Abstract
Objective
Epilepsy is a chronic neurological condition that significantly increases risk of injury and premature death. Rates of mental illness are also disproportionately high in those with epilepsy, which can be attributed in part to the stress and stigma associated with epilepsy. Psychiatric conditions generally complicate the management of epilepsy, and understanding how psychiatric comorbidity affects use of crisis-based health resources could inform care approaches that help improve epilepsy care. To better understand effects of psychiatric comorbidity on epilepsy burden, we conducted a 5-year retrospective analysis of data from a large safety-net healthcare network and compared the occurrence of negative health events (NHEs), defined as emergency department (ED) visits and hospitalizations, among individuals with epilepsy and mental illness (E–MI) vs. those with epilepsy alone (E).
Methods
Electronic health record (EHR) data from a large Midwestern U.S. safety-net healthcare system were queried to identify a study population of adults ≥18 years with a diagnosis of epilepsy, with or without mental illness. We assessed demographic and clinical characteristics for each of the 5 years and compared NHEs between subgroups with E–MI vs. E. An additional analysis focused on those individuals who remained in the healthcare system over the entire 5-year study time frame (January, 2010 to December, 2014). Annual and cumulative NHE counts and hospital length of stay for individuals with E–MI and E were assessed, as were hospital discharge diagnoses.
Results
The number (approximately 2000) and demographic characteristics of individuals with epilepsy who received care each year of the study period was relatively consistent. In 2014, mean age of individuals with epilepsy was 48 (range: 18–95), 48.2% were women, 51.5% were White, 37.9% were African-American, and 8.6% were Hispanic. In 2014, there were 1616 (78.6%) individuals in the subgroup with E and 439 (21.4%) in the subgroup with E–MI. Most clinical and demographic variables between the subgroups with E–MI and E were similar, except that individuals with E–MI were less likely to be employed or commercially insured. Overall, NHEs were common, with over 1/4 (27.5%) of all individuals with epilepsy having an ED visit during the year, 13.7% having hospitalization, and 34.2% having either an ED visit or hospitalization. Individuals with E–MI had significantly more NHEs compared to individuals with epilepsy only, as evidenced by higher rates of any NHE (p < .001), ED visits (p < .001), and hospitalizations (p < .001). The cumulative differential in ED and hospital use between subgroups with E–MI and E was substantial over a 5-year time period. While most NHEs were directly related to seizures for the overall group, substance-use complications appeared as a top reason for hospitalization only in the group with E–MI.
Conclusions
Individuals with E–MI made up just over 20% of all people with epilepsy in a safety-net system and had higher rates of NHEs than those without mental illness. Better and earlier identification of individuals with E–MI, assistance with self-management including helping individuals to optimize ambulatory care settings as opposed to the ED, and treatment for substance use disorders could eventually reduce NHEs in this vulnerable subgroup of individuals with epilepsy.
Keywords: Epilepsy, Seizures: Mental Illness, Comorbidity, Health-care utilization
1. Introduction
Epilepsy is a chronic neurological condition with a lifetime prevalence of 1.8% [1]. Epilepsy significantly increases risk of injury and premature death [2,3], and standardized mortality ratios as high as 2.4 to 5.6 have been reported for epilepsy-related accidental deaths, while approximately 30% of people with epilepsy experience seizure-related injury. In addition to injury and death, recurrent seizures are often associated with psychological comorbidity [4,5]. Rates of mental illness (MI), such as schizophrenia, bipolar disorder, and recurrent or severe depression, are disproportionately high in those with epilepsy. It is estimated that 20–30% of people with epilepsy have comorbid mental illnesses [6–12]. Depression is particularly common [13], and individuals with epilepsy are 4 times more likely to be hospitalized with depression than those without epilepsy [14]. Rates of psychotic illness in epilepsy are 6–12 times higher than in the general population, with a prevalence of 7–8% [15]. Additionally, rates of bipolar disorder in people with epilepsy may be as high as 12% [16,17].
A recent survey [18] noted that almost half of people with epilepsy reported having a seizure in the previous 3 months. Risk factors for ongoing seizures and poorly controlled epilepsy include comorbidities, as well as medication non-adherence and poor social support. Individuals of lower socioeconomic status may be particularly likely to have negative health events (NHEs), such as frequent or poorly controlled seizures and hospitalizations [19]. Some reports note that having a psychiatric condition may lower seizure threshold or increase risk of treatment-resistant epilepsy [20,21]. Heavy stigma burden, lack of support, social disadvantages (e.g., unemployment), and isolation may result in poorer outcomes for persons with epilepsy and comorbid mental illnesses. The risk for suicide among people with epilepsy is 5 times the rate of the general population, possibly because of under-treated mental illness, suboptimal health self-management, or the synergistic negative effects of E–MI comorbidity [22–24].
An Institute of Medicine (IOM) report on epilepsy noted that addressing comorbidity burden, particularly with respect to mental disorder, is a priority area [25]. Since 2009, the Centers for Disease Control and Prevention (CDC) Prevention Research Centers' Managing Epilepsy Well (MEW) Network has focused its efforts on addressing mental health issues in epilepsy [26,27]. Supplementing this effort, in order to better understand the association of psychiatric conditions with health care utilization among persons with epilepsy, we conducted a 5-year retrospective analysis of data from a large safety-net healthcare network and compared the occurrence of NHEs, defined as emergency department (ED) visits and hospitalizations, among individuals with epilepsy and MI and those with epilepsy alone (E).
2. Methods
2.1. Overview
This 5-year retrospective medical record review used data from a single healthcare system's inpatient, outpatient, and emergency department (ED) settings to compare the occurrence of negative health events (NHEs) in people with epilepsy and comorbid mental illness vs. people with epilepsy not diagnosed with mental illness. Because the healthcare system is a safety-net system, it provides services to individuals regardless of their ability to pay. Safety-net care systems generally serve higher proportions of minorities and individuals of lower socioeconomic status than most commercially insured networks. In addition to NHEs, the analysis compared demographic and clinical characteristics of E–MI vs. E. The study was approved by the local Institutional Review Board (IRB).
2.2. Study population and time frame
Electronic health record (EHR) data from a large Midwestern U.S. safety-net healthcare system were queried to identify a study population of adults, aged 18 and above, with a diagnosis of epilepsy and with at least 1 visit to the care system's primary care or neurology outpatient or inpatient settings within the past 5 years. The EHR system used was Epic, one of the most widely used systems nationally, accounting for 20.3% of the EHR market share [28]. Epilepsy cases were identified based upon an epilepsy diagnosis (ICD-9-CM codes 345.1–345.91) on the EHR problem list. Mental disorder cases were identified by having a mental disorder on the EHR problem list (ICD-9-CM codes 295–296.89) or by being prescribed a medication that is generally only used to treat a chronic bipolar or psychotic disorder (antipsychotic drugs or lithium). The study time frame of observation was January 2010 to December 2014.
2.3. Demographic and clinical variables
We assessed age (in years), gender, race, insurance status, employment status, and number of neurology and primary care visits. Patient addresses were geo-coded to obtain the census block group, which was then used to retrieve the median neighborhood income and high school graduation rate. Income and education results were altered by a random amount to obscure the exact residence location. Once the census data were coded and complete, the dataset was entirely de-identified, and only de-identified data were used for analysis purposes.
2.4. Negative health events (NHEs) and health resource use
Negative health event occurrence was defined as emergency department (ED) visits and/or hospitalization. If an individual was admitted to the hospital from the ED, only the hospitalization was counted as an NHE. For individuals who had hospitalizations, hospital discharge diagnosis was assessed.
2.5. Data analysis
Demographics and clinical characteristics of the sample in each of the 5 years of the study time frame were summarized by means and standard deviations for continuous variables and by frequency and proportions for categorical variables. As some individuals dropped in and out of treatment, or did not stay in the care system consistently, each year was treated as a discrete sample. The subgroups of individuals with E–MI and E were described separately. To compare the subgroups with E–MI and E, two-sample Mann–Whitney U tests were used for count data and Fisher's exact test for binary outcomes.
An additional analysis was conducted, focusing on those individuals who remained in the healthcare system over the entire 5-year study time frame (from January 2010 to December 2014). The annual and cumulative NHE counts for individuals with E–MI and E were assessed, as was hospital discharge diagnosis. For NHE counts as the dependent variable (each year, over the 5 years), a negative binomial count regression model was fitted. Subject-level random effects were included to account for within-subject correlation. Covariates that were considered were age, race, gender, employment status, and insurance status, as well as an indicator variable for E–MI status. The latter variables were based on 2014 data. Primary focus was the effect of E–MI status on NHE counts. For the employment variable: subjects were considered as employed if their status in the database was listed as full time, part time, or self-employed; they were considered as not employed if their status was either not employed, retired, full- or part-time student. For the race variable, declined and unavailable were considered in one group as unknown. SPSS v. 22 (IBM Corp. released 2013. IBM Statistical Package for the Social Sciences Statistics for Windows, version 22.0. Armonk, NY: IBM Corp.) was used for data analysis.
3. Results
3.1. Overall sample description
As noted in Fig. 1, the number of unique individuals with epilepsy who received care each year of the 5-year study period was very consistent at approximately 2000 persons annually, ranging from 1976 (in 2011) to 2055 (in 2014). Fig. 1 also illustrates the proportion of individuals with MI vs. those who did not have MI in each year of the study period. On average, individuals with MI comprised just over 20% of all patients with epilepsy. Demographic and clinical variables also remained fairly consistent across the 5-year period; Table 1 illustrates these variables in the last year of the study time frame (2014). In the 2014 sample, mean age of all individuals with epilepsy was 48 (SD: 14.6, range: 18–95), 48.2% were women, 51.5% were White, 37.9% were African-American, and 8.6% were Hispanic. The majority of individuals were insured by national or state-funded health plans (Medicare and Medicaid). The biggest change in demographic/clinical variables over the 5-year period was in insurance status. Rates of being uninsured dropped substantially from 2010 to 2014, with 19.1% in 2010, 19.1% in 2011, 17.1% in 2012, 5.6% in 2013, and 4.2% in 2014, while Medicaid insurance rates increased. Only 20.4% of the entire sample was employed, and most lived in limited financial circumstances, with a mean neighborhood household income of U.S. $38,400/year.
Fig. 1.
Patients included in study by year.
Table 1.
Demographic and clinical variable for all patients with a diagnosis of epilepsy (ICD-9-CM 345.1–345.91) and one or more visits to primary care or neurology department from January 1, 2014–December 31, 2014.a
| Demographic | All | Epilepsy only (E) |
Epilepsy + MI (E–MI) |
||||
|---|---|---|---|---|---|---|---|
| All | No NHE | With NHE | All | No NHE | With NHE | ||
| Sample Size | 2055 | 1616 | 1132 | 484 | 439 | 220 | 219 |
| Mean age, years (SD) | 48.0 (14.6) | 48.2 (15.1) | 48.5 (15.2) | 47.6 (14.6) | 47.1 (12.6) | 47.5 (12.7) | 46.6 (12.4) |
| Gender (% female) | 48.2 | 48.1 | 47.0 | 50.8 | 49.4 | 50.9 | 48.0 |
| Race, % | |||||||
| White | 51.5 | 50.6 | 52.6 | 45.9 | 54.9 | 55.0 | 54.8 |
| African-American | 37.9 | 39.2 | 37.9 | 42.2 | 33.3 | 36.8 | 29.7 |
| Hispanic | 8.6 | 8.0 | 7.0 | 10.3 | 10.9 | 7.7 | 14.2 |
| Other/unknown | 2 | 2.2 | 2.5 | 1.6 | 0.9 | 0.5 | 1.3 |
| Insurance Status, % | |||||||
| Commercial | 14.9 | 17.5 | 20.7 | 9.9 | 5.7 | 8.2 | 3.2 |
| Medicare | 36.1 | 35.0 | 36.4 | 31.8 | 40.1 | 41.4 | 38.8 |
| Medicaid (includes Waiver program) | 44.8 | 42.9 | 38.7 | 52.7 | 51.7 | 48.2 | 55.3 |
| Uninsured | 4.2 | 4.6 | 4.2 | 5.6 | 2.5 | 2.3 | 2.7 |
| Employment status, (% employed) | 20.4 | 24.0 | 26.0 | 19.4 | 7.0 | 5.4 | 8.7 |
| Mean neighborhood household income, $ (IQR) | 38,400 (21,600–49,300) | 38,800 (21,900–49,800) | 41,700 (23,500–53,800) | 32,000 (20,000–40,900) | 36,700 (20,800–46,800) | 38,850 (22,100–50,100) | 34,600 (20,500–43,250) |
| Mean neighborhood hs graduation rate, % (IQR) | 80.7 (72–91) | 80.7 (72–91) | 82.4 (74–92) | 76.7 (67–87) | 80.7 (73–90) | 82.8 (76–90) | 78.5 (70–89) |
| ED visit, % | 27.5 | 23.8 | – | 79.6 | 41.0 | – | 82.2 |
| Hospitalization, % | 13.7 | 11.6 | – | 38.8 | 21.4 | – | 42.9 |
| Any NHEb, % | 34.2 | 30.0 | – | 100 | 50.0 | – | 100 |
| Mean NHEs (SD) | 0.9 (2.4) | 0.7 (1.6) | – | 2.3 (2.3) | 1.8 (4.0) | – | 3.5 (5.1) |
If patient was admitted from ED, only hospital admission is counted.
Limited to those age ≥18 who had made at least one visit to either primary care or neurology within the past 1 year.
NHE = Negative health event (ED visit + hospitalization).
In the 2014 sample, there were 1616/2055 (78.6%) individuals in the subgroup with E and 439/2055 (21.4%) in the subgroup with E–MI. Most clinical and demographic variables between the subgroups with E–MI and E were similar, except that individuals with E–MI appeared less likely to be employed (p = .001) and less likely to have commercial health insurance (p = .007), as assessed through chi-squared tests.
3.2. NHEs and resource use
As noted in Table 1, NHEs were common in this sample of individuals with epilepsy. Over 1/4 (27.5%) of all individuals with epilepsy had an ED visit during the year, 13.7% were hospitalized, and 34.2% had either an ED visit or hospitalization. Mean number of NHEs overall was 0.9 (SD: 2.4), with a range of 0–31 NHEs. Particularly striking is the fact that half (50%) of individuals with E–MI had at least 1 NHE over the course of a year. Individuals with E–MI had significantly more NHEs compared to individuals with epilepsy only, as evidenced by higher rates of any NHE (p < .001), ED visits (p < .001), and hospitalizations (p < .001). We consider multivariate analysis of NHE counts below.
There were 981 individuals with epilepsy [including 198 (20.2%) with E–MI] who remained in the health system for the entire 5-year duration of the study observation period. Table 2 illustrates annual NHE occurrence in this sample of 981 individuals during each of the 5 study years, categorized by NHEs overall, ED visits, and hospitalizations.
Table 2.
Annual NHEs among individuals with epilepsy who received care in a large safety-net healthcare system from January 2010–December 2O14.a
| Outcome measure | All | Year |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 |
2011 |
2012 |
2013 |
2014 |
||||||||||||
| E | E–MI | p-Value | E | E–MI | p-Value | E | E–MI | p-Value | E | E–MI | p-Value | E | E–MI | p-Value | ||
| NHE (%) | 37.8 | 35.3 | 47.3 | <.01b | 32.7 | 51.6 | <.01b | 32.0 | 49.3 | <.01b | 30.3 | 47.4 | <.01b | 28.2 | 46.2 | <.01b |
| ED (%) | 30.1 | 27.6 | 39.5 | <.01b | 25.6 | 41.6 | <.01b | 23.9 | 41.0 | <.01b | 24.2 | 37.5 | <.01b | 22.2 | 36.6 | <.01b |
| Mean ED visits | 0.57 | 0.53 | 0.87 | <.01c | 0.51 | 1.2 | <.01c | 0.43 | 1.0 | <.01c | 0.44 | 0.83 | <.01c | 0.41 | 0.99 | <.01c |
| Hospitalization (%) | 13.4 | 12.6 | 16.0 | <.01b | 10.4 | 24.7 | <.01b | 11.2 | 22.2 | <.01b | 11.0 | 20.2 | <.01b | 10.8 | 17.3 | <.01b |
| Mean hospitalizations | 0.21 | 0.20 | 0.29 | <.01c | 0.19 | 0.36 | <.01c | 0.17 | 0.40 | <.01c | 0.17 | 0.31 | <.01c | 0.15 | 0.30 | <.01c |
Individuals who received healthcare during each of the 5 study years (N= 981).
Fisher's exact sig. (2-sided).
Mann–Whitney U test (2-sided).
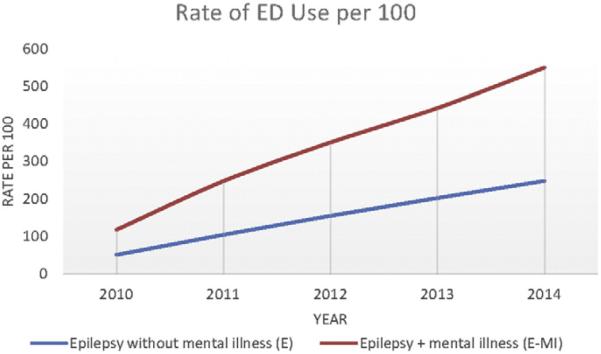
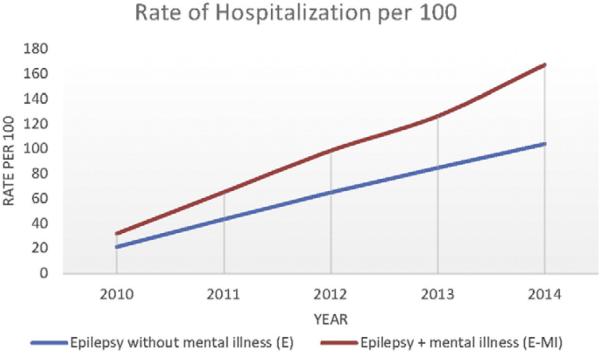
For the 981 individuals who utilized the healthcare system every year over the 5-year period, we fit a mixed model negative binomial regression of the NHE count data. In this model there was a highly significant difference between the groups with E–MI and E, with the group with E–MI having higher expected NHE counts (p < .001). Significant covariates were race (p < .001), age (p = .003), employment status (p < .001), and insurance status (p < .001). Hispanics had significantly higher expected NHE counts relative to Whites, while African-Americans did not. Younger subjects and those with Medicaid also had higher expected NHE counts. For these same individuals who used the healthcare system over the 5-year period, Figs. 2 and 3 illustrate cumulative differences in groups with E–MI vs. E expressed as rates of ED visits and rates of hospitalizations per 100 individuals.
Fig. 2.
Cumulative differences in ED visits.
Fig. 3.
Cumulative differences in hospitalizations.
Table 3 illustrates the top reasons for hospital discharge among the 981 individuals with epilepsy who used the safety-net healthcare system over a 5-year period (number of cumulative admissions and % of all hospitalizations for each subgroup: all individuals with epilepsy, subgroup with E–MI, and subgroup with E). Negative health events in this sample were mostly related to having epilepsy or seizures. In both groups with E and E–MI, epilepsy or seizures were the top hospitalization discharge diagnosis. But a major difference in reasons for hospitalization in groups with E–MI vs. E were hospitalizations for alcohol or other drug intoxication/poisoning, which were in the top 5 causes of hospitalization for the group with E–MI but did not appear in the top 5 list of hospitalizations for those people with epilepsy who did not have MI.
Table 3.
Most common hospital discharge diagnoses for individuals with epilepsy who remained in a safety-net healthcare system over a 5-year time period.
| All individuals with epilepsy | Individuals with epilepsy and no MI | Individuals with E–MI | |
|---|---|---|---|
| Sample size | 981 | 783 | 198 |
| Total discharges | 1010 | 707 | 303 |
| 1 or more hospitalizations, N (%) | 381 | 277 (35.4) | 104 (52.5) |
| Top 5 discharge diagnostic categories, N (%)a | Epilepsy – 178 (17.6) | Epilepsy – 115 (16.3) | Epilepsy – 63 (20.8) |
| Septicemia – 32 (3.2) | Septicemia – 30 (4.2) | Alcohol-related disorder – 13 (4.3) | |
| Pneumonia – 28 (2.8) | Pneumonia – 23 (3.3) | Skin infection – 11 (3.6) | |
| Diabetes – 24 (2.4) | Dysrhythmia – 21 (3.0) | Poisoning by meds or drugs – 10 (3.3) | |
| Dysrhythmia – 23 (2.3) | CHF – 18 (2.6) | COPD – 9 (3.0) |
MI: Mental illness.
E–MI: Epilepsy + comorbid mental illness.
COPD: Chronic obstructive pulmonary disease.
CHF: Congestive heart failure.
Discharge diagnoses grouped using Clinical Classifications Software (CCS) for ICD-9-CM as suggested by Cowen ME, Dusseau DJ, Toth BG, et al. Casemix adjustment of managed care claims data using the clinical classifications for health policy research method. Medical Care, 1998, 36:1108–1113.
4. Discussion
The results of this 5-year retrospective EHR-facilitated analysis of individuals with epilepsy cared for in a large safety-net healthcare system in the U.S. suggested that, while annual overall rates of using the ED or hospital for crisis care were relatively high at almost 38%, individuals with psychiatric comorbidity had significantly more NHEs compared to individuals with epilepsy that was not complicated by mental illness. Our findings are consistent with other reports noting that psychiatric comorbidity substantially increases healthcare use in people with epilepsy [29,30].
Wilner and colleagues [29] recently reported that, in a study involving 6621 individuals with epilepsy from eight commercial health plans, 50% of men and 43% of women with epilepsy had 1 or more comorbidities. Psychiatric diagnoses were the top comorbidity for both women and men in this commercially-insured sample. Among women, top comorbidities were psychiatric diagnosis (16%), hypertension (12%), asthma (11%), hyperlipidemia (11%), and headache (7%). Among men, top comorbidities were psychiatric diagnosis (15%), hyperlipidemia (12%), hypertension (12%), asthma (8%), and diabetes (5%). In the study by Wilner, having one comorbidity approximately tripled the health-care costs compared to individuals without comorbidity. Individuals with epilepsy in our safety-net care system sample had higher rates of psychiatric diagnoses than those in the report by Wilner [29], which may be reflective of differences in commercially insured vs. publicly insured groups. An additional important difference in our sample compared to the report by Wilner and colleagues [29] is the fact that our safety-net health system sample focused on those with more severe mental illness, such as chronic psychosis or bipolar disorder, vs. a broader (and potentially less disabling) group of mental conditions, such as anxiety or mild depression.
In our sample, approximately 1 in 5 individuals with epilepsy had psychiatric comorbidity, and individuals with E–MI had more NHEs overall, including more ED visits and more hospitalizations. This difference was sustained over a 5-year period, and the group with E–MI consistently had greater service use, including more ED visits and more hospitalizations. Over time, individuals with E–MI appear to have more health-related complications and use a disproportionate amount of health care services than people with epilepsy and no psychiatric comorbidity.
The majority of individuals with epilepsy in our sample received healthcare that was publicly funded (Medicare or Medicaid). Consistent with the general population served by this safety-net system, nearly 80% of all patients with epilepsy in this sample were not employed, and most lived in poor neighborhoods with relatively low annual household income levels. The number of individuals who were uninsured decreased over the 5-year study time period from around 20% to around 5%, which is consistent with what occurred in this safety-net system generally and was mainly driven by policy changes supported by the Affordable Care Act, including a federally approved Medicaid Waiver initiative in the health system in 2013 and state Medicaid expansion in 2014. In our setting, Hispanic patients with epilepsy [31] experienced a disproportionate amount of NHEs. It is possible that at least some of the ethnic disparity could have been due to care access based upon insurance coverage, and perhaps NHE occurrence will change in the future with the newly expanded healthcare coverage.
Negative health events in this sample were mostly related to having epilepsy or seizures. But in the group with E–MI, some health-related problems appeared to be related to the highly destructive effects of psychiatric illness, including poor epilepsy management and unhealthy behaviors, such as use of drugs or alcohol. It is known that the use of both legal (alcohol) and illicit substances (cocaine, etc.) may reduce effectiveness of antiepileptic drugs or may be associated with a lifestyle that interferes with appropriate management of epilepsy [32]. Alcohol, cannabis, and cocaine have negative effects on cognitive functioning, such as decreased verbal fluency and working memory, as well as deleterious effects on decision-making [33].
There are a number of clinical implications inherent in our study findings. First, recognition of comorbid psychiatric illness appears to be a crucial factor in being able to reduce the use of crisis care by individuals with epilepsy. Routine screening for psychiatric illness and possibly tapping into the capacity of an EHR to help identify individuals with comorbidity may be reasonable methods to augment ongoing clinical assessment for depression, bipolar mania, or other psychiatric symptoms that are typical in serious mental illness. Second, once psychiatric comorbidity is identified, individuals with E–MI may benefit from targeted self-management approaches that address depression, stress, and other common emotional problems in people with epilepsy [26,27,34–36]. The MEW Network has been a leader in developing care approaches that specifically address depression and other mental illnesses in people with epilepsy, who may be doubly-stigmatized because of having two health conditions that can be associated with social isolation and exclusion [27]. Aspects of self-management that may be particularly important in people with epilepsy and comorbid psychiatric symptoms include adherence to both antiepileptic drugs and medications for treatment of mental disorders and communication with providers to preemptively manage side effects and adverse events in ambulatory settings as opposed to ED care [36]. As evidenced by our findings, care in those with E–MI needs to include attention to the use of substances that may further complicate the course of epilepsy, as well as addressing the social determinants of health, such as unemployment, insurance, and disability status that can contribute to or exacerbate the negative effects of mental disorders.
Our study has a number of limitations that may affect interpretation or generalizability of findings, such as a relatively limited sample size and a single health system setting. A retrospective medical record study such as ours may have a number of liabilities such as the possibility of incomplete records, misdiagnosis, miscoding, and other data entry problems. It is possible that some E–MI was undetected, as chart diagnoses may underreport stigmatizing conditions such as mental illnesses. Our reliance on categorical EHR data (e.g., demographic variables and billing codes) did not allow us to identify details that might have been included in the narrative clinical record, such as whether epilepsy was primary or secondary to comorbidities or whether seizures were possibly nonepileptic in nature. Additionally, some patients may have been treated for NHEs in different care settings, and the NHEs were, thus, not captured in this database. This would be particularly likely for NHEs where it might be expected that emergency medical services would take patients to the closest ED, regardless of whether it was in the patients' usual care setting or not. Finally, the study methodology did not permit a finer-grained analysis of the wide variety of negative health outcomes and complications that can happen to individuals with epilepsy, such as seizures that do not result in hospitalization or ED visit and suicide or suicide attempts that do not come to the attention of medical professionals. While these are all potentially important limitations, the strengths of this analysis include the 5-year time frame that facilitated observation of the consistency of findings and the fact that the safety-net system covers a relatively wide geographic setting with multiple clinical sites, increasing the likelihood that patients would use the health system for most, if not all, of their healthcare needs. An additional strength of the analysis is that it includes a substantial portion of minorities with epilepsy, a feature not found in some other samples with epilepsy [37].
5. Conclusion
In conclusion, among individuals with epilepsy who use a safety-net health system, approximately 1/3 of individuals will have an NHE over a 1-year time period. Individuals with E–MI have even higher rates of NHEs than those who do not have psychiatric comorbidity, and the cumulative differential in ED and hospital use is substantial over a 5-year time period. Optimizing screening and appropriate treatment for mental disorder may help in identifying individuals with E–MI who could benefit from epilepsy self-management. Care approaches should include a focus on using ambulatory rather than ED settings for healthcare decision-making and management of substance use disorders. Specialized care approaches that address the unique challenges and problems of people with E–MI might be able to eventually reduce NHEs in this vulnerable subgroup of individuals with epilepsy.
Acknowledgment
This study was supported by a grant from the Centers for Disease Control and Prevention (CDC) grant CDC U48DP005030 SIP14-007 (PI Sajatovic).
The authors would like to acknowledge the helpful suggestions of Rosemarie Kobau, Matthew Zack, and Niu Tian from the Centers for Disease Control and Prevention (CDC).
Disclosure Martha Sajatovic has current research grants from the following institutions: Pfizer, Merck, Ortho-McNeil Janssen, Janssen, Reuter Foundation, Woodruff Foundation, Reinberger Foundation, National Institute of Health (NIH), and Centers for Disease Control and Prevention (CDC). She acts as a consultant for Bracket, Prophase, Otsuka, Pfizer, Amgen, and Sunovion. Dr. Sajatovic receives royalties from Springer Press, Johns Hopkins University Press, Oxford Press, UpToDate, and Lexicomp. She is currently involved in CME activities with the following organizations: American Physician's Institute, MCM Education, and CMEology.
References
- [1].Centers for Disease Control and Prevention Epilepsy in adults and access to care — United States 2010. Morb Mortal Wkly Rep. 2012;61:909–13. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6145a2.htm?s_cid=mm6145a2_e. [PubMed] [Google Scholar]
- [2].Lhatoo SD, Sander JW. Cause-specific mortality in epilepsy. Epilepsia. 2005;46(Suppl. 11):36–9. doi: 10.1111/j.1528-1167.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- [3].Logroscino G, Hesdorffer DC, Cascino G, Hauser WA, Coeytaux A, Galobardes B, et al. Mortality after a first episode of status epilepticus in the United States and Europe. Epilepsia. 2005;46(Suppl. 11):46–8. doi: 10.1111/j.1528-1167.2005.00409.x. [DOI] [PubMed] [Google Scholar]
- [4].Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72:184–91. doi: 10.1002/ana.23601. [DOI] [PubMed] [Google Scholar]
- [5].DiIorio C, Osborne Shafer P, Letz R, Henry T, Schomer DL, Yeager K. The association of stigma with self-management and perceptions of health care among adults with epilepsy. Epilepsy Behav. 2003;4:259–67. doi: 10.1016/s1525-5050(03)00103-3. [DOI] [PubMed] [Google Scholar]
- [6].Vuilleumier P, Jallon P. Epilepsy and psychiatric disorders: epidemiological data. Rev Neurol (Paris) 1998;154:305–17. [PubMed] [Google Scholar]
- [7].Barry JJ, Lembke A, Gisbert PA, Gilliam F. Affective Disorders in Epilepsy. In: Ettinger AB, Kanner AM, editors. Psychiatric Issues in Epilepsy: A Practical Guide to Diagnosis and Treatment. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 203–47. [Google Scholar]
- [8].Jobe PC, Dailey JW, Wernicke JF. A noradrenergic and serotonergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol. 1999;13:317–56. doi: 10.1615/critrevneurobiol.v13.i4.10. [DOI] [PubMed] [Google Scholar]
- [9].Jobe PC. Common pathogenic mechanisms between depression and epilepsy: an experimental perspective. Epilepsy Behav. 2003;4(Suppl. 3):S14–24. doi: 10.1016/j.yebeh.2003.08.020. [DOI] [PubMed] [Google Scholar]
- [10].Tellez-Zenteno JF, Patten SB, Jette N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–44. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- [11].Ettinger A, Reed M, Cramer J. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63:1008–14. doi: 10.1212/01.wnl.0000138430.11829.61. [DOI] [PubMed] [Google Scholar]
- [12].Kobau R, DiIorio CA, Price PH, Thurman DJ, Martin LM, Ridings DL, et al. Prevalence of epilepsy and health status of adults with epilepsy in Georgia and Tennessee: Behavioral Risk Factor Surveillance System, 2002. Epilepsy Behav. 2004;5:358–66. doi: 10.1016/j.yebeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [13].Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46:1133–9. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- [14].Mendez MF, Cummings JL, Benson DF. Depression in epilepsy. Significance and phenomenology. Arch Neurol. 1986;43:766–70. doi: 10.1001/archneur.1986.00520080014012. [DOI] [PubMed] [Google Scholar]
- [15].Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(Suppl. 10):S2–S20. doi: 10.1111/j.1528-1157.1999.tb00883.x. [DOI] [PubMed] [Google Scholar]
- [16].Mula M, Schmitz B, Jauch R, Cavanna A, Cantello R, Monaco F, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13:658–61. doi: 10.1016/j.yebeh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [17].Ettinger AB, Reed ML, Goldberg JF, Hirschfeld RM. Prevalence of bipolar symptoms in epilepsy vs other chronic health disorders. Neurology. 2005;65:535–40. doi: 10.1212/01.wnl.0000172917.70752.05. [DOI] [PubMed] [Google Scholar]
- [18].Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults—19 states, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5706a1.htm. [PubMed] [Google Scholar]
- [19].Begley C, Basu R, Lairson D, Reynolds T, Dubinsky S, Newmark M, et al. Socioeconomic status, health care use, and outcomes: persistence of disparities over time. Epilepsia. 2011;52:957–64. doi: 10.1111/j.1528-1167.2010.02968.x. [DOI] [PubMed] [Google Scholar]
- [20].Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–6. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- [21].Kanner AM, Byrne R, Chicharro A, Wuu J, Frey M. A lifetime psychiatric history predicts a worse seizure outcome following temporal lobectomy. Neurology. 2009;72:793–9. doi: 10.1212/01.wnl.0000343850.85763.9c. [DOI] [PubMed] [Google Scholar]
- [22].Rafnsson V, Olafsson E, Hauser WA, Gudmundsson G. Cause-specific mortality in adults with unprovoked seizures. A population-based incidence cohort study. Neuroepidemiology. 2001;20:232–6. doi: 10.1159/000054795. [DOI] [PubMed] [Google Scholar]
- [23].Wiegartz P, Seidenberg M, Woodard A, Gidal B, Hermann B. Co-morbid psychiatric disorder in chronic epilepsy: recognition and etiology of depression. Neurology. 1999;53:S3–8. [PubMed] [Google Scholar]
- [24].Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62:258–61. doi: 10.1212/01.wnl.0000103282.62353.85. [DOI] [PubMed] [Google Scholar]
- [25].Epilepsy across the spectrum: promoting health and understanding. National Academy of Sciences; Washington DC: 2012. http://books.nap.edu/openbook.php?record_id=13379. [PubMed] [Google Scholar]
- [26].DiIorio CK, Bamps YA, Edwards AL, Escoffery C, Thompson NJ, Begley CE, et al. The prevention research centers' managing epilepsy well network. Epilepsy Behav. 2010;19:218–24. doi: 10.1016/j.yebeh.2010.07.027. [DOI] [PubMed] [Google Scholar]
- [27].National Center for Chronic Disease Prevention and Health Promotion . Managing epilepsy well network: putting collective wisdom to work for people with epilepsy. Centers for Disease Control and Prevention; Atlanta, GA: 2013. http://www.cdc.gov/epilepsy/pdfs/mew-booklet-tagged-508.pdf. [Google Scholar]
- [28].Ritchie J. EMR market share. 2013 Available from: http://www.emrandhipaa.com/james/2013/07/18/emr-market-share-2/
- [29].Wilner AN, Sharma BK, Soucy A, Thompson A, Krueger A. Common comorbidities in women and men with epilepsy and the relationship between number of comorbidities and health plan paid costs in 2010. Epilepsy Behav. 2014;32:15–20. doi: 10.1016/j.yebeh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- [30].Cardarelli WJ, Smith BJ. The burden of epilepsy to patients and payers. Am J Manag Care. 2010;16:S331–6. [PubMed] [Google Scholar]
- [31].Overall JA, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- [32].Leach JP, Mohanraj R, Borland W. Alcohol and drugs in epilepsy: pathophysiology, presentation, possibilities, and prevention. Epilepsia. 2012;53(Suppl. 4):48–57. doi: 10.1111/j.1528-1167.2012.03613.x. [DOI] [PubMed] [Google Scholar]
- [33].Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–32. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- [34].Ciechanowski P, Wagner E, Schmaling K, Schwartz S, Williams B, Diehr P, et al. Community-integrated home-based depression treatment in older adults: a randomized controlled trial. JAMA. 2004;291:1569–77. doi: 10.1001/jama.291.13.1569. [DOI] [PubMed] [Google Scholar]
- [35].Ciechanowski P, Chaytor N, Miller J, Fraser R, Russo J, Unutzer J, et al. PEARLS depression treatment for individuals with epilepsy: a randomized controlled trial. Epilepsy Behav. 2010;19:225–31. doi: 10.1016/j.yebeh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- [36].Sajatovic M. Second Partners Against Mortality in Epilepsy (PAME) Meeting. Minneapolis, MN: 2014. The role of self-management in managing epilepsy and reducing complications. [Google Scholar]
- [37].Dowell K. Epilepsy Foundation Consumer Needs Assessment 2013. EvalSolutions; Mt. Airy, MD: 2013. [Google Scholar]