Abstract
Background
A human immunodeficiency virus (HIV) vaccine that limits disease and transmission is urgently needed. This clinical trial evaluated the safety and immunogenicity of an HIV vaccine that combines a plasmid-DNA priming vaccine and a modified vaccinia virus Ankara (MVA) boosting vaccine.
Methods
Forty healthy volunteers were injected with DNA plasmids containing gp160 of HIV-1 subtypes A, B, and C; rev B; p17/p24 gag A and B, and RTmut B by use of a needle-free injection system. The vaccine was administered intradermally or intramuscularly, with or without recombinant granulocyte macrophage colony-stimulating factor, and boosted with a heterologous MVA containing env, gag, and pol of CRF01A_E. Immune responses were monitored with HIV-specific interferon (IFN)-γ and interleukin (IL)–2 ELISpot and lymphoproliferative assays (LPAs).
Results
Vaccine-related adverse events were mild and tolerable. After receipt of the DNA priming vaccine, 11 (30%) of 37 vaccinees had HIV-specific IFN-γ responses. After receipt of the MVA boosting vaccine, ELISpot assays showed that 34 (92%) of 37 vaccinees had HIV-specific IFN-γ responses, 32 (86%) to Gag and 24 (65%) to Env. IFN-γ production was detected in both the CD8+ T cell compartment (5 of 9 selected vaccinees) and the CD4+ T cell compartment (9 of 9). ELISpot results showed that 25 (68%) of 37 vaccinees had a positive IL-2 response and 35 (92%) of 38 had a positive LPA response. Of 38 subjects, a total of 37 (97%) were responders. One milligram of HIV-1 DNA administered intradermally was as effective as 4 mg administered intramuscularly in priming for the MVA boosting vaccine.
Conclusion
This HIV-DNA priming–MVA boosting approach is safe and highly immunogenic.
Trials registration
International Standard Randomised Controlled Trial number: ISRCTN32604572.
With an estimated 5 million new cases of HIV infection each year, the majority in sub-Saharan Africa, new preventive strategies are needed to reduce HIV transmission [1]. One such preventive strategy would be an effective prophylactic vaccine, but developing such a vaccine has proven difficult [2]. HIV is highly variable and is an elusive target for neutralizing antibodies [3]. Recombinant monomeric envelope proteins proved to be immunogenic, but gave no protection in 2 phase III studies performed in the Unites States and Thailand [4]. The difficulties of eliciting broadly neutralizing antibodies to HIV led to alternative vaccine approaches that focused on the induction of cell-mediated immune responses. Studies in nonhuman primate models, which use HIV-DNA and/or simian immunodeficiency virus (SIV)–DNA vaccines and live vector-based vaccines (e.g., adenovirus serotype 5 [Ad5] or recombinant modified vaccinia virus Ankara [MVA]) in priming-boosting vaccination regimens, have shown that this approach is effective in reducing challenge virus replication and preventing the development of simian HIV (SHIV)–induced disease [5–7]. DNA-based and Ad5 vector–based HIV-1 vaccine candidates have shown immunogenicity in phase I clinical trials, and HIV-1 DNA priming and Ad5 or poxvirus boosted vaccine regimens are evaluated in phase I or phase II clinical trials [8–11]. However, vaccination with a clade B, Ad5-based, HIV-1 vaccine in a phase IIB clinical trial, STEP, was recently discontinued because the vaccine was not effective. In that trial, there was a trend towards an increased rate of HIV acquisition among vaccinees with preexisting Ad5 antibody titers over 200 [12, 13]. Current HIV vaccine development efforts are employing additional methods to increase the breadth of the immune response by increasing the number of included genes and subtypes and by evaluating other vectors.
Although the primary aim of a phase I trial is safety, it is important to learn as much as possible regarding immunogenicity. Adjuvants and novel delivery modes are needed to improve the immunogenicity of DNA. Granulocyte macrophage colony-stimulating factor (GM-CSF) was shown to enhance HIV-1 DNA–induced immune responses in animals and responses to hepatitis B virus vaccines in human clinical trials [14–18]. Intra-dermal (ID) vaccine delivery has also been shown to increase immunogenicity [19].
This descriptive phase I clinical trial evaluated the safety and immunogenicity of 4 modes of delivery for a multigene, multiclade HIV-1 DNA priming vaccine followed by a heterologous MVA boosting vaccine. It provided guidance for an ongoing phase I/II trial in Dar es Salaam, Tanzania.
METHODS
Study design
Forty healthy volunteers at low risk for HIV-1 infection were recruited into the DNA priming phase of the study. Two received only 1 DNA vaccination. The remaining 38 volunteers were rerandomized for receipt of an HIV-1 MVA boosting vaccine. The first volunteer was enrolled on 16 February 2005 and the last scheduled follow-up visit was performed on 6 September 2006.
Volunteers were randomized to 4 different treatment arms (table 1). HIV-1 DNA vaccinations were given with the Biojector 2000 (Bioject Medical Technologies) on days 0, 30, and 90. The GM-CSF protein adjuvant, sargramostim (Leukine; Berlex), was used in combination with HIV-1 DNA in treatment groups C and D (table 1). The volunteers were block rerandomized to receive either a single ID boosting vaccination of 107 pfu of HIV-1 MVA by needle over the deltoid muscle or a single intra-muscular (IM) vaccination of 108 pfu of HIV-1 MVA by needle injection in the left deltoid muscle 6 months after the last DNA vaccination.
Table 1.
Characteristics of 4 treatment groups in an HIV vaccine study that combined DNA priming vaccinations and a modified vaccinia virus Ankara (MVA) boosting vaccination.
| Treatment group | DNA priming vaccination | MVA boosting vaccination, left arm | |
|---|---|---|---|
| Left arm, env and reva (adjuvant) | Right arm, gag and RTmuta | ||
| A (n = 10) | 3 ID injections of 0.1 mL; total, 0.6 mg DNA (none) | 2 ID injections of 0.1 mL; total, 0.4 mg DNA | Group A1 (n = 5), IM injection of 108 pfu Group A2 (n = 5), ID injection of 107 pfu |
| B (n = 10) | 1 IM injection of 1.0 mL; total, 2.0 mg DNA (none) | 1 IM injection of 0.9 mL; total, 1.8 mg DNA | Group B1 (n = 5), IM injection of 108 pfu Group B2 (n = 5), ID injection of 107 pfu |
| Cb (n = 9) | 3 ID injections of 0.1mL; total, 0.6 mg DNA (GM-CSF protein, 150 μg SC via needle under ID injection site) | 2 ID injections of 0.1 mL; total 0.4 mg DNA | Group C1 (n = 5), IM injection of 108 pfu Group C2b (n = 4), ID injection of 107 pfu |
| Db (n = 9) | 1 IM injection of 0.6 mL; total, 1.2 mg DNA (GM-CSF protein, 150 μg IM at IM injection site) | 1 IM injection of 0.45 mL; total, 0.9 mg DNA | Group D1b (n = 4), IM injection of 108 pfu Group D2 (n = 5), ID injection of 107 pfu |
NOTE. DNA priming vaccinations were administered at days 0, 30, and 90. The MVA boosting vaccination was administered at month 9. GM-CSF, granulocyte macrophage colony stimulating factor (sargramostim); ID, intradermal; IM, intramuscular; SC, subcutaneous.
With respect to DNA vaccinations, all IM injections were administered with a needle-free injection system (Biojector 2000; Bioject Medical Technologies). All ID injections were administered with the same system, except for GM-CSF, which was administered SC with a needle.
One volunteer who received only 1 DNA injection was not included in the analysis. See Methods for details.
Safety evaluations, including physical examinations and laboratory tests, were performed before and at each vaccination, 2 weeks after each vaccination, and 3 months after the last DNA or MVA vaccination. Volunteers with active hepatitis B, hepatitis C, or syphilis infection were excluded at recruitment. A 12-lead electrocardiogram (ECG) was performed before and 2 weeks after the MVA vaccination.
Vaccines
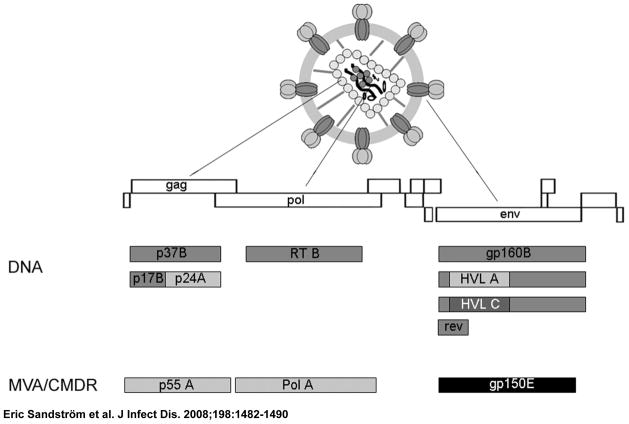
(figure 1) The development and expression of the HIV-1 envelope genes env A, env B, and env C (pKCMVgp160 A, B, and C); rev (pKCMVrev); RT (reverse transcriptase pKCM-VRTmut); gag A; and gag B (pKCMVp37BA and B) have been described elsewhere [15, 16, 20]. The vaccine immunogens are encoded in expression vector pKCMV, which contains the promoter sequence from human cytomegalovirus, the poly(A) signal from human papilloma virus 16, the Escherichia coli origin of replication, and a kanamycin-resistance gene [20]. The pKCMVgp160B encodes gp160 of subtype B, a fusion protein of gp120 and gp41, while the pKCMVgp160B/A and pKCMV gp160B/C encode chimeric gp160B proteins with the hypervariable loops (V1–V5) exchanged for subtype A or C sequences, respectively. PKCMVp37 B encodes p17 and p24 of HIV subtype B, whereas in pKCMVp37BA, the p24 component was exchanged for p24 subtype A. Regulatory protein Rev is native, while the enzyme RT has been mutated in its enzyme-active site; both are derived from HIV subtype B. The 7 plasmids, expressing 9 different HIV-1 genes, were delivered as 2 entities— one containing the gag p37 (BA and B) and RT genes and the other containing gp160 (A, B, and C) and rev genes—to minimize interference between antigens [21]. The DNA vaccine was produced by Vecura.
Figure 1.
Schematic representation of the HIV-1 virion, its genes, and the plasmid and modified vaccinia Ankara (MVA) inserts of the HIV Immunogenicity Study vaccine
Development and characterization of MVA-Chiang Mai double recombinant (MVA-CMDR) (HIV-1 MVA), will be described elsewhere by one of the authors (P.E.). In brief, MVA-CMDR expresses HIV-1 subtype E Env and subtype A Gag/Pol from Thai isolates CM235 and CM240, respectively, both under control of the early/late mH5 promoter. Mutations were engineered to enhance expression and safety. Thus, the cytoplasmic tail of Env was truncated, and the RNaseH and integrase genes were entirely deleted. In addition, the active site of RT contains a mutation that abolishes enzymatic activity. Efficient protein expression, processing, and function were verified in vitro in seed lots of virus as well as in the vaccine product. The genetic stability of the inserts was demonstrated by DNA sequencing, plaque immunostaining, and polymerase chain reaction analysis of the vaccine product. The vaccine was produced by the Walter Reed Army Institute of Research Pilot Bioproduction Facility, Forest Glen, Maryland.
Cell preparation
Whole blood samples for analysis of immune responses were collected in cell preparation tubes (CPT Vacutainer tubes; BD) and processed within 6 h, in accordance with the manufacturer’s instructions. The yield and viability of peripheral blood mononuclear cells (PBMCs) were determined by using a NucleoCounter (ChemoMetec A/S). Fresh PBMCs were used for ELISpot and lymphoproliferation assays (LPAs). The remaining cells were cryopreserved.
ELISpot assays
ELISpot assays were performed using the h-interferon (IFN)–γ ELISpotPLUS kit and the h-interleukin (IL)–2 ELISpot kit in a 2-step detection system, in accordance with the manufacturer’s instructions (Mabtech). PBMCs in complete RPMI medium with fetal calf serum were stimulated for 20 h at 37°C in triplicate wells (200,000 cells/well) with HIV-1 peptide pools (table 2) (5μg/mL, except for the Gag WR pool, which was used at 1 μg/mL); a CEF peptide pool (5 μg/mL) composed of 23 peptides from cytomegalovirus, Epstein-Barr virus, and influenza virus [22]; or phytohemagglutinin ([PHA] 5μg/mL). RPMI medium in triplicate wells was used as a background control. Spot-forming cells were measured in an automated microscope (Zeiss). Results were expressed as the mean number of spot-forming cells per 106 PBMCs without subtracting background reactivity. ELISpot responses were considered positive if the number of spot-forming cells was ≥4 times the background (i.e., RPMI medium only) and >55 sfc/106 PBMCs [23]. Samples with RPMI medium reactivity >100 sfc/106 PBMCs were excluded from analyses.
Table 2.
HIV-1–specific peptide pools used in ELISpot assays of peripheral blood mononuclear cells from study volunteers who received HIV vaccine.
| Peptide pool | Protein | Peptide number | Clade | Source |
|---|---|---|---|---|
| Gag Ia | p17 | 1–26 | B | Thermo Scientific |
| Gag IIa | p24 | 27–71 | A | Thermo Scientific |
| Env Ia | gp120, including V1 and V2 | 1–50 | A/B | Thermo Scientific |
| Env IIa | gp120, including V3-V5 | 51–100 | A | Thermo Scientific |
| Env IIIa | gp41 | 101–169 | B | Thermo Scientific |
| RT I | pol | 1–55 | B | NIBSC |
| RT II | pol | 56–110 | B | NIBSC |
| Gag WRb | p6, p7, p17, p24 | 1–160 | A | WRAIR |
NOTE. MVA, modified vaccinia virus Ankara; NIBSC, National Institute for Biological Standards and Control; WRAIR, Walter Reed Army Institute of Research.
DNA vaccine clade A–specific and clade B–specific peptides. All peptides were 15-mers with 10-aa overlap.
The peptides included in the Gag WR pool were specific for the HIV inserts in MVA and were 15-mers with an 11-aa overlap. This peptide pool was included in assays of samples obtained at the time of HIV-1 MVA boosting vaccination and 2 weeks after HIV-1 MVA boosting vaccination.
The HIV-1 specific peptide pools used in the ELISpot assays are shown in table 2. In pretrial testing of the Gag and Env peptide pools, 1 of 30 seronegative blood donors showed reactivity to 1 of the peptide pools, Gag WR. None of 21 seronegative blood donors showed reactivity to the RT I or II pools.
For determination of CD8+ and CD4+ cell responses, CD8+ cell populations were depleted from cryopreserved PBMCs by using magnetic Dynabeads (Dynal Biotech). Depletion was assessed by flow-cytometric analysis with Cell-Quest software (BD Biosciences) following staining with anti-CD4 and anti-CD8 antibodies (BD Biosciences). The median percentage (range) of CD8+ cells before depletion was 14.2% (9.2%–49.4%), and after depletion, it was 1.0% (<0.01%–2.4%). Cell concentrations were adjusted to give 200,000 cells per well before use in the IFN-γ ELISPOT assay.
Lymphoproliferation assay (LPA)
PBMCs were cultured in triplicate with or without HIV antigen or PHA in complete medium in 96-well flat bottomed plates at 37°C, in 7.5% CO2, for 6 days (cultures with and without HIV antigen) and 2 days (cultures with and without PHA), and thereafter pulsed with 1 μCi (37kBq) per well of tritiated thymidine ([3H]-thymidine) for 6 h. The antigens used at a final concentration of 2.5 μg/mL were aldrithiol-2 (AT-2)–treated HIV-1MN and SUPT1 micro-vesicles (control; kindly provided by Dr. J. Lifson at SAIC Frederick). Stimulation indices (SI) were calculated by dividing the mean incorporation of [3H]-thymidine in antigen-stimulated wells by the mean incorporation in control wells. SI ?8 was considered a positive result, based on analyses of results from 27 normal blood donors (SI, mean + 3SD, 7.95).
Antibody assays
HIV antibodies were tested by use of a commercial EIA (IMx HIV-1/HIV-2 III Plus; Abbott). Samples that were reactive by EIA were tested by Western blot analysis (HIV blot 2.2; Genelabs Diagnostics). Antibodies to Gag (p55; kindly provided by Dr. S. Barnett of Chiron) and gp160 (Advanced Biotechnologies) were also tested by use of in-house EIAs [16].
Statistical analysis
Clinical and vaccine safety laboratory data were entered in Södersjukhuset hospital’s computerized patient-registry system under the national identification code and full name. Study data were entered under study code and initials on clinical report forms. Specimens for immunological and virological studies were sent under study code to the Swedish Institute for Infectious Disease Control, which remained blinded to the randomization. Clinical and vaccine safety laboratory data were entered in Access, and immunological laboratory data were entered in Excel (Microsoft). Volunteer data and immunological data were analyzed in SPSS (version 15.0; SPSS) under study code. Most data are presented without statistical analysis because this is a descriptive, hypothesis-generating, study. Cross-tabulations were evaluated with Fisher’s exact test or the χ2 test, as appropriate. The ELISpot responses were compared by using the Mann-Whitney test. Pearson’s correlation coefficient was calculated for IL-2 ELISpot and LPA vs. IFN-γ ELISpot.
Ethical approval
The protocols and products were approved by the Karolinska Institute, regional ethics committees, and the Swedish Medical Products Agency. Informed consent was obtained from all participants.
RESULTS
Forty volunteers entered the trial. Because of a regulatory restriction, only women who were unable to become pregnant were eligible, and despite extending the age range, only 7 women were finally included (table 3). This caused the men and women in the study to be unevenly distributed by age as well as by sex, because the females were significantly older, P < .001. Two individuals received only 1 DNA vaccination; a man (25 years old) in group C defaulted from later visits and a woman (59 years old) in group D discontinued the study due to adverse events (AEs) related to vaccine–GM-CSF.
Table 3.
Sex and age of study volunteers who received HIV vaccine.
| Vaccine group | Male | Female | ||
|---|---|---|---|---|
|
| ||||
| n | Age Median (range) | n | Age Median (range) | |
| A | 9 | 26.2 (20.5–62.8) | 1 | 40.7 |
| B | 7 | 30.8 (27.0–39.3) | 3 | 55.0 (53.7–57.4) |
| C | 8a | 28.3(21.1–43.6) | 1 | 59.0 |
| D | 8 | 39.7 (20.2–57.4) | 1a | 59.5 |
| A+B+C+D | 32 | 29.8 (20.2–62.8) | 6 | 55.7 (40.7–59.5) |
Two subjects, one in each group, did not receive all immunizations and were excluded from this table.
Safety
The DNA vaccine was generally well tolerated. In groups A and B (the groups that received vaccine not adjuvanted with GM-CSF), 4 volunteers developed 8 grade-2 AEs; in groups C and D (which received vaccine adjuvanted with GM-CSF), 2 volunteers developed systemic grade-3 AEs, and 7 developed 15 grade-2 AEs dominated by flu-like symptoms, all probably or possibly related to the vaccine.
The HIV-1 MVA boosting vaccination, given either as an ID or IM injection, was equally well tolerated. Mild local irritation dominated with ID injections, whereas mild systemic reactions were more frequent after IM injections. One volunteer reported a grade-2 event, fatigue, after an IM MVA vaccination.
There was no influence of the vaccinations on hemoglobin level; white blood cell, neutrophil, lymphocyte, or platelet count; aspartate aminotransferase level; alanine aminotransferase level; alkaline phosphatase level; bilirubin level; creatinine level; or fasting blood glucose level. No vaccinee had a 4-fold sustained increase from baseline in creatinine kinase level. There were no increases in anti-nuclear antibodies after DNA plasmid vaccinations or changes in ECG after MVA vaccination (data not shown).
Vaccine-induced T cell responses
The IFN-γ ELISpot assay was performed on fresh PBMCs before vaccination, 2 weeks after the third HIV-1 DNA immunization, at the time of the HIV-1 MVA boosting vaccination, and 2 weeks after the boosting vaccination (figure 2). None of the vaccinees had a positive response to any of the HIV-specific peptide pools tested before vaccination (data not shown). Two weeks after the third HIV-1 DNA vaccination, 11 (30%) of 37 evaluable vaccinees had a positive IFN-γ ELISpot response to ≥1 HIV-specific peptide pool (figure 2A). One volunteer was excluded from this analysis due to high background reactivity. The HIV-1 MVA boosting vaccine increased the HIV-specific IFN-γ ELISpot response rate to 92% (34 of 37 vaccinees). Broad IFN-γ ELISpot reactivity was seen in response to multiple peptide pools (figure 2B). Thirty-two vaccinees (86%) responded to Gag, 24 (65%) to Env, and 22 (59%) to both Gag and Env peptide pools (table 4). Only 1 vaccinee responded to RT (not shown). Two weeks after the third HIV-1 DNA vaccination, the highest frequency of IFN-γ ELISpot responses was observed in the vaccinees who had received the highest dose of the HIV-1 DNA vaccine IM (group B): in this group, 7 of 9 vaccinees responded, compared with 3 of 10 in group A, 1 of 9 in group C, and 0 of 9 in group D (figure 2A). However, the lower-dose ID injections of the HIV-1 DNA vaccine (group A) were found to prime as well as the IM injections (group B) with respect to IFN-γ responses after the HIV-1 MVA boosting vaccination (figure 2B).
Figure 2.
Interferon (IFN)–γ ELISpot responses in vaccinees, grouped according to mode of delivery of the HIV-1 DNA vaccinations and the HIV modified vaccinia virus Ankara (MVA) vaccine. A, ELISpot results 2 weeks after the third DNA vaccination in the 4 groups randomized to receive DNA vaccine intradermally (ID) or intramuscularly (IM), with or without granulocyte macrophage colony-stimulating factor (GM-CSF). B, ELISpot results in the same 4 groups 2 weeks after the MVA boosting vaccination. C and D compare the ELISpot results for vaccinees who received the MVA boosting vaccine ID (C) with those for vaccinees who received the boosting vaccine 108 pfu IM (D) after DNA priming by any of the modes of delivery (ID or IM, with 107 pfu or without GM-CSF). Horizontal black dashed line, minimum level for responders; horizontal colored lines, median values. The numbers in parentheses are the number of vaccinees with a positive response to a peptide pool (i.e., vaccinees with >4 times the background number of spot-forming cells [RPMI medium only] and >55 sfc/106 peripheral blood mononuclear cells [PBMCs]). The magnitude of IFN-γ ELISpot responses—expressed as the median (25th–75th percentile) number of sfc/106 PBMCs—in responders after boosting with MVA IM (D) was 358 sfc/106 PBMCs (146–928 sfc/106PBMCs ) in response to the Gag WR peptide pool, 133 sfc/106 PBMCs (79–166 sfc/106 PBMCs) to the Env I pool, and 145 sfc/106 PBMCs (75–383 sfc/106 PBMCs) to the Env III pool. The corresponding values in responders after boosting with MVA ID (C) were 140 sfc/106 PBMCs (90–290 sfc/106 PBMCs) in response to Gag WR, 110 sfc/106 PBMCs (84–136 sfc/106 PBMCs) to Env I, and 113 sfc/106 PBMCs (73–178 sfc/106 PBMCs) to Env III.
Table 4.
Summary of interferon γ ISpot peptide pool reactivities in vaccinees 2 weeks after receipt of HIV-1 modified vaccinia virus Ankara boosting vaccination.
| Peptide pool | Responders, no. (%) |
|---|---|
| Gag I | 11 (30) |
| Gag II | 24 (65) |
| Gag I or Gag II | 24 (65) |
| Gag WR | 32 (86) |
| Env I | 18 (49) |
| Env II | 3 (8) |
| Env III | 15 (41) |
| Any Env | 24 (65) |
| Only Gag | 9 (24) |
| Only Env | 2 (5) |
| Gag and Env | 22 (59) |
| Gag or Env | 34 (92) |
The addition of recombinant GM-CSF to the ID injection of HIV-1 DNA (group C) seemed to decrease the magnitude of the response to both the Gag and Env peptide pools, compared with ID injection of HIV-1 DNA alone (group A). GM-CSF administered IM did not compensate for the lower dose of DNA, 2 mg, administered to group D, compared with 3.8 mg administered to group B.
For the HIV-1 MVA boosting vaccination, the volunteers were rerandomized 6 months after the last HIV-1 DNA vaccination to receive either an ID injection of 107 pfu HIV-1 MVA or an IM injection of 108 pfu HIV-1 MVA. The response was better, both in magnitude and with respect to the number of IFN-γ responders, after an IM boosting vaccination with the higher dose of HIV-1 MVA (figure 2C and 2D). The magnitude of the IFN-γ ELISpot response to the Gag WR peptide pool was significantly higher in the vaccinees who received a high dose of HIV-1 MVA IM (median response, 358 sfc/106 PBMCs), compared with the group that received the low dose of HIV-1 MVA vaccine ID (median response, 140 sfc/106 PBMCs) (P = .010).
Age influenced the overall response, as demonstrated by IFN-γ ELISpot responses to the WR Gag peptide pool. No high responders were seen among persons >40 years old. The overall lower magnitude of cellular immune responses in persons with a history of vaccinia vaccination seemed to be related to age (figure 3). Age also covaried with sex, though responses were not selectively lower in women when compared to men of a similar age (data not shown).
Figure 3.
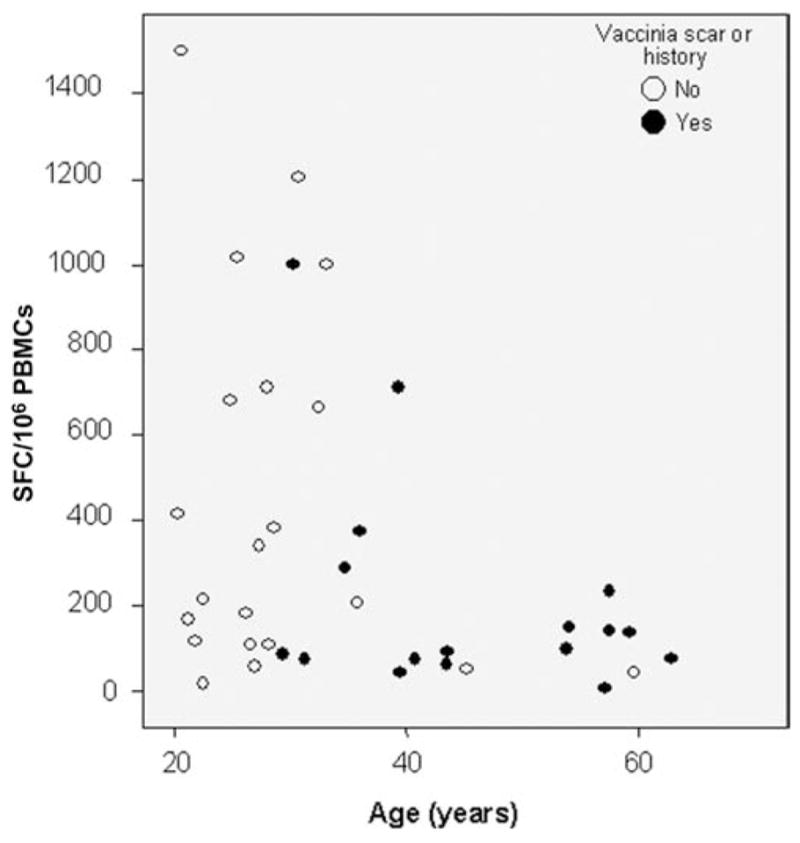
Interferon (IFN)–γ ELISpot response to the HIV-1 Gag WR peptide pool 2 weeks after the HIV-1 modified vaccinia virus Ankara boosting vaccination, according to age and previous smallpox vaccination status. PBMCs, peripheral blood mononuclear cells.
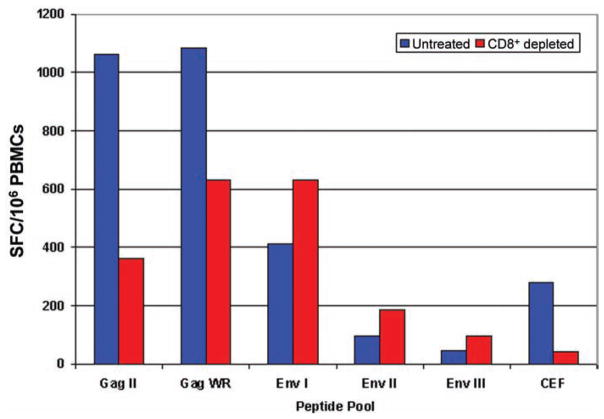
CD8+ and CD4+ T cell responses to Gag were determined by analyzing cryopreserved PBMCs— collected 2 weeks after the HIV-1 MVA boosting vaccination—with the IFN-γ ELISpot assay before and after CD8+ cell depletion. Nine vaccinees with high fresh-cell ELISpot results and sufficient numbers of cryopreserved cells were selected. Five of them displayed decreased Gag reactivity after CD8+ T cell depletion, indicating both CD8+ and CD4+ T cell responses to Gag. The other 4 vaccinees had an increased response to Gag after CD8+ T cell depletion, indicating that the Gag-specific responses were exclusively CD4+– cell mediated in these vaccinees (figure 4).
Figure 4.
Results from a representative CD8+ T cell–depletion experiment. Cryopreserved peripheral blood mononuclear cells (PBMCs) from a volunteer were tested in an interferon (IFN)–γ ELISPOT assay of samples obtained 2 weeks after receipt of the modified vaccinia virus Ankara boosting vaccination.
HIV-specific T cell responses were also measured by an IL-2 ELISpot assay and an LPA. Two weeks after the HIV-1 MVA boosting vaccination, 25 (68%) of 37 vaccinees had a positive IL-2 response, whereas a positive LPA response was detected in 35 (92%) of 38 vaccinees. Altogether, 37 (97%) of the 38 vaccinees had a positive HIV-specific response as determined by either IFN-γ ELISpot or LPA. The nonresponder was a 57-year-old, vaccinia-vaccinated man who received IM DNA priming vaccinations adjuvanted with GM-CSF and boosted with 107 pfu HIV-1 MVA administered ID.
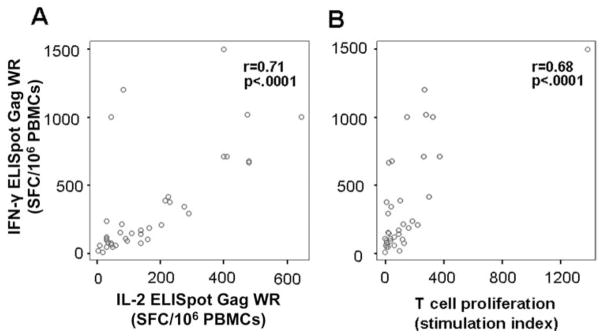
IFN-γ and IL-2 ELISpot responses to the Gag WR peptide pool (r = 0.71) were correlated, as well as Gag-specific IFN--γ ELISpot responses and LPA responses to the AT-2–treated HIV-1 antigen (r = 0.68) (figure 5).
Figure 5.
Correlation between response in ELISpot testing for interferon (IFN)–γ and interleukin (IL)–2 production (A) and between IFN-γ ELISpot response and T cell proliferation as measured by 3H-thymidine incorporation (B). Pearson’s correlation coefficient was used for r and P values. PBMCs, peripheral blood mononuclear cells.
Vaccine-induced antibody responses
Testing of samples from the 38 vaccinees by use of a routine HIV antibody EIA after the HIV MVA boosting vaccination showed positive reactivity in 7 vaccinees. In Western blot analysis, all 7 samples reacted with Gag p24, 1 sample reacted with Gag p17, and 1 sample reacted with Env gp120 (data not shown).
Testing by in-house EIA revealed moderate titers (50–10,200) of anti-Gag antibodies in 21 of 37 vaccinees and anti-gp160 antibodies in 1 of 37 vaccinees (data not shown). Neutralization assays were not performed because of the low anti-Env reactivity.
DISCUSSION
The primary aim of this descriptive phase I trial was to study safety and immunogenicity and to guide us in designing a phase I/II trial in Tanzania of this multigene, multiclade HIV-1 DNA priming–MVA boosting vaccine regimen, focusing on modes of DNA vaccine delivery. Furthermore, the vaccine was designed to be suitable for a subsequent phase I/II trial in Tanzania by including antigens from prevalent clades in East Africa. The study showed that this vaccine regimen was safe and highly immunogenic. Altogether, 97% of the vaccinees developed HIV-specific cellular immune responses.
Overall the HIV-1 DNA and MVA vaccines were well tolerated. However, administration of GM-CSF, although tolerable, was associated with a greater number of local and systemic AEs, which agrees with other observations [18]. In previous studies, the number of ID Biojector injections was limited to 3 because it was feared that a larger number would not be tolerated [24]. To deliver a 1-mg dose of DNA, 5 ID injections were considered plausible and safe, as had been shown in rhesus macaques [19]. Our experience confirms the feasibility of multiple ID DNA Biojector injections.
Three immunizations with HIV-1 DNA alone induced HIV-specific IFN-γ ELISpot responses in 30% of the vaccinees. The highest frequency and magnitude of IFN-γ ELISpot responses were found in individuals given the highest dose of HIV-1 DNA IM. However, a subsequent boosting vaccination with HIV-1 MVA demonstrated that the lower ID dose of HIV-1 DNA gave similar results after receipt of the boosting vaccination. GM-CSF did not have an adjuvant effect.
The most effective route of MVA administration is not known. Because it has been shown for other antigens that ID delivery can compensate for lower antigen doses, we explored whether ID administration of 107 pfu HIV-1 MVA would be as efficacious as IM administration of 108 pfu HIV-1 MVA. The latter turned out to be associated with stronger responses. Previous receipt of vaccinia vaccinations, given before 1976, did not seem to affect the subject’s ability to respond to HIV antigens after the boosting vaccination with the recombinant HIV MVA.
Whether priming and boosting should be done with matched or mismatched HIV sequences is a matter of debate. A recent study in mice has shown that priming and boosting with mismatched HIV vaccines of different clades predominantly induced T cell responses to conserved epitopes [25]. In the present study, the HIV-1 DNA priming vaccine and the HIV-1 MVA boosting vaccine were not matched. A high rate of immunogenicity was seen in response to HIV peptide pools not matched to the boosting immunogen, indicating the feasibility of heterologous boosting.
The immune responses induced by HIV-1 DNA and MVA were balanced, in that the IFN-γ ELISpot assay demonstrated a high response rate against Gag (32 [86%] of 37 vaccinees), as well as Env (24 [65%] of 37 vaccinees). The induction of a Gag response in vaccinees could be favorable, because recent data from a large cohort study in South Africa and a smaller study in Tanzania indicated that Gag-specific immune responses were associated with lower viral loads [26, 27].
The primary immunological end point in the present study was the responsiveness determined by use of the IFN-γ ELISpot assay, which is the best standardized assay used internationally for measuring HIV vaccine–induced immune responses [23, 28–30]. Fresh PBMCs were used in the immunological assays (except for the determination of CD8+ and CD4+ T cell responses) because we had observed that cryopreserved PBMCs gave lower levels of HIV-specific reactivity in the IFN-γ ELISpot assay (data not shown). The high response rate demonstrated by the IFN-γ ELISpot assay (34 [92%] of 37 vaccinees) was corroborated by the high response rate in the LPA (35 [92%] of 38 vaccinees).
The IFN-γ ELISpot response rate in our study is similar to that recently demonstrated in a EuroVacc phase I priming-boosting vaccine trial (18 [90%] of 20 vaccinees) that used recombinant DNA and vaccinia vector NYVAC expressing HIV-1 env and gag/pol/nef [31]. However, the DNA-NYVAC study showed an Env-dominant response while our study showed more balanced responses to Env.
Previous phase I/II clinical trials that used low doses of HIV-1 DNA and MVA encoding HIV-1 clade A Gag p24/p17 sequences and a string of CD8+ T cell epitopes showed a low frequency (<15%) of IFN-γ ELISpot responses [32]. A subsequent small trial that used higher doses of these vaccine constructs induced IFN-γ ELISpot responses in 4 of 8 vaccinees [33].
Determination of CD8+ and CD4+ T cell responses by intracellular cytokine staining was not included in the present study. However, IFN-γ ELISpot testing before and after CD8+T cell depletion in 9 selected vaccinees showed CD8+ T cell responses to Gag in 5 subjects and CD4+ T cell responses in all subjects.
The failure of the STEP study has renewed the interest in alternative viral boosting strategies, including the use of poxviruses to induce T cell–mediated immunity [34]. Considering that several factors were suboptimal, the demonstration of strong immunogenicity in this trial is promising. The ongoing phase I/II HIV DNA–MVA vaccine trial in Tanzania was informed by this study and designed accordingly. In that study, HIV-1 DNA vaccinations are given ID or IM without GM-CSF, the HIV-1 MVA boosting vaccination is administered IM (108 pfu), and age >40 years is an exclusion criterion.
HIV IMMUNOGENICITY STUDY 01/02 TEAM
In addition to the authors, the team included Ulrika Edbäck, Gunnel Engström, Lindvi Gudmundsdotter, Eva Hansson-Pilainen, Maria Isaguliants, Katarina Karlén, Anne Kjerrström, and Erik Rollman, of the Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, and the Swedish Institute for Infectious Disease Control; Pontus Blomberg, of Vecura at Karolinska University Hospital; and Ronny Ask, Stefan Ekroth, Lars Eriksson, Inger Petz, and Karin Reinhard, of Venhälsan, Södersjukhuset, Stockholm, Sweden.
Acknowledgments
Financial support: European Union (INCO-DEV A4 ICFP501A4PR03 to E.S., AVIP 503487 to B.W.); Swedish International Development Cooperation Agency (Sida); Department of Research Cooperation (SAREC) (SWE-2004-120 to C.N., HIV2004-000809 to G.B., 2004:813 to G.B.); Swedish Research Council (Vetenskapsrådet, K2004-16x-07743-19 to B.W.); Läkare mot AIDS Forskningsfond (04-050301 and 01-051101 to E.S.). Construction of the HIV-1 modified vaccinia virus Ankara was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and the US Military HIV Research Program (to B.M., P.E., D.B., and N.M.). The production costs were funded by the US Military HIV Research Program, Walter Reed Army Institute of Research.
We extend special thanks to all the dedicated volunteers; to Inger Petz, Karin Reinhard, and Ronnie Ask for their devoted clinical work; to Viveca Holmberg, for excellent handling of the EU administration; to Professor Sari Ponzer, for generously supporting the trial at crucial moments; to Marta Christensson, Karolinska University Laboratory, for analysis of ANA; and to Rana Alizadeh, Södersjukhuset AB, for ECG evaluations. We also thank Katarina Karlén, Ulrika Edbäck, Eva Hansson-Pilainen, and Lindvi Gudmundsdotter for excellent technical assistance; Gunnel Engström for stringent work with documentation and vaccine DNA analysis; Pontus Blomberg, of Vecura, for devoted work on plasmid production; the Data and Safety Monitoring Committee; Peter Liljeström, Knut Lidman, and Patrik Olin, for conscientious oversight; and Anita Swedendal for excellent drug handling.
Footnotes
The views and opinions expressed herein do not necessarily reflect those of the US Army or the Department of Defense.
Potential conflicts of interest: R.S. is currently an officer, employee and shareholder of Bioject. All other authors report no relevant conflicts of interest.
Presented in part: 5th Annual International AIDS Vaccine Conference, Amsterdam, Holland, 29 August–1 September 2006 (oral abstract session 3:2); 6th Annual International AIDS Vaccine Conference, Seattle, Washington, 20–23 August 2007 (abstract OA02-03).
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) [Accessed 8 September 2008];AIDS Epidemic Update. 2006 Dec; Available at: http://www.unaids.org/en/HIV_data/epi2006.
- 2.Johnston MI, Fauci AS. An HIV vaccine— evolving concepts. N Engl J Med. 2007;356:2073–81. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 3.Phogat S, Wyatt RT, Karlsson Hedestam GB. Inhibition of HIV-1 entry by antibodies: potential viral and cellular targets. J Intern Med. 2007;262:26–43. doi: 10.1111/j.1365-2796.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert PB, Peterson ML, Follmann D, et al. Correlations between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis. 2005;191:666–77. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 5.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:S25–S32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 6.Letvin NL, Mascola JR, Sun Y, et al. Preserved CD4+ ntral memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–33. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mäkitalo B, Lundholm P, Hinkula J, et al. Enhanced cellular immunity and systemic control of SHIV infection by combined parenteral and mucosal administration of a DNA prime MVA boost vaccine regimen. J Gen Virol. 2004;85:2407–19. doi: 10.1099/vir.0.79869-0. [DOI] [PubMed] [Google Scholar]
- 8.Graham BS, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006;194:1650–60. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catanzaro AT, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catanzaro AT, Roederer M, Koup RA, et al. Phase 1 clinical evaluation of a six-plasmid multiclade HIV-1 DNA vaccine candidate. Vaccine. 2007;25:4085–92. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 11.International AIDS Vaccine Initiative (IAVI) [Accessed 8 September 2008];IAVI Database of AIDS vaccines in human trials. Available at: http://www.iavireport.org/trialsdb.
- 12.Cohen J. AIDS research: promising AIDS vaccine’s failure leaves field reeling. Science. 2007;318:28–29. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. AIDS research: did Merck’s failed HIV vaccine cause harm? Science. 2007;318:1048–49. doi: 10.1126/science.318.5853.1048. [DOI] [PubMed] [Google Scholar]
- 14.Rollman E, Hinkula J, Arteaga J, et al. Multi-subtype gp160 DNA immunization induces broadly neutralizing anti-HIV antibodies. Gene Ther. 2004;11:1146–54. doi: 10.1038/sj.gt.3302275. [DOI] [PubMed] [Google Scholar]
- 15.Bråve A, Ljungberg K, Boberg A, et al. Multigene/multisubtype HIV-1 vaccine induces potent cellular and humoral immune responses by needle-free intradermal delivery. Mol Ther. 2005;12:1197–205. doi: 10.1016/j.ymthe.2005.06.473. [DOI] [PubMed] [Google Scholar]
- 16.Bråve A, Boberg A, Gudmundsdotter L, et al. A new multiclade DNA prime/recombinant MVA boost vaccine induces broad and high levels of HIV-1 specific CD8+ T-cell and humoral responses in mice. Mol Ther. 2007;15:1724–33. doi: 10.1038/sj.mt.6300235. [DOI] [PubMed] [Google Scholar]
- 17.Hasan MS, Agosti JM, Reynolds KK, Tanzman E, Treanor JJ, Evans TG. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination of healthy adults. J Infect Dis. 1999;180:2023–6. doi: 10.1086/315129. [DOI] [PubMed] [Google Scholar]
- 18.Cruciani M, Mengoli C, Serpelloni G, Mazzi R, Bosco O, Malena M. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination: a meta-analysis. Vaccine. 2007;25:709–18. doi: 10.1016/j.vaccine.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Amara RR, Villinger F, Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 20.Ljungberg K, Rollman E, Eriksson L, Hinkula J, Wahren B. Enhanced immune responses after DNA vaccination with combined envelope genes from different subtypes. Virology. 2002;302:44–57. doi: 10.1006/viro.2002.1547. [DOI] [PubMed] [Google Scholar]
- 21.Bråve A, Ljungberg K, Boberg A, et al. Reduced cellular immune responses following immunization with a multi-gene HIV-1 vaccine. Vaccine. 2006;24:4524–6. doi: 10.1016/j.vaccine.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Currier JR, Kuta EG, Turk E, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 2002;260:157–72. doi: 10.1016/s0022-1759(01)00535-x. [DOI] [PubMed] [Google Scholar]
- 23.Dubey S, Clair J, Fu T-M, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr. 2007;45:20–7. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 24.Epstein JE, Gorak EJ, Charoenvit Y, et al. Safety, tolerability, and lack of antibody responses after administration of PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum Gene Ther. 2002;13:1551–60. doi: 10.1089/10430340260201644. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Ren L, Huang X, et al. Sequential priming and boosting with heterologous HIV immunogens predominantly stimulated T cell immunity against conserved epitopes. AIDS. 2006;20:2293–303. doi: 10.1097/QAD.0b013e328010ad0c. [DOI] [PubMed] [Google Scholar]
- 26.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ cell responses to different HIV proteins have discordant association with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 27.Geldmacher C, Currier JR, Herrmann E, et al. CD8 T cell recognition of multiple epitopes within two Gag regions is associated with maintenance of a low steady-state viremia in HIV-1 seropositives. J Virol. 2007;81:2440–8. doi: 10.1128/JVI.01847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bull M, Lee D, Stucky J, Chiu, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox JH, DeSouza M, Ratto-Kim S, Ferrari G, Weinhold K, Birx DL. Cellular immunity assays for evaluation of vaccine efficacy. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of clinical laboratory immunology. 7. Washington, DC: American Society for Microbiology Publications; 2006. pp. 301–314. [Google Scholar]
- 30.Gotch F, Holmes H, Imami N. The importance of standardisation of laboratory evaluations in HIV vaccine trials. Microbes Infect. 2005;7:1424–32. doi: 10.1016/j.micinf.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Harari A, Bart PA, Stöhr W, et al. An HIV-1 clade C DNA prime, NY-VAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanke T, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA-and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol. 2007;88:1–12. doi: 10.1099/vir.0.82493-0. [DOI] [PubMed] [Google Scholar]
- 33.Goonetilleke N, Moore S, Dally L, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)- specific T cells capable of proliferation in healthy subjects using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ cell epitopes. J Virol. 2006;80:4717–28. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekaly R-P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development. J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]