Abstract
The combination of optogenetics with functional magnetic resonance imaging is a promising tool to study the causal relationship between specific neuronal populations and global brain activity. We employed this technique to study the brain response to recruitment of glutamatergic neurons in the mouse hippocampus. The light-sensitive protein channelrhodopsin-2 was expressed in α-CamKII-positive glutamatergic neurons in the left hippocampus (N = 10). Functional magnetic resonance imaging was performed during local laser stimulation, with stimulus duration of 1 second. The hemodynamic response to these stimuli was analyzed on a whole-brain level. In a secondary analysis, we examined the impact of the stimulation locus on the dorso-ventral axis within the hippocampal formation. The hemodynamic response in the mouse hippocampus had an earlier peak and a shorter duration compared to those observed in humans. Photostimulation was associated with significantly increased blood oxygen level-dependent signal in group statistics: bilaterally in the hippocampus, frontal lobe and septum, ipsilaterally in the nucleus accumbens and contralaterally in the striatum. More dorsal position of the laser fiber was associated with a stronger activation in projection regions (insular cortex and striatum). The characterization of brain-region-specific hemodynamic response functions may enable more precise interpretation of future functional magnetic resonance imaging experiments.
Keywords: Functional magnetic resonance imaging, optogenetics, hemodynamics, hippocampus, glutamate
Introduction
The hippocampus (HC) appears to play a fundamental role for large-scale synchronization of brain activity, with hippocampal theta oscillations as a key mechanism.1,2
Altered connectivity between brain regions, in turn, is hypothesized to be a pathophysiological hallmark of neuropsychiatric disorders, in particular schizophrenia3 and autism.4,5
Accordingly, exploring the functional connectivity of the HC is likely of paramount importance for understanding these disorders.
In this study, we probed whether we can use a combination of optogenetics and functional magnetic resonance imaging (opto-fMRI) to study activation of remote brain regions through optogenetics in the scanner and examined the underlying hemodynamic response. Towards this aim, we used recently developed tools for selective excitation of neurons through viral expression of light-activatable opsins (channelrhodopsin-2 [ChR2])6; a technique that can be also applied while imaging rodents in MRI.7 Opto-fMRI provides a promising tool to bridge the gap between cellular and systems neuroscience. This is because optogenetics, on the one hand, allows for neuronal stimulation with high cellular specificity and high temporal resolution. fMRI, on the other hand, can detect responses in all areas of the brain and is translatable to human studies and task-based fMRI.8
In the first opto-fMRI study, Deisseroth and colleagues7 could show that light-driven activation of neurons in the primary motor cortex in rats increased the blood oxygen level-dependent (BOLD) signal either by perisomatic or axonal stimulation. Subsequently, two opto-fMRI studies examined the HC.7,9 Abe and colleagues10 targeted the dentate gyrus (DG) in rats (N = 3) with BOLD increases in the ipsilateral DG around the site of stimulation, in a caudal region of the ipsilateral DG, in the cornu ammonis (CA) and caudate-putamen; yet statistical thresholds were chosen very liberally (p < .01, uncorrected), and group statistics were not performed. A second study in rats compared stimulation of the dorsal versus intermediate HC with a range of different laser frequencies.9 While optogenetic stimulation of the dorsal HC resulted predominantly in activation at the site of stimulation, excitation of the intermediate HC recruited widespread cortical networks. Higher frequencies tended to yield a more widespread and prolonged BOLD activation. Few other opto-fMRI studies exist in mice,11–13 rats14,15 and primates.16,17 Of note, Kahn and colleagues12,13 provided evidence that the BOLD signal correlates closely with neuronal action potential activity (rather than with local field potential, LFP), and that the relationship between BOLD signal and spiking activity is approximately linear. However, another study15 found that upon optogenetic or sensory stimulation, BOLD response correlated better with LFP than with action potentials (multi-unit activity). This study also found that the kinetics of the hemodynamic response were similar between sensory and optogenetic stimulation. Optical intrinsic signal imaging revealed that the relationship between hemodynamics and electrophysiological data is strongly dependent on the duration of the stimulus and that no single hemodynamic response function (HRF) can describe this relationship for all stimulus durations.18
There is also evidence from a recent opto-fMRI study in rats that there can be a mismatch between the BOLD signal and the underlying neural activity: The BOLD signal was spatially more extended and had its maximum in a different cortical layer (layers II/III vs. IV) when compared to the electrophysiological recording.14
In the present study, we used opto-fMRI to study the connectivity of the hippocampal formation in mice (N = 10). We used transgenic animals expressing Cre recombinase under the α-Ca2+-calmodulin-dependent protein kinase II (α-CamKII) promoter to achieve specificity for glutamatergic neurons. The injected adenoviral construct contained the gene coding for the light-sensitive channel (ChR2) in a reverse orientation so that only following recombination via Cre recombinase (expressed specifically in glutamatergic neurons), ChR2 was expressed.
Previous work has shown that even in the absence of Channelrhodopsin, laser administration can cause BOLD signal changes (both negative and positive), and that this effect is most likely due to heating-induced T1 and T2* relaxation changes.19 Therefore, as a control investigation, we studied potential ChR2-independent laser effects on the BOLD signal in wild-type mice, at varying laser powers.
We hypothesized that optogenetic stimulation of the HC would lead to regional BOLD activation within the hippocampal formation, and more distal activation, including cortical, especially prefrontal, areas. Since we used a relatively short stimulus (1 s), our design allowed us to study the HRF in mouse HC. The HRF can be described as the temporal pattern of vascular and blood oxygenation changes (reflected by the BOLD signal) in response to a brief period of neuronal activity. Knowledge about the HRF is crucial for interpretation of fMRI findings. Opto-fMRI provides an exciting opportunity for investigating the HRF, because optogenetic stimulation (as opposed to sensory or electrical stimulation) leads to selective activation of ChR+ glutamatergic neurons.
Surprisingly, little is known about the HRF in non-human species like mice, because most studies investigating the vascular or BOLD response to sensory20–23 or optogenetic7,9–11,13,14 stimulation have used stimuli longer than 10 s, which is long compared to the hemodynamic response and therefore provides only limited information about the HRF.
Methods
Animals
We used heterozygous transgenic male mice (α-CamKII-Cre) expressing Cre recombinase driven by the α-Ca2+-calmodulin-dependent protein kinase II (α-CamKII) promoter (TgCre4).24 The genetic background was C57BL/6NCrl (Charles River, Wilmington, MA). For control experiments, C57BL/6NCrl wild-type mice were used; 2–3 mice were housed per cage and were kept in a standard 12-h light/dark cycle and given food and water ad libitum. All procedures were conducted according to the regulations covering animal experimentation within the European Union (European Communities Council Directive 86/609/EEC) and within the German Animal Welfare Act. The study is reported with the Animals Research: Reporting in Vivo Experiments (ARRIVE) guidelines for research involving animals.25 Experiments were approved by the German animal welfare authorities (Regierungspräsidium Karlsruhe).
To generate cell-type-specific expression of ChR2, we injected a Cre-inducible recombinant AAV vector (rAAV1/2-FLEX-ChR2(H134R):YFP)26 into heterozygous α-CamKII-Cre mice. Cre-dependent recombinant AAV vectors were produced with AAV1/2 coat proteins and purified with heparin columns to a final viral concentration of approx. 1016 genome copies/mL.27 Mice aged >8 weeks were anesthetized with isoflurane; 0.25 µL of purified virus was injected into the left dorsal HC (from bregma: posterior 2.2 mm, lateral 1.5 mm; ventral 1.5 mm from brain surface). In the same surgery session, a mono fiber-optic cannula (Doric Lenses, Québec, Canada) was implanted stereotaxically in the left HC and fixed with dental cement for laser stimulation. The implanted fiber cannula had a fiber core diameter of 300 µm and a numerical aperture of 0.37. Mice recovered for at least 21 days before scanning.
Expression analysis
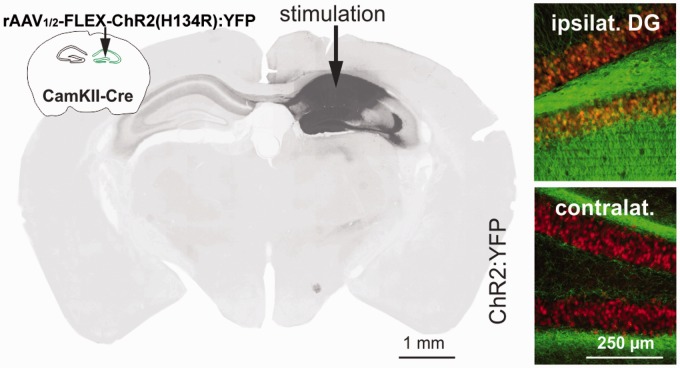
Several days after scanning, mice were given an overdose of isoflurane and perfused transcardially with PBS at 37 ℃ followed by perfusion with 4% PFA and post-fixed in 4% PFA for 12 h at 4 ℃. Sections were stained with a fluorescence Nissl staining kit (Invitrogen). Sections were mounted with Fluoromount (Sigma) and analyzed using a Neurolucida System (Microbrightfield) attached to an epifluorescence microscope (Zeiss Imager, 20× objective). Some of the mice had died after the scanning session and before histology could be performed. Cause of death remained unknown for these animals but since there were several days between scanning and death, it was plausibly unrelated to anesthesia/scanning and did not affect the measurements. Among the five animals from which we obtained expression patterns of ChR2:YFP after scanning, all displayed a similar expression both in the more medial aspects of the CA and throughout the DG, as shown in Figure 1.
Figure 1.
The light-activated protein channelrhodopsin-2 (ChR2) fused to yellow fluorescent protein (YFP) was selectively expressed in α-CamKII-Cre+ neurons in the left hippocampus through unilateral infection with a Cre-dependent AAV (rAAV1/2-FLEX-ChR2(H134R):YFP). Left: The image displays ipsilateral expression of ChR2:YFP in neurons of the cornu ammonis and dentate gyrus and contralateral axonal projections. Right: Ipsilaterally, a neuronal Nissl counterstain (red) visualizes the granule cell layer of the dentate gyrus and ChR2:YFP (green) expression co-localized in somata in the dentate gyrus, while on the contralateral site ChR2:YFP was confined to fibers.
We compared the activation patterns between the animals that could be analyzed histologically and the animals that died before histological analysis could be performed. As can be seen in supplementary Figure 1, the activation patterns were similar between the two groups. A two-sample t-test between the groups yielded no significant voxels at p < .001, minimum cluster size k > 10.
Laser
Transistor-transistor logic (TTL) pulse driven laser pulses were delivered from 300 µm multimode optical fibers (Doric Lenses, Québec, Canada) coupled to a 25 mW, 473 nm, diode-pumped, solid-state laser (Shanghai Laser & Optics Century, Shanghai, China). The laser power was measured with a power meter (Thorlabs, Newton, New Jersey, USA) in a condition in which the laser light was continuously on. For photostimulation during scanning, laser pulses were controlled via 10 kHz TTL modulation from a Master-8 (A.M.P.I., Jerusalem, Israel), that in turn was initially triggered by the first scanner pulse of each Echo Planar Imaging (EPI) sequence.
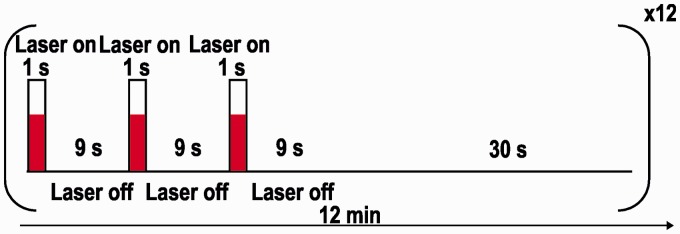
During the measurements, 5 ms light pulses were delivered at 40 Hz for 1 s. This was done three times within 30 s, followed by 30 s without stimulation (see Figure 2).
Figure 2.
Laser stimulation paradigm: 12 burst stimulation sequences, each consisting of three 1-s stimuli with an 9 s interstimulus interval followed by a 30-s rest period. Pulse duration was 5 ms and repetition frequency 40 Hz.
To investigate heating effects, four mice without virus injection were measured each with varying laser powers (Paverage). The time-averaged laser power was increased between the measurements in three steps: 1 mW, 2 mW and 3 mW. These mean power values were calculated by averaging the power delivered during the 1 s stimulation interval. In the main part of the study, we set the laser power to Paverage = 3 mW.
Scanner
A 94/20 Bruker Biospec MRI scanner (9.4 T; Bruker Ettlingen, Germany) with Avance III hardware, BGA12S gradient system with the maximum strength of 705 mT/m and running Paravision 5.1 software was used for all experiments.
Transmission and reception were attained using a linear whole-body volume transmitter coil combined with an anatomically shaped surface receive mouse brain coil (with a hole for the optical fiber).
Scanning procedures
Mean weight at the time of scanning was 25 ± 1 g (range: 24–26 g). To investigate possible heating effects due to the laser power, animals without prior virus injection were scanned in a control experiment (N = 4). In the main study, we investigated 10 α-CamKII:Cre+ mice with hippocampal ChR2-YFP expression.
For preparations, the mice were initially anesthetized with 4% isoflurane (Baxter Deutschland GmbH, Unterschleissheim, Germany) in a mixture of N2 (70 %) and O2 (30 %). The anesthesia was reduced to ∼1.5% isoflurane for positioning in the scanner (head first, prone) and adjustments. During the acquisition of anatomical high-resolution T2 weighted images, a bolus of 0.4 mg/kg medetomidine (Domitor®, Janssen-Cilag, Neuss; 0.07 mg/kg) was applied and the isoflurane stepwise decreased. Ten minutes after the bolus, a continuous dose of 0.8 mg/(kg*h) medetomidine was started and continued throughout the fMRI experiments, which were started 30 min after bolus injection.
To reverse the sedative effect and compensate for the fluid loss during the experiments, Atipamezol (Antisedan®, Janssen-Cilag, Neuss; 1 mg/kg) and ∼0.3 mL of saline were injected subcutaneously after the final experiment.
During all measurements the animal body temperature was measured with a rectal probe and kept at 36 ℃ using warm water circulation pads. Breathing and cardiac rates were monitored using a respiration pad placed beneath the chest (Small Animal Instruments Inc., NY, USA) and a pulse oximeter attached to the tail. Signals were recorded (10 ms resolution) using a signal breakout module (Small Animal Instruments Inc., NY, US).
fMRI acquisitions
In addition to standard localizer images, high-resolution T2-weighted 3D brain images were acquired (TR/TEeff = 1200/50 ms, 192 × 225 matrix, FOV = 15 × 17.5 mm2, 96 slices, 0.156 mm slice thickness) to achieve a robust normalization of the functional data to a digitized mouse brain atlas.28
Functional data were acquired using a T2*-weighted EPI sequence with 720 volumes over 12 min with the following parameters: 17 slices (0.6 mm thickness, 0.1 mm gap), TR/TE = 1 s/16 ms, 96 × 96 matrix, field of view of 25 × 25 mm2. In addition, a B0-fieldmap (TR 20 ms, TE (dual echo) 1.7/5.7 ms, flip angle 20°, 64 × 64 × 64 matrix) was recorded before every EPI sequence to correct for geometrical distortions.
Image data processing
All data was processed using FMRIB Software Library (FSL, version 4.1) (http://www.fmrib.ox.ac.uk/fsl), SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) as well as our own Matlab (http://www.mathworks.com/products/matlab/) scripts. Three-dimen-sional anatomical T2-weighted images were brain-extracted. The functional images were corrected for geometrical distortions using the acquired B0-maps, realigned and coregistered to the brain-extracted T2-weighted anatomical images. Subsequently, a correction for physical noise was conducted using the Aztec29 software availing the RETROICOR30 method with the physiological data sampled during the measurements. Functional data were slice time corrected and normalized to a Paxinos space digital atlas using the normalization parameters of the 3D-structural images.28 In the last preprocessing step, the normalized data were smoothed with a 0.6-mm isotropic Gaussian kernel.
To investigate the accurate HRF, the statistical analysis of the stimulation was first done with a finite impulse response (FIR) basis function (9 s length, order 9). The results of this analysis were used to adjust the canonical HRF basis function for the subsequent general linear model analysis. Movement parameters were used as covariates in all first-level statistics. For group statistics, first-level beta images of each subject were then analyzed in second-level designs (one-sample t-tests and ANOVA). Statistical threshold was set to p < .05 (FWE-corrected) for first level statistics, and p < .001 (uncorrected), voxel size > 10 voxels for group statistics.
For display purposes (see Figure 4g), the average time course over all ‘activated’ voxels was extracted for each animal and then averaged over all mice, using the significant voxels from the group statistics as a mask.
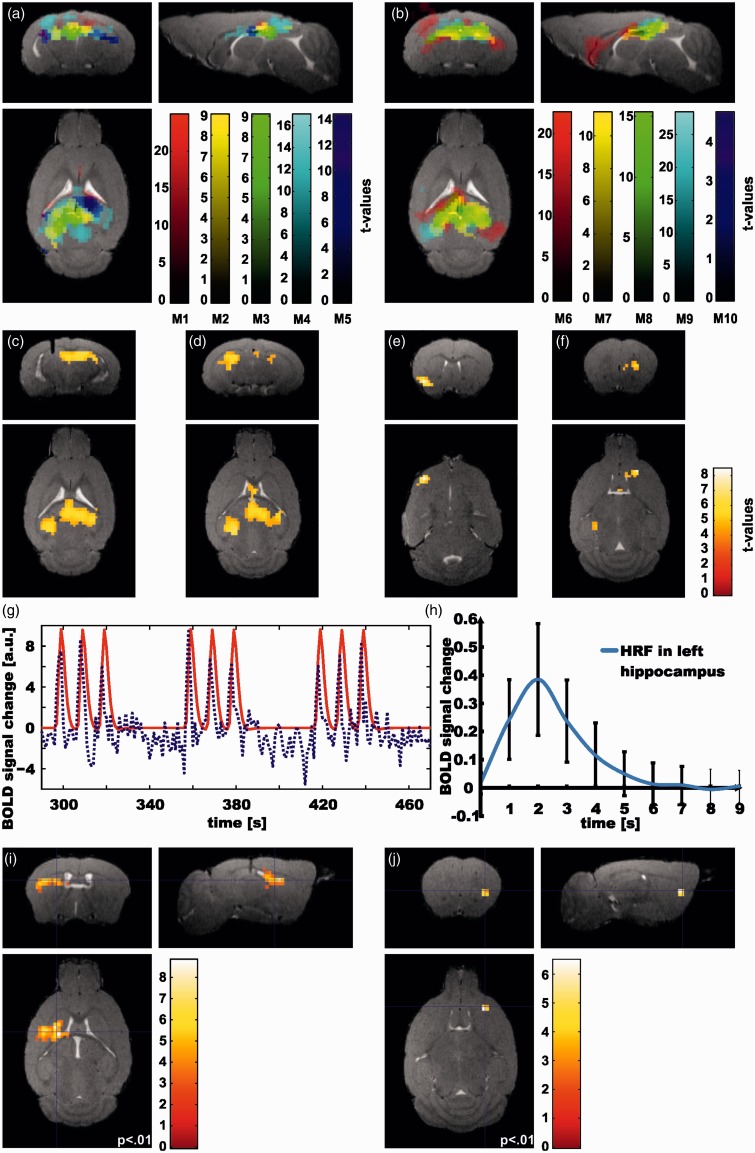
Figure 4.
BOLD responses to optogenetic laser stimulation: (a) First-level statistics for single mice (mouse 1 – mouse 5). All significant voxels are shown (p < .05, FWE corrected), color-coded according to t value. For each mouse, a different color scale is used. (b) 1st level statistics for single mice (mouse 6–mouse 10); otherwise, same display method as in (a). (c–f) Significant BOLD signal change as evidenced by group statistics (N = 10). Here, statistical threshold was set to p < .001, minimum cluster size k = 10 voxels. Coordinates of peak voxel (anterior-right-ventral, in mm): (c) −2.6, 1.1, 1.5; (d) −4.2, −1.8, 2.1; (e) 0.7, −2.8, 4.2; (f) 1.5, 1.6, 2.7. (g) BOLD signal time course averaged over all animals masked by the significant voxels from the group statistics. (h) BOLD response to a single burst stimulation averaged over all onsets and activated voxels in the ipsilateral hippocampus by finite impulse response (FIR) analysis, which corresponds to the hemodynamic response function. (i) Negative correlation of fiber depth and BOLD signal change for whole brain (p < .001, pFWEclust < .005). (j) Negative correlation between fiber depth and BOLD signal change masked with prefrontal lobe (peak voxel significant at p < .05 FWE-corrected). For display the threshold was set to a lower threshold (p < .01).
Histological analyses showed a rather widespread ChR2 expression (Figure 1). Also, the position of the laser fiber in the dorso-ventral axis varied considerably between animals. We therefore performed multiple regression analyses of first level beta images of each subject to study the effect of the fiber position within the hippocampal formation. Therefore, in a secondary analysis, we used the tissue penetration depth of the fiber tip, measured from the dorsal brain surface in the normalized images, as a covariate. We performed the analysis using an inclusive mask of the frontal lobe to test our hypothesis of the frontal response to hippocampal activation. Secondly, the analysis was done without mask to investigate whole-brain effects.
Results
Control experiment
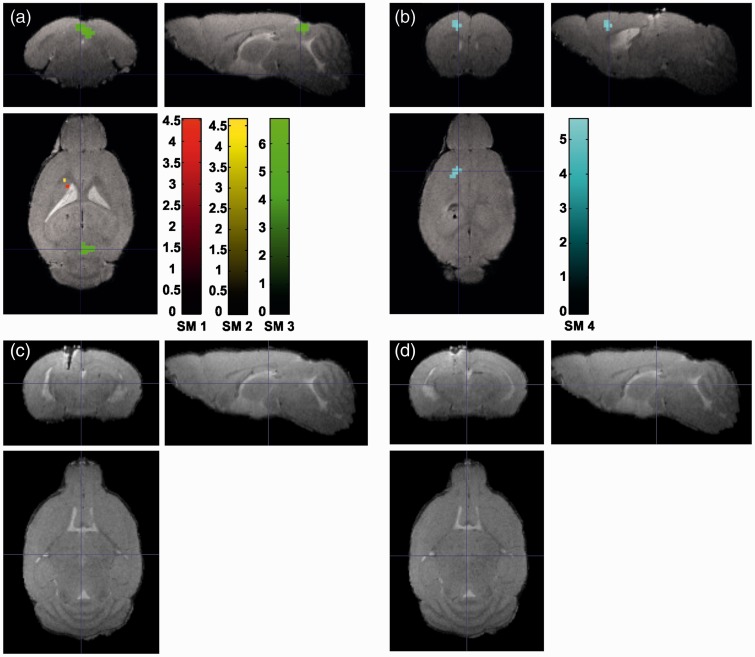
The experiment with wild-type animals without virus injection showed no laser heating effects in the proximity of the fiber tip for all investigated laser powers (p < .05 FWE-corrected). At a laser power of Paverage = 1 mW (Figure 3a), we observed an activation in the superior colliculus in one mouse; two other animals showed single dispersed activated voxels. One animal showed a deactivation in the frontal lobe (Figure 3b). No BOLD signal change was found in sham animals for the higher (2 mW (Figure 3c) and 3 mW (Figure 3d)) laser powers.
Figure 3.
BOLD signal change as a function of laser power in four wild-type mice (SM1, SM2, SM3, SM4) without virus injection. For each mouse a different color scale is used (p < .05 FWE-corrected). (a) Activation and (b) deactivation for 1 mW stimulation laser power. (c) No BOLD signal change for 2 mW and (d) for 3 mW.
BOLD response to laser stimulation in ChR2 animals
The HRF following the 1 s burst stimulation was assessed first in single animals using an FIR basis function as described in the methods section with nine estimators each 1-s long covering the time after the burst. The beta images for each regressor were further analyzed in a second-level ANOVA group statistic (N = 10). Compared to the canonical HRF in humans, the results demonstrate a much shorter response function with a maximum at 2 s after the stimulus onset and return to baseline at 6 s (Humans: maximum at 6 s and undershoot at 16 s, Figure 4h).
For further analysis, we therefore used an adjusted canonical HRF with peak amplitude at 2 s as a basis function.
The individual animals all showed a strong BOLD response in the ipsi- and contralateral HC, while they differed in other regions across the animals (Figure 4a and b).
As shown in Figure 4(c–f), there were four significant clusters at the group level. The range of the first cluster extends in the medial ipsi- and contralateral HC with the maximum of the activation in the contralateral HC. The second is positioned ipsilaterally in the lateral hippocampal regions and ranges to pre-para subiculum and corpus callosum. The smaller clusters are located anteriorly in the ipsilateral frontal lobe and nucleus accumbens and medially in the contralateral frontal lobe, lateral septum and striatum. Time courses of each mouse were extracted from all clusters significant in the group statistics. The mean of these time courses over all animals is shown in Figure 4(g) illustrating the immediate BOLD signal increase occurring with every stimulation onset.
The mean dorsal position of the fiber tip was located –1.47 ± 0.31 mm (mean ± SD) from dorsal brain surface (N = 10). For three mice the fiber tip was located in the CA1 and for three other animals in the DG; in the remaining four mice, it was located between the CA1 region and the upper boarder of DG. In the regression analysis using a frontal lobe mask,28 a cluster in the contralateral insula was seen (p < .01, k = 10, peak voxel pFWE < 0.05; Figure 4j), such that a more dorsal fiber position (stimulating a larger portion of the CA1 region) was associated with stronger BOLD response in this region.
In the whole-brain analysis (i.e. without a-priori mask), we detected a negative correlation between the fiber depth and the BOLD signal change in a cluster in the ipsilateral striatum (and including smaller parts of the corpus callosum and parietal lobe) (p < .001, pFWEclust < .005; Figure 4i).
Discussion
We report about the first opto-fMRI study applying hippocampal stimulation in mice. In response to laser stimulation in the left HC, a robust hippocampal BOLD activation was observed. Activation in other areas was more heterogeneous but some regions (frontal lobe, septum, striatum) were significantly activated in the group analysis.
As a first important result, we noted that the HRF in the mouse HC had a peak around 2 s post stimulation onset, and returned to baseline after around 6 s. This corresponds to the hemodynamic response observed after optogenetic stimulation in the mouse somatosensory cortex12 and is also similar to the BOLD response to a series of short (0.3 ms) electrical forepaw stimulati in rats,31 but much shorter than the response to optogenetic stimulation in the rat somatosensory cortex, although the stimulus duration was also longer in that experiment.15 Interestingly, the timing of the BOLD response seen in this and our own work is in accordance with a more foundational study of vascular diameter changes in response to sensory stimuli:32 arteriole diameter increased with a peak at around 2–3 s after stimulation onset, and the response lasted around 5–6 s in total.
When comparing these different studies, it is important to note the respective type of anesthetic used, since they can have vasodilatory (isoflurane, used by Kahn et al.12) and vasoconstrictive (medetomidine, used in our study) effects. Indeed, a recent study found the anesthetic urethane to significantly decrease the arterial response to vibrissae stimulation.32 In light of these differences, it is noteworthy that the hemodynamics observed in our experiments is so similar to the data by Kahn et al.12 It must be noted as a limitation of our study that we could not directly assess the effect of medetomidine anesthesia on BOLD dynamics.
Overall, our findings are in agreement with previous evidence that the HRF varies between species (with the BOLD response in humans peaking at around 4–5 s and lasting around 10–16 s,33–36 but much faster and shorter responses in rats31 and mice12). It is plausible to speculate that the shorter duration of the HRF is due to the smaller cardiovascular system in mice including a much higher heart rate compared to humans.
Further, while it has been suggested that the HRF may vary between brain regions,35 our data suggest that the HRF is approximately similar in mouse HC (our data) and somatosensory cortex.12
Anatomically, besides the local BOLD response within the HC, projection regions of HC showed activation during stimulation (striatum, frontal cortex, septum and nucleus accumbens). Single animal statistics indicated relatively constant activation in the ipsilateral HC but high variability for more distant regions (Figure 4a–b). A reasonable explanation could be a slight variation in the locus of stimulation, especially since the HC is a highly complex structure with several small subdivisions (see below).
In order to further investigate the effect of the locus of stimulation, we performed a regression analysis using the position of the fiber tip in the dorso-ventral axis as the covariate of interest. We found that more dorsal stimulation (corresponding to stimulation in the CA1) was associated with stronger BOLD activation in the striatum, parietal lobe, insula and corpus callosum. This is biologically plausible since the CA1 provides the main hippocampal output to other brain regions.
An alternative explanation would be that the more dorsal the fiber tip, the more hippocampal neurons are stimulated (since the light propagates through the tissue in a ventral direction), and therefore dorsal illumination causes a more widespread BOLD response.
Our results show reasonable anatomical correspondence to the existing opto-fMRI literature in some respects: both previous studies stimulating the HC9,10 – which were, however, performed in rats – reported BOLD activation in the ipsilateral HC as the most prominent finding. As in our study, this was not restricted to the region directly around the site of stimulation, but extended to other hippocampal areas. In our study, specifically, BOLD activation extended posteriorly to the subiculum and laterally to the CA3 on the group level. Keeping in mind the organization of the HC (flow of information: entorhinal cortex - DG - CA3 - CA1 - subiculum - entorhinal cortex),37 it is plausible that excitation in one hippocampal region should lead to activation in the downstream areas.
Secondly, we also found a contralateral hippocampal BOLD signal. Abe et al.10 found contralateral activation in the HC in one out of three mice, and in a recent study by Weitz and colleagues,9 contralateral BOLD change was dependent on the site of stimulation. The commissure of fornix (also called the hippocampal commissure) may be the anatomical basis of this contralateral activation.
Frontal activation was seen bilaterally, which may reflect the importance of the hippocampal-prefrontal network, which has been well described in rodents38 and has many times been linked to psychiatric disorders both in humans39–41 and in animal models.42
There is some anatomical plausibility also for the BOLD signal increases in the septum and the nucleus accumbens: both regions receive direct projections from the HC via the fornix, which is its main efferent pathway.43
While some activation clusters seen in our study can be explained in anatomical terms, it must be noted that other regions receiving (indirect) input from the HC (e.g. the anterior thalamus) did not show BOLD signal change. Also, as demonstrated previously,9 hippocampal optogenetic stimulation can yield BOLD activation also in regions that have no direct fiber connection to the site of stimulation. Due to the relatively low temporal resolution of BOLD fMRI, we cannot distinguish activations resulting from mono- or polysynaptic pathways.
Several limitations of our study must be noted. Firstly, our control experiment investigating the heating effects included only a small number of animals, and no laser powers higher than the power in the main experiment were used. Another limitation is that we did not have histological analyses for all animals, which means that virus expression in these animals could not be verified. Only ChR2+ animals exhibited similar local BOLD activation; this effect was independent of whether histology was obtained or not from these animals.
Due to the limitations inherent in fMRI technology, we cannot distinguish whether the vascular changes responsible for the BOLD signal change are directly caused by the stimulated glutamatergic neurons or by downstream effects (e.g. activity of innervated excitatory or inhibitory neurons or glial cells). Also, the HRF defined in our study need not correspond to an HRF elicited by peripheral sensory stimulation or, for example, memory tasks, since in each case different neuronal populations may be active.
In conclusion, we could demonstrate that optogenetic stimulation of the mouse HC yields local, regional and also distal BOLD signal increase. In particular, we showed that the anatomical pattern of the BOLD activation depends on the precise locus of stimulation, with more dorsal stimulation (corresponding to the CA1 region) yielding stronger stimulation in distal projection regions (insular cortex and striatum).
Our data help define the HRF in the mouse HC, indicating that it has an earlier peak and a shorter overall duration compared to human data. This is highly relevant for the interpretation of mouse fMRI studies.
Supplementary Material
Acknowledgment
We thank Felix Hörner and Claudia Falfan-Melgoza for their excellent technical assistance.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Bernstein Center for Computational Neurosciences (German Ministry of Education and Research BMBF, 01GQ1003B) to AS and a DFG Emmy-Noether-Grant KE1661/1-1 to WK.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
PL planned and performed the experiments, analyzed data and wrote the manuscript. CCvH analyzed data and wrote manuscript. WW-F planned and performed the experiments, analyzed data and wrote the manuscript. WK manufactured virus, performed surgery and histology and wrote the manuscript. AS planned the experiment, analyzed data and wrote the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull 2008; 34: 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirota A, Montgomery S, Fujisawa S, et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 2008; 60: 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 2009; 35: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 2007; 17: 103–111. [DOI] [PubMed] [Google Scholar]
- 5.Melillo R, Leisman G. Autistic spectrum disorders as functional disconnection syndrome. Rev Neurosci 2009; 20: 111–131. [DOI] [PubMed] [Google Scholar]
- 6.Boyden ES, Zhang F, Bamberg E, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Durand R, Gradinaru V, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 2010; 465: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wentz CT, Oettl LL, Kelsch W. Optogenetics in psychiatric animal models. Cell Tissue Res 2013; 354: 61–68. [DOI] [PubMed] [Google Scholar]
- 9.Weitz AJ, Fang Z, Lee HJ, et al. Optogenetic fMRI reveals distinct, frequency-dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage 2015; 107: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe Y, Sekino M, Terazono Y, et al. Opto-fMRI analysis for exploring the neuronal connectivity of the hippocampal formation in rats. Neurosci Res 2012; 74: 248–255. [DOI] [PubMed] [Google Scholar]
- 11.Desai M, Kahn I, Knoblich U, et al. Mapping brain networks in awake mice using combined optical neural control and fMRI. J Neurophysiol 2011; 105: 1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn I, Desai M, Knoblich U, et al. Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J Neurosci 2011; 31: 15086–15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn I, Knoblich U, Desai M, et al. Optogenetic drive of neocortical pyramidal neurons generates fMRI signals that are correlated with spiking activity. Brain Res 2013; 1511: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, van Zijl P, Thakor N, et al. Study of the spatial correlation between neuronal activity and BOLD fMRI responses evoked by sensory and channelrhodopsin-2 stimulation in the rat somatosensory cortex. J Mol Neurosci 2014; 53: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iordanova B, Vazquez AL, Poplawsky AJ, et al. Neural and hemodynamic responses to optogenetic and sensory stimulation in the rat somatosensory cortex. J Cereb Blood Flow Metab 2015; 35: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerits A, Farivar R, Rosen BR, et al. Optogenetically induced behavioral and functional network changes in primates. Curr Biol 2012; 22: 1722–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohayon S, Grimaldi P, Schweers N, et al. Saccade modulation by optical and electrical stimulation in the macaque frontal eye field. J Neurosci 2013; 33: 16684–16697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji L, Zhou J, Zafar R, et al. Cortical neurovascular coupling driven by stimulation of channelrhodopsin-2. PLoS One 2012; 7: e46607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christie IN, Wells JA, Southern P, et al. fMRI response to blue light delivery in the naive brain: Implications for combined optogenetic fMRI studies. Neuroimage 2013; 66: 634–641. [DOI] [PubMed] [Google Scholar]
- 20.Schroeter A, Schlegel F, Seuwen A, et al. Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage 2014; 94: 372–384. [DOI] [PubMed] [Google Scholar]
- 21.Dalkara T, Irikura K, Huang Z, et al. Cerebrovascular responses under controlled and monitored physiological conditions in the anesthetized mouse. J Cereb Blood Flow Metab 1995; 15: 631–638. [DOI] [PubMed] [Google Scholar]
- 22.Woolsey TA, Rovainen CM, Cox SB, et al. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cerebral Cortex 1996; 6: 647–660. [DOI] [PubMed] [Google Scholar]
- 23.Bosshard SC, Baltes C, Wyss MT, et al. Assessment of brain responses to innocuous and noxious electrical forepaw stimulation in mice using BOLD fMRI. PAIN® 2010; 151: 655–663. [DOI] [PubMed] [Google Scholar]
- 24.Mantamadiotis T, Lemberger T, Bleckmann SC, et al. Disruption of CREB function in brain leads to neurodegeneration. Nat Gen 2002; 31: 47–54. [DOI] [PubMed] [Google Scholar]
- 25.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardin JA, Carlen M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009; 459: 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wieland S, Du D, Oswald MJ, et al. Phasic dopaminergic activity exerts fast control of cholinergic interneuron firing via sequential NMDA, D2, and D1 receptor activation. J Neurosci 2014; 34: 11549–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorr AE, Lerch JP, Spring S, et al. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 2008; 42: 60–69. [DOI] [PubMed] [Google Scholar]
- 29.van Buuren M, Gladwin TE, Zandbelt BB, et al. Cardiorespiratory effects on default-mode network activity as measured with fMRI. Hum Brain Mapp 2009; 30: 3031–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000; 44: 162–167. [DOI] [PubMed] [Google Scholar]
- 31.Hirano Y, Stefanovic B, Silva AC. Spatiotemporal evolution of the functional magnetic resonance imaging response to ultrashort stimuli. J Neurosci 2011; 31: 1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drew PJ, Shih AY, Kleinfeld D. Fluctuating and sensory-induced vasodynamics in rodent cortex extend arteriole capacity. Proc Natl Acad Sci USA 2011; 108: 8473–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp 1997; 5: 329–340. [DOI] [PubMed] [Google Scholar]
- 34.Henson R, Friston K. Convolution models for fMRI. In: Friston K, Ashburner J, Kiebel S, Nichols T, Penny W. (eds). Statistical parametric mapping: The analysis of functional brain images, London: Academic Press, 2007, pp. 178–192. [Google Scholar]
- 35.Rosen BR, Buckner RL, Dale AM. Event-related functional MRI: past, present, and future. Proc Natl Acad Sci USA 1998; 95: 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puckett AM, Mathis JR, DeYoe EA. An investigation of positive and inverted hemodynamic response functions across multiple visual areas. Hum Brain Mapp 2014; 35: 5550–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral D, Witter M. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 1989; 31: 571–591. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz AJ, Gass N, Sartorius A, et al. The low-frequency blood oxygenation level-dependent functional connectivity signature of the hippocampal-prefrontal network in the rat brain. Neuroscience 2013; 228: 243–258. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatr 2005; 62: 379–386. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Shu N, Liu Y, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res 2008; 100: 120–132. [DOI] [PubMed] [Google Scholar]
- 41.Goveas J, Xie C, Wu Z, et al. Neural correlates of the interactive relationship between memory deficits and depressive symptoms in nondemented elderly: resting fMRI study. Behav Brain Res 2011; 219: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigurdsson T, Stark KL, Karayiorgou M, et al. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 2010; 464: 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amaral D, Witter M. Hippocampal formation. In: Paxinos G. (ed). The rat nervous system, San Diego: Academic Press, 1995, pp. 443–493. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.