Infectious gastroenteritis is a common, acute illness that is characteristically self-limiting, but it can become debilitating and life-threatening in immunocompromised patients.1 Noroviruses are major pathogens among the microbes associated with gastroenteritis in both immunocompetent and immunocompromised hosts1–4 (Table 1). In the United States, noroviruses are the single most common cause of acute gastroenteritis in adults that results in a visit to the hospital emergency department,2 and they are second only to rotaviruses as a major cause of severe diarrhea in infants and young children.5 In developing countries, noroviruses are estimated to cause more than 200,000 deaths annually among children younger than 5 years of age, and it is predicted that these viruses will become the predominant cause of diarrhea in all age groups worldwide once rotavirus infection is controlled through vaccination.6
Table 1.
Infectious Causes of Gastroenteritis.
| Type of Pathogen | Immunocompetent Hosts | Immunocompromised Hosts |
|---|---|---|
| Viruses | Norovirus | Norovirus |
| Rotavirus | Rotavirus | |
| Astrovirus | Astrovirus | |
| Adenovirus | Adenovirus | |
| Herpesvirus | ||
| Human immunodeficiency virus (AIDS enteropathy) |
||
| Cytomegalovirus | ||
| Bacteria |
Escherichia
coli (pathogenic) |
E. coli (pathogenic) |
| Salmonella spp. | Salmonella spp. | |
| Campylobacter jejuni | C. jejuni | |
| Clostridium difficile | C. difficile | |
| Shigella spp. | Shigella spp. | |
| Chlamydia trachomatis | ||
| Mycobacterium tuberculosis, M. avium complex | ||
| Parasites | Cryptosporidium spp. | Cryptosporidium spp. |
| Entamoeba histolytica | E. histolytica | |
| Giardia lamblia | G. lamblia | |
| Cystoisospora belli | ||
| Blastocystis hominis | ||
| Cyclospora spp. | ||
| Strongyloides stercoralis | ||
| Fungi | Microsporidium spp. | |
| Histoplasma spp. | ||
| Candida spp. |
Noroviruses are increasingly recognized as an important cause of chronic gastroenteritis in immunocompromised patients, as reflected by the growing number of clinical case reports.7–9 A comparison of the known features of norovirus gastroenteritis in immunocompetent versus immunocompromised hosts highlights the potentially serious outcome of this illness in persons who cannot adequately clear the virus (Table 2). The purpose of this review is to summarize recent developments in norovirus research that are relevant to the prevention and management of norovirus gastroenteritis in immunocompromised patients.
Table 2.
Characteristics of Norovirus Gastroenteritis in Immunocompetent versus Immunocompromised Hosts.
| Characteristic | Immunocompetent Hosts | Immunocompromised Hosts |
|---|---|---|
| Prevalence | Leading cause of gastroenteritis worldwide | Not established; estimated at about 17 to 18% |
| Seasonality | Peak in winter months | Year-round |
| Clinical features | Acute onset, duration of 24 to 48 hr | Acute onset, indefinite duration |
| Viral shedding | 20 to 40 days | Weeks to years |
| Level of virus | 108 to 109 genome copies per gram of stool | 105 to 108 genome copies per gram of stool, depending on level of immunosuppressive therapy |
| Evolution of virus in host | Small number of stable variants | Markedly diverse variants |
| Tissue tropism | Small intestine | Small intestine |
| Complications | Dehydration | Dehydration, malnutrition, dysfunction of intestinal barrier |
| Treatment | Infection is usually self-limiting; rehydration, if needed | No virus-specific treatment is available; supportive care, adjustment of immunosuppressive therapy |
| Prognosis | Usually excellent, but the infection can be life-threatening | Poor to excellent; chronic infection is common |
NOROVIRUS CLASSIFICATION AND STRUCTURE
Noroviruses are small, nonenveloped viruses with a single-stranded RNA genome that make up the genus norovirus of the family Caliciviridae.4 They are divided into six major genogroups designated GI through GVI. GI and GII contain the majority of norovirus strains associated with human disease and are further divided into about 30 genotypes.10 A single genotype, GII.4, has been associated with the majority of global outbreaks since the mid-1990s, when active surveillance with molecular diagnostic techniques was initiated.11,12
The norovirus genome encodes seven nonstructural and two structural proteins (Fig. 1).5,13 The majority of reverse-transcriptase–polymerase-chain-reaction (RT-PCR) diagnostic assays target the RNA polymerase region of the genome because of its higher sequence conservation among strains. VP1 is the major structural protein of the virus that self-assembles into viruslike particles (VLPs), which are being evaluated as possible vaccines; VP2 is a minor structural protein.5,14,15 Noroviruses bind saccharides of the human histo–blood group antigens (HBGAs) within their VP1 protruding 2 (P2) domain,13 a proposed mechanism for facilitating viral entry into the epithelial cells of the gastrointestinal tract (Fig. 1). It is thought that susceptibility to norovirus in humans is determined by allelic variation of HBGAs,13 with each norovirus strain presenting a characteristic HBGA-binding profile. Thus, a certain genetic background might confer resistance to the infection, as in the case of persons classified as nonsecretors (i.e., persons in whom these carbohydrates are not expressed on the surface of intestinal epithelial cells), who are resistant to infection with Norwalk virus, a GI.1 strain.13
Figure 1. Genomic Organization and Atomic Structure of the Norovirus Capsid.
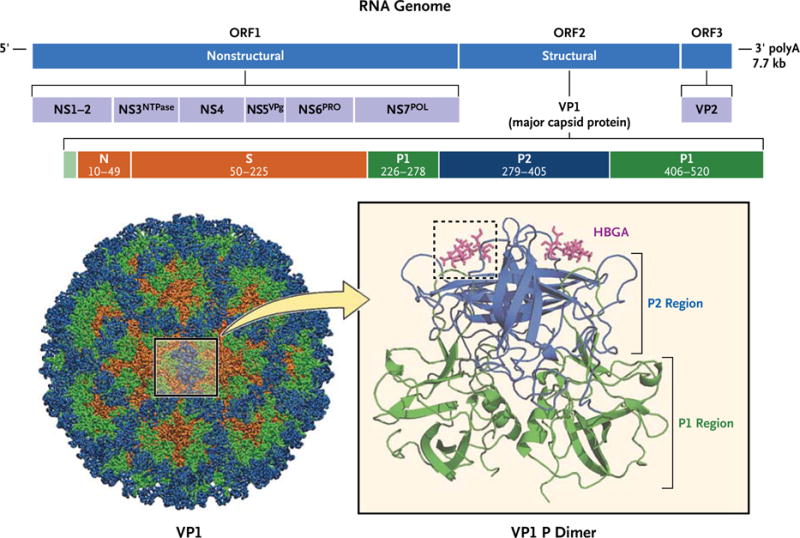
The RNA genome of the prototype norovirus strain, Norwalk virus (shown at the top), is organized into three open reading frames (ORF1, ORF2, and ORF3) that encode the designated nonstructural and structural proteins. Most diagnostic primers used in reverse-transcriptase–polymerase-chain-reaction assay target conserved areas in the RNA-dependent RNA polymerase region (NS7POL). VP1, the major capsid protein (shown below), is further organized into the N-terminal (N), shell (S), and protruding (P) domains defined by the indicated VP1 amino acid residues. The P2 region of the P domain (blue) is exposed on the surface of the capsid protein and is the site where histo–blood group antigens (HBGAs) (magenta) interact with the virion (dashed box).5,13
NOROVIRUSES IN IMMUNOCOMPROMISED PATIENTS
Prolonged norovirus and illness have been reported in persons who are immunosuppressed as a result of congenital immunodeficiency, immunosuppressive therapy for the purpose of maintaining an organ allograft, cancer chemotherapy, and infection with the human immunodeficiency virus (HIV).16,17 Immunocompromised patients can be exposed to noroviruses from many sources, including family members, health care workers, contaminated food or water, and the environment (including nosocomial sources).5 The overall incidence of norovirus gastroenteritis in hospital and community settings has not yet been determined. An increasing number of studies show that immunosuppressive therapy is a risk factor for norovirus infection. According to one report, 18% of patients who underwent allogeneic hematopoietic stem-cell transplantation (HSCT) contracted norovirus over a 1-year period, many after they had received intensified immunosuppressive regimens for suspected graft-versus-host disease (GVHD).18 A 2-year survey of renal-transplant recipients showed that 17% of the patients were chronically infected with norovirus and had intermittent diarrhea.19
Noroviruses are highly resistant to harsh environmental conditions, and the infectious oral dose is estimated to be less than 20 viral particles.4 In immunocompetent adults, norovirus gastroenteritis is characteristically acute (24 to 48 hours in duration) and self-limiting, but in immunocompromised adults, the disease can become chronic and can persist for weeks to years5,9 (Table 2). A marked predominance of wintertime norovirus infections has been widely described in the general population,5 and common names are winter-vomiting disease and stomach flu. In contrast, in a case–control study of children with cancer9 and in a case series of patients who had undergone HSCT,18 the rate of illness was reported to be unchanged throughout the year.
It is not yet clear whether noroviruses are transmissible to immunocompetent adults from patients with chronic viral shedding, the latter having been proposed as a possible reservoir of novel genetic variants.20 Surveillance studies suggest that most nosocomial norovirus infections are acquired in the community; nosocomial outbreaks in which persons with immunodeficiency disorders are the source are rare.21,22 Vomiting and diarrhea have been linked to high viral loads in patients undergoing immunosuppressive therapy, whereas asymptomatic shedding was associated with lower viral loads.9 These findings, as well as those involving immunocompetent hosts, suggest that in most cases the virus is transmitted from symptomatic persons, even when high levels of the virus are shed in the stool for prolonged periods after symptoms have resolved.23
Chronic norovirus gastroenteritis can present specific clinical challenges in patients with an impaired immune response, as compared with immunocompetent hosts. For example, norovirus-induced diarrhea in immunosuppressed renaltransplant recipients is characterized by dramatic weight loss and lasts considerably longer than treatable bacteria- or parasite-induced diarrhea (i.e., an average of 9 months vs. 1 month).24 The malnutrition, dehydration, and altered intestinal mucosal barrier associated with prolonged norovirus-related diarrhea can increase morbidity and worsen the outcome of the underlying disease.21 Noroviruses were reported to be the cause of death in one patient 49 days after the onset of symptoms21 and in another patient after 1 year of unresolved gastroenteritis.18
DIAGNOSIS OF NOROVIRUS GASTROENTERITIS
It is difficult to diagnose norovirus gastroenteritis on the basis of clinical features alone. Diarrhea is a common complication in transplant recipients17: gastroenteritis develops in 80% of patients who have undergone allogeneic HSCT, as a result of conditioning therapy, GVHD, drugs, or infectious agents.25 Symptoms of acute norovirus disease can include diarrhea, fever, and projectile vomiting, in contrast to the characteristic combination of diarrhea and nausea (without vomiting) observed in GVHD.5,18 Although a provisional diagnosis might be made, use of a reliable diagnostic assay is crucial to distinguish infectious diarrhea from clinical complications such as graft rejection and GVHD, since these conditions require diametrically opposed approaches to management (i.e., decreasing immunosuppression in infectious diarrhea and increasing it in graft rejection and GVHD).26
Noroviruses are shed in stool, and norovirus-specific antigens and RNA can be detected in stool samples. Regular or quantitative real-time RT-PCR assay is the most widely used laboratory method for diagnosing norovirus gastroenteritis, but several other assays are now available.4,27 Computed tomography has been reported to aid in discriminating between norovirus infection and GVHD, since norovirus-infected patients have pronounced bowel-wall edema restricted to the small intestine, which is infrequently seen in patients with intestinal cytomegalovirus infection or GVHD.21,28 Precise and timely diagnosis of norovirus infection by means of laboratory testing is essential in intestinal-transplant recipients, since the pathological characteristics of norovirus infection are similar to those seen in allograft rejection, with chronic inflammatory change, apoptotic cells, and blunted villi.22 The diagnosis of gastrointestinal GVHD, a well-described complication of HSCT, also relies on histopathological findings that could be mistaken for those associated with norovirus infection, in which numerous apoptotic bodies are observed.29
NOROVIRUS DIVERSIT Y AND EVOLUTION IN IMMUNOCOMPROMISED PATIENTS
The diversity of norovirus genotypes circulating in the community, where GII.4 is most prevalent, is reflected in the genotypes detected in persons with an impaired immune system.18,30,31 There have been no reported strain differences among the norovirus genotypes with regard to symptoms, severity, or progression to chronicity in immunocompromised hosts.
Intrahost variation and evolution of the viral genome over extended periods of shedding have been studied in detail in several patients persistently infected with norovirus.19,30–34 An analysis of the viral variants present in the stool of infected persons showed that in immunocompetent persons in whom the infection resolved during the acute phase, a single major variant predominated, whereas immunocompromised patients with chronic norovirus infection had a diverse viral population (Fig. 2).34 These data indicate that a chronic infection without immune pressure allows the generation of a diverse norovirus population in the host; however, at present there is no epidemiologic evidence to suggest that these variants become prevalent as epidemic strains in the community. Despite the increased viral heterogeneity generated during a chronic norovirus infection, the amino acid residues that interact with HBGA ligands remain conserved — a finding that is consistent with the proposed importance of this interaction in the binding of virus to intestinal epithelial cells.35
Figure 2. Distributions of Norovirus Variants in Representative Immunocompetent and Immunocompromised Hosts.
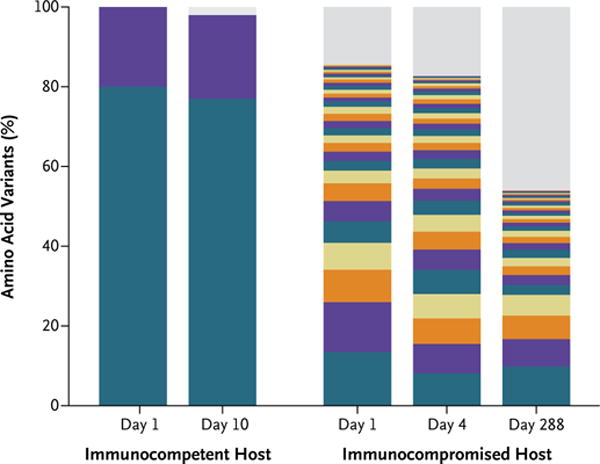
The bar graph shows the frequency distribution of VP1 norovirus variants, identified through next-generation sequencing, in an immunocompetent host and an immunocompromised host. Each unique variant is represented by a different color. Given the limited color palette, repeated colors represent distinct variants. Low-frequency variants, with an estimated frequency of occurrence below the detection threshold (2%), are shown in gray. Only two variants were detected in the immunocompetent host, and they remained stable throughout the 10 days of infection. No predominant variant was observed in the immunocompromised host. Instead, numerous low-frequency variants coexisted, and their prevalences varied over the course of the infection. Adapted from Bull et al.34
The shedding of norovirus by immunocompromised patients for an extended period of time has provided a unique opportunity to monitor the effects of genetic changes that lead to the accumulation of alterations in the amino acid makeup of the virus.19 Evolution of the norovirus genome in patients infected for extended periods is relatively rapid (3.3% amino acid substitutions per year), considering that GII.4 noroviruses have been shown to have accumulated only a 10% amino acid difference in their viral capsid after circulating in the community for 31 years.36 Accurate determination of a substitution rate could be useful in assessing whether a patient with chronic shedding continues to have the same norovirus strain or has been reinfected with a new strain; it may also help track norovirus transmission among immunocompromised patients in a common setting. This “time-clock” approach may prove useful in establishing the role of nosocomial transmission in immunocompromised patients and in evaluating the efficacy of treatment because knowledge of the rate at which genetic differences are generated can be used to determine whether a persistent strain is under investigation or a new strain has been introduced.19
PREVENTION AND TREATMENT OF NOROVIRUS INFECTION IN IMMUNOCOMPROMISED PATIENTS
No vaccines or specific antiviral agents are currently available to prevent or treat norovirus infection, but progress has recently been made in vaccine development.14,15 Norovirus vaccines have been tested in both humans and chimpanzees, and the results of these studies were used to determine correlates of protection and duration of immune response.14,15 It has also been reported that both T-cell and B-cell responses are necessary to clear norovirus. In a mouse model, CD4+ and CD8+ cells were required for clearance of murine norovirus in the intestine.37,38 Norovirus clearance in patients with chronic infection has been shown to be associated with the recovery of T cells25; in one study, symptoms improved in patients with HIV infection who had an increased CD4+ count.39
Currently, the treatment of patients with norovirus gastroenteritis is primarily supportive and focuses on prevention and reversal of dehydration. Chronic norovirus infections in transplant recipients may also require the adjustment of immunosuppressive therapy during prolonged illness.24 Passive antibody therapies have been tested in individual case studies, and evidence of their efficacy in treating patients with norovirus gastroenteritis is mostly anecdotal. Oral administration of breast milk or immune globulin has yielded mixed results, probably reflecting differences in the quality and quantity of norovirus-specific antibodies in the treatment administered.7,32 Both immune globulin and breast milk have been administered successfully through a duodenal tube (in an attempt to bypass the adverse acidic environment of the stomach) to treat prolonged norovirus infection in a heart-transplant recipient, but this approach failed to clear norovirus in a patient with agammaglobulinemia.40,41
Certain commonly used antiviral drugs such as ribavirin have failed to clear norovirus in chronically infected patients.40 Nitazoxanide (an antiprotozoal drug) was reported to significantly reduce the time to resolution of symptoms related to both rotavirus- and norovirus-induced diarrhe in immunocompetent patients,42 and symptoms of severe norovirus gastroenteritis were described as markedly reduced after 1 day of treatment in a patient who underwent HSCT.43 However, quantification of the exact genomic load in this patient was not reported, and viral shedding persisted for a month after treatment. Further testing will be needed to determine the efficacy of this drug in immunocompromised patients.
Finally, it has been suggested that the class of immunosuppressive drugs provided might affect the clearance of norovirus, since certain drugs also have antiviral properties.30,44 A significant increase in the antiviral properties of the immunosuppressive therapy administered (as measured by the incidence of cytomegalovirus infections in immunodeficient patients) was observed only when a switch was made from an antimetabolite (azathioprine or mycophenolate) to a mammalian target of rapamycin (mTOR) inhibitor (sirolimus or everolimus).45 The incidence of norovirus gastroenteritis in patients being treated with different types of immunosuppressive agents will require more study.
CONCLUSIONS
Given the substantial toll that noroviruses can take on the prognosis for and quality of life of patients with a deficient immune response, appropriate measures should be taken to reduce the risk of norovirus infection. First and foremost, rigorous personal hygiene, especially hand-washing, is the single most effective measure to combat norovirus transmission. This measure is crucial, given the fact that 80% of hospital surfaces were found to be contaminated with 21 different noroviruses during environmental surveillance in a unit for children with immunodeficiency disorders.46 Immunocompromised patients should avoid contact with persons who are acutely ill with gastroenteritis and should follow guidelines designed to prevent infections with enteric pathogens.47 Such patients should consume foods considered to be safe according to the principles designed to minimize the risk of foodborne diseases.48,49 Although it is prudent to isolate patients who have chronic norovirus infection, the virulence of noroviruses shed in the stool in such patients has been called into question, given the absence of reported secondary cases. Finally, norovirus testing can now be included in the care of immunocompromised patients with acute or chronic gastroenteritis of unknown cause. Widespread use of diagnostic assays and continued research will help clarify the precise disease burden and epidemiologic features of norovirus infection in this population and will improve the clinical care of those infected.
Acknowledgments
We thank Dr. Albert Z. Kapikian for his critical review and suggestions and Dr. Colleen Hadigan for expert clinical advice.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
No potential conflict of interest relevant to this article was reported.
References
- 1.Krones E, Hogenauer C. Diarrhea in the immunocompromised patient. Gastroenterol Clin North Am. 2012;41:677–701. doi: 10.1016/j.gtc.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Bresee JS, Marcus R, Venezia RA, et al. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis. 2012;205:1374–81. doi: 10.1093/infdis/jis206. [DOI] [PubMed] [Google Scholar]
- 3.Capizzi T, Makari-Judson G, Steingart R, Mertens WC. Chronic diarrhea associated with persistent norovirus excretion in patients with chronic lymphocytic leukemia: report of two cases. BMC Infect Dis. 2011;11:131. doi: 10.1186/1471-2334-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–85. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green KY. Caliciviridae: the noroviruses. In: Knipe DM, editor. Fields virology. 5th. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 949–79. [Google Scholar]
- 6.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–31. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florescu DF, Hermsen ED, Kwon JY, et al. Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr Transplant. 2011;15:718–21. doi: 10.1111/j.1399-3046.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 8.Henke-Gendo C, Harste G, Juergens-Saathoff B, Mattner F, Deppe H, Heim A. New real-time PCR detects prolonged norovirus excretion in highly immunosuppressed patients and children. J Clin Microbiol. 2009;47:2855–62. doi: 10.1128/JCM.00448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig A, Adams O, Laws HJ, Schroten H, Tenenbaum T. Quantitative detection of norovirus excretion in pediatric patients with cancer and prolonged gastroenteritis and shedding of norovirus. J Med Virol. 2008;80:1461–7. doi: 10.1002/jmv.21217. [DOI] [PubMed] [Google Scholar]
- 10.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–23. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J Infect Dis. 1999;179:1334–44. doi: 10.1086/314783. [DOI] [PubMed] [Google Scholar]
- 12.Lindesmith LC, Donaldson EF, Lobue AD, et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 2008;5(2):e31. doi: 10.1371/journal.pmed.0050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan M, Jiang X. Norovirus-host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol. 2011;19:382–8. doi: 10.1016/j.tim.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bok K, Parra GI, Mitra T, et al. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc Natl Acad Sci U S A. 2011;108:325–30. doi: 10.1073/pnas.1014577107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armbrust S, Kramer A, Olbertz D, Zimmermann K, Fusch C. Norovirus infections in preterm infants: wide variety of clinical courses. BMC Res Notes. 2009;2:96. doi: 10.1186/1756-0500-2-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishman JA. Infections in immunocompromised hosts and organ transplant recipients: essentials. Liver Transpl. 2011;17(Suppl 3):S34–S37. doi: 10.1002/lt.22378. [DOI] [PubMed] [Google Scholar]
- 18.Roddie C, Paul JP, Benjamin R, et al. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis. 2009;49:1061–8. doi: 10.1086/605557. [DOI] [PubMed] [Google Scholar]
- 19.Schorn R, Höhne M, Meerbach A, et al. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin Infect Dis. 2010;51:307–14. doi: 10.1086/653939. [DOI] [PubMed] [Google Scholar]
- 20.Sukhrie FH, Siebenga JJ, Beersma MF, Koopmans M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J Clin Microbiol. 2010;48:4303–5. doi: 10.1128/JCM.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz S, Vergoulidou M, Schreier E, et al. Norovirus gastroenteritis causes severe and lethal complications after chemotherapy and hematopoietic stem cell transplantation. Blood. 2011;117:5850–6. doi: 10.1182/blood-2010-12-325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman SS, Chatterjee NK, Fuschino ME, et al. Characteristics of human calicivirus enteritis in intestinal transplant recipients. J Pediatr Gastroenterol Nutr. 2005;40:328–33. doi: 10.1097/01.mpg.0000155182.54001.48. [DOI] [PubMed] [Google Scholar]
- 23.Sukhrie FH, Teunis P, Vennema H, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis. 2012;54:931–7. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- 24.Roos-Weil D, Ambert-Balay K, Lanternier F, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92:61–9. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 25.Saif MA, Bonney DK, Bigger B, et al. Chronic norovirus infection in pediatric hematopoietic stem cell transplant recipients: a cause of prolonged intestinal failure requiring intensive nutritional support. Pediatr Transplant. 2011;15:505–9. doi: 10.1111/j.1399-3046.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 26.Alkhouri N, Danziger-Isakov L. Norovirus and severe chronic gastroenteritis in pediatric stem cell transplantation: the plot thickens. Pediatr Transplant. 2011;15:671–2. doi: 10.1111/j.1399-3046.2011.01522.x. [Erratum, Pediatr Transplant 2011; 15:766.] [DOI] [PubMed] [Google Scholar]
- 27.Kirby A, Iturriza-Gómara M. Norovirus diagnostics: options, applications and interpretations. Expert Rev Anti Infect Ther. 2012;10:423–33. doi: 10.1586/eri.12.21. [DOI] [PubMed] [Google Scholar]
- 28.Tajiri H, Kiyohara Y, Tanaka T, Etani Y, Mushiake S. Abnormal computed tomography findings among children with viral gastroenteritis and symptoms mimicking acute appendicitis. Pediatr Emerg Care. 2008;24:601–4. doi: 10.1097/PEC.0b013e3181850cc8. [DOI] [PubMed] [Google Scholar]
- 29.Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Mod Pathol. 2011;24:117–25. doi: 10.1038/modpathol.2010.163. [DOI] [PubMed] [Google Scholar]
- 30.Boillat Blanco N, Kuonen R, Bellini C, et al. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl Infect Dis. 2011;13:213–5. doi: 10.1111/j.1399-3062.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 31.Siebenga JJ, Beersma MF, Vennema H, van Biezen P, Hartwig NJ, Koopmans M. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J Infect Dis. 2008;198:994–1001. doi: 10.1086/591627. [Erratum, J Infect Dis 2008;198:1575.] [DOI] [PubMed] [Google Scholar]
- 32.Nilsson M, Hedlund KO, Thorhagen M, et al. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J Virol. 2003;77:13117–24. doi: 10.1128/JVI.77.24.13117-13124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann D, Hutzenthaler M, Seebach J, et al. Norovirus GII.4 and GII.7 capsid sequences undergo positive selection in chronically infected patients. Infect Genet Evol. 2012;12:461–6. doi: 10.1016/j.meegid.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Bull RA, Eden JS, Luciani F, McElroy K, Rawlinson WD, White PA. Contribution of intra- and interhost dynamics to norovirus evolution. J Virol. 2012;86:3219–29. doi: 10.1128/JVI.06712-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlsson B, Lindberg AM, Rodriguez-Díaz J, Hedlund KO, Persson B, Svensson L. Quasispecies dynamics and molecular evolution of human norovirus capsid P region during chronic infection. J Gen Virol. 2009;90:432–41. doi: 10.1099/vir.0.005082-0. [DOI] [PubMed] [Google Scholar]
- 36.Boon D, Mahar JE, Abente EJ, et al. Comparative evolution of GII.3 and GII.4 norovirus over a 31-year period. J Virol. 2011;85:8656–66. doi: 10.1128/JVI.00472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 2008;4(12):e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chachu KA, Strong DW, LoBue AD, Wobus CE, Baric RS, Virgin HW., IV Antibody is critical for the clearance of murine norovirus infection. J Virol. 2008;82:6610–7. doi: 10.1128/JVI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wingfield T, Gallimore CI, Xerry J, et al. Chronic norovirus infection in an HIV-positive patient with persistent diarrhoea: a novel cause. J Clin Virol. 2010;49:219–22. doi: 10.1016/j.jcv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 40.van de Ven AA, Douma JW, Rademaker C, et al. Pleconaril-resistant chronic parechovirus-associated enteropathy in agammaglobulinaemia. Antivir Ther. 2011;16:611–4. doi: 10.3851/IMP1792. [DOI] [PubMed] [Google Scholar]
- 41.Ebdrup L, Bottiger B, Molgaard H, Laursen AL. Devastating diarrhoea in a heart-transplanted patient. J Clin Virol. 2011;50:263–5. doi: 10.1016/j.jcv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Rossignol JF, El-Gohary YM. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Aliment Pharmacol Ther. 2006;24:1423–30. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 43.Siddiq DM, Koo HL, Adachi JA, Viola GM. Norovirus gastroenteritis successfully treated with nitazoxanide. J Infect. 2011;63:394–7. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelen MA, Gunia S, Stypmann J. Elimination of norovirus in a chronic carrier under immunosuppression after heart transplantation — effect of everolimus. Transpl Int. 2011;24(11):e102–e103. doi: 10.1111/j.1432-2277.2011.01330.x. [DOI] [PubMed] [Google Scholar]
- 45.Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–48. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 46.Xerry J, Gallimore CI, Cubitt D, Gray JJ. Tracking environmental norovirus contamination in a pediatric primary immunodeficiency unit. J Clin Microbiol. 2010;48:2552–6. doi: 10.1128/JCM.00066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozicki M, Steffen R, Schar M. ‘Boil it, cook it, peel it or forget it’: does this rule prevent travellers’ diarrhoea? Int J Epidemiol. 1985;14:169–72. doi: 10.1093/ije/14.1.169. [DOI] [PubMed] [Google Scholar]
- 49.Koo HL, DuPont HL. Noroviruses as a potential cause of protracted and lethal disease in immunocompromised patients. Clin Infect Dis. 2009;49:1069–71. doi: 10.1086/605558. [DOI] [PubMed] [Google Scholar]


