Abstract
Introduction:
The essential oil Mentha x villosa (MVEO) has a wide range of actions, including antibacterial, antifungal, antiprotozoal and schistosomicidal actions. The present study aimed to investigate the ultrastructural changes of MVEO on the tegument of adult Schistosoma mansoni.
Materials and Methods:
Different concentrations of MVEO were tested on S. mansoni adult worms in vitro. Ultrastructural changes on the tegument of these adult worms were evaluated using scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Results:
The MVEO caused the death of all worms at 500 μg mL-1 after 24 h. After 24h of 500 μg mL-1 MVEO treatment, bubble lesions were observed over the entire body of worms and they presented loss of tubercles in some regions of the ventral portion. In the evaluation by TEM, S. mansoni adult worms treated with MVEO, 500 μg mL-1, presented changes in the tegument and vacuoles in the syncytial matrix region. Glycogen granules close to the muscle fibers were visible.
Conclusion:
The ability of MVEO to cause extensive ultrastructural damage to S. mansoni adult worms correlates with its schistosomicidal effects and confirms earlier findings with S. mansoni.
Keywords: Schistosomicidal activity, Schistosoma mansoni, Mentha x villosa
INTRODUCTION
Schistosomiasis is a neglected disease widespread worldwide and poses a major public health problem. It is caused by parasitic trematode flatworms of theSchistosoma genus; moreover, S. mansoni is the only species found in Brazil1 , 2.
The treatment of schistosomiasis is based on the use of praziquantel (PZQ); however, this drug seems ineffective against juvenile stages of S. mansoniand its extensive use in mass treatment of populations in schistosomiasis risk areas have favored the emergence of refractory strains of S. mansoni to conventional treatment with PZQ3.
Therefore, the search for new drugs that can act against S. mansonibecomes relevant, and tools such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) have been employed to study the effects of compounds on the tegument of many helminths, especially S. mansoni 4. In this context, the search for natural bioactive compounds against S. mansoni becomes an interesting alternative2.
Mentha x villosaHudson (Lamiaceae) has been used in traditional medicine due to its antiparasitic activity. It is known popularly as "hortelã-rasteira", "hortelã comum", or "hortelã-da-folha-miúda"5. Giamebil(r)is a commercial formulation presenting amebicidal (Entamoeba histolytica) and giardicidal (Giardia lamblia) activities, having as its active ingredient the dry extract from the leaves and stem of M. x villosa 6. Recent studies have also demonstrated the efficacy ofM. x villosa against Trichomonas vaginalis 7.
Essential oils (EOs) and extracts of aromatic plants have been recognized for many years as a great source of pharmaceutical agents and food additives8. Some studies show different biological effects caused by the M. x villosa essential oil (MVEO): antimicrobial9, hypotensive and bradycardiac10 , 11, cardiovascular12 - 14, larvicidal15, antinociceptive16, cytotoxic, antitumor 17 and schistosomicidal activities 18,
Recent studies developed by our research group have demonstrated the in vitro schistosomicidal activity of MVEO18. However, there are no studies showing ultrastructural changes inS. mansoni adult worms after incubation with MVEO.
The aim of this study was to evaluate the ultrastructural changes in S. mansoni male worms after in vitro incubation with MVEO; the results shown here are supported by TEM and SEM.
MATERIALS AND METHODS
Ethics statement: All experiments involving the use of experimental animals were performed in accordance to the ethical standards of theFundação Oswaldo Cruz and were approved by the Animal Experimentation Ethics Committee (No. 06/2010).
Botanical material: Fresh leaves of the species M. x villosa were used. They were gathered from the Medicinal Plants Garden of the Laboratório de Tecnologia Farmacêutica, Universidade Federal da Paraíba between April and June 2011. They were identified and authenticated by Dr. F. J. Abreu Matos (Laboratório de Produtos Naturais, Universidade Federal do Ceará) and Dr. Raymond Harley of the Royal Botanic Gardens, Kew, England. A voucher specimen was deposited in the Prisco Bezerra Herbarium of the Federal University of Ceará (N. 14996).
Preparation of samples: To extract MVEO, 10 kg of leaves were steam-distilled for 8 h. The oil obtained (0.1%) was dried over anhydrous sodium sulfate in the usual manner and stored at 4 °C. We used a gas chromatograph coupled to a mass spectrometer (Shimadzu QP-5000) under the following analytical conditions: capillary column, OV-5 (30m × 0.25 mm × 0.25 μm); injector (Ohio Valley Specialty Chemical, Inc.), 240 °C; detector, 230 °C; electron impact, 70 eV; gas drag, He; flow, 1.0 mL/min; split, 1/20; program temperature, 60 °C - 240 °C at 3 °C/min; and solution injection volume, 1 μL (1 μL of essential oil per 1 mL of ethyl acetate). The compounds were identified by comparing their mass spectra using the GC MS database system (Nist 62 lib.) and the Kovats retention index. The compounds were dissolved in 100% dimethyl sulfoxide (DMSO)18.
Praziquantel was commercially available through Sigma-Aldrich (Sigma chemical, St Louis, MO, USA) with purity of 99.9%.
Obtaining and maintenance of S. mansoni adult worms: The BH S. mansoni strain (Belo Horizonte, Minas Gerais, Brazil) was used throughout this study. This strain was maintained in Biomphalaria glabrata snails and Swiss Webster mice in a laboratory at the Centro de Pesquisas Aggeu Magalhães of Fundação Oswaldo Cruz. Female Swiss Webster mice weighing 20 ± 5 grams were used as the definitive host, and were infected transcutaneously with about 120 cercariae of the BH strain, -as previously described18, using the tail immersion technique. The animals were exposed for 1 h to the cercariae and they were subsequently kept under controlled temperature and light conditions. Furthermore, they had access to food and water ad libitum 19.
After fifty-five days of infection, S. mansoni adult worms were recovered from the mice by perfusion, washed in RPMI 1640 medium buffered with HEPES (20 mM), pH 7.5, supplemented with penicillin (100 IU mL-1), streptomycin (100 µg mL-1), and 10% fetal bovine serum (Gibco), and placed in petri dishes containing 2 mL of sterile culture medium20.
In vitro studies of S. mansoni adult worms: To assess the damage to the tegument, adult worms of S. mansoni were recovered from the hepatic portal system of the infected mice and left for a period of 2 h to adapt to the culture medium. MVEO isolate and compound was added in varying concentrations: a) MVEO (5, 10, 100, 250, and 500 µg mL-1). Then, the worms were incubated at 37 °C in an atmosphere containing 5% CO2 18.
As controls, S. mansoni adult worms were incubated in the presence of 1.6% DMSO in RPMI 1640 (negative control) or exposed to 0.5 µg mL-1PZQ (positive control). All experiments were performed with three replicates. The final volume in each well was 2 mL. The parasites were collected and monitored for routine processing with SEM and TEM at 24, 48, 72, 96, and 120 h intervals. The worms were considered dead when there was no motion detected after 3 minutes of observation. SEM and TEM were used as tools to evaluate the morphological changes inS. mansoni adult worms after in vitroexposure.
Transmission Electron Microscopy (TEM): S. mansoni adult worms in each group were fixed (2.5% glutaraldehyde in sodium cacodylate buffer 0.1 M, pH 7.4). After fixation, they were washed with sodium cacodylate buffer 0.1 M, pH 7.4, and postfixed with 1% osmium tetroxide (OsO4), in the same buffer, for 2 h in the dark. Then, samples were washed, counterstained block with 5% uranyl acetate in water. Dehydration was performed in a series of increasing acetone (30, 50, 70, 90 and 3 x 100%) for 30 min, each at room temperature and followed by embedding in Embed 812/Araldite resin (Electron Microscopy Sciences, Hartfield, PA) at 70 °C for 48 h. Semi-thin sections were stained with toluidine blue for morphological observation, while ultrathin sections were subsequently contrasted in uranyl acetate for 1 h and lead citrate for 10 min, and observations done in TEM (TEM 100CXII JEOL).
Scanning Electron Microscopy (SEM): The worms were incubated for 24 h and, after their death, they were washed with sodium cacodylate buffer (pH = 7.2), fixed with 2.5% glutaraldehyde (pH = 7.4) during 24 h, and then fixed with 1% osmium tetroxide for 1 h. The samples were dehydrated by an increasing amount of ethanol solution, dried in a critical point dryer, and then mounted on stubs and coated with gold using a sputter coater. The material was examined under a JEOL - 5600 LV microscope.
RESULTS AND DISCUSSION
The tegument of S. mansoni is an important structure for its survival since it is involved in nutrient absorption, secretion of metabolites, osmotic balance, and parasitic defense against the host immune system; this structure is an important target for drug action21. Some studies have documented damages to the tegument of S. mansoni caused by synthetic22 - 24 and natural25 - 26 antischistosomal compounds.
Ultrastructural analysis was performed on male worms for two reasons: females are frequently in contact with the host microenvironment and studies in the literature have shown that soft tissue alterations are more pronounced in males than in female worms27.
Ultrastructural analysis of MVEO-induced surface damage in S. mansoni
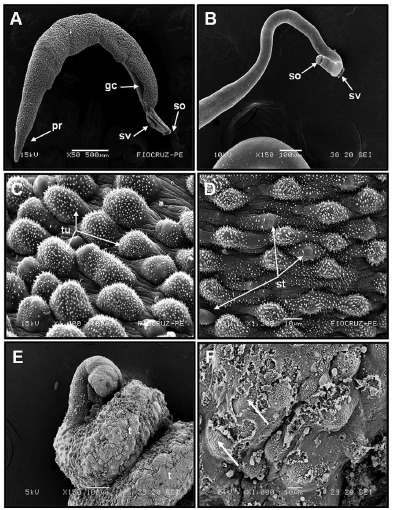
Scanning Electron Microscopy (SEM): Control groups were not affected for up to five days of observation and all worms exhibited vigorous activity. It can be seen that male worms of S. mansoni in the control group presented the tegument covered with tubercules and tiny projections (spines). The back was long and contained the gynecophoral canal (gc). The area between the oral and ventral suckers did not have any tubercles (tu), spines (sp) or sensory papillae (Fig. 1A-1B). The presence of a large number of tubercles with typical spines (Fig. 1C) as well as sensory papillae (st) (Fig. 1D) was observed.
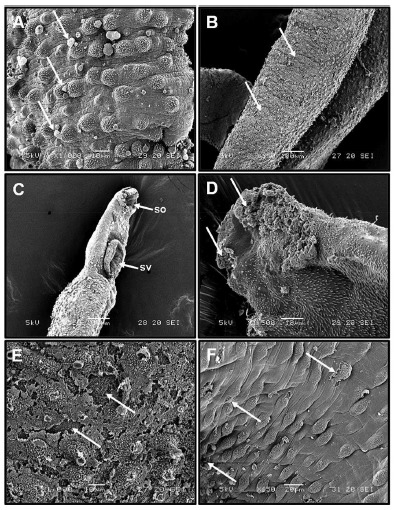
Fig. 1. (A-D) Electromicrographs of adult worms of S. mansoni without treatment. (A) Gynecophoric canal (gc), thinner portion of the worms located in the posterior region (pr), (B) while in the anterior region are located the oral (so) and ventral (sv) suckers. (C) In the tegument of male worms the presence of tubercles (tu) with spines was observed.(D) The presence of a large number of tubercles with typical spines, randomly distributed throughout the body (st) was identified. (E-F) Electromicrographs of adult male worms ofS. mansoni treated with PZQ (0.5 µg mL-1). (E) Adult worms presenting winding body and extensive destruction of the tegument (t). (F)Severe damage on the tegument with loss of spines and extensive ulceration with muscle exposition (arrows).
In assessing the viabitility of the worms treated with PZQ, it was observed the death of all worms after 24h of incubation. Using SEM, it was identified that theS. mansoni adult worms treated with PZQ (0.5 µg mL-1) showed spiraled body (Fig. 1E). In the tegument there was destruction of tubercules and spines, and many regions with ulceration (Fig. 1F).
After 24 h of MVEO (500 µg mL-1) treatment, bubble lesions were spread over the entire body of the worms (Fig. 2A), and the worms showed loss of tubercules in some regions of the ventral portion (Fig. 2B). After 48 h of incubation at 250 µg mL-1, death of worms was observed, destroyed the oral sucker had been destroyed and the ventral sucker contracted (Fig. 2C). Tegument lesion severity increased after 72 h of MVEO treatment (100 µg mL-1), which caused the basal membrane to become unprotected (Fig. 2D). Lower concentrations (5 and 10 µg mL-1) were unable to cause mortality of S. mansoniadult worms after 120 h of exposure; however, changes in the tegument of the worms were recorded. At a concentration of 10 µg mL-1, tegument erosion can be visualized at higher magnification (Fig. 2E) and in the worms treated with 5 µg mL-1 there was destruction of some tubercules (Fig. 2F).
Fig. 2. (A-F). Electromicrographs of S. mansoniadult male worms of treated with different concentrations of MVEO.(A) After 24 h of MVEO (500 µg mL-1)treatment, bubble lesions were spread over the entire body of the worms (arrow). (B) Ventral portion of the adult worms ofS. mansoni after 24h of incubation with MVEO (500 µg mL-1). The loss of tubercules in some regions was observed (arrows). (C) Anterior region of the adult male worms 48 h after incubation with 250 µg mL-1 of MVEO. Destruction of the oral (os) and ventral (vs) suckers. (D) Tegument lesion severity increased (arrows) after 72 h of MVEO treatment (100 µg mL-1). (E) Tegument erosion (arrows) can be visualized at a higher magnification with no spines after 96 h of exposure to 10 µg mL-1 of MVEO. (F) Destruction of some tubercules after 120 h of incubation with 5 µg mL-1of MVEO (arrows).
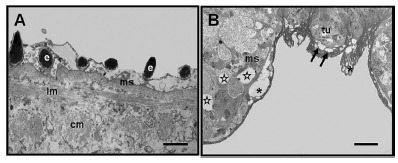
Transmission Electron Microscopy (TEM): The ultrastructural evaluation of S. mansoni adult worms by TEM revealed the presence of spines, characteristical matrix syncytial, and circular and longitudinal muscles in the subtegumentary region of the worms (Fig. 3A). The TEM analysis of S. mansoni adult worms treated with PZQ showed many changes like vacuoles in tuber, presence of vesicles in the syncytial matrix, and mesenchymal vacuolization (Fig. 2B).
Fig. 3. (A-B). Electromicrographs of S. mansoni adult worms visualized by TEM. (A) In the control group, observe the spines (e). In the tegument, it is possible to identify the matrix syncytial (ms) and, in the subtegumentary region, it is possible to visualize the circular (cm) and longitudinal (lm) muscles. (B) In the group treated with PZQ (0.5 µg mL-1) vacuoles (arrows) are observed in the tubercules (tu), presence of vesicles (asterisks) in the matrix syncytial (ms) and vacuolated mesenchymal (stars). Bars = 1µm.
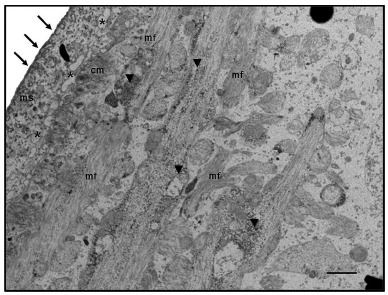
In the evaluation by TEM, the S. mansoni adult worms treated with MVEO (500 µg mL-1) presented changes in the tegument and presence of vacuoles in the syncytial matrix region. It was visible the presence of glycogen granules close to the muscle fibers (Fig. 4). Essential oils are highly enriched with compounds termed terpenoids that possess several biological properties such as schistosomicidal activity21 - 25 , 28 - 30. In recent years, a number of studies have been developed through in vitro screening using essential oils, extracts, and bioactive compounds from medicinal plants27 , 29 - 32 ,to identify a leading substance that can be used in preclinical trials for the treatment of experimental schistosomiasis 33 - 36.
Fig. 4. Electromicrographs of adult worms of S. mansonitreated with MVEO (500 μg mL-1). Observe changes in the tegument (arrows) and vacuoles (asterisks). In the matrix syncytial (ms), it is even possible to identify accumulation of glycogen granules (arrowheads) around muscle fibers (mf) and circular muscle (cm). Bar = 1μm.
Generally, there was a marked difference between the morphology of worms treated with PZQ compared with MVEO, and compounds used individually. In the macroscopic examination, the S. mansoni adult worms, when exposed to PZQ, presented muscle contractions causing them to stay retracted or twisted. However, this behavior was not observed in the worms treated with MVEO or their constituents.
The adult worms incubated for 24 h with MVEO (500 µg mL-1) showed damaged tegument and exposed musculature in some worms. These findings were identified by LORSUWANNARAT et al.37 when testing plumbagin (100 µg mL-1) in S. mansoni adult worms. EISSA et al.24 paid attention to this same finding when evaluating the effect of miltefosine (10 µg mL-1) on S. mansoni adult worms; however, the authors made observations after 120 h of incubation. LIMA et al.4, when assessing the effects of allicin on the tegument of S. mansoni, describe the occurrence of ulceration on the parasite tegument after 120 minutes of incubation with 20 µg mL-1 of MVEO. Previously, BERTÃO et al 38 also evaluated the effects of miltefosine onS. mansoni adult worms by testing 200 µM and found the same results; however, they used only 12 h of incubation.
In the present study, after 48 h of incubation with 250 µg mL-1 of MVEO, morphological changes in the oral sucker and ventral suckers of S. mansoni adult worms were observed. Comparable to our findings, ALBUQUERQUE et al.22describe similar changes by treating S. mansoni with (Z)-3-(4-chloro-benzyl)-5-(4-nitro-benzylidene)-imidazolidine-2,4-dione (120 µg mL-1); however, this data was observed following five days of incubation. OLIVEIRA et al.39 draw attention to the occurrence of damage in the oral sucker ofS. mansoni adult worms after exposure to the essential oil ofBaccharis trimera (130 mg mL-1) after 24 h of exposure. NEVES et al.2 only observed the contraction of the ventral sucker when evaluating a derivative of thioxo-imidazolidine (100 µM) on S. mansoni adult worms after 3 h of incubation. KEISER et al.40 also reported erosion of the tegument of female worms after exposure to mefloquine (10 µg mL-1) after 1 h of incubation. Our results show that the lowest concentration of MVEO (5 and 10 µg mL-1) caused less damage to soft tissue compared to the highest concentrations. The worms incubated in these concentrations generally showed destruction of tubercules with no spines. The same finding was observed by MANNECK et al.41 when assessing the effects of mefloquine (10 µg mL-1) on the tegument of S. mansoni adult worms. Recently, NEVES etal.2, while evaluating a thioxo-imidazolidine, determined that after less than 1 h, adultS. mansoni vesicles showed that the increased number of these vesicles was proportional to the time of evaluation.
The mechanism by which MVEO exerts its in vitro anti-S. mansoni action is unclear. However, it has been reported that, because of the great different classes of compounds, usually essential oils may have no specific cellular target. Essential oils are typical lipophilic compounds, thus, the chemical substances of the oil, may pass through the cell wall, tegument, and cytoplasmic membrane damaging their structures and cellular membranes, which may lead to cellular lysis23. Regarding the therapeutic benefits of essential oils, so far, there are no studies that can give us a clear idea, or be accurate, about the mode of action. However, some effects are associated with loss of ions and reduction of membrane potential, as well as collapse of the proton pump and depletion of the ATP pool23. Furthermore, it has to be kept in mind that essential oils are complex mixtures of volatile constituents biosynthesized by plants28. The present study showed that these MVEO are capable of producing a range of ultrastructural changes in the S. mansoni tegument. Therefore, considering the anti-S. mansoni action of MVEO, it may be possible that the activity of its main constituents can be modulated by molecules present in the essential oil.
CONCLUSIONS
The ability of MVEO to cause extensive ultrastructural damage to S. mansoni adult worms correlates with its schistosomicidal effects and confirms earlier findings with S. mansoni.
ACKNOWLEDGMENTS
The authors thank LTF/UFPB, LIKA/UFPE and Núcleo de Plataformas Tecnológicas (NPT) of Aggeu Magalhães Research Center of the Oswaldo Cruz Foundation for supporting the experiments and CAPES for a scholarship.
References
- 1.Neves BJ, Andrade CH, Cravo PVL. Natural products as leads in schistosome drug discovery. Molecules. 2015;20:1872–1903. doi: 10.3390/molecules20021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neves JK, Lima MCA, Pereira VRA, De Melo CML, Peixoto CA, Pitta IR. Antischistosomal action of thioxo-imidazolidine compounds: an ultrastructural and cytotoxicity study. Exp Parasitol. 2011;128:82–90. doi: 10.1016/j.exppara.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Liang YS, Coles GC, Doenhoff MJ, Southgate VR. Susceptibility to praziquantel of male and female cercariae of praziquantel-resistant and susceptible isolates of Schistosoma mansoni. Int J Parasitol. 2001;31:202–207. doi: 10.1016/s0020-7519(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 4.Lima CM, Freitas FI, Morais LC, Cavalcanti MG, Silva LF, Padilha RJ. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Rev Soc Bras Med Trop. 2011;44:327–330. doi: 10.1590/s0037-86822011005000023. [DOI] [PubMed] [Google Scholar]
- 5.Matos FJA, Machado MIL, Craveiro AA, Alencar JW, Barbosa JM, Cunha EVL, Himura CH. Plants used in traditional medicine of China and Brazil. J Essent Oil Res. 1991;2:13–16. doi: 10.1590/s0074-02761991000600006. [DOI] [PubMed] [Google Scholar]
- 6.Teles NSB, Fechine FV, Viana FAC, Viana IOL, Nascimento DF, Leite ALAS. Evaluation of the therapeutic efficacy of Mentha crispa in the treatment of giardiasis. Contemp Clin Trials. 2011;32:809–813. doi: 10.1016/j.cct.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Moraes ME, Cunha GH, Bezerra MM, Fechine FV, Pontes AV, Andrade WS. Efficacy of the Mentha crispa in the treatment of women with Trichomonas vaginalis infection. Arch Gynecol Obstet. 2012;286:125–130. doi: 10.1007/s00404-012-2251-4. [DOI] [PubMed] [Google Scholar]
- 8.Joy B, Rajan A, Abraham E. Antimicrobial activity and chemical composition of essential oil from Hedychium coronarium. Phytother Res. 2007;21:439–443. doi: 10.1002/ptr.2091. [DOI] [PubMed] [Google Scholar]
- 9.Arruda TA, Antunes RMP, Catão RMR, Lima EO, Sousa DP, Nunes XP. Preliminary study of the antimicrobial activity of Mentha x villosa Hudson essential oil, rotundifolone and its analogues. Rev Bras Farmacogn. 2006;16:307–311. [Google Scholar]
- 10.Guedes DN, Silva DF, Barbosa-Filho JM, Medeiros IA. Calcium antagonism and the vasorelaxation of the rat aorta induced by rotundifolone. Braz J Med Biol Res. 2004;37:1881–1887. doi: 10.1590/s0100-879x2004001200014. [DOI] [PubMed] [Google Scholar]
- 11.Guedes DN, Silva DF, Barbosa-Filho JM, Medeiros IA. Endothelium-dependent hypotensive and vasorelaxant effects of the essential oil from aerial parts of Mentha x villosa in rats. Phytomedicine. 2004;11:490–497. doi: 10.1016/j.phymed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Lahlou S, Carneiro-Leão RF, Leal-Cardoso JH. Cardiovascular effects of the essential oil of Mentha x villosa in DOCA-salt-hypertensive rats. Phytomedicine. 2002;9:715–720. doi: 10.1078/094471102321621313. [DOI] [PubMed] [Google Scholar]
- 13.Lahlou S, Carneiro-Leão RF, Leal-Cardoso JH, Toscano CF. Cardiovascular effects of the essential oil of Mentha x villosa and its main constituent, piperitenone oxide, in normotensive anaesthetised rats: role of the autonomic nervous system. Planta Med. 2001;67:638–643. doi: 10.1055/s-2001-17352. [DOI] [PubMed] [Google Scholar]
- 14.Lahlou S, Magalhães PJC, Carneiro-Leão RFL, Leal-Cardoso JH. Involvement of nitric oxide in the mediation of the hypotensive action of the essential oil of Mentha x villosa in normotensive conscious rats. Planta Med. 2002;68:694–699. doi: 10.1055/s-2002-33797. [DOI] [PubMed] [Google Scholar]
- 15.Lima TC, da Silva TK, Silva FL, Barbosa-Filho JM, Marques MO, Santos RL. Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere. 2014;104:37–43. doi: 10.1016/j.chemosphere.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Sousa PJC, Linard CFBM, Azevedo-Batista D, Oliveira AC, Coelho-de-Souza AN, Leal-Cardoso JH. Antinociceptive effects of the essential oil of Mentha x villosa leaf and its major constituent piperitenone oxide in mice. Braz J Med Biol Res. 2009;42:655–659. doi: 10.1590/s0100-879x2009000700010. [DOI] [PubMed] [Google Scholar]
- 17.Amaral RG, Fonseca CS, Silva TK, Andrade LN, França ME, Barbosa-Filho JM. Evaluation of the cytotoxic and antitumour effects of the essential oil from Mentha x villosa and its main compound, rotundifolone. J Pharm Pharmacol. 2015 doi: 10.111/jphp.12409. [DOI] [PubMed] [Google Scholar]
- 18.Matos-Rocha TJ, Cavalcanti MGS, Barbosa-Filho JM, Lúcio ALSC, Veras DL, Feitosa APS. In vitro evaluation of schistosomicidal activity of essential oil of Mentha x villosa and some of its chemical constituents in adult worms of Schistosoma mansoni. Planta Med. 2013;79:1307–1312. doi: 10.1055/s-0033-1350732. [DOI] [PubMed] [Google Scholar]
- 19.Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 20.Aires AL, Ximenes EC, Silva RA, Barbosa VX, Góes AJ, Peixoto CA. Ultrastructural analysis of β-lapachone-induced surface membrane damage in male adult Schistosoma mansoni BH strain worms. Exp Parasitol. 2014;142:83–90. doi: 10.1016/j.exppara.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Faghiri Z, Skelly PJ. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake. FASEB J. 2009;23:2780–2789. doi: 10.1096/fj.09-130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albuquerque MCP, Pitta MGR, Irmão JI, Peixoto CA, Malagueño E, Santana JV. Tegumental alterations in adult Schistosoma mansoni treated with imidazolidine derivatives. Lat Am J Pharm. 2007;26:65–69. [Google Scholar]
- 23.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 24.Eissa MM, EL-Azzouni MZ, Amer EI, Baddour NM. Miltefosine, a promising novel agent for schistosomiasis mansoni. Int J Parasitol. 2011;41:235–242. doi: 10.1016/j.ijpara.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Esperandim VR, da Silva FD, Sousa RKC, Magalhães LG, Medeiros SJ, Pauletti PM. In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Med. 2013;79:1653–1655. doi: 10.1055/s-0033-1351022. [DOI] [PubMed] [Google Scholar]
- 26.Godinho LS, Aleixo de Carvalho LS, Barbosa de Castro CC, Dias MM, Pinto PF, Crotti AE. Anthelmintic activity of crude extract and essential oil of Tanacetum vulgare (Asteraceae) against adult worms of Schistosoma mansoni. Scientific WorldJournal. 2014;(2014):460342–460342. doi: 10.1155/2014/460342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mostafa OM, Soliman MI. Experimental use of black-seed oil against Schistosoma mansoni in albino mice: II. Surface topography of adult worms. Egypt J Med Lab Sci. 2002;11:79–85. [Google Scholar]
- 28.Moraes J, de Oliveira RN, Costa JP, L A, Junior, de Sousa DP, Freitas RM. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl Trop Dis. 2014;8:4. doi: 10.1371/journal.pntd.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moraes J de, Nascimento C, Lopes PO, Nakano E, Yamaguchi LF, Kato MJ. Schistosoma mansoni: in vitro schistosomicidal activity of piplartine. Exp Parasitol. 2011;127:357–364. doi: 10.1016/j.exppara.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Guimarães MA, de Oliveira RN, Véras LM, Lima DF, Campelo YD, Campos SA. Anthelmintic activity in vivo of epiisopiloturine against juvenile and adult worms of Schistosoma mansoni. PLoS Negl Trop Dis. 2015;9:4. doi: 10.1371/journal.pntd.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magalhães LG, Kapadia GJ, da Silva Tonuci LR, Caixeta SC, Parreira NA, Rodrigues V. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol Res. 2010;106:395–401. doi: 10.1007/s00436-009-1674-8. [DOI] [PubMed] [Google Scholar]
- 32.Moraes J, Nascimento C, Yamaguchi LF, Kato MJ, Nakano E. Schistosoma mansoni: in vitro schistosomicidal activity and tegumental alterations induced by piplartine on schistosomula. Exp Parasitol. 2012;132:222–227. doi: 10.1016/j.exppara.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Sass DC, Morais GO, Miranda RA, Magalhães LG, Cunha WR, dos Santos RA. Structurally modified natural sesquiterpene lactones constitute effective and less toxic schistosomicidal compounds. Org Biomol Chem. 2014;12:7957–7964. doi: 10.1039/c4ob00426d. [DOI] [PubMed] [Google Scholar]
- 34.Santos AF, Fonseca AS, César FA, de Azevedo Albuquerque MCP, Santana JV, Santana AEG. A penta-substituted pyridine alkaloid from the rhizome of Jatropha elliptica (Pohl) Muell. Arg. is active against Schistosoma mansoni and Biomphalaria glabrata. Parasitol Res. 2014;113:1077–1084. doi: 10.1007/s00436-013-3743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva AP, Silva MP, Oliveira CG, Monteiro DC, Pinto PL, Mendonça RZ. Garcinielliptone FC: antiparasitic activity without cytotoxicity to mammalian cells. Toxicol In Vitro. 2015;29:681–687. doi: 10.1016/j.tiv.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Veras LM, Guimaraes MA, Campelo YD, Vieira MM, Nascimento C, Lima DF. Activity of epiisopiloturine against Schistosoma mansoni. Curr Med Chem. 2012;19:2051–2058. doi: 10.2174/092986712800167347. [DOI] [PubMed] [Google Scholar]
- 37.Lorsuwannarat N, Saowakon N, Ramasoota P, Wanichanon C, Sobhon P. The anthelmintic effect of plumbagin on Schistosoma mansoni. Exp Parasitol. 2013;133:18–27. doi: 10.1016/j.exppara.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Bertão HG, Silva RAR, Padilha RJR, Albuquerque MCPA, Rádis-Baptista G. Ultrastructural analysis of miltefosine-induced surface membrane damage in adult Schistosoma mansoni BH strain worms. Parasitol Res. 2012;110:2465–2473. doi: 10.1007/s00436-011-2786-5. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira RN, Rehder VLG, Oliveira ASS, Montanari I, Júnior, Carvalho JE, Ruiz ALTG. Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp Parasitol. 2012;132:135–143. doi: 10.1016/j.exppara.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J. Mefloquine an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3:4. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manneck T, Haggenmüller Y, Keiser J. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology. 2010;137:85–98. doi: 10.1017/S0031182009990965. [DOI] [PubMed] [Google Scholar]


