Abstract
The use of a monoclonal antibody to block the neurite outgrowth inhibitor Nogo-A has been of great interest for promoting axonal recovery as a treatment for spinal cord injury. While several cellular and non-cellular assays have been developed to quantify the bioactive effects of Nogo-A signaling, demand still exists for the development of a reliable approach to characterize the effectiveness of the anti-Nogo-A antibody. In this study, we developed and validated a novel cell-based approach to facilitate the biological quantification of a Nogo-A antibody using PC-12 cells as an in vitro neuronal cell model. Changes in the mRNA levels of the neuronal differentiation markers, growth-associated protein 43 and neurofilament light-polypeptide, suggest that activation of the Nogo-A pathway suppresses axonal growth and dendrite formation in the tested cell line. We found that application of anti-Nogo-A monoclonal antibody can significantly enhance the neuronal maturity of PC-12 cells by blocking the Nogo-A inhibitory effects, providing enhanced effects on neural maturity at the molecular level. No adverse effects were observed on cell viability.
Keywords: Nogo-A, bioactivity, neuroregeneration, PC-12 cells
Introduction
Spinal cord injury (SCI) affects approximately 12,500 people each year in the United States1 and often results in partial or complete loss of function below the initial site of the injury, permanently removing the patient’s ability to perform basic daily activities. The loss of function occurs by disrupting signal transduction mechanisms of both ascending and descending neuronal tracts in the gray matter of the spinal cord. After the primary compression or contusion injury, a secondary injury cascade occurs leading to the formation of a glial scar, an increase in cell death at the injury site, and an upregulation of inhibitory factors that prevent functional recovery.2,3 Although there is no cure for SCI yet, a better understanding of the molecular and cellular mechanisms post-injury, with advances in the fields of biomaterials and tissue engineering, has led to important new discoveries.4
It is well established that neurons of the central nervous system (CNS) can extend neurites after injury when offered a permissive environment.5,6 The endogenous inhibition of this system has been recognized by cell surface receptor and myelin interactions.7 Since 2005, the transmembrane protein Nogo-A has been recognized as one of the primary contributors for neurite outgrowth inhibition.8,9 This finding has been further supported by the application of specific antibodies against Nogo-A using in vitro as well as in vivo models.10 These findings demonstrated that a Nogo-A antibody functions by blocking the interaction of Nogo-A with the Nogo receptor and co-receptors p75, TROY, and LINGO-1 located on neurons from the CNS.11–13
Various anti-Nogo-A antibodies have been engineered and reported in the literature.14,15 One of the most commonly studied Nogo-A antibodies is the mouse monoclonal antibody 11c7, which was raised against a peptide corresponding to aminoacids 623–640 of the rat Nogo-A protein sequence,8 which are within the central inhibitory domain.16 The in vitro effects of the 11c7 antibody have been previously shown by Western blot, as well as indirect immunoassays utilizing variety of cell-based assays.17,18
While several cellular and non-cellular assays have been established to quantify the effects of Nogo-A activation,8,19 the development of a reliable approach to characterize the antibody is important for SCI recovery. Whereas the delivery of anti-Nogo-A has been of great interest for neuroregeneration, our long-term goal is to develop a localized delivery strategy for this antibody with the goal of facilitating neuroplasticity at the injury site.20–23 The main objective herein is to report for the first time a systematic approach to evaluate the anti-inhibitory effects of the Nogo-A antibody 11c7 on the nerve growth factor (NGF)-induced neuronal-like differentiation of the pheochromocytoma cell line, PC-12. By following this approach, a new in vitro cell-based assay to facilitate quantification of an anti-Nogo-A antibody has been demonstrated.
Description of methods
Materials
The mouse monoclonal antibody 11c7 against Nogo-A was generously provided by Novartis Pharma AG (Basel, Switzerland). Dulbecco’s phosphate buffered saline (PBS; pH 7.4) was purchased from Invitrogen (GIBCO Invitrogen Laboratories, NY, USA). All buffers were prepared using water distilled and deionized using a Millipore Milli-Q UF Plus at 18 MΩ resistance (Millipore, Bedford, MA, USA).
Cell culture
The rat adrenal pheochromocytoma cell line, PC-12, was purchased from the American-type culture collection (CRL-1721, ATCC, Rockville, MD, USA), cultured in RPMI 1640 with UltraGlutammine media (LONZA, Basel, Switzerland), and supplemented with 10% fetal bovine serum (FBS), 5% horse serum (HS), and 1% penicillin/streptomycin (P/S) on BD-Biocoat™ collagen-coated plates (BD Biosciences, Oxford, UK). Cells were incubated in an atmosphere containing 5% CO2 at 37°C.
Effects of Nogo-A peptide and Nogo-A monoclonal antibody on proliferation of PC-12 cells
PC-12 cells were examined for proliferation through the active incorporation of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) dye (Invitrogen). They were seeded at a cell density of 2000 per well into a 96-well plate and grown overnight prior to receiving treatment. On day 2, cells were treated with 100 ng/mL NGF (R&D Systems, Minneapolis, MN, USA) for 2 h followed by incubation with a recombinant Nogo-A peptide (corresponding to aminoacids 566–748 of the human protein, thus expected to block the 11c7 antibody; R&D Systems) using 1 µg/mL. The cells were then treated with 1, 3, and 12 µg/mL of Rat Nogo-A monoclonal antibody 11c7. PC-12 cells were incubated for 6 days for to monitor axonal growth and neurite maturity. At pre-determined times, cells were treated with 0.5 mg/mL of MTT in complete media for 2 h. The media was then completely removed and replaced with 100 µL of dimethyl sulfoxide (DMSO) for 30 min and the plate was then measured on a Synergy H4 plate reader at optical density (OD) of 570 nm.
Differentiation and characterization of neurite outgrowth
PC-12 cells were seeded on collagen-type IV-coated 24-well plates (BD-Biocoat) at a cell density of 4000 cells/cm2 and grown overnight prior to receiving treatment. On day 2, cells were treated with 100 ng/mL NGF (R&D Systems) for 2 h followed by incubation with a recombinant Nogo-A peptide (R&D Systems) using 25, 100, and 1000 ng/mL. The cells were then treated with 1, 3, and 12 µg/mL of Rat Nogo-A monoclonal antibody 11c7. PC-12 cells were incubated for 6 days for to monitor axonal growth and neurite maturity.
Immunostaining
At the end of the incubation period, PC-12 cells were stained with an alpha-tubulin antibody (DM1A; Thermo Fisher Scientific, Waltham, MA, USA) to assess the effects of the Nogo-A peptide and the Nogo-A antibody on cytoskeletal elements. Cells were seeded into chambered glass slides and stained for actin and tubulin using Alexa Fluor-555 Phalloidin (Invitrogen) as previously described.24 Treated cells were prepared for staining according to the manufacturer’s recommendations. Briefly, cells were fixed within paraformaldehyde (4%) in PBS for 10 min, followed by permeabilization with 0.2% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) in PBS for 10 min at room temperature (RT). Cells were then blocked for 30 min at RT in 1% bovine serum albumin (BSA; Sigma-Aldrich) diluted in PBS and containing 0.05% Tween-20 (Sigma-Aldrich), followed by 1-h incubation with the alpha-tubulin antibody at RT. Cells stained with phalloidin, a bicyclic peptide with high affinity for filamentous actin (F-actin), were fixed and permeabilized as described earlier. PC-12 cells were blocked in 1% BSA in PBS for 10 min at RT followed by a 20-min incubation with 2.5% phalloidin in PBS. Cells were then mounted using Prolong Gold (Invitrogen) with 4′,6-diamidino-2-phenylindole (DAPI) and allowed to dry overnight before examination with an A1 confocal laser microscopy (Nikon, Eclipse Ti, Tokyo, Japan).
Morphological evaluation and neurite outgrowth assay
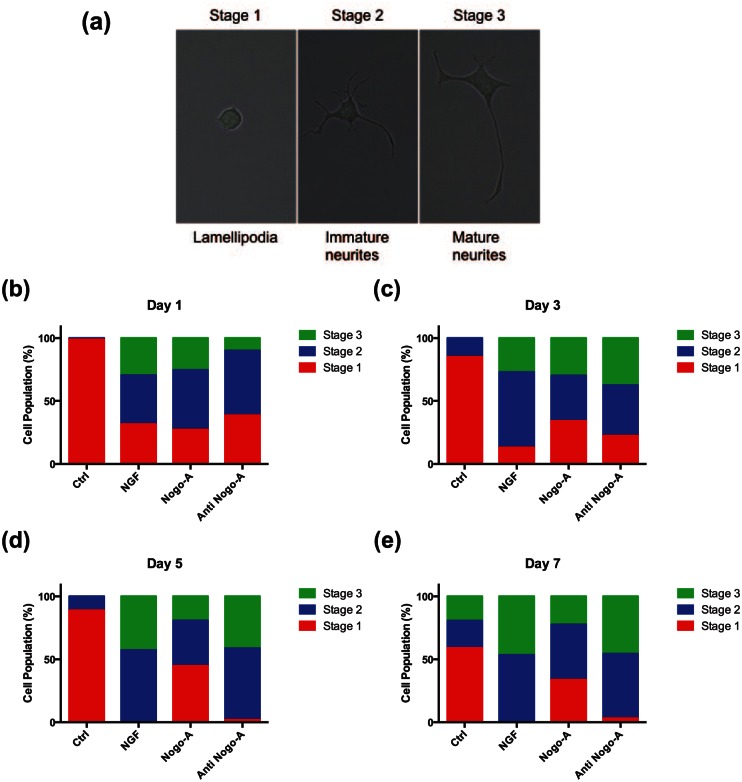
The cellular assay for determining neurite outgrowth was performed as previously described25 with certain modifications. Briefly, the morphology and neurite outgrowth of PC-12 cells were used to evaluate the bioactivity of NGF, Nogo-A, and Nogo-A antibody. PC-12 cells responded reversibly to NGF by differentiating into a neuronal phenotype with neurite extension. At days 1, 3, 5, and 7, the percentage of neurite-bearing cells was determined by counting ~100 cells in random fields using a Nikon Eclipse 80i and the measuring tool in NIS element software (Nikon). For quantification purposes we considered neurite-bearing cells as those with processes greater than, or equal to, the cell body diameter. Furthermore, for the first time we used well-characterized steps of neuronal development as a tool to distinguish three major stages of neuron polarized structures in order to differentiate cell stages from each other as rounded cells with uncoordinated lamellipodia (stage 1), cells with sprouting of several minor neurites smaller than or equal to the cell body diameter (stage 2), and cells with elongated neurites larger than the cell body (stage 3).
Reverse transcription polymerase chain reaction
PC-12 cells were seeded at 8 × 105 cells/600 mm2 into a 100-mm BD-Biocoat dish (Corning, NY, USA) and grown overnight in Roswell Park Memorial Institute (RPMI) 1640 with UltraGlutammine media (LONZA) supplemented with 10% FBS, 5% HS, and 1% P/S. After 24 h, cells were treated with 100 ng/mL NGF. On day 3, cells were treated for 2 h with 1, 3, and 12 µg/mL of anti-Nogo-A. Following this treatment, cells were treated with 1000 ng/mL of the recombinant Nogo-A peptide.
Total RNA extraction was performed using TRIzol® (Life Technologies, Carlsbad, CA, USA). RNA retro-transcription was performed using SuperScript® II Reverse Transcriptase (Life Technologies, Carlsbad, CA, USA). Neuron growth-associated protein 43 (GAP43) and neurofilament light (NFL) gene expression analysis was performed using StepOnePlus™ Real-Time PCR (Applied Biosystems®, Foster City, CA, USA) and TaqMan® Fast Advanced Master Mix (Life Technologies). The rat probes used for this analysis were Rn01474579_m1 (GAP43) and Rn00582365_m1 (NFL; Life Technologies); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as expression housekeeping control (Rn01775763_g1; Life Technologies).
Statistical analysis
All data presented graphically are the mean values of at least three trials and results from each experiment were compiled from at least 100 cells per well in eight randomly selected fields of view. All the data were analyzed by one-way factorial analysis of variance (ANOVA) and multiple comparisons. Significant effects of treatment were defined using Scheffe’s method as post-hoc test.
Results and discussion
To confirm the effect of Nogo-A and anti-Nogo-A antibody on PC-12 cells viability, MTT assay was performed. The results of proliferation study indicated that NGF enhanced cell proliferation of PC-12 cells by day 7. Moreover, Nogo-A peptide interaction reduced cell proliferation, while the Nogo-A antibody in a dose-dependent manner from 1 to 12 µg/mL enhanced cellular proliferation at days 3 and 7. Specifically, anti-Nogo-A antibody at a concentration of 12 µg/mL had the strongest stimulatory effect to promote cell growth (Figure 1).
Figure 1.
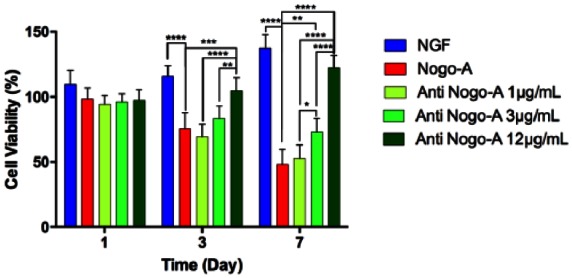
Effects of Nogo-A and anti-Nogo-A antibody on PC-12 proliferation. PC-12 cells were stimulated by NGF and post-treated with Nogo-A at 1 µg/mL concentration and post-treated with anti-Nogo-A at 1, 3, and 12 µg/mL concentrations. Statistically significant differences were *p < 0.05, **p < 0.01, and ***p < 0.001 in each group with respect to the Nogo-A peptide group.
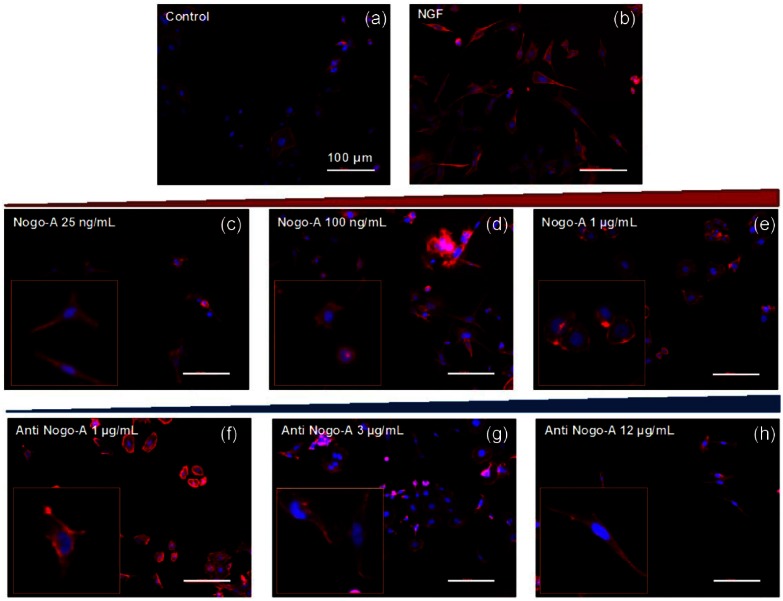
In agreement with previously published studies,8,19 PC-12 cells differentiated neurites over collagen-coated substrates in the presence of NGF (Figure 2(a) and (b)). The inhibition of neurite outgrowth appeared to be directly correlated, in a dose-dependent manner (after 3 days), with the presence of a recombinant Nogo-A peptide, at concentrations varying from 25 ng/mL to 1 µg/mL (Figure 2(c)–(e)). A Nogo-A concentration of 1 µg/mL appeared to exhibit the highest inhibition over 3 days (Figure 2(e)). This finding was in agreement with previous reports of neurite inhibition in the presence of adsorbed myelin proteins17 and suggest the interaction of the peptide with other co-receptors in the Nogo-A pathway required for outgrowth. On the other hand, the inhibitory effects of the Nogo-A peptide were blocked when cells were treated with the Nogo-A antibody in a dose-dependent manner from 1 to 12 µg/mL on differentiated PC-12 cells (Figure 2(f)–(h)). Cells treated with the highest concentration of the Nogo-A antibody appeared to display the greatest neurite outgrowth among all groups (Figure 2(h)).
Figure 2.
Immunofluorescent images of PC-12 cells stained for actin and tubulin using Alexa Fluor-555 Phalloidin and for cell nuclei using DAPI. (a) untreated, (b) NGF treated, (c) pretreated with NGF and post-treated with Nogo-A 25 ng/mL, (d) pretreated with NGF and post-treated with Nogo-A 100 ng/mL, (e) pretreated with NGF and post-treated with Nogo-A 1 µg/mL, (f) pretreated with NGF and Nogo-A 1 µg/mL and post-treated with anti-Nogo-A 1 µg/mL, (g) pretreated with NGF and Nogo-A 1 µg/mL and post-treated with anti-Nogo-A 3 µg/mL, and (h) pretreated with NGF and Nogo-A 1 µg/mL and post-treated with anti-Nogo-A 12 µg/mL. All scale bars represent 100 µm.
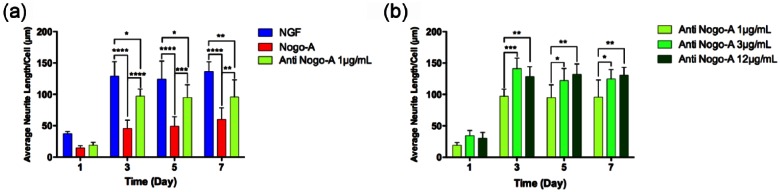
To further quantitatively verify the previous observations, we evaluated the morphological changes of the cells and characterized the average neurite length by measuring their neurite outgrowth under different incubation conditions. Figure 3(a) demonstrates that, while NGF-treated cells exhibited the highest neurite outgrowth by day 3 (average neurite length = 136 µm/cell), Nogo-A peptide-treated cells reduced neurite outgrowth by approximately 60% after the same incubation period. In addition, the anti-Nogo-A antibody specifically blocked the inhibitory effects of Nogo-A peptide on PC-12 neurite outgrowth in a consistent timely manner. These findings agreed with previous studies on the known presence of the Nogo-A protein in myelinated axons and its mechanism of action in terms of binding to the Nogo-A receptor for blocking cellular interactions involved in the outgrowth of neurites.9,26
Figure 3.
Average changes in neurite length. (a) Average PC-12 cell neurite length per cell stimulated by NGF, and post-treated with Nogo-A and anti-Nogo-A 1 µg/mL. (b) Average PC-12 cell neurite length per cell stimulated by NGF with Nogo-A and post-treated with anti-Nogo-A at 1, 3, and 12 µg/mL concentrations.
Our observations were further supported by testing three concentrations of anti-Nogo-A antibody and evaluating the effects in a dose-dependent manner. Average neurite length of PC-12 cells was determined over 7 days of treatment to verify the optimum concentration of Nogo-A antibody required to block the neurite growth inhibitory effects of the Nogo-A peptide. As shown in Figure 3(b), anti-Nogo-A concentrations of ⩾3 µg/mL exhibited the maximum neurite outgrowth, but there was no significant difference in Nogo-A inhibition reduction and neurite outgrowth between 3 and 12 µg/mL anti-Nogo-A concentrations over 7 days. Cells pretreated with these concentrations exhibited similar average neurite outgrowth length per cell compared to the NGF-treated group (Supplementary Figure S1).
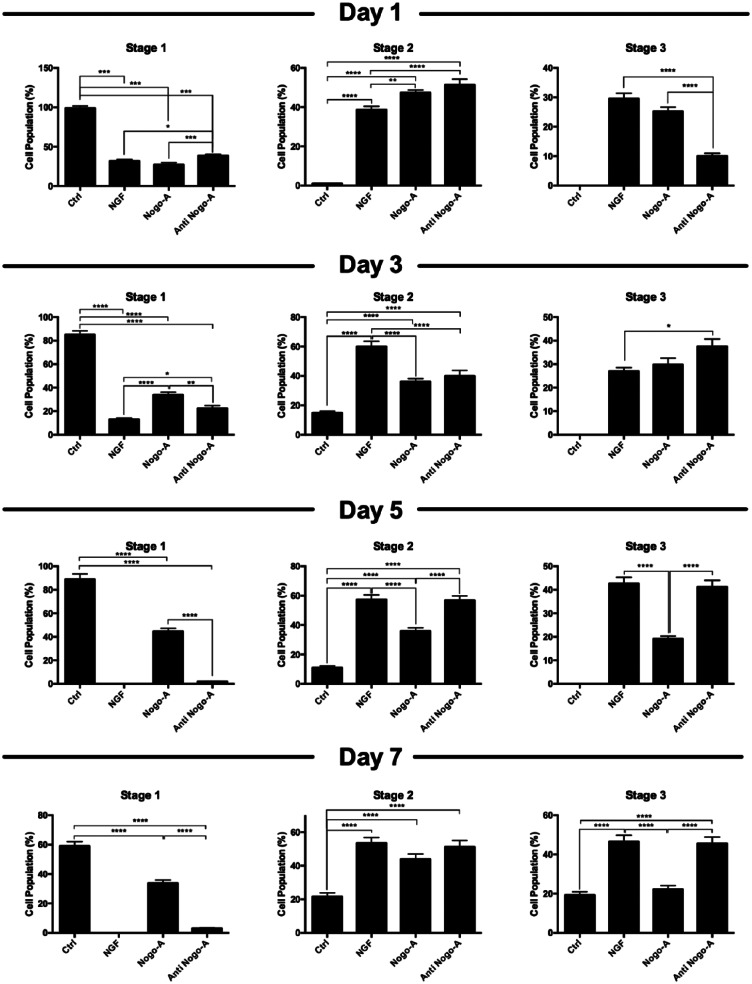
We performed morphological evaluation of neurite-bearing cells to observe other anti-inhibitory effects of anti-Nogo-A. Three major stages of neuronal development were used as a template to differentiate cell populations from each other in the different treatment groups (Figure 4(a)). When comparing the response of the Nogo-A peptide and the Nogo-A antibody groups to NGF treatment cells, no distinctive stage pattern among cell population distributions was observed at day 1, and all three groups had similar percentages for all three differentiation stages (Figure 4(b)). On days 5, however, both NGF and Nogo-A antibody-treated groups appeared to have a higher percentage of stage 2 and stage 3 cell populations and statistically significant when compared to the Nogo-A peptide group (Figure 4(c); Supplementary Figure S2). On and after day 5 of incubation, we observed a shift in cell population stages for the Nogo-A peptide group, while both NGF and Nogo-A antibody groups revealed similar trends for the development of neuronal stages (Figure 4(d)). This anti-inhibitory effect of the Nogo-A antibody was also observed at day 7. As shown in Figure 4(e) and Supplementary Figure S2, approximately equal populations of cells at stages 2 and 3 were found in both NGF and Nogo-A antibody-treated groups, while the Nogo-A-treated cells appeared to have the largest percentage of stage 1 cells among all groups. Overall, the pretreatment of PC-12 cells with a Nogo-A antibody was adequately effective to block the inhibitory effects of the Nogo-A peptide on allowing the neurite length outgrowth as observed for the NGF-treated group.
Figure 4.
Morphological changes of PC-12 cells leading to different neuronal stages. (a) Three differential stages for neurite outgrowth and neuronal polarization were observed in cultured PC-12 cells. Stage changes in neurite outgrowth of PC-12 cells stimulated with NGF, and post-treated with Nogo-A peptide, or a combination of the Nogo-A peptide and the Nogo-A antibody at days (b) 1, (c) 3, (d) 5, and (e) 7.
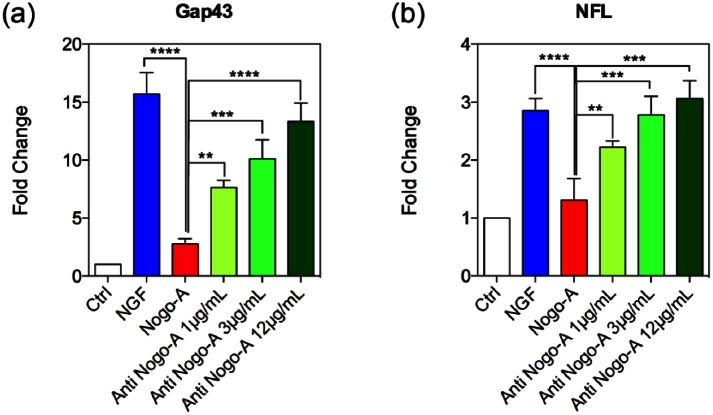
Finally, we determined the expression levels of GAP43 and NFL and evaluated whether the Nogo-A antibody enhances neuronal maturity of PC-12 cells in a manner similar to the observation with NGF after Nogo-A peptide application. The neuronal-like differentiation in the Nogo-A peptide group was also compared to NGF and Nogo-A antibody treatment alone. Expression levels were characterized from identical number of viable cells. Our results suggest that the activation of neurotrophin receptors after NGF treatment was able to induce a neuronal-like differentiation of PC-12 cells. Based upon the changes in GAP43 expression levels, by inhibiting Nogo-A peptide inhibitory effects with the Nogo-A antibody, we observed a significant boost in the differentiation capabilities of these cells, which furthermore appeared to be directly produced in a dose-dependent manner (Figure 5(a)). In the case of NFL expression, an increase in the levels of this gene was also observed when the PC-12 cells were treated with NGF to trigger the neuron-like differentiation. Cells treated with the Nogo-A peptide showed significant changes in expression, which occurred in a short period of time, resulting in a significant decrease in NFL expression.27 In addition, the messenger levels obtained after Nogo-A antibody treatments at different concentrations showed a restoration of gene expression levels in a dose-dependent manner (Figure 5(b)). While a concentration of 1 µg/mL of the Nogo-A antibody was enough to restore differentiation in a minor extent as when compared with the Nogo-A peptide-treated group, higher concentrations of the antibody were nonetheless successful for allowing similar NFL gene expression levels as those observed for the NGF-treated group (Figure 5(b)).
Figure 5.
Gene expression changes for neuronal markers. PC-12 cells’ mRNA expression levels of (a) Gap43 and (b) NFL, showing neural maturity during culture stimulated by NGF, and post-treated with Nogo-A peptide or three different concentrations of Nogo-A antibody. The intensities for the GAP43 and NFL genes in the Nogo-A antibody-treated groups were significantly upregulated when compared to the Nogo-A peptide group. Statistically significant differences were *p < 0.05, **p < 0.01, and ***p < 0.001 in each group with respect to the Nogo-A peptide group.
Our established in vitro assay using PC-12 cells here is a feasible method to study the bioactive effects of the monoclonal Nogo-A antibody. However, it is important to bear in mind the limitation of these cells since they have some genetic abnormalities, which may not reflect the in vivo phenomenon. Future studies need to be focused on neural stem cells and neural precursor cells to fully evaluate these effects.
Conclusion
We have developed and validated a new in vitro cell-based assay to facilitate quantification of bioactive effects of the mouse monoclonal Nogo-A antibody 11c7 using a PC-12 cell line. Our results show that the Nogo-A antibody can enhance the maturity of PC-12 cells into a neuronal-like phenotype without creating any adverse effects for survival. The quantitative evaluation of Nogo-A bioactivity, using morphological and neurite outgrowth assessment, validates the enhancing effects of the Nogo-A antibody to promote neural maturity, neurite growth, and further substantiates this assay at the molecular levels by assessing changes in GAP43 and NFL, markers of neuronal differentiation.
Acknowledgments
The authors acknowledge Dr. Kemi Cui and HMRI Microscopy core, and thank Megan Livingston for her assistance in editing this publication.
Supplementary Information
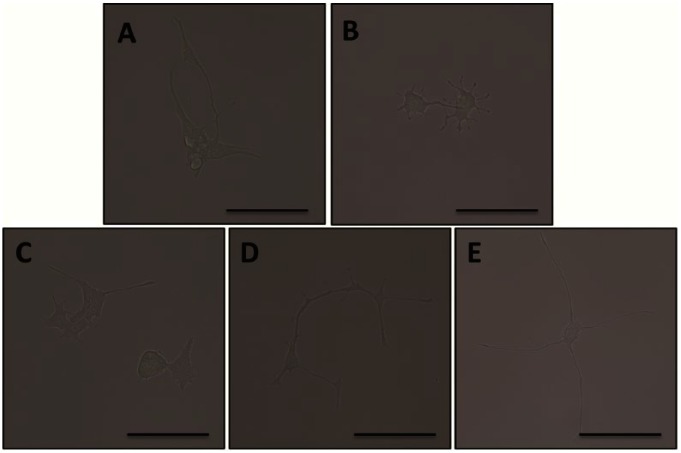
Supplementary Figure S1.
Bright field images of PC-12 cells at day 5 treated with (A) NGF; (B) pretreated with NGF and post-treated with Nogo-A; (C) pretreated with NGF and Nogo-A and post-treated with anti-Nogo-A 1 μg/mL; (D) pretreated with NGF and Nogo-A and post-treated with anti-Nogo-A 3 μg/mL; (E) pretreated with NGF and Nogo-A and post-treated with anti-Nogo-A 12 μg/mL. All scale bars represent 100 μm.
Supplementary Figure S2.
Morphological changes of PC-12 cells leading to different neuronal stages. (A) Three differential stages for neurite outgrowth and neuronal polarization were observed in cultured PC-12 cells; stage changes in neurite outgrowth of PC-12 cells stimulated with NGF, and post-treated with Nogo-A peptide, or a combination of the Nogo-A peptide and the Nogo-A antibody at days 1, 3, 5, and 7. Statistically significant differences were *p < 0.05, **p < 0.01, and ***p < 0.001 in each group.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge the generous support by the Brown Foundation (Project ID: 18130011), the Cullen Trust for Health Care Foundation (Project ID: 18130015), the William Randolph Hearst Foundation (Project ID: 18130017), and the George J. and Angelina P. Kostas Charitable Foundation (Project ID: 18130016).
References
- 1. Center NSCIS. Spinal cord injury facts and figures at a glance. J Spinal Cord Med 2013; 36(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003; 41(7): 369–378. [DOI] [PubMed] [Google Scholar]
- 3. Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996; 76(2): 319–370. [DOI] [PubMed] [Google Scholar]
- 4. Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp 2011; 71(2): 281–299. [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Cooke MJ, Shoichet MS. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol 2012; 30(1): 55–63. [DOI] [PubMed] [Google Scholar]
- 6. Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature 2000; 407(6807): 963–970. [DOI] [PubMed] [Google Scholar]
- 7. Fournier AE, Grandpre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 2001; 409(6818): 341–346. [DOI] [PubMed] [Google Scholar]
- 8. Oertle T, Van Der Haar ME, Bandtlow CE, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci 2003; 23(13): 5393–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pernet V, Schwab M. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res 2012; 349(1): 97–104. [DOI] [PubMed] [Google Scholar]
- 10. Hubli M, Bolliger M, Dietz V. Neuronal dysfunction in chronic spinal cord injury. Spinal Cord 2011; 49(5): 582–587. [DOI] [PubMed] [Google Scholar]
- 11. Wong ST, Henley JR, Kanning KC, et al. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci 2002; 5(12): 1302–1308. [DOI] [PubMed] [Google Scholar]
- 12. Wang KC, Kim JA, Sivasankaran R, et al. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 2002; 420(6911): 74–78. [DOI] [PubMed] [Google Scholar]
- 13. Mi S, Lee X, Shao Z, et al. Lingo-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 2004; 7(3): 221–228. [DOI] [PubMed] [Google Scholar]
- 14. Tsai S-Y, Papadopoulos CM, Schwab ME, et al. Delayed anti-Nogo-A therapy improves function after chronic stroke in adult rats. Stroke 2011; 42(1): 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider MP, Schwab ME, Kessler TM. Anti-Nogo-A antibody: a treatment option for neurogenic lower urinary tract dysfunction? BJU Int 2015; 115: 16–17. [DOI] [PubMed] [Google Scholar]
- 16. Gonzenbach RR, Schwab ME. Disinhibition of neurite growth to repair the injured adult CNS: focusing on Nogo. Cell Mol Life Sci 2008; 65(1): 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubin BP, Spillmann AA, Bandtlow CE, et al. Inhibition of pc12 cell attachment and neurite outgrowth by detergent solubilized CNS myelin proteins. Eur J Neurosci 1995; 7(12): 2524–2529. [DOI] [PubMed] [Google Scholar]
- 18. Brösamle C, Huber AB, Fiedler M, et al. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci 2000; 20(21): 8061–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Douglas Baumann M, Austin JW, Fehlings MG, et al. A quantitative ELISA for bioactive anti-Nogo-A, a promising regenerative molecule for spinal cord injury repair. Methods 2009; 47(2): 104–108. [DOI] [PubMed] [Google Scholar]
- 20. Fan D, De Rosa E, Murphy MB, et al. Mesoporous silicon-PLGA composite microspheres for the double controlled release of biomolecules for orthopedic tissue engineering. Adv Funct Mater 2012; 22(2): 282–293. [Google Scholar]
- 21. Murphy MB, Khaled SM, Fan D, et al. A multifunctional nanostructured platform for localized sustained release of analgesics and antibiotics. Eur J Pain 2011; 5(S2): 423–432. [Google Scholar]
- 22. Minardi S, Pandolfi L, Taraballi F, et al. PLGA-mesoporous silicon microspheres for the in vivo controlled temporospatial delivery of proteins. ACS Appl Mater Interfaces 2015; 7(30): 16364–16373. [DOI] [PubMed] [Google Scholar]
- 23. Yazdi IK, Ziemys A, Evangelopoulos M, et al. Physicochemical properties affect the synthesis, controlled delivery, degradation and pharmacokinetics of inorganic nanoporous materials. Nanomedicine 2015; 10(19): 3057–3075. [DOI] [PubMed] [Google Scholar]
- 24. Martinez JO, Boada C, Yazdi IK, et al. Short and long term, in vitro and in vivo correlations of cellular and tissue responses to mesoporous silicon nanovectors. Small 2013; 9(9–10): 1722–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Govek E-E, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev 2005; 19(1): 1–49. [DOI] [PubMed] [Google Scholar]
- 26. Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol 2014; 27: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong N, Pu J, Zhao H, et al. Knocking-down of Nogo-A gene expression in PC12 cell line by plasmid-based RNAI. J Huazhong Univ Sci Technolog Med Sci 2007; 27(4): 433–436. [DOI] [PubMed] [Google Scholar]