res. Brain Res. Protoc. 2004;13(June):76. doi: 10.1016/j.brainresprot.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Workman A.D., Charvet C.J., Clancy B., Darlington R.B., Finlay B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci.: Off. J. Soc. Neurosci. 2013;33(April 24):7368. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. human Gene Ther. Clin. Dev. 2015;12(February) doi: 10.1089/humc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z. A double-injection model of intracerebral hemorrhage in rabbits. J. Clin. Neurosci.: Off. J. Neurosurg. Soc. Australasia. 2009;16(April):545. doi: 10.1016/j.jocn.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Zervas M., Walkley S.U. Ferret pyramidal cell dendritogenesis: changes in morphology and ganglioside expression during cortical development. J. Comp. Neurol. 1999;413(October):429. doi: 10.1002/(sici)1096-9861(19991025)413:3<429::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Zhang K., Sejnowski T.J. A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Nat. Aca. Sci. U. S. A. 2000;97(May 9):5621. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Chen Y., Xu Y., Pi G. Effect of intrauterine infection on brain development and injury. Int. J. Dev. Neurosci.: Off. J. Int. Soc. Dev. Neurosci. 2013;31(November):543. doi: 10.1016/j.ijdevneu.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Zitzow L.A. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 2002;76(May):4420. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]