Abstract
This study investigated the effect of intranasal administration of progesterone on the early brain mitochondrial respiratory chain dysfunction and oxidative damage after transient middle cerebral occlusion in male and female mice. We showed that progesterone (8 mg/kg at 1 h post-middle cerebral occlusion) restored the mitochondrial reduced glutathione pool and the nicotinamide adenine dinucleotide-linked respiration in both sexes. Progesterone also reversed the decrease of the flavin adenine dinucleotide-linked respiration, which was only observed in females. Our findings point to a sex difference in stroke effects on the brain respiratory chain and suggest that the actions of progesterone on mitochondrial function may participate in its neuroprotective properties.
Keywords: Mitochondria, oxidative phosphorylation, oxidative stress, progesterone, sex difference
Introduction
Neuroprotective approaches by progesterone are a promising way to reduce lesion volume and improve functional outcome after stroke. Progesterone has pleiotropic effects, and some targets have already been identified such as inflammation, edema or apoptotic gene expression.1 We postulated that the mitochondrial respiratory chain (RC) and oxidative stress could be targets of progesterone or its metabolites and of their neuroprotective effects after cerebral ischemia. Indeed, RC impairments are centrally involved in ischemia-induced cell death. The large reduction of blood flow during ischemia limits the availability of glucose and oxygen, which reduces the activity of the oxidative phosphorylation system (OXPHOS) and leads to a lack of adenosine triphosphate (ATP). Moreover, as a principal generator of reactive oxygen species (ROS), RC largely participates in the reperfusion-induced injury.2 Progesterone has been shown to enhance brain mitochondrial energy metabolism and to decrease oxidative stress in ovariectomized rats3 and reversed the decrease of mitochondrial respiration rate following traumatic brain injury (TBI).4
Increasing evidence indicates that sex influences not only the incidence but also the physiopathological mechanisms of stroke.5 Therefore, taking the sex effect into account in experimental studies is essential, especially as sex-dependent differences have been described in brain mitochondrial function.6
The aim of this study was to evaluate the effect of intranasal progesterone administration on mitochondrial RC function and oxidative stress after a transient focal cerebral ischemia in males and females.
Materials and methods
Animals and middle cerebral occlusion
Three-month-old female and male C57BL6J mice (Janvier, le Genest-Saint-Isle, France) were housed in a temperature-controlled room on a 12-h light, 12-h dark cycle. The estrus cycle was assayed by vaginal smear examination over 10 days to check cycling regularity. The classification was proestrus, estrus, metestrus and diestrus. Females in the diestrus stage on the day of the experiment were selected.
One-hour middle cerebral occlusion (MCAO) followed by reperfusion was realized according to Frechou et al.7 The criterion of exclusion was a decrease of blood flow less than 50%. On this basis, no animals were excluded. Sham-operated mice underwent the same surgical procedure except that no filament was inserted. Experimenters were blinded to the experimental conditions of mice. Mice were randomly and blindly assigned to either progesterone (8 mg/kg in oleogel), or vehicle-treated (oleogel) groups (n = 5/group). Administrations were performed intranasally immediately on reperfusion. This formulation of progesterone is efficient to rapidly deliver progesterone to blood and brain (13 ± 2.5% and 9.5 ± 0.9% of the initial dose respectively after 10 min of administration), and the reached brain levels did not vary significantly over time (2–120 min).8
Ethical statement
Procedures concerning animal care and use were carried out in accordance with national guidelines (authorization 94-345 to R.G., animal facility approval 94-043-13), French ethical laws (Act 87-848 and Act 2013-11) and European Communities Council Directive (86/609/EEC); and have been approved by the ethical committee of French Ministry of Higher Education and Research C2EA-26 (project 2014_029). All experiments were performed following the ARRIVE guidelines (www.nc3rs.org.uk).
Isolation of mitochondria-enriched fraction
Mice were decapitated 6 h after MCAO (i.e. 5 h after reperfusion), and the forebrain minus the cerebellum was rapidly dissected on ice. The mitochondria-enriched fraction was immediately isolated from the freshly dissected ischemic (ipsilateral) and contralateral hemispheres.6 Based on the cytochrome c oxidase (also called complex IV, CIV) activity pelleted in the mitochondrial fraction, the yield was 46% ± 7% of total mitochondria. Only 1.9 ± 0.3% of the total activity of CIV in presence of 2.5 mM laurylmaltoside was measured in the absence of this detergent, indicating that the mitochondrial outer membrane was intact. The purity of the mitochondrial fraction (75 ± 3%) was evaluated by the percentage of a cytosolic enzyme activity (the lactate dehydrogenase, LDH) in the mitochondrial pellet fraction. The purity and yield of mitochondria isolation were consistent between contralateral and ischemic hemispheres (T test, p > 0.05) and similar in all individual mouse preparations (ANOVA, p > 0.05).
Mitochondrial oxygen consumption
Mitochondrial oxygen consumption was measured polarographically in the freshly prepared mitochondria-enriched fraction.6 The rate of oxygen consumption was calculated from the slope of the response of mitochondria to the successive administration of substrates and expressed as nmol O2/min/mg protein.
Enzymatic activities
RC complexes (CI, CII, CIII and CIV), LDH, isocitrate dehydrogenase (IDH), α-ketoglutarate dehydrogenase (AKGDH), malate dehydrogenase (MDH) and pyruvate dehydrogenase complex (PDHc) activities were spectrophotometrically measured at 37℃.6 All enzymatic activities were expressed as nmol/min/mg protein.
RC complexes quantity and integrity
Blue-Native polyacrylamide gel electrophoresis followed by Western-Blot analysis was performed on a part of mitochondria-enriched fraction.9 Solubilized OXPHOS (5 µg) proteins were loaded on a 4–16% gradient acrylamide non-denaturing gel (Invitrogen, CA, USA). Immunoblotting was performed with monoclonal antibodies (Abcam, MA, USA) against CI (subunit GRIM19, 1:1200), CII (subunit SDHA, 1:2400), CIII (subunit UQCRC2, 1: 3000) or CIV (subunit MT-CO1, 1:2000). The secondary antibody was a peroxidase-conjugated anti-mouse immunoglobulin G (IgG) (1:4000, Sigma, MO, USA). Signals were detected using an Amersham ECL plus revelation kit (GE Healthcare, NJ, USA) with a G:BOX Chemi system (Syngene, MD, USA). Quantification was performed using ImageJ software (NIH, MD, USA).
Oxidative stress markers
Reduced glutathione (GSH) and oxidized glutathione (GSSG) concentrations were measured by reverse phase HPLC coupled with electrochemical detection.6 Limits of quantification were 0.6 μM for GSH and 0.9 μM for GSSG. Activities of the mitochondrial tricarboxylic acid (TCA) cycle enzymes aconitase and fumarase were spectrophotometrically measured, and the ratio of the activities of aconitase to fumarase was calculated.6 A decrease of the aconitase to fumarase activity ratio is a functional indicator of ROS production: the iron–sulfur cluster in the catalytic site of aconitase renders it very susceptible to inactivation by ROS whereas the activity of fumarase is ROS-insensitive.
Statistical analysis
For each analysis, the ratio between the ipsilateral and the contralateral hemisphere was calculated. Data were expressed as mean ± SEM and were analyzed by a commercially available program (GraphPad Prism 4.01, Graphpad Inc., CA, USA). One-way ANOVA analysis followed by Tukey post-hoc test was performed, and p values < 0.05 were considered statistically significant.
Results
Mitochondrial oxidative stress
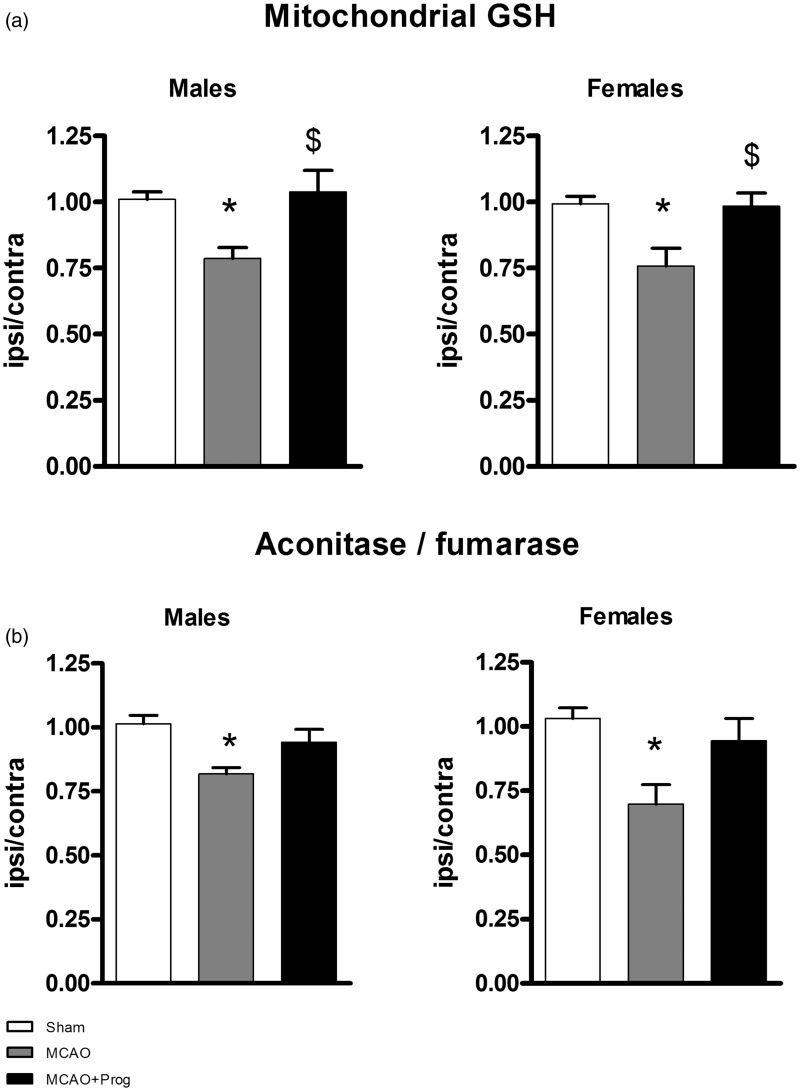
Ischemia reduced the GSH mitochondrial pool in males and females (respectively−23%, p < 0.05 and−24%, p < 0.05 versus Sham) and progesterone treatment restored it ( + 23%, p < 0.05 in males and + 22%, p < 0.05 in females versus MCAO) (Figure 1(a)). Mitochondrial GSSG was below the limit of quantification for all samples. Ischemia-induced oxidative damages in males and females as shown by a decrease of the aconitase to fumarase activities ratio (respectively −19%, p < 0.05 in males and −34%, p < 0.05 in females versus Sham). Progesterone did not significantly re-establish a normal ratio ( + 13%, p = 0.09 in males and + 25%, p = 0.07 in females versus MCAO) (Figure 1(b)).
Figure 1.
Brain mitochondrial oxidative stress markers in three-month-old male and female mice: effect of 1-h MCAO and intranasal progesterone administration. (a) Mitochondrial reduced glutathione (GSH) concentration and (b) aconitase to fumarase activity ratio. Mitochondria were isolated 6 h after transient MCAO (i.e. 5 h after reperfusion), from ischemic and contralateral hemispheres. The experimental groups were: Sham operation (Sham), MCAO + placebo administration (MCAO), and MCAO + progesterone administration (MCAO + Prog).
Results are expressed in (a) as nmol/mg protein (GSH) and in (b) as a ratio of aconitase to fumarase activity and the ratio between ischemic to contralateral hemispheres were calculated for each animal. Data represent the mean ± SEM of the ratio ipsilateral/contralateral (n = 5 animals per group).
*Sham versus MCAO (*p < 0.05); $ MCAO versus MCAO+Prog ($p < 0.05).
Mitochondrial respiration
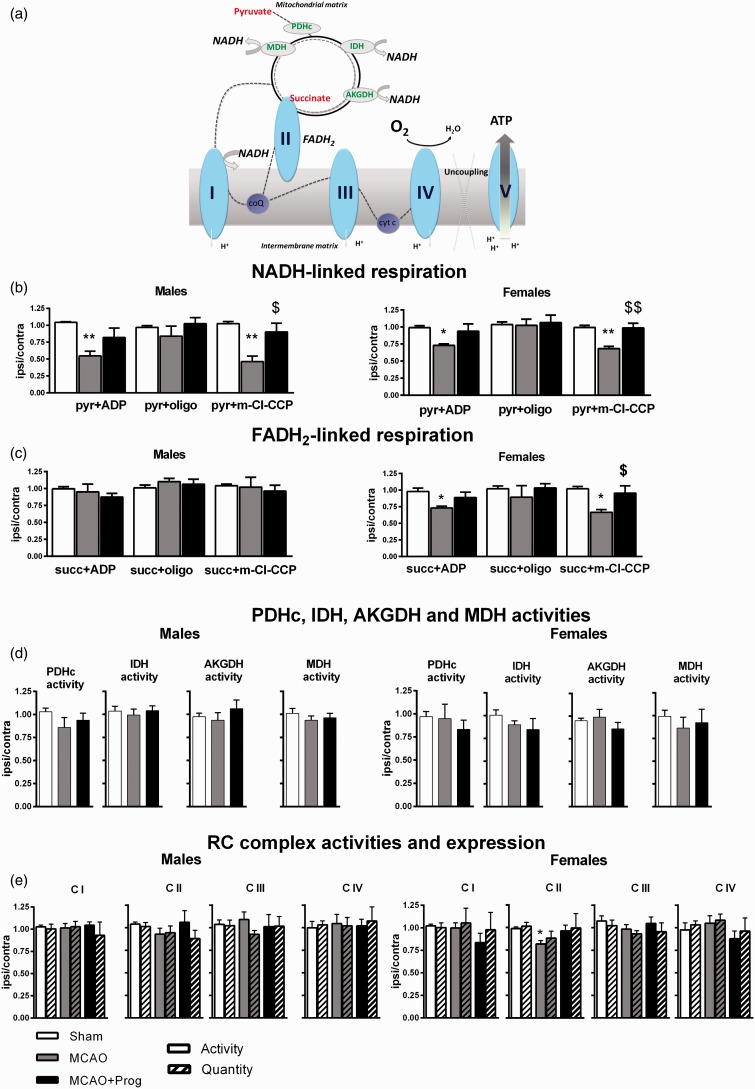
Mitochondrial oxygen consumptions using pyruvate (nicotinamide adenine dinucleotide(NADH)-linked respiration) and succinate (flavin adenine dinucleotide (FADH2)-linked respiration) were measured with adenosine diphosphate (ADP) (stimulation), oligomycin (ATP-synthase inhibitor) and m-chlorophenylhydrazone (m-Cl-CCP, uncoupler) (Figure 2(a)).
Figure 2.
Brain oxidative phosphorylation system function in three-month-old male and female mice: effect of 1-h MCAO and intranasal progesterone administration. (a) NADH-linked stimulated respiration was measured by addition of pyruvate-malate + ADP. The pyr + ADP oxidation rate depends on the activities of PDHc, TCA cycle enzymes, CI, CIII and CIV and on the phosphorylating (Continued) Figure 2. Continued. apparatus efficiency. The ATP synthase (or complex V) was then inhibited by the addition of oligomycin, to evaluate proton leakage (pyr + oligo). Uncoupled respiration was measured by the addition of m-chlorophenylhydrazone (pyr + m-Cl-CCP). FADH2-linked stimulated respiration was measured by addition of succinate in presence of ATP, rotenone (CI inhibitor) and ADP. The succ + ADP oxidation rate depends on the activities of CII, CIII and CIV and of the phosphorylating apparatus efficiency. The ATP synthase was then inhibited (succ + oligo) and m-Cl-CCP was added (succ + m-Cl-CCP). (b) Oxygen consumption rates in presence of pyruvate (NADH-linked respiration), (c) Oxygen consumption rates in presence of succinate (FADH2-linked respiration), (d) PDHc, IDH, AKGDH and MDH activities and (e) RC complexes activities and expression. Mitochondria were isolated 6 h after transient MCAO (i.e. 5 h after reperfusion), from ischemic and contralateral hemispheres. The experimental groups were: Sham operation (Sham), MCAO + placebo administration (MCAO) and MCAO + progesterone administration (MCAO + Prog).
Results are expressed as nmol O2/min/mg protein and the ratio between ischemic to contralateral hemispheres was calculated for each animal. Data represent the mean ± SEM of the ratio ipsilateral/contralateral (n = 5 animals per group).
* Sham versus MCAO (* p < 0.05, ** p < 0.01); $ MCAO versus MCAO + Prog ($p < 0.05, $$ p < 0.01).
MDH: malate dehydrogenase; PHDc: pyruvate dehydrogenase complex; IDH: isocitrate dehydrogenase; AKGDH: α-ketoglutarate dehydrogenase; ATP: adenosine triphosphate; FADH: Flavin adenine dinucleotide; NADH: nicotinamide adenine dinucleotide.
NADH-linked respiration
Pyr + ADP and pyr + m-Cl-CCP oxidation rates were significantly decreased after ischemia in males (−45%, p < 0.01 and −54%, p < 0.01 versus Sham) and in females (−27%, p < 0.05 and −32%, p < 0.01 versus Sham). Pyr + oligo oxidation rates were not affected by ischemia, indicating that proton leak was not modified. Progesterone administration after MCAO significantly increased pyr + m-Cl-CCP oxidation rate ( + 44%, p < 0.05 in males; + 30% in females p < 0.01 versus MCAO) (Figure 2(b)).
FADH2-linked respiration
Succinate oxidation rates were not modified by ischemia and by progesterone treatment in males. By contrast, succ + ADP and succ + m-Cl-CCP oxidation rates decreased after MCAO in females (−25%, p < 0.05 and −36%, p < 0.05 versus Sham). Progesterone restored succ + m-Cl-CCP oxidation rate ( + 29%, p < 0.05 versus MCAO) (Figure 2(c)).
PDHc and TCA cycle enzymes
PDHc activity, the first step of pyruvate oxidation, was not significantly modified by ischemia or by progesterone administration. No significant effects were observed for IDH, AKGDH and MDH activities, the TCA cycle enzymes involved in NADH/NAD recycling (Figure 2(d)).
RC complexes
In males, neither activity nor quantity and integrity of the CI, CII, CIII and CIV were modified by ischemia and by progesterone treatment. In females, the CII activity decreased after MCAO (−17%, p < 0.05 versus Sham), without change in CII quantity and integrity. Progesterone treatment did not significantly restore C II activity ( + 15% versus MCAO, p = 0.09) (Figure 2(e)).
Discussion
This is the first study reporting the effect of progesterone treatment on mitochondrial respiration and oxidative stress after stroke. Intranasal administration was used because of its effective and easy way of administration, compatible with an emergency exigency.7
GSH is the major anti-oxidant factor in brain. The mitochondrial pool of GSH is crucial for neuronal survival, and is selectively decreased after ischemia.10 We demonstrated that progesterone significantly restores the mitochondrial GSH pool 6 h after a transient ischemia in both males and females. This restoration could contribute to limit oxidative damages, and consequently may increase neuronal survival.
Progesterone restores NADH-linked respiration in males and in females. Only the uncoupled respiration (pyr + m-Cl-CCP) was enhanced by progesterone, indicating that progesterone acts on the oxidative capacity of the RC rather than on the phosphorylating apparatus. The exploration of the activity of the enzymes involved in pyruvate oxidation and NADH/NAD recycling showed no effect of stroke or progesterone. Western-Blot analysis in non-denaturing conditions excluded a modification of the integrity and the quantity of the RC complexes. Taken together, our findings suggest that ischemia-induced oxidative stress may alter respirasome (supercomplexes I-III-IV) assembly and that progesterone administration preserves it. Indeed, NADH-linked electron flux efficacy also depends on the respirasome organization. In addition, it has been proposed that this organization is altered by oxidative stress.11 Indeed, in heart ischemia, a decrease of the respiration was associated with a modification of the respirasome assembly without change of enzymatic activities.12 The anti-oxidant effect of progesterone could protect mitochondrial membrane from lipid peroxidation, as already described for cell membrane lipid peroxidation,13 and preserve respirasome assembly after stroke.11 It has also been suggested in a model of TBI that anti-oxidant progesterone properties is a potential mechanism to explain the influence of progesterone on mitochondrial respiration.4
Our study also points to a sex difference in the effects of stroke on mitochondrial respiration. The FADH2-linked stimulated and uncoupled respirations were decreased specifically in females, and this decrease can be attributed to a downregulation of the CII activity. In some tissues, the activity of CII has been shown to be increased by the deacetylase NAD-dependent Sirtuin 3 and the Fgr-kinase; and both systems can be stimulated by oxidative stress.14–16 Nevertheless, most of the studies were performed in males. We could postulate that a sex difference in the activities of Sirtuin 3 or Fgr-kinase after ischemia could be involved in the observed dimorphic effect. Interestingly, an interaction between Sirtuin 3 and the poly-ADP-ribose polymerase pathway (PARP-1) was described in cortical neuron injury.14 PARP-1 is also a NAD-dependent enzyme and its activation effect showed a well-described sex difference in stroke.5 In addition, we showed that progesterone fully rescues the uncoupled FADH2-linked respiration. The precise mechanism remains to be determined, as progesterone has only a slight and statistically not significant effect on CII activity. Effects of progesterone on other targets (the quinone pool, assembly of the super-complex III2-IV1-2) could be potential mechanisms.
The beneficial effects of post-ischemia progesterone administration were most exclusively demonstrated in males. Except one study which showed that progesterone acts differently on lesion volume and neurological score in ageing and ovariectomized females.17 We demonstrated a selective effect of ischemia and progesterone treatment on FADH2-linked respiration. This observation may contribute to explaining sex differences in stroke outcome. Further studies are necessary to determine the exact impact of hormonal status on this sex difference.
In conclusion, our findings indicate that mitochondria constitute an essential target for the neuroprotective effect of progesterone and emphasize the necessity to include both males and females in experimental models of stroke.
Acknowledgements
We thank F. Rousseau and Y. Sandre for their technical help. We thank Dr. Krzysztof Rajowski for critical reading and English editing of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Inserm and the Mattern Foundation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
AS and RG contributed equally to this work. PG: conception and design, experimental procedures, analysis and interpretation of the results, writing the article. MF: experimental procedures, analysis and interpretation of the results, critical revision of the article. MS: obtained funding, analysis and interpretation of the results, critical revision of the article. PT: obtained funding, analysis and interpretation of the results, critical revision of the article. CM: conception and design, experimental procedures, critical revision of the article. AS: conception and design, analysis and interpretation of the results, critical revision of the article. Overall responsibility. RG: conception and design, analysis and interpretation of the results, critical revision of the article. Overall responsibility.
References
- 1.Gibson CL, Coomber B, Rathbone J. Is progesterone a candidate neuroprotective factor for treatment following ischemic stroke? Neuroscientist 2009; 15: 324–332. [DOI] [PubMed] [Google Scholar]
- 2.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 2010; 1802: 80–91. [DOI] [PubMed] [Google Scholar]
- 3.Irwin RW, Yao J, Hamilton RT, et al. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 2008; 149: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson CL, Puskar A, Hoffman GE, et al. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol 2006; 197: 235–243. [DOI] [PubMed] [Google Scholar]
- 5.McCullough LD, Zeng Z, Blizzard KK, et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 2005; 25: 502–512. [DOI] [PubMed] [Google Scholar]
- 6.Gaignard P, Savouroux S, Liere P, et al. Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 2015; 156: 2893–2904. [DOI] [PubMed] [Google Scholar]
- 7.Frechou M, Zhang S, Liere P, et al. Intranasal delivery of progesterone after transient ischemic stroke decreases mortality and provides neuroprotection. Neuropharmacology 2015; 97: 394–403. [DOI] [PubMed] [Google Scholar]
- 8.Ducharme N, Banks Wa, Morley JE, et al. Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration. Eur J Pharmacol 2010; 641: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wittig I, Braun H-P, Schägger H. Blue native PAGE. Nat Protoc 2006; 1: 418–428. [DOI] [PubMed] [Google Scholar]
- 10.Anderson MF, Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem 2002; 81: 541–549. [DOI] [PubMed] [Google Scholar]
- 11.Lenaz G, Baracca A, Barbero G, et al. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta 2010; 1797: 633–640. [DOI] [PubMed] [Google Scholar]
- 12.Rosca MG, Vazquez EJ, Kerner J, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 2008; 80: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal R, Medhi B, Pathak A, et al. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J Pharm Pharmacol 2008; 60: 731–737. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Lu HF, Alano CC. Neuronal sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS One 2011; 6: e14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acín-Pérez R, Carrascoso I, Baixauli F, et al. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab 2014; 19: 1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimen H, Han MJ, Yang Y, et al. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 2010; 49: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain 2011; 134: 2125–2133. [DOI] [PubMed] [Google Scholar]