Abstract
Purpose
To explore the effects of osmoprotectants on pro-inflammatory mediator production in primary human corneal epithelial cells (HCECs) exposed to hyperosmotic stress.
Methods
HCECs cultured in iso-osmolar medium (312mOsM) were switched to hyperosmotic media with or without prior incubation with 2–20mM of L-carnitine, erythritol or betaine for different time periods. The mRNA expression and protein production of pro-inflammatory markers in HCECs were evaluated by RT-qPCR and ELISA.
Results
Hyperosmolar media significantly stimulated the mRNA and protein expression of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, and chemokines, IL-8, CCL2 and CCL20 in HCECs in an osmolarity dependent manner. The stimulated expression of these pro-inflammatory mediators was significantly but differentially suppressed by L-carnitine, erythritol or betaine. L-carnitine displayed the greatest inhibitory effects and down-regulated 54–77% of the stimulated mRNA levels of TNF-α (down from 12.3 to 5.7 fold), IL-1β (2.2 to 0.9 fold), IL-6 (7.3 to 2.9 fold), IL-8 (4.6 to 2.0 fold), CCL2 (15.3 to 3.5 fold) and CCL20 (4.1 to 1.5 fold) in HCECs exposed to 450mOsM. The stimulated protein production of TNF-α, IL-1β, IL-6 and IL-8 was also significantly suppressed by L-carnitine, erythritol and betaine. L-carnitine suppressed 49–79% of the stimulated protein levels of TNF-α (down from 81.3 to 17.4pg/ml), IL-1β (56.9 to 29.2pg/ml), IL-6 (12.8 to 4.6ng/ml), and IL-8 (21.2 to 10.9ng/ml) by HCECs exposed to 450mOsM. Interestingly, hyperosmolarity stimulated increase in mRNA and protein levels of TNF-α, IL-1β and IL-6 were significantly suppressed by a TRPV1 activation inhibitor capsazepine.
Conclusions
L-carnitine, erythritol and betaine function as osmoprotectants to suppress inflammatory responses via TRPV1 pathway in HCECs exposed to hyperosmotic stress. Osmoprotectants may have efficacy in reducing innate inflammation in dry eye disease.
Keywords: Corneal epithelial cells, hyperosmolarity, osmoprotectant, pro-inflammatory cytokines, chemokines
INTRODUCTION
As defined by the International Dry Eye Workshop, dry eye is a multifactorial disease of the tears and ocular surface with symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is often accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.1 Comparison of age-specific data on the prevalence of dry eye from large epidemiological studies reveals a range of about 5% to over 35% at various ages.2,3 People with dry eye are significantly more likely than people without dry eye to report problems with reading, performing professional work, computer use, television watching, and daytime and nighttime driving.4 Chronic dry eye has been demonstrated to cause ocular surface inflammation, evidenced by an increase in pro-inflammatory cytokines and chemokines in the tear fluid, increased expression of immune activation and adhesion molecules by the conjunctival epithelium and increased number of T lymphocytes in the conjunctiva.5–8
Dry eye, developed from decreased tear secretion or excessive tear evaporation, is known to result in a hyperosmolar tear film.9 Hyperosmolarity has been shown to be a potent pro-inflammatory stress. There is increasing evidence that osmotic stress, caused by increased extracellular osmolarity, is a highly relevant challenge to normal cell function in a variety of tissues, including human conjunctival10–12 and corneal epithelial cells.13–18 The importance of inflammation in the pathogenesis of the ocular surface disease of dry eye, termed keratoconjunctivitis sicca, is underscored by the clinical improvement seen with anti-inflammatory therapies such as cyclosporine A, corticosteroids and doxycycline.19
L-Carnitine, gamma-trimethyl-beta-hydroxybutyrobetaine, is a small molecule widely present in all cells from prokaryotic to eukaryotic ones.20 Study on the expression and localization of carnitine/organic cation transporters in human corneal and conjunctival epithelia suggests that L-carnitine could serve as an osmoprotectant in dry eye disease and play an important role during oxidation-induced ocular disorder.21,22 Erythritol, a four-carbon polyol, is a biological sweetener with applications in food and pharmaceutical industries. It is also used as a functional sugar substitute in special foods for people with diabetes and obesity because of its unique nutritional properties. Betaine, a metabolite of plant and animal tissues, is considered as an osmoprotectant against salt and temperature stress in plants.23 It has been reported that betaine stabilizes cells volume and protects against apoptosis in human corneal epithelial cells under hyperosmotic stress.24 We have observed that L-carnitine and erythritol protected human corneal epithelial cells against MAP kinase activation by hyperosmotic stress.25
Tear osmolarity is one of the best single metrics both to diagnose and classify dry eye disease.26 Tear hyperosmolarity is regarded as a key factor initiating ocular surface inflammation, cell damage and irritation symptoms in dry eye disease.1 Our previous studies showed that hyperosmotic stress stimulated the expression of pro-inflammatory mediators.14,27 However, the mechanisms remain to be elucidated. This study was to investigate whether the transient receptor potential vanilloid channel type 1 (TRPV1) activation induces hyperosmotic effects on proinflammatory cytokine and chemoattractant release, and further explore the effects of osmoprotectants (L-carnitine, erythritol and betaine) on pro-inflammatory mediators by evaluating the ability to suppress their production in primary human corneal epithelial cells (HCECs) exposed to hyperosmotic stress.
MATERIALS AND METHODS
Materials and reagents
Cell culture dishes, plates, centrifuge tubes, and other plastic ware were purchased from BD Biosciences (Lincoln Park, NJ); Dulbecco modified Eagle medium (DMEM), Ham F-12, amphotericin B, and gentamicin were from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). L-carnitine, erythritol, betaine and capsazepine were from Sigma-Aldrich (St. Louis, MO). Human TNF-α, IL-1β, IL-6 and IL-8 ELISA kits were from Biolegend (San Diego, CA). RNeasy Plus Mini RNA extraction kit from Qiagen (Valencia, CA); Ready-To-Go-Primer First-Strand Beads were from GE Healthcare (Piscataway, NJ); TaqMan gene expression assays and real-time PCR master mix were from Applied Biosystems (Foster City, CA).
Primary cultures of human corneal epithelial cells and cell treatment
Fresh human corneoscleral tissues (<72 hours after death) not suitable for clinical use, from donors aged 19 to 67 years, were obtained from the Lions Eye Bank of Texas (Houston, TX). Primary HCECs were cultured in 12-well plates using explants from corneal limbal rims in a supplemented hormonal epidermal medium (SHEM) containing 5% FBS using our previous methods.28 Confluent primary corneal epithelial cultures in 14–18 days were switched to an equal volume (0.5 mL/well) of serum-free medium (SHEM without FBS) for 24 hours, and then treated for 4 or 24 hours with different osmolar media (312, 400, 450 and 500 mOsM), which was achieved by adding 0, 44, 69 or 94 mM sodium chloride (NaCl), with or without one hour prior incubation with different concentrations (2, 10 or 20mM) of L-carnitine, erythritol or betaine, as well as capsazepine. The osmolarity of the culture media was measured by a vapour pressure osmometer in the Body Fluid Chemistry Clinical Laboratory of the Methodist Hospital (Houston, TX). The cells treated for 4 hours were lysed in RLT buffer from Qiagen RNeasy Plus Mini kit for RNA extraction. The cells treated for 24 hours were used for ELISA of cytokine proteins. All experiments were repeated at least 4 times. The conditioned media were collected and centrifuged, and the supernatants were stored at −80 °C before use.
RNA extraction, reverse transcription, and quantitative real-time PCR (RT-qPCR)
Total RNA was extracted with a RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, quantified with a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Wilmington, DE), and stored at −80°C before use. The first strand cDNA was synthesized by RT from 1.0 μg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described.29,30 Quantitative real-time PCR was performed in a Mx3005P QPCR System (Stratagene, La Jolla, CA) with 20 μl reaction volume containing 5 μl of cDNA, 1 μl gene expression assay and 10 μl gene expression master mix (TaqMan; ABI). TaqMan gene expression assays used for this study were: GAPDH (Hs99999905_m1), TNF-α (Hs00174128_m1), IL-1β (Hs01555413_m1), IL-6 (Hs00174131_m1), IL-8 (Hs00174103_m1), CCL2 (Hs00234140_ml) and CCL20 (Hs01011368_m1). The thermocycler parameters were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A nontemplate control was included to evaluate DNA contamination. The results were analyzed by the comparative threshold cycle (Ct) method and normalized by GAPDH as an internal control.31
Enzyme-linked immunosorbent assay (ELISA)
Double-sandwich ELISA for human TNF-α, IL-1β, IL-6 and IL-8 was performed, according to the manufacturer’s protocol and our previous publication.32 In Brief, the protein standards of TNF-α, IL-1β, IL-6 and IL-8 were diluted from stock solution. Add 50 μl Assay Buffer A to each well and 50 μl standard dilutions or samples to the appropriate wells. Seal and incubate the plates at room temperature for 2 hours with shaking at 200 rpm. Wash the plates with Washing Buffer 4 times. Add 100 μl of the Detection Antibodies for human TNF-α, IL-1β, IL-6 or IL-8, seal the plates and incubate for 1 hour with shaking. Discard the contents and wash the plates 4 times with Washing Buffer. Add 100 μl of Avidin-HRP B solution to each well, seal and incubate the plates for 30 minutes while shaking. Wash the plate one more time, add 100 μl of Substrate Solution to each well and incubate for 15 minutes in the dark. Stop the reaction by adding 100 μl of Stop Solution to each well. Absorbance was read at a wavelength of 450 nm by a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Student’s t-test was used to compare differences between two groups. One-way ANOVA test was used to make comparisons among three or more groups, followed by Dunnett’s post-hoc test. P <0.05 was considered statistically significant.
RESULTS
Increased production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in HCECs exposed to hyperosmotic stress
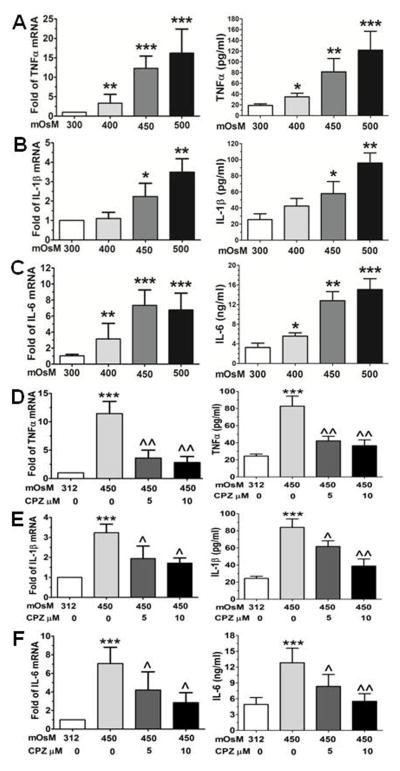
Primary HCECs cultured in SHEM with iso-osmolarity (312 mOsM) were switched to hyperosmotic media (400, 450 or 500 mOsM) by adding 44, 69 or 94 mM of NaCl for 4–24 hours for evaluation of mRNA expression and protein production. As shown in Fig 1, the mRNA expression and protein production of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were significantly increased in HCECs exposed to hyperosmotic stress, mostly in an osmolarity dependent fashion. As determined by RT-qPCR, the increased hyperosmolarity (400, 450 or 500 mOsM) significantly stimulated TNF-α mRNA expression to 3.22±2.30, 12.31±3.14 and 16.22±6.16 fold respectively (P<0.01, P<0.001, and P<0.001, n=5, respectively). These increased mRNA levels were confirmed by ELISA that showed the osmolarity-dependently increased TNF-α protein levels in the medium supernatants from 18.9±3.0 pg/ml in iso-osmolar medium to 34.8±6.8, 81.3±24.9 and 121.7±35.1 pg/ml respectively in hyperosmotic media with 400, 450 and 500 mOsM (P<0.05, P<0.01, and P<0.001, n=5), respectively (Fig 1A). IL-1β mRNA expression was significantly increased to 2.2±0.7 and 3.5±0.7 fold (P<0.05 and P<0.01, n=5, respectively) by hyperosmotic media (450 or 500mOsM), and these increased mRNA levels were accompanied by stimulated IL-1β protein concentrations in the medium supernatants (56.9±14.7 and 95.9±12.6 pg/ml, P<0.05 and P<0.01, n=5 respectively), as compared to their control levels of 25.5±7.4 pg/ml in iso-osmolar medium (Fig 1B). IL-6 is another important inflammatory cytokine stimulated by hyperosmotic stress. The mRNA expression of IL-6 by HCECs was significantly increased to 3.1±1.9, 7.3±1.9 and 6.8±2.1 with P<0.01, P<0.001, and P<0.001 respectively when exposed to 400, 450 and 500 mOsM media. Similar pattern to the mRNA expression, HCECs in hyperosmotic media (400, 450 and 500 mOsM) produced significantly higher levels of IL-6 protein at osmolarity-dependent manner in the culture media (5.6±0.7, 12.8±1.8 and 15.1±2.2 ng/ml with P<0.05, 0.01, and 0.001, n=4, respectively) when compared with control levels at 3.2±0.9 ng/ml (Fig 1C).
Fig. 1.
Increased mRNA expression and protein production of cytokines in primary human corneal epithelial cells (HCECs) by hyperosmotic stress. The cells exposed to hyperosmolar media for 4 h were lysed for mRNA expression by RT-qPCR (left column) and the supernatants of conditioned media from cell cultures exposed to hyperosmolar media for 24 h were collected for ELISA (right column) to determine protein production of TNF-α (A), IL-1β (B) and IL-8 (C). To investigate a role of TRPV1 activation in expression of TNF-α (D), IL-1β (E) and IL-8 (F), the cells were prior treated with a TRPV1 inhibitor capsazepine (CPZ, 5 and 10 μM) before exposed to hyperosmotic media (D–F). Each bar in the diagrams represents mean ± SD of five independent experiments. *P<0.05; **P<0.01, ***P<0.001, n=4, compared with 312mM controls; ^P<0.05; ^^P<0.01, n=4, compared with 450mM stimulated levels.
To identify whether hypertonic stress promotes expression and production of the inflammatory cytokine through transient receptor potential vanilloid channel type 1 (TRPV1) signaling pathway activation, the cells were prior treated with a TRPV1 inhibitor capsazepine before exposed to hypertonic media. Interestingly, the hyperosmolarity (450 mOsM) stimulated mRNA levels of TNF-α, IL-1β and IL-6 were significantly suppressed by capsazepine (CPZ, 5–10 μM). These inhibitory effects of capsazepine were confirmed at protein levels when evaluated by ELISA (Fig 1, D–F).
Increased production of chemokines (IL-8, CCL2 and CCL20) in HCECs exposed to hyperosmotic stress
Interestingly, we observed similar stimulatory effects of hyperosmolarity on certain inflammation-associated chemokines, IL-8, CCL2 and CCL20 in HCECs. As shown in Fig 2A, hyperosmotic media (400, 450 or 500mOsM) significantly stimulated CXC chemokine IL-8 mRNA expression to 3.1±1.3, 4.6±1.6 and 4.9±1.6 with P<0.01, 0.001, and 0.001, n=4, respectively. The production of IL-8 protein in the supernatants of culture media was also significantly stimulated from normal control levels (6.2±1.0 ng/ml) to 15.6±2.8, 21.2±3.1 and 24.6±4.7 ng/ml (P<0.01, P<0.001, and P<0.001, n=4, respectively). Chemokine (C-C motif) ligand 2 (CCL2) and 20 (CCL20) were also significantly stimulated by hyperosmotic stress (400, 450 and 500 mOsM). CCL2 mRNA expression was increased to 6.1±3.6, 15.3±8.6 and 13.1±7.4 fold with P<0.01, 0.001, and 0.001, n=4, respectively (Fig 2B), and CCL20 transcripts increased to 2.4±1.3, 4.1±1.6 and 3.0±1.7 fold (P<0.05, P<0.001 and P<0.01, n=4) respectively (Fig 2C).
Fig. 2.
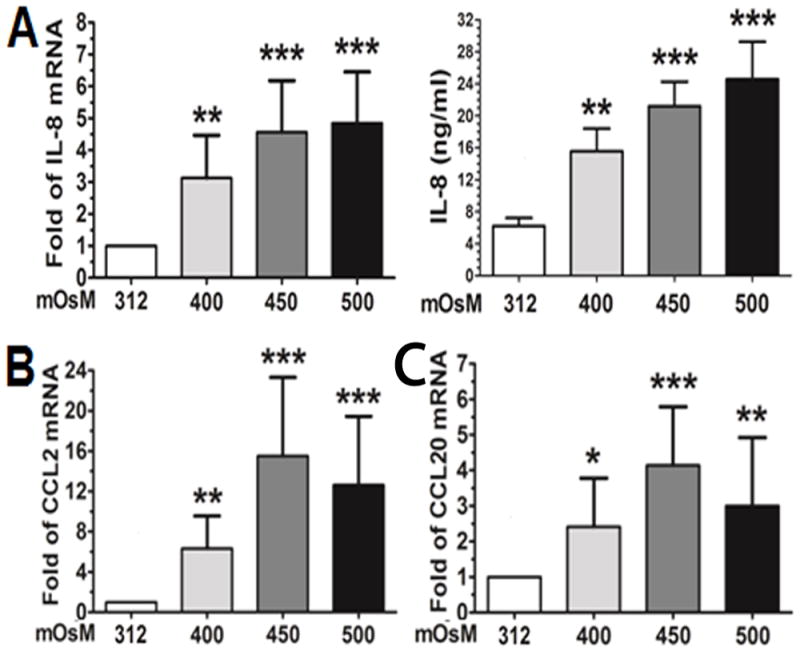
Increased mRNA expression and protein production of chemokines in HCECs by hyperosmotic stress. The cells exposed to hyperosmolar media for 4 h were lysed for mRNA expression by RT-qPCR, and the supernatants of conditioned media from cell cultures exposed to hyperosmolar media for 24 h were collected for ELISA to determine protein production for IL-8 (A), CCL2 (B) and CCL20 (C). Each bar in the diagrams represents mean ± SD of four independent experiments. *P<0.05; **P<0.01, ***P<0.001.
Effects of L-carnitine, erythritol and betaine on pro-inflammatory cytokine production in HCECs exposed to hyperosmotic stress
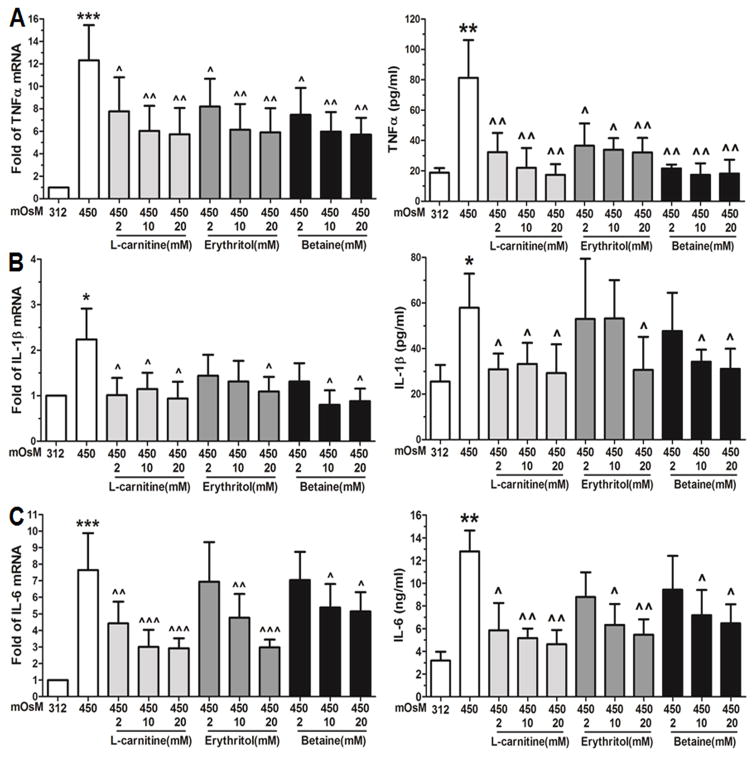
Osmoprotectants, such as L-carnitine, allows Gram-negative bacteria such as Escherichia coli to grow in high osmolarity condition.33 To explore the potential effects of these osmoprotectants, primary HCECs in iso-osmolar medium were switched to hyperosmotic medium for 4 h for mRNA expression or for 24 h for protein expression, with or without 1 hour prior incubation of different concentrations (2, 10 or 20mM) of L-carnitine, erythritol or betaine. As shown in Fig 3, the stimulated expression and production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) by hyperosmotic stress (450 mOsM) was significantly but differentially suppressed by L-carnitine, erythritol or betaine, mostly in a concentration-dependent fashion. Taking the stimulated mRNA levels (12.3 fold) by 450mOsM hyperosmolarity as a positive control, TNF-α expression was significantly decreased to 7.7-5.7, 8.2-5.9, and 7.5-5.6 fold (n=4) by L-carnitine, erythritol, and betaine, respectively, at different concentrations (2–20mM) (Fig 3A). These reductions were confirmed at protein levels by ELISA. TNF-α concentration in culture medium from the stimulated levels (81.3±24.9 pg/ml) by 450 mOsM decreased to 32.3-17.4, 36.6-32.2, and 21.7-17.4 pg/ml (n=4) by 2–20 mM of L-carnitine, erythritol and betaine respectively (Fig 3A). L-carnitine and betaine showed stronger reductions than erythritol.
Fig. 3.
Suppressive effects of L-carnitine, erythritol and betaine on cytokine production in HCECs exposed to hyperosmotic stress. HCECs in normal iso-osmolar SHEM (312mOsM) were switched to 450mOsM hyperosmolar medium with or without 1 h prior addition of L-carnitine, erythritol or betaine at different concentration (2–20 mM). The cells exposed to hyperosmolar medium for 4 h were lysed for mRNA expression by RT-qPCR, and the supernatants of conditioned media from cell cultures exposed to hyperosmolar media for 24 h were collected for ELISA to determine protein production of TNF-α (A), IL-1β (B) and IL-8 (C). Each bar in the diagrams represents mean ± SD of four independent experiments. *P<0.05; **P<0.01, ***P<0.001, as compared with 312 mOsM; ^P<0.05; ^^P<0.01, ^^^P<0.001, as compared with 450 mOsM.
Similar to TNF-α, the stimulated mRNA (2.3 fold) and protein levels (56.9 pg/ml) of IL-1β by 450 mOsM hyperosmolarity were significantly reduced to 1.2-0.9 fold and 33.3-29.2 pg/ml, respectively by 2–20 mM of L-carnitine; to 1.1 fold and 30.7 pg/ml by 20mM erythritol; or to 1.3-0.8 fold and 34.2-31.1 pg/ml, respectively by 10–20 mM betaine (Fig 3B). L-carnitine displayed the strongest suppressive effects among the three. As shown in Fig 3C, the stimulated mRNA and protein levels of IL-6 by 450 mOsM were also significantly inhibited by prior treatment with 2–20 mM of L-carnitine, or 10–20 mM of erythritol or betaine.
Effects of L-carnitine, erythritol and betaine on chemokine expression in HCECs exposed to hyperosmotic stress
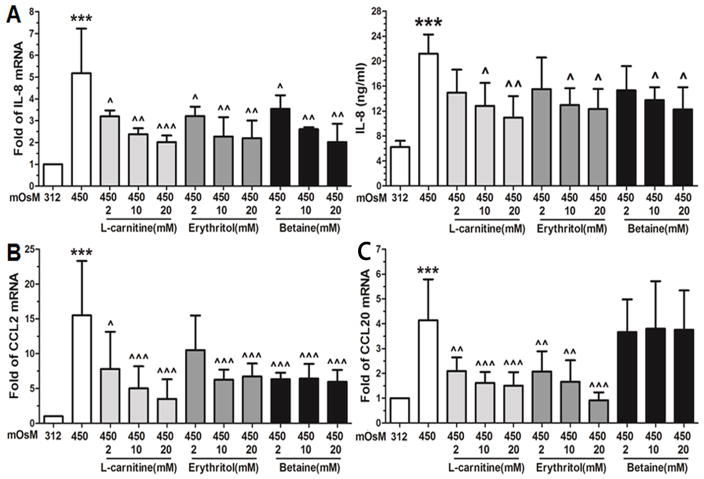
Similar to cytokines, we observed the effects of these osmoprotectants on the stimulated production of chemokines (IL-8, CCL2 and CCL20) in HCECs exposed to hyperosmotic stress. The IL-8 mRNA levels was stimulated to 4.6±1.6 fold by 450 mOsM hyperosmotic medium, and significantly decreased to 3.2-2.0, 3.2-2.2, and 3.6-2.0 fold (n=4) by 2–20 mM of L-carnitine, erythritol and betaine, respectively (Fig 4A). ELISA results further confirmed their suppressive effects at protein levels. IL-8 protein from 6.2±1.0 ng/ml in the iso-osmolar medium increased to 21.2±3.1 ng/ml by 450 mOsM. L-carnitine, erythritol or betaine at 10–20 mM significantly reduced the stimulated levels to 12.8-10.9, 13.0-12.3 and 13.8-12.3 ng/ml (n=4), respectively (Fig 4A). Furthermore, we observed the differential effects on mRNA expression of CCL2 and CCL20 by these osmoprotectants. The stimulated mRNA levels (15.3±8.6 fold) of CCL2 by 450 mOsM were significantly reduced to 7.8-3.5 or 6.4-5.9 fold by 2–20 mM of L-carnitine or betaine (Fig 4B), and to 6.3–6.7 fold by 10–20 mM of erythritol. Differentially, the stimulated CCL20 mRNA expression was reduced by L-carnitine and erythritol at 2–20 mM, but not by betaine (Fig 4C).
Fig. 4.
Suppressive effects of L-carnitine, erythritol and betaine on chemokine expression in HCECs exposed to hyperosmotic stress. HCECs in iso-osmolar SHEM (312mOsM) were switched to 450mOsM hyperosmolar medium with or without 1 h prior addition of L-carnitine, erythritol or betaine at different concentration (2–20 mM). The cells exposed to hyperosmolar medium for 4 h were lysed for mRNA expression of IL-8 (A), CCL2 (B) and CCL20 (C) by RT-qPCR, and the supernatants of conditioned media from cell cultures exposed to hyperosmolar media for 24 h were collected for ELISA to determine IL-8 protein production (A). Each bar in the diagrams represents mean ± SD of four independent experiments. *P<0.05; **P<0.01, ***P<0.001, as compared with 312 mOsM; ^P<0.05; ^^P<0.01, ^^^P<0.001, as compared with 450 mOsM.
DISCUSSION
Increasing evidence shows that hyperosmotic stress is a highly relevant challenge to normal cell function in a variety of tissues including ocular surface. An increased tear osmolarity has been recognized as a hallmark of dry eye disease; and it appears to have an important role in the pathogenesis of ocular surface damage. Tear osmolarity has been reported to be the single best marker for dry eye disease.26,34 Tear film hyperosmolarity may cause pathological changes in the corneal epithelium, such as increased desquamation, decreased intercellular connections, blunting and loss of microplicae, cell membrane disruptions and cellular swelling with decreased cytoplasmic density.35 Our previous studies demonstrated that hyperosmotic stress not only stimulated the expression and production of pro-inflammatory cytokines IL-1β and TNF-α, chemokine IL-8 and matrix metalloproteinases (MMP) 9 by human corneal epithelial cells in vitro,13,14 but also stimulated the expression of these pro-inflammatory mediators in experimental dry eye mouse model in vivo.27,29,36 Other investigators also showed that hyperosmolarity induced the pro-inflammatory cytokine and chemokines IL-6, IL-8 and monocyte chemotactic protein-1 (MCP-1) in cultured human corneal epithelial cells.17,37
Many studies on hyperosmolarity have compared the media supplemented with NaCl and sucrose, and got very similar results. We and others also showed that hyperosmolarity by NaCl or sucrose have similar inflammatory effects that activated MAP kinases and stimulated inflammatory cytokines.14,25,38 Pan and colleagues37 revealed that TRPV1 activation is required for hyperosmolarity-stimulated inflammatory cytokine release in SV40 adenovirus-immortalized HCECs exposed to higher sucrose. These authors further showed that the increases occurred through NF-κB activation. Using primary HCECs exposed to higher salt media, we aslo showed that the hyperosmolarity stimulated mRNA expression and protein production of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, were significantly suppressed at both mRNA and protein levels by capsazepine, a TRPV1 inhibitor (Fig 1, D–F). Thus, there is no doubt that hyperosmolarity triggers the inflammatory reactions. Further studies are interesting and necessary to explore the mechanism by which TRPV1 activation leads to inflammatory response by HCECs exposed to hyperosmolarity.
In physiological condition of the ocular surface, the corneal epithelial cells are more possible to be exposed to tear NaCl rather than sucrose. Gilbard emphasized years ago that tear hyperosmolarity in dry eye is due to elevated sodium ion concentration.39,40 His group has used isotonic or hypotonic saline drops to treat patients with keratoconjunctivitis sicca.41,42 The hyperosmolarity model we used makes more similar to dry eye patients in reality. Simulating the dry eye condition by increasing the NaCl concentration in medium is more representative of this disease than a rise in sucrose concentration. In the present study, primary HCECs in normal osmolar medium (312mOsM) were switched to hyperosmolar media with additional NaCl. We found that hyperosmotic stress (400, 450 or 500 mOsM) significantly stimulated the mRNA expression and/or protein production of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 (Fig 1), and chemokines, IL-8, CCL2 and CCL20 (Fig 2), mostly in an osmolarity dependent fashion.
Current dry eye therapies include tear supplementation, tear retention, tear stimulation, biological tear substitutes, anti-inflammatory therapy, essential fatty acids, and environmental strategies.43 A number of therapies have been shown to improve dry eye symptoms. It is obvious that these therapies would relieve symptoms and improve quality of life. However many of them are palliative rather than disease-modifying. Several surveys found that treatment often did not provide adequate symptom relief or prevent disease progression.44 Therefore, new preventive and therapeutic approaches need to be developed to treat the patients with dry eye syndrome. Osmoprotectants are potential candidates because of their abilities to mitigate the effects of hyperosmolar stress.
Study of osmotic adaptation in prokaryotic cells led to the notion that carnitine can serve as osmoprotectant.45 The tear L-carnitine levels in dry eye patients were found to be lower than that in healthy subjects,46 suggesting that carnitine solutions may protect against the adverse effects of tear hyperosmolarity. The structure of the osmolyte erythritol was found to resemble mannitol, a well-known antioxidant,47 and oxidative damage has been implicated in the pathogenesis and development of dry eye.48,49 And recently, L-carnitine was found to protect the corneal epithelial cells from apoptosis induced by hyperosmotic stress.50 Betaine, a metabolite of plant and animal tissues, is considered a protectant against salt and temperature stress in plants.23 L-carnitine, erythritol, and/or betaine have been reported to serve as osmoprotectants for hyperosmotic stress20,23,51 and inflammatory conditions.51,52 L-carnitine therapy has been shown considerable promise in addressing a variety of disease states that involve both primary and secondary carnitine deficiency, cardiovascular disorders, and other diseases.53–57
However, a few studies have investigated the important role and molecular mechanism of these osmoprotectants in hyperosmolarity mediated inflammatory ocular surface disease. Our group has identified that L-carnitine and erythritol, alone or in combination, significantly suppressed the activation of c-Jun N-terminal kinases (JNK) and p38 MAP kinases in human corneal epithelial cells exposed to hyperosmolar media.25 Compare to controls, 10mM L-carnitine or 40 mM erythritol significantly lowered the levels of the phosphorylated JNK and p38 MAP kinases in HCECs stimulated by hyperosmotic media. They also suppressed the ratios of phosphorylated to total MAP kinases to barely detectable levels in cells cultured in isotonic media. The present study further explored that osmoprotectants, such as L-carnitine, erythritol and betaine, exhibit significantly and differentially suppressive effects on production of pro-inflammatory cytokines and chemokines in HCECs exposed to hyperosmotic stress.
Primary HCECs in normal osmolar medium (312mOsM) were exposed to hyperosmolar media (450mOsM), with or without 1 hr prior incubation with different concentrations (2–20mM) of L-carnitine, erythritol or betaine. Interestingly, the dramatically stimulated mRNA expression of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, by hyperosmotic stress was significantly reduced by L-carnitine, erythritol or betaine, mostly in an osmolarity dependent manner. L-carnitine appeared to have the greatest suppressive activity, as shown in Fig 3. The reductions of these three pro-inflammatory cytokines were further confirmed at the protein levels by ELISA. TNF-α, IL-1β and IL-6, the major pro-inflammatory cytokines, have commonly found to increase significantly in tear, cornea and conjunctiva. These innate inflammatory mediators may cause ocular surface disease and participate in induction of T help adaptive responses in human dry eye patients,58–60 as well as in experimental animal models.27,29,61–63
Chemokines are also important mediators involved in the pathogenesis of inflammatory and immune disorders of ocular surface in dry eye.30,63,64 IL-8, known as neutrophil chemotactic factor, is produced by macrophages, epithelial and other cells. IL-8 mediates the immune reaction in the innate immune system response. CCL2, also referred to as monocyte chemotactic protein-1 (MCP-1) belongs to the C-C motif chemokine family. CCL2 recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation. CCL20, also known as macrophage inflammatory protein-3 (MIP3A), is strongly chemotactic for lymphocytes (particular Th 17 cells), and weakly attracts neutrophils. CCL20 is implicated in the formation and function of mucosal lymphoid tissues via chemoattraction of lymphocytes and dendritic cells towards the epithelial cells surrounding these tissues.65 These 3 chemokines have been found to be higher in tear and conjunctiva, and correlated to the severity of dry eye disease in human patients.59,66–69 In this study, we showed that the mRNA expression and/or protein production of IL-8, CCL2 and CCL20 was significantly stimulated in HCECs exposed to hyperosmotic media (400–500 mOsM) (Fig 1). Interestingly, the stimulated levels of mRNA and/or protein of these 3 chemokines were dramatically reduced by prior incubation with L-carnitine, erythritol or betaine at 2–20 or 10–20 mM (Fig 4). L-carnitine showed the greatest suppressive activity among the three.
At this time, relatively little is known about the exact mechanism of action for the observed effects of osmoprotectants in the suppression of pro-inflammatory mediators. L-carnitine has been shown to be taken up by corneal and conjunctival cells.21,22 This class of small organic osmolyte has been reported to have multiple stabilizing effects on protein and metabolic function, but may provide a primary benefit by not binding to or interfering with cellular macromolecules under conditions of increased concentration, unlike the effects of electrolytes – a function described as being a “compatible” solute.70 Based on TRPV1 activation study, our findings suggest that these osmoprotectants could induce such protection through attenuating hyperosmotic-induced TRPV1 activation. This is possible since carnitine and capsazepine both blocked hyperosmotic-induced responses.
In conclusion, our findings demonstrate that hyperosmotic stress stimulates the expression and production of a variety of pro-inflammatory mediators in HCECs. L-carnitine, erythritol and betaine serve as osmoprotectants that suppress the inflammatory responses in HCECs exposed to hyperosmotic stress. L-carnitine was noted to have the greatest osmoprotective effect among the three. These anti-inflammatory osmoprotectants may have potential therapeutic efficacy in treating dry eye disease. Further studies are necessary to investigate the mechanism through which these osmoprotectants elicit anti-inflammatory effect against the hyperosmotic stress faced by ocular surface epithelium.
Acknowledgments
Grant support: National Eye Institute, National Institutes of Health grants EY11915 (SCP) and Core Grant for Vision Research EY002520, Allergan Inc, Eye Bank Association of America (DQL), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Abbreviations
- HCECs
Human corneal epithelial cells
- SHEM
Supplemented hormonal epidermal medium
- JNK
c-Jun N-terminal kinases
- MAPK
Mitogen-activated protein kinase
- TRPV1
transient receptor potential vanilloid channel type 1
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 3.Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 4.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflugfelder SC. Differential diagnosis of dry eye conditions. Adv Dent Res. 1996;10:9–12. doi: 10.1177/08959374960100011801. [DOI] [PubMed] [Google Scholar]
- 6.Lemp MA. Evaluation and differential diagnosis of keratoconjunctivitis sicca. J Rheumatol Suppl. 2000;61:11–14. [PubMed] [Google Scholar]
- 7.Brignole F, Pisella PJ, Goldschild M, De Saint JM, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci. 2000;41:1356–1363. [PubMed] [Google Scholar]
- 8.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 9.Pflugfelder SC, Stern ME, Beuerman R. The Problem. In: Pflugfelder SC, Stern ME, Beuerman R, editors. Dry Eye and the Ocular Surface. New York: Marcel Dekker; 2004. [Google Scholar]
- 10.Versura P, Profazio V, Schiavi C, Campos EC. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Invest Ophthalmol Vis Sci. 2011;52:5488–5496. doi: 10.1167/iovs.11-7215. [DOI] [PubMed] [Google Scholar]
- 11.Bellotti M, Bast W, Berra A, Bonetto FJ. Effects of osmolarity on human epithelial conjunctival cells using an electrical technique. Graefes Arch Clin Exp Ophthalmol. 2011;249:1875–1882. doi: 10.1007/s00417-011-1723-8. [DOI] [PubMed] [Google Scholar]
- 12.Julio G, Lluch S, Pujol P, Merindano MD. Effects of tear hyperosmolarity on conjunctival cells in mild to moderate dry eye. Ophthalmic Physiol Opt. 2012;32:317–323. doi: 10.1111/j.1475-1313.2012.00915.x. [DOI] [PubMed] [Google Scholar]
- 13.Li D-Q, Chen Z, Song XJ, Luo L, Pflugfelder SC. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- 14.Li D-Q, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz L, Guais A, Pooya M, Abolhassani M. Is inflammation a consequence of extracellular hyperosmolarity? J Inflamm (Lond) 2009;6:21. doi: 10.1186/1476-9255-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Tong L, Li Z, et al. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49:539–549. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavet ME, Harrington KL, Ward KW, Zhang JZ. Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells. Mol Vis. 2010;16:1791–1800. [PMC free article] [PubMed] [Google Scholar]
- 18.Png E, Samivelu GK, Yeo SH, Chew J, Chaurasia SS, Tong L. Hyperosmolarity-mediated mitochondrial dysfunction requires Transglutaminase-2 in human corneal epithelial cells. J Cell Physiol. 2011;226:693–699. doi: 10.1002/jcp.22389. [DOI] [PubMed] [Google Scholar]
- 19.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Am J Ophthalmol. 4 A.D;137:337–342. [Google Scholar]
- 20.Peluso G, Barbarisi A, Savica V, et al. Carnitine: an osmolyte that plays a metabolic role. J Cell Biochem. 2000;80:1–10. doi: 10.1002/1097-4644(20010101)80:1<1::aid-jcb10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Garrett Q, Xu S, Simmons PA, Vehige J, Flanagan JL, Willcox MD. Expression and localization of carnitine/organic cation transporter OCTN1 and OCTN2 in ocular epithelium. Invest Ophthalmol Vis Sci. 2008;49:4844–4849. doi: 10.1167/iovs.07-1528. [DOI] [PubMed] [Google Scholar]
- 22.Xu S, Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Transport of L-carnitine in human corneal and conjunctival epithelial cells. Mol Vis. 2010;16:1823–1831. [PMC free article] [PubMed] [Google Scholar]
- 23.Eklund M, Bauer E, Wamatu J, Mosenthin R. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 2005;18:31–48. doi: 10.1079/NRR200493. [DOI] [PubMed] [Google Scholar]
- 24.Garrett Q, Khandekar N, Shih S, et al. Betaine stabilizes cell volume and protects against apoptosis in human corneal epithelial cells under hyperosmotic stress. Exp Eye Res. 2013;108:33–41. doi: 10.1016/j.exer.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Corrales RM, Luo L, Chang EY, Pflugfelder SC. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea. 2008;27:574–579. doi: 10.1097/ICO.0b013e318165b19e. [DOI] [PubMed] [Google Scholar]
- 26.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–798. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 27.de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 30.Yoon KC, de Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 31.Ma P, Bian F, Wang Z, et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009;50:2702–2709. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li DQ, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–1325. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verheul A, Wouters JA, Rombouts FM, Abee T. A possible role of ProP, ProU and CaiT in osmoprotection of Escherichia coli by carnitine. J Appl Microbiol. 1998;85:1036–1046. doi: 10.1111/j.1365-2672.1998.tb05269.x. [DOI] [PubMed] [Google Scholar]
- 34.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 35.Gilbard JP, Carter JB, Sang DN, Refojo MF, Hanninen LA, Kenyon KR. Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology. 1984;91:1205–1212. doi: 10.1016/s0161-6420(84)34163-x. [DOI] [PubMed] [Google Scholar]
- 36.Luo L, Li D-Q, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 37.Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liedtke CM, Cole TS. Activation of NKCC1 by hyperosmotic stress in human tracheal epithelial cells involves PKC-delta and ERK. Biochim Biophys Acta. 2002;1589:77–88. doi: 10.1016/s0167-4889(01)00189-6. [DOI] [PubMed] [Google Scholar]
- 39.Gilbard JP, Rossi SR. Changes in tear ion concentrations in dry-eye disorders. Adv Exp Med Biol. 1994;350:529–533. doi: 10.1007/978-1-4615-2417-5_89. [DOI] [PubMed] [Google Scholar]
- 40.Gilbard JP, Ross SR, Gray KL. Mechanisms for increased tear film osmolarity. In: Cavanagh HD, editor. The Cornea: Transactions of the World Congress on the Cornea III. New York: Raven Press; 1988. pp. 5–7. [Google Scholar]
- 41.Gilbard JP, Farris RL. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 1979;97:1642–1646. doi: 10.1001/archopht.1979.01020020210003. [DOI] [PubMed] [Google Scholar]
- 42.Gilbard JP. Topical therapy for dry eyes. Trans Ophthalmol Soc U K. 1985;104(Pt 4):484–488. [PubMed] [Google Scholar]
- 43.Pflugfelder SC, Geerling G, Kinoshita S, et al. Management and therapy of dry eye disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:163–178. doi: 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 44.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14:S102–S106. [PubMed] [Google Scholar]
- 45.Verheul A, Wouters JA, Rombouts FM, Abee T. A possible role of ProP, ProU and CaiT in osmoprotection of Escherichia coli by carnitine. J Appl Microbiol. 1998;85:1036–1046. doi: 10.1111/j.1365-2672.1998.tb05269.x. [DOI] [PubMed] [Google Scholar]
- 46.Pescosolido N, Imperatrice B, Koverech A, Messano M. L-carnitine and short chain ester in tears from patients with dry eye. Optom Vis Sci. 2009;86:E132–E138. doi: 10.1097/OPX.0b013e318194e767. [DOI] [PubMed] [Google Scholar]
- 47.den Hartog GJ, Boots AW, Adam-Perrot A, et al. Erythritol is a sweet antioxidant. Nutrition. 2010;26:449–458. doi: 10.1016/j.nut.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Uchino Y, Kawakita T, Miyazawa M, et al. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS One. 2012;7:e45805. doi: 10.1371/journal.pone.0045805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pescosolido N, Imperatrice B, Karavitis P. The aging eye and the role of L-carnitine and its derivatives. Drugs R D. 2008;9(Suppl 1):3–14. doi: 10.2165/0126839-200809001-00002. [DOI] [PubMed] [Google Scholar]
- 50.Khandekar N, Willcox MD, Shih S, Simmons P, Vehige J, Garrett Q. Decrease in hyperosmotic stress-induced corneal epithelial cell apoptosis by L-carnitine. Mol Vis. 2013;19:1945–1956. [PMC free article] [PubMed] [Google Scholar]
- 51.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morita Y, Tadokoro S, Sasai M, Kitamoto D, Hirashima N. Biosurfactant mannosyl-erythritol lipid inhibits secretion of inflammatory mediators from RBL-2H3 cells. Biochim Biophys Acta. 2011;1810:1302–1308. doi: 10.1016/j.bbagen.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Goa KL, Brogden RN. l-Carnitine. A preliminary review of its pharmacokinetics, and its therapeutic use in ischaemic cardiac disease and primary and secondary carnitine deficiencies in relationship to its role in fatty acid metabolism. Drugs. 1987;34:1–24. doi: 10.2165/00003495-198734010-00001. [DOI] [PubMed] [Google Scholar]
- 54.Pepine CJ. The therapeutic potential of carnitine in cardiovascular disorders. Clin Ther. 1991;13:2–21. [PubMed] [Google Scholar]
- 55.Brass EP. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin Ther. 1995;17:176–185. doi: 10.1016/0149-2918(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 56.Pons R, De Vivo DC. Primary and secondary carnitine deficiency syndromes. J Child Neurol. 1995;10(Suppl 2):S8–24. [PubMed] [Google Scholar]
- 57.Ferrari R, Merli E, Cicchitelli G, Mele D, Fucili A, Ceconi C. Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: a review. Ann N Y Acad Sci. 2004;1033:79–91. doi: 10.1196/annals.1320.007. [DOI] [PubMed] [Google Scholar]
- 58.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjogren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- 59.Jones DT, Monroy D, Ji Z, Pflugfelder SC. Alterations of ocular surface gene expression in Sjogren’s syndrome. Adv Exp Med Biol. 1998;438:533–536. doi: 10.1007/978-1-4615-5359-5_75. [DOI] [PubMed] [Google Scholar]
- 60.Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19:492–496. doi: 10.1097/00003226-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Pflugfelder SC, de Paiva CS, Tong L, Luo L, Stern ME, Li D-Q. Stress-activated Protein Kinase Signaling Pathways in Dry Eye and Ocular Surface Disease. Ocul Surf. 2005;3:S154–S157. doi: 10.1016/s1542-0124(12)70244-6. [DOI] [PubMed] [Google Scholar]
- 62.Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26:579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 63.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011;17:533–542. [PMC free article] [PubMed] [Google Scholar]
- 65.Enriquez-de-Salamanca A, Calonge M. Cytokines and chemokines in immune-based ocular surface inflammation. Expert Rev Clin Immunol. 2008;4:457–467. doi: 10.1586/1744666X.4.4.457. [DOI] [PubMed] [Google Scholar]
- 66.Narayanan S, Miller WL, McDermott AM. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest Ophthalmol Vis Sci. 2006;47:2445–2450. doi: 10.1167/iovs.05-1364. [DOI] [PubMed] [Google Scholar]
- 67.Na KS, Mok JW, Kim JY, Rho CR, Joo CK. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5443–5450. doi: 10.1167/iovs.11-9417. [DOI] [PubMed] [Google Scholar]
- 68.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 69.Huang JF, Zhang Y, Rittenhouse KD, Pickering EH, McDowell MT. Evaluations of tear protein markers in dry eye disease: repeatability of measurement and correlation with disease. Invest Ophthalmol Vis Sci. 2012;53:4556–4564. doi: 10.1167/iovs.11-9054. [DOI] [PubMed] [Google Scholar]
- 70.Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]