Abstract
Purpose
To examine the anatomical variation of normal human collector channel orifices and their relationship with Schlemm's canal.
Methods
Ten human anterior segments fixed by immersion or perfusion were dissected radially and further divided by fine dissection into corresponding inner and outer wall segments. The tissues were dehydrated, critical-point dried, sputter coated, and examined by scanning electron microscopy. Images were obtained at magnifications from ×200 to ×10,000. Selected radial collector channel regions were processed for plastic embedding.
Results
Two classes of collector channel orifices were identified. Simple oval orifices (54.7 ± 4.6–μm diameter) were lined with endothelial cells and most often occurred on a planar region of Schlemm's canal outer wall. Complex orifices (62.7 ± 3.4–μm diameter) were often found associated with septal columns and bridges, and typically covered with flap-like structures (10–40 μm) that extended between the inner and outer wall and over the collector channel orifices. Both simple and complex orifices had complete or partial lip-like rims. In orifices with partial rims, a trough-like groove was often visible on the outer wall surface opposite the lip. Transected septa and inner and outer wall adhesion sites were often found in association with complex collector channel orifices.
Conclusions
Collector channel orifice structure varied from simple ovals to complex tethered flaps and bridges. Collector channel orifices with complex flaps connect the inner and outer walls of Schlemm's canal, and may serve to enhance and regulate aqueous outflow in these regions.
Keywords: collector channel, trabecular meshwork, Schlemm's canal, aqueous humor dynamics, conventional outflow pathway
Intraocular pressure (IOP) is determined by the relationship between the amount of aqueous humor produced, the drainage of aqueous humor through the outflow pathways and episcleral venous pressure. Increased outflow resistance to aqueous humor drainage results in elevated IOP,1 which is the most prevalent and only treatable risk factor for glaucoma.2 In humans, the majority of aqueous humor is removed from the anterior chamber by way of the trabecular meshwork. Considerable attention has been directed toward the juxtacanalicular region of the trabecular meshwork and the adjacent basement membrane of Schlemm's canal as the main site of outflow resistance in both normal and glaucoma eyes.3–5 However, up to 50% of the resistance to aqueous flow occurs in the aqueous outflow pathway distal to the trabecular meshwork.6,7 This includes Schlemm's canal and the collector channels that join Schlemm's canal to intrascleral and episcleral vessels.
Collector channels are endothelial-lined openings that originate in the outer wall of Schlemm's canal and act as conduits to pass aqueous fluid to intrascleral and episcleral vessels leading to the venous system. The number of collector channels varies in humans, but generally is in the range of 24 to 35 per eye,8–10 and are distributed in a random manner around the circumference of Schlemm's canal outer wall. Recent work by our laboratory confirmed the variation and number within normal eyes and observed increased collector channel numbers in POAG eyes under elevated pressure.11 The openings (or orifices) of collector channels have been described as ovals, ovals with aqueous bridges,10 and ovals with sieve-like coverings.12 “Torus- or lip-like ridges” have also been observed by transmission electron microscopy.13 In addition to collector channels, a variety of intraluminal structures have been reported. These structures include septa that are adjacent to collector channel orifices10 and tubule-like structures.14 Septa are column-like structures, 30 to 50 μm in diameter, which are made up of a collagenous core lined by Schlemm's canal endothelium. Septa connect to both the inner and outer wall of Schlemm's canal and are thought to be important for maintaining the patency of Schlemm's canal during high pressure elevation.14 Tubule-like structures 10 to 12 μm in diameter span the inner and outer wall and are found throughout Schlemm's canal. While the function of tubule-like structures are not known, these structures have been shown to transport aqueous, pigment, and red blood cells (Martin E, et al. IOVS 2012;53:ARVO E-Abstract 3261).14
Interest in understanding the role of collector channels in outflow resistance has increased due to reports of preferential flow patterns within the trabecular meshwork, changes in collector channels with pressure (Zhou Z, et al. IOVS 2015:56;ARVO E-Abstract 3259), and pathologic changes in collector channels found in glaucoma (Gong H, et al. IOVS 2007;48:ARVO E-Abstract 2079).11,15–19 Because collector channels and the structures associated with them have not been consistently described in the human eye, we examined the intraluminal surface morphology of Schlemm's canal with special reference to associated anatomical structures such as septa, tubule-like structures, and endothelial bridges that span collector channel orifices in normal human eyes. Studying the anatomical characteristics of collector channel orifices in conjunction with the associated septa and tubule-like structures will help to understand the structure–function relationships in aqueous humor outflow.
Methods
Human Eyes
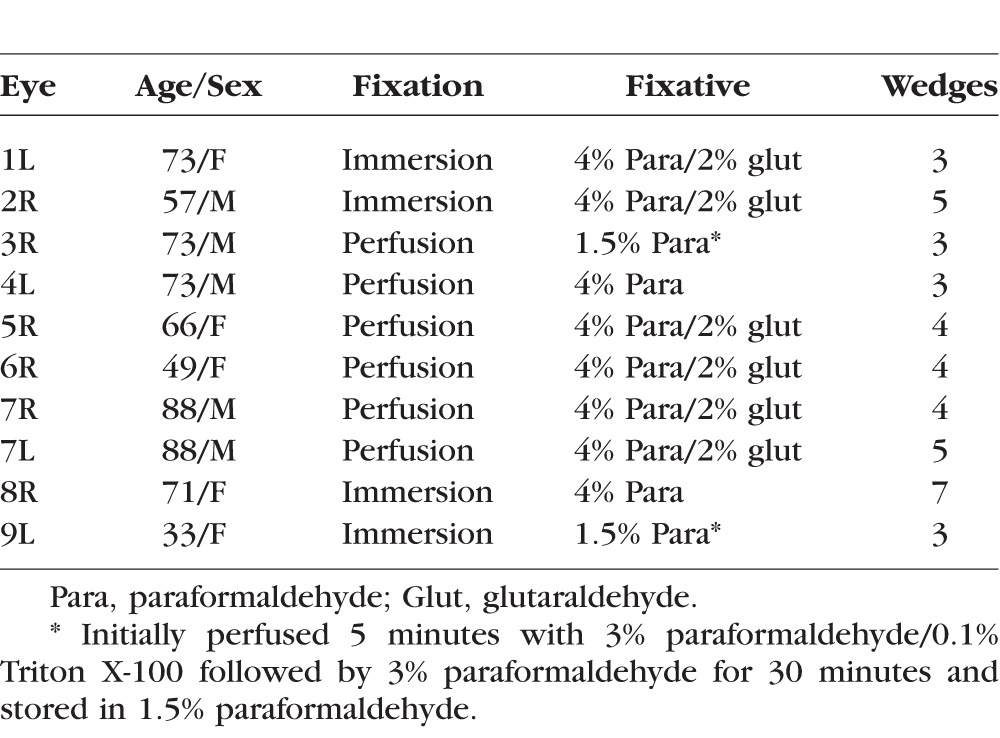
Use of human donor eyes was approved by the Mayo Clinic (Rochester, MN, USA) institutional review board and conformed to the ethical guidelines defined by the Declaration of Helsinki. Ten archived normal human eyes stored in different fixative solutions were used for this study (Table). Four eyes, mean age 58 (range, 33–73), were immersion fixed in either 2% or 4% paraformaldehyde in 0.1 M phosphate buffer. Six additional eyes, mean age 73 (range, 49–88) were perfusion fixed at 10 mm Hg in either 4% paraformaldehyde, 4% paraformaldehyde/2% glutaraldehyde, or in a light fixation regimen in which 3% paraformaldehyde/0.1% Triton X-100 (5 minutes) was followed by 3% paraformaldehyde (30 minutes) with storage in 1.5% paraformaldehyde. All perfusion fixations were preceded by two hours of initial perfusion with Dulbecco's Modified Eagle's Media (DMEM; Mediatech, Inc., Herndon, VA, USA) at 10 mm Hg to assure the anterior segment outflow channels remained open.20
Table.
Demographics of Normal Human Eyes
Tissue Dissection
Anterior segments were removed from all specimens and dissected into 1-mm radial wedges preserving the complex flaps and adjacent tissue. Three to seven wedges were preferentially selected from each eye by laying the 1-mm radial sections flat on a glass slide in a drop of PBS and using transillumination on a dissecting scope to visualize collector channels, septa, and other prominent outer wall features (n = 41). These wedges were finely dissected into corresponding inner and outer wall specimens. Briefly, with the radial wedge lying flat on a wax dissecting surface, a vertical cut was made at the anterior limit of Schlemm's canal. This separated the inner and outer wall anteriorly. An angular cut was made from the posterior aspect of Schlemm's canal extending to the edge of the ciliary body remnant. Any connections between the inner wall and outer wall were gently severed with a beaver blade to allow complete separation of the canal. The remnant of the ciliary body was then used to transfer the specimens so the endothelial surface was left undisturbed. The inner and outer wall portions were each processed for scanning electron microscopy.
Scanning Electron Microscopy
Tissue corresponding to inner and outer walls of each wedge were rinsed in 0.1 M phosphate buffer (pH 7.2) and post-fixed in a phosphate buffered 1% osmium tetroxide solution for 1 hour. The specimens were dehydrated in a graded series of acetone solutions (50%–100%), critical point dried, mounted, and sputter coated with gold. Specimens were examined in a JEOL 6510 scanning electron microscope (Peabody, MA, USA) at 10 to 15 KV at magnifications from ×200 to ×10,000. Collector channel orifices were measured at the widest measureable diameter. This included orifices, which were transected in preparation or partially obstructed from view by flaps. For example, the diameter of orifices with oval shapes was measured on the long axis.
Orifice Analysis
A total of 52 visible orifices were examined and categorized as either simple or complex. For each orifice, the largest measureable diameter was measured using a linear distance application that is included in ANALYZE software (Mayo Clinic, Rochester, MN, USA). The measurements of diameter were carried out on four separate occasions by one of the authors (MB) and on one occasion by another of the authors (CH). The five independent measurements were averaged to obtain representative diameter values for each orifice. The intraclass correlation coefficient for the intraobserver measurements was 0.96 (95% coefficient intervals (CI): 0.94–0.98) and for interobserver measurements was 0.98 (95% CIs: 0.96–0.99).
Correlative Light Microscopy
Representative wedges were prepared for light microscopy to examine complex orifice features. The wedges were dehydrated in ethanol and embedded in Epon-Araldite. Serial 1-μm sections were cut and stained with 1% Toluidine Blue.
Statistical Analysis
Orifice diameters are reported as the mean ± SEM. Unpaired t-tests were performed to examine whether fixation (immersion versus perfusion) or type of collector channel (simple versus complex) were statistically different. Comparisons with a P less than 0.05 were considered to be significantly different. The reproducibility of the collector channel diameter measurements between intraobserver and interobserver was investigated using intraclass correlation coefficients. This was completed both within the four measurements from the first observer, and between the observers. The range of this statistical summary is from zero to one with a zero implying no reproducibility and 1 being perfect reproducibility. Additionally, 95% CIs were provided for the estimates.
Results
Collector channel orifices were evaluated and split into two groups: simple or complex. Of the 52 orifices examined, 19 (37%) had simple oval openings in the outer wall of Schlemm's canal while 33 (63%) were complex due to bridge-like coverings, flap-like structures, or association with septal columns. The long axis of the oval orifices corresponded to the long axis of Schlemm's canal.
Simple Collector Channel Orifices
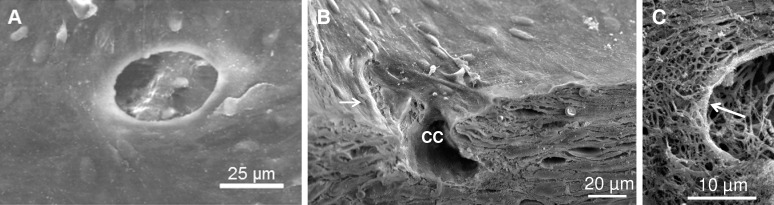
Simple orifices had an oval appearance with a 54.7 ± 4.6 μm diameter opening (n = 19, range, 20–232 μm). No difference in diameter size was identified between immersion (n = 12) or perfusion fixed tissue (n = 7; P = 0.25). In regions that were torn or cut during preparation, the orifices were lined with a single thin layer of outer wall endothelial cells that appeared narrow in width (5–10 μm) and elongated (50–100 μm), with slightly raised edges at the junction of adjacent cells (Fig. 1A). Simple orifices were most often seen in planar regions in the middle or anterior portion of Schlemm's canal. Six orifices had a thickened endothelial rim that surrounded the entire circumference at the junction with Schlemm's canal (Fig. 1A). Seven orifices had a partial lip-like rim that was located opposite to a smooth sloping entrance and appeared to funnel fluid downward into the collector channel (Fig. 1B). A partially denuded simple orifice revealed an extensive branching of extracellular matrix with a dense circumferential fibrous appearance at the edge of the orifice (Fig. 1C). Four of 19 simple orifices were located within 100 to 300 μm of a complex orifice.
Figure 1.
Various types of simple collector channel (CC) orifices in the outer wall of Schlemm's canal. (A) Representative image showing a simple orifice with thickened rim surrounding entire CC opening. (B) Transected orifice showing lip-like rim (white arrow) in association with a CC. (C) Underlying matrix of orifice rim denuded of endothelium. Arrow points to circumferential fibers at orifice rim.
Complex Collector Channel Orifices
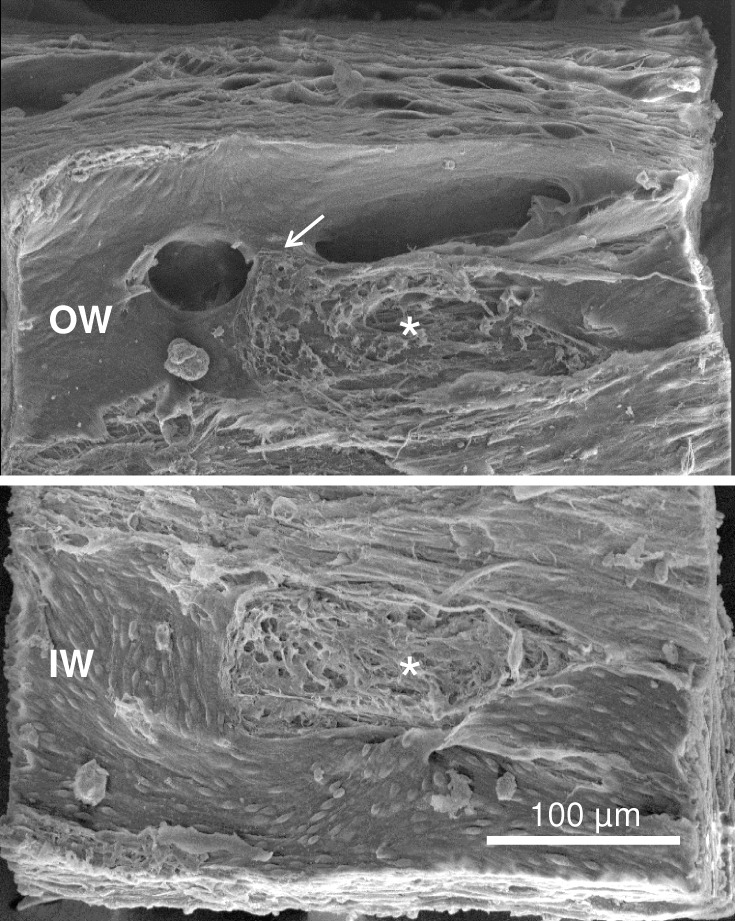
Complex orifices were similar in structure and size to the simple orifice with an average mean diameter of 62.7 ± 3.4 μm (n = 33, range, 20–236 μm; P = 0.16) with no size difference noted between immersion (n = 10) or perfusion fixed tissue (n = 23; P = 0.82). The complex orifices were most often located in the middle and posterior portions of Schlemm's canal. During gross dissection, large intrascleral vessels were observed posterior to Schlemm's canal within the sclera adjacent to the scleral spur. These vessels in some cases extended further posteriorly terminating on the surface of the sclera beneath the posterior portion of the ciliary body. A variety of structures were associated with the complex orifices. Sixteen of 33 complex collector channel orifices were elongated and were spanned by a wide bridge-like structure dividing the orifice. The bridge-like structure appeared to be a support for maintaining orifice patency. The bridge-like structures appeared to be covering either one or in some cases two collector channel orifices (Fig. 2). The bridge structures were lined with outer wall endothelial cells oriented parallel to the span of the bridge crossing the orifice. The bridges had an underlying fibrous matrix core that was continuous with the underlying connective tissue.
Figure 2.
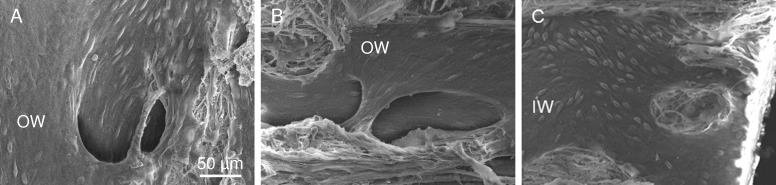
Complex orifice with bridge. Elongated orifice with overlying bridge (arrow). Outer wall (OW, top) and inner wall (IW, bottom) are joined at corresponding oval adhesion sites (asterisks). Inner wall cells appeared rounded and elevated in contrast to OW cells that appeared elongated and flat.
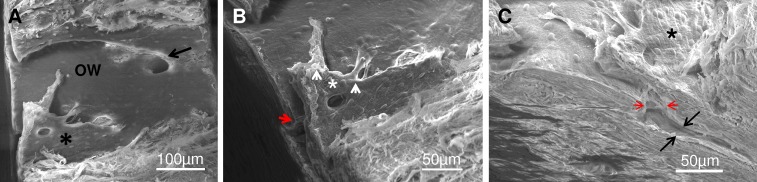
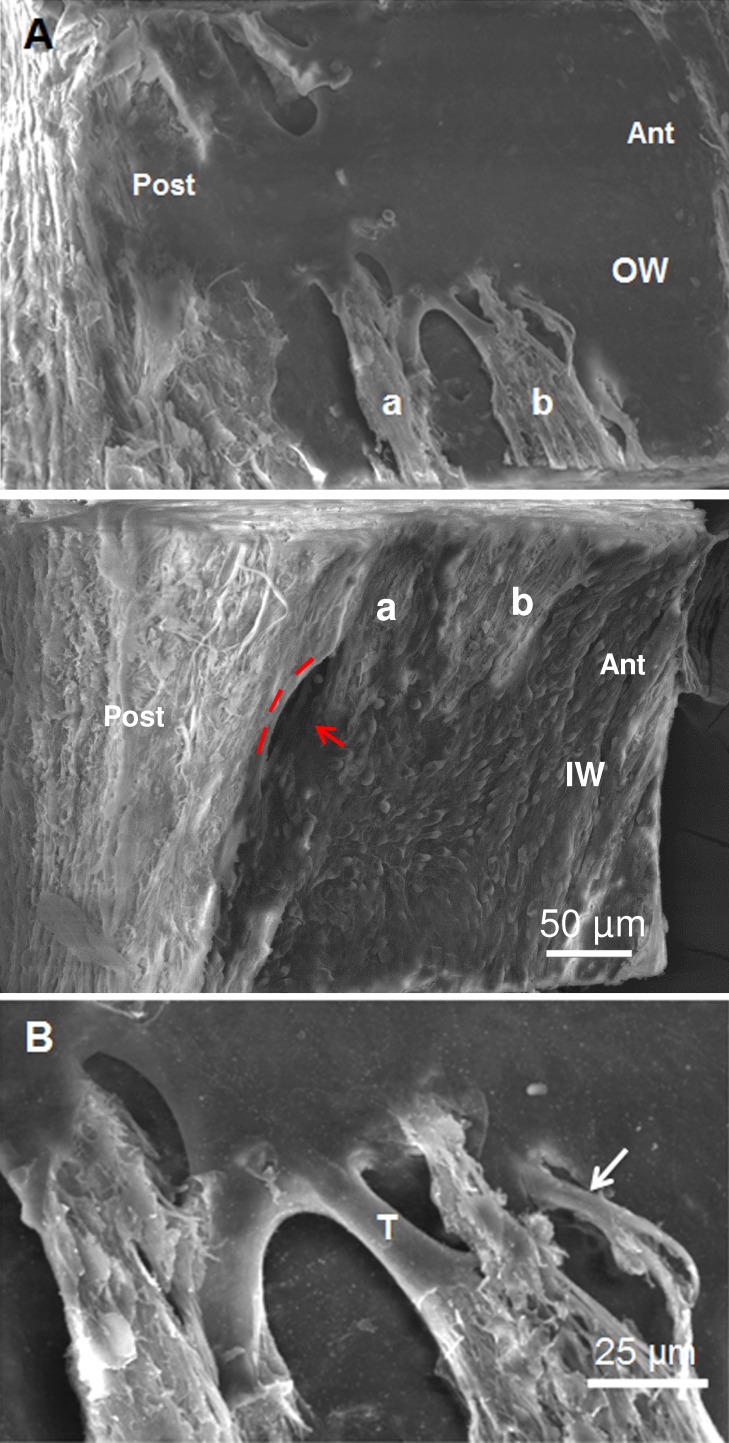
In addition to bridge-like structures, twelve complex orifices had flap-like structures (Figs. 3, 4). These flap-like structures extended between the outer and inner walls with a luminal surface lined by endothelial cells that were oriented in relation to the shape of the particular structure. Various configurations of endothelial–lined extensions with diameters of 10 to 20 μm were present that fanned over the orifice and anchored the flaps to the inner and outer wall (Figs. 3, 4). The flap with its extensions covered not only the orifice proper (Fig. 3C) but extended distally beyond it for some distance (Fig. 3B). Magnification of a tethered region (Fig. 4B) showed that these extensions commonly formed “U”-shaped outer wall connections and contained an underlying fibrous core. In some instances, tubule-like structures could be identified. The extensions and tubular-like structures could also be observed in gross dissections (Fig. 5A). Serial sections of the wedge (Fig. 5B) facing the transected area shown in Figure 4B confirmed the presence and complexity of the flap-like structures associated with the orifices. In these sections, the connective tissue core in the flaps and extensions was continuous with the juxtacanalicular tissue.
Figure 3.
Complex CC showing relationship to adjacent simple CC, flap covering orifice and intrascleral CC. (A) Low power of OW showing complex orifice with flap (asterisk) and simple orifice (arrow). (B) Higher magnification of complex flap with endothelial cells covering its surface (asterisk) and two flap “anchors” extending from its edge (arrowheads). Note on the longer flap a collagenous core is visible. Flap covers entrance to CC in the sclera (red arrow head) and distally extends from the orifice 175 μm. (C) Side view showing the orifice neck (red arrowheads) and CC proper (black arrows). Complex flap is visible over the CC orifice in the OW with extension into the CC proper in the sclera.
Figure 4.
Complex CC orifice associated with flaps. (A) Corresponding OW (top) and IW (bottom) showing flap-structure on OW overlying orifice at the junctional region of the IWs and OWs (red arrow). Lip of the orifice (IW panel, middle) is delineated by three red dashes on the top and a red arrow, which points to the darkened orifice region. Extensions and corresponding anchor sites on IWs and OWs are labeled a and b. Anterior and posterior are labeled in OW and IW wedges. (B) Enlargement of Figure 4A OW (top) near region labelled “b” showing underlying fibrous structure of anchoring tether (T) and tubule-like structure (arrow).
Figure 5.
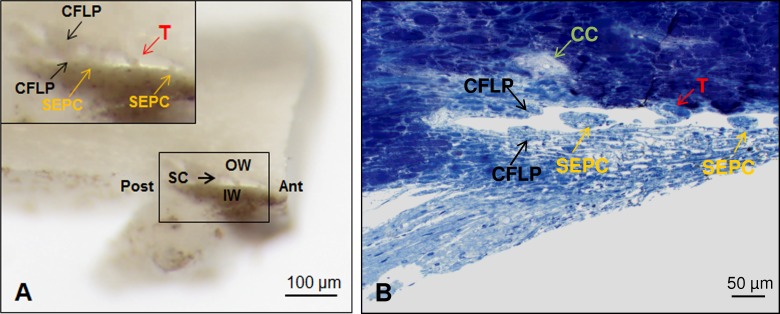
Gross dissection of complex flap in Schlemm's canal and correlative plastic section. (A) Gross dissection of radial wedge of anterior segment showing trabecular meshwork, IW, OW, and Schlemm's canal (SC). Anterior (Ant) and posterior (Post) are noted in the overview of the gross dissection. Inset (black square) shows complex U-shaped flap (CFLP, black arrows) spanning IW and OW with septal columns (SEPC, yellow arrows) and tubule-like structure (T, red arrow) in the posterior portion of Schlemm's canal. (B) Correlative plastic section of SC in same orientation as Figure 5A with transected portions of complex flap (CFLP, black arrows) on IW and OW, SEPC (yellow arrows) on the IW and funnel-shaped attachment of tubule-like structure (T, red arrow) on the OW. Scleral bed of CC in sclera is indicated by green arrow.
Five of the complex orifices were associated with septal columns. Septal columns in association with collector channels were visible in gross dissections before the separation of the inner and outer wall (Fig. 5A).
Schlemm's Canal in Region of Collector Channel Orifices
The endothelial cells of the outer wall were mostly flat and elongated with the longitudinal axes of the outer wall endothelial cells frequently oriented toward the orifices, giving an impression of streaming in both simple and complex collector channels (Figs. 1B, 6A, 6B). In contrast, the endothelial cells of the inner wall were more rounded, slightly narrower (4- to 6-μm wide), shorter in length (40–60 μm), and often bulging toward the lumen of Schlemm's canal (Figs. 2, 6C). The junction between cells varied from slightly raised edges to grooves or fissures between the cells. The elevated cells were more numerous in areas of the inner wall directly underneath both simple and complex collector channel orifices, giving the inner wall a cobblestone appearance. Inner wall endothelium formed complex folds, pockets, and clefts in areas opposite the collector channel orifices. Plaque-like areas (50–200 μm) containing fibrous material were found on corresponding inner and outer walls, most likely representing areas that were in close apposition. Small delicate tubular-shaped structures were often seen in the gross dissections in the anterior and middle portion of Schlemm's canal (Fig. 5) but were not found intact in SEM preparations.
Figure 6.
Schlemm's canal in region of CC orifices. (A) Streaming alignment of OW cells into a complex orifice. (B) Outer wall cells stream over a complex bridge toward the orifice. (C) Inner wall cells with prominent bulging nuclei surround remnant of septal column and show cell alignment near orifice.
Discussion
The results of the present study indicate that collector channel orifices have diverse morphologic appearances ranging from simple oval openings to complex orifice structures. Complex orifices were observed frequently with bridge-like structures, tethered flaps and in association with septal columns. In addition, complex orifice flap-like structures were connected to the inner and the outer wall of Schlemm's canal. It is unlikely that the complex orifices were a result of tissue preparation because they were observed in eyes that were immersion fixed and also in eyes that were perfusion fixed. There was considerable variation in orifice diameters, which ranged between 20 and 110 μm. These values were consistent with those previously reported.9,10,21 The orifice diameters were similar in both simple and complex orifices.
While single flaps and bridging sieve-like structures have been described,10,12 multiple flap-like connections to the inner and outer wall of Schlemm's canal have not been previously reported. This could be due in part to the use of routine dissection and the representative sampling techniques used for light, transmission, and scanning microscopy. In our study, collector channel regions were preferentially selected by dissection and preserved with surrounding tissue to get a 3-dimensional analysis of collector channel regions. Collector channels in radial wedges greater than 50 μm in size were visible in the outer wall. It is interesting to note that many collector channels associate with large intrascleral vessels or connect to ciliary vessels. It is interesting to speculate that different orifice structures (simple versus complex) may relate to specific connections within the venous system and indicate a systematic hierarchy of moving aqueous fluid between different vascular beds. Answers to the exact functions of these complex collector channels will rely on further studies of the outflow system to definitively establish the nature of downstream connections.
Complex collector channels with flap-like extensions connecting the inner and outer wall may also function similar to valves found in veins and lymph vessels by limiting fluid backflow. In this regard, the flaps could potentially prevent aqueous fluid that had passed into the collector channels from returning to Schlemm's canal when pressures in Schlemm's canal are similar to episcleral venous pressure. It is known that reflux of blood occurs when the IOP is lower than episcleral venous pressure in the range of 8 to 9 mm Hg.22 When episcleral venous pressure is higher than Schlemm's canal pressure, these flaps may leak, causing blood reflux into Schlemm's canal. Ramos et al.23 suggested that Schlemm's canal endothelium share many morphologic and functional characteristics with endothelium of both blood and lymphatic vessels.24 Recent work has shown the presence of prospero homeobox protein 1 (PROX1), a protein responsible for regulating lymphatic development, and forkhead transcription factor FOXC2, a lymphatic valve marker protein, in Schlemm's canal endothelial cells of lymphatic and blood vasculature reporter mice.25 Additionally, Park et al.25 found that Schlemm's canal cells also expressed integrin α9 and had vascular endothelium-cadherin (VE-cadherin) containing junctions similar to collecting lymphatics. These findings along with new work demonstrating the responsiveness of Schlemm's canal development in mouse, zebrafish, and human eye tissue to VEGF-C, a lymphangiogenic growth factor, suggests that Schlemm's canal has lymphatic characteristics and may function as a rudimentary lymphatic vessel.26
Li et al.27 using a primate model system and phase sensitive optical coherence tomography (PhS-OCT) have observed that the trabecular meshwork and Schlemm's canal are sensitive to pulse-induced movement. Using PhS-OCT with defined parameters of pressure and pulsed-flow demonstrated dilation of Schlemm's canal, distension of trabecular meshwork, and increased visibility of collagenous structures within the canal and at collector channel ostia.28 These observations suggest that a mechanism, similar to the valve-like function proposed here for complex flaps, may be necessary to keep orifices patent by being readily deformable to compensate for rapid pressure changes as found during head movement, blinking, and sneezing,29 and may also function in response to transient movement in normal IOP.14 In the present study, we observed adhesion areas and septal columns where the inner and outer walls were in close contact. Presumably these adhesion sites represented deformation of inner wall toward the outer wall. The septal columns maintained Schlemm's canal patency especially in the vicinity of collector channel orifices, preventing herniation of the inner wall into the collector channel. Smit and Johnstone30 suggested that extensions bridging the inner and outer wall may serve as tethers that maintain the shape of Schlemm's canal.
The flap-like extensions of complex collector channels may relay extracellular matrix tension or stiffness through Schlemm's canal endothelial cells by coupling the matrix of the inner and outer wall. This could occur via the elastin fiber system, which connects to the actin cytoskeleton of Schlemm's canal cells through connecting fibrils.31 Regions where the endothelium was disrupted or missing on flaps or orifices, revealed a matrix with a mesh pattern similar to that reported for the elastin fiber system of rat venules.32 The appearance of the extracellular mesh was also similar to that observed by scanning electron microscopy in preparations from our laboratory where the inner wall endothelium of Schlemm's canal was removed in anterior segment culture with varying concentrations of ethacrynic acid.33 Further correlative histologic and immunologic labeling will extend these observations.
Additional hints of coupling that may relay matrix tension or stiffness was observed in the mirror alignment of outer wall cells in regions of collector channels, which are contiguous in flaps, septums, and tubule-like structures. The alignment of endothelial cells was apparent in our study with outer wall endothelial cells aligned with one another toward collector channel orifices. The endothelial cell alignment appears to depend on transendothelial flow of aqueous humor. Its associated pressure gradient and the alignment of F-actin in the endothelial cells appears to provide critical mechanical reinforcement as noted by Ethier et al.34 Notably the actin cytoskeleton is linked to the elastin system, which may act in concert to sense changes in the extracellular matrix environment along with integrins and other signaling proteins.
Variations in collector channel orifice structure imply a possible regulatory function through linkage to the distal outflow system. In addition, the presence of different types of collector channels may compensate for changing conditions in fluid flow and pressure dynamics adjacent to the orifices and in Schlemm's canal at large. In aging or disease, if one collector channel orifice becomes blocked or a region of Schlemm's canal becomes adherent, other collector channels would be available. Taken together, the anatomical structures identified in this study indicate a possible regulatory role in fluid dynamics by Schlemm's canal by virtue of collector channels and their associated structural entities such as flap-like structures, septa, and tubule-like structures. Further studies are warranted to explore the distal outflow pathway, its structures and its role in aqueous humor dynamics.
Acknowledgments
Supported in part by National Eye Institutes research Grant EY 21727 (Bethesda, MD, USA); Mayo Foundation (Rochester, MN, USA); and Research to Prevent Blindness, Inc. (New York, NY, USA; Department of Ophthalmology, Mayo Clinic is the recipient of an unrestricted grant).
Disclosure: M.D. Bentley, None; C.R. Hann, None; M.P. Fautsch, None
References
- 1. Chader GJ. Key needs and opportunities for treating glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 2456–2460. [DOI] [PubMed] [Google Scholar]
- 2. Quigley HA,, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson M. What controls aqueous humor outflow? Exp Eye Res. 2006; 82: 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maepea O,, Bill A. The pressures in the episcleral veins, Schlemm's canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Exp Eye Res. 1989; 49: 645–663. [DOI] [PubMed] [Google Scholar]
- 5. Maepea O,, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992; 54: 879–883. [DOI] [PubMed] [Google Scholar]
- 6. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783–801. [DOI] [PubMed] [Google Scholar]
- 7. Maepea O. Pressures in the anterior ciliary arteries choroidal veins and choriocapillaris. Exp Eye Res. 1992; 54: 731–736. [DOI] [PubMed] [Google Scholar]
- 8. Ashton N. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. Part I. Aqueous veins. Br J Ophthalmol. 1951; 35: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dvorak-Theobald G. Further studies on the canal of Schlemm. Its anastomoses and anatomic relations. Am J Ophthalmol. 1955; 39: 65–89. [DOI] [PubMed] [Google Scholar]
- 10. Rohen JW,, Rentsch FJ. Uber den Bau des Schlemmschen Kanals und seiner AbfluBwege beim Menschen. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1968; 176: 309–329. [DOI] [PubMed] [Google Scholar]
- 11. Hann CR,, Vercnocke AJ,, Bentley MD,, Jorgensen SM,, Fautsch MP. Anatomic changes in Schlemm's canal and collector channels in normal and primary open-angle glaucoma eyes using low and high perfusion pressures. Invest Ophthalmol Vis Sci. 2014; 55: 5834–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann F,, Dumitrescu L. Schlemm's Canal under the scanning electron microscope. Ophthalmol Res. 1971; 2: 37–45. [Google Scholar]
- 13. Rohen J,, Rentsch F. Electronmicroscopic studies on the structure of the outer wall of Schlemm's canal, its outflow channels and age changes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1969; 177: 1–17. [DOI] [PubMed] [Google Scholar]
- 14. Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004; 13: 421–438. [DOI] [PubMed] [Google Scholar]
- 15. Chi HH,, Katzin HM,, Teng CC. Primary degeneration in the vicinity of the chamber angle; as an etiologic factor in wide-angle glaucoma. II. Am J Ophthalmol. 1957; 43: 193–203. [DOI] [PubMed] [Google Scholar]
- 16. Teng CC,, Paton RT,, Katzin HM. Primary degeneration in the vicinity of the chamber angle; as an etiologic factor in wide-angle glaucoma. Am J Ophthalmol. 1955; 40: 619–631. [DOI] [PubMed] [Google Scholar]
- 17. Hann CR,, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009; 50: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melamed S,, Epstein DL. Alterations of aqueous humour outflow following argon laser trabeculoplasty in monkeys. Br J Ophthalmol. 1987; 71: 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Battista SA,, Lu Z,, Hofmann S,, Freddo T,, Overby DR,, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008; 49: 5346–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parc CE,, Johnson DJ,, Brilakis HS. Giant vacuoles are found preferentially near collector channels. Invest Ophthalmol Vis Sci. 2000; 41: 2984–2990. [PubMed] [Google Scholar]
- 21. Hann CR,, Fautsch MP. The elastin fiber system between and adjacent to collector channels in the human juxtacanalicular tissue. Invest Ophthalmol Vis Sci. 2011; 52: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnstone MA,, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973; 75: 365–383. [DOI] [PubMed] [Google Scholar]
- 23. Ramos RF,, Hoying JB,, Witte MH, Daniel Stamer W. Schlemm's canal endothelia, lymphatic, or blood vasculature? J Glaucoma. 2007; 16: 391–405. [DOI] [PubMed] [Google Scholar]
- 24. Karpinich NO,, Caron KM. Schlemm's canal: more than meets the eye lymphatics in disguise. J Clin Invest. 2014; 124: 3701–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park DY,, Lee J,, Park I,, et al. Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity. J Clin Invest. 2014; 124: 3960–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aspelund A,, Tammela T,, Antila S,, et al. The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest. 2014; 124: 3975–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li P,, Reif R,, Zhi Z,, et al. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo nonhuman primate eyes. J Biomed Opt. 2012; 17: 076026. [DOI] [PubMed] [Google Scholar]
- 28. Hariri S,, Johnstone M,, Jiang Y,, et al. Platform to investigate aqueous outflow system structure and pressure-dependent motion using high-resolution spectral domain optical coherence tomography. J Biomed Opt. 2014; 19: 106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coleman DJ,, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969; 82: 637–640. [DOI] [PubMed] [Google Scholar]
- 30. Smit BA,, Johnstone MA. Effects of viscoelastic injection into Schlemm's canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology. 2002; 109: 786–792. [DOI] [PubMed] [Google Scholar]
- 31. Rohen JW,, Futa R,, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981; 21: 574–585. [PubMed] [Google Scholar]
- 32. Shinaoka A,, Momota R,, Shiratsuchi E,, et al. Architecture of the subendothelial elastic fibers of small blood vessels and variations in vascular type and size. Microsc Microanal. 2013; 19: 406–414. [DOI] [PubMed] [Google Scholar]
- 33. Bahler CK,, Hann CR,, Fautsch MP,, Johnson DH. Pharmacologic disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 2246–2254. [DOI] [PubMed] [Google Scholar]
- 34. Ethier CR,, Read AT,, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J. 2004; 87: 2828–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]