Abstract
Nanotechnology could provide a new complementary approach to treat coronary artery disease (CAD) which is now one of the biggest killers in the Western world. The course of events, which leads to atherosclerosis and CAD, involves many biological factors and cellular disease processes which may be mitigated by therapeutic methods enhanced by nanotechnology. Nanoparticles can provide a variety of delivery systems for cargoes such as drugs and genes that can address many problems within the arteries. In order to improve the performance of current stents, nanotechnology provides different nanomaterial coatings, in addition to controlled-release nanocarriers, to prevent in-stent restenosis. Nanotechnology can increase the efficiency of drugs, improve local and systematic delivery to atherosclerotic plaques and reduce the inflammatory or angiogenic response after intravascular intervention. Nanocarriers have potential for delivery of imaging and diagnostic agents to precisely targeted destinations. This review paper will cover the current applications and future outlook of nanotechnology, as well as the main diagnostic methods, in the treatment of CAD.
Keywords: : atherosclerosis, coronary artery disease, nanocarriers, nanotechnology, restenosis, stent coatings, vulnerable plaque
Coronary artery disease (CAD) describes a disease process in which atherosclerotic plaque accumulates in the lining of coronary artery, producing a narrowing of the lumen of the artery, reducing the compliance of the vessel wall and gradually or suddenly causing a loss of blood supply to a portion of the myocardium [1]. Angina or chest pain that can occur either after exercise or even at rest results, when blood supply to the heart muscle is seriously restricted. Another major problem occurs when a coronary thrombosis suddenly blocks the blood supply to the heart, precipitating a heart attack or a myocardial infarction. The commonest cause of this sudden thrombosis is the rupture of an atherosclerotic plaque in a coronary artery [2]. The plaques that are prone to rupture have certain characteristics which are described as ‘vulnerable’. These characteristics include the presence of a thin collagen cap, a lipid-rich interior, a high metabolic rate, many activated macrophages, a high degree of inflammation, a necrotic core resulting from macrophage apoptosis and a content rich in tissue factor that precipitates the actual thrombosis [3]. Recent reviews have widened the concept of vulnerability to heart attack to include vulnerable blood (prone to thrombosis) and vulnerable myocardium (prone to fatal arrhythmia). Therefore, the term ‘vulnerable patient’ may be more appropriate to describe the high likelihood of developing cardiac events in the near future [4].
Atherosclerosis is a thickening of the arterial vessel wall that becomes inflamed due to atheromatous plaque formation [5]. The propagation of the lesions in atherosclerosis leads to angiogenesis or the formation of new blood vessels within the artery wall similar to that seen in growth of cancerous tumors. Increased metabolic activity of the growing plaque requires a higher supply of nutrients and oxygen to the underlying proliferating cells. To satisfy this nutritional need, endothelial cells rapidly proliferate and form atypical blood vessels that are defective and immature. This state alters the dynamics of macromolecular transport to and from the lesion, and is responsible for the enhanced permeability and retention (EPR) effect that allows accumulation of systemically delivered macromolecules [6]. Furthermore, tissue inflammation causes constant leukocyte recruitment through the release of proinflammatory cytokines. As leukocytes traverse through the endothelium into the extravascular space, the transport of circulating nanomaterials is facilitated by the leaky endothelial cell-to-cell junctions. This condition increases endothelial permeability and allows for selective delivery of therapeutic nanocarriers in the inflamed area [7].
Restenosis occurs after balloon angioplasty has been employed to widen the arterial lumen, where the vessel closes upon itself again, caused by a different mechanism than the original atherosclerosis [1]. This pathological state caused by balloon angioplasty has been attributed to three different processes: the elastic response that occurs after the overstretching of the vessel, neointimal formation and chronic remodeling [8].
Current therapies for atherosclerosis focus on lessening the burden of atherosclerotic plaque, stabilizing vulnerable plaques defined as those plaques likely to rupture and cause thrombosis [9]. To reduce the risk of in-stent thrombosis and/or restenosis caused by bare metal stents, new types of stents were designed that function as drug-eluting stents (DES). A wide range of dissimilar classes of drugs have been tested with DES in order to prevent smooth muscle cell (SMC) growth and proliferation including anticancer and anti-inflammatory agents. Another approach is the modulation of gene expression with plasmid DNA or RNA interference to generate an imbalance in local concentrations of specific signaling molecules that can inhibit the growth of certain cells, while promoting the growth of others [1].
Therapeutic agents
Drugs that can be used to combat restenosis can be classified into four groups: anti-inflammatory, antithrombogenic, antiproliferative and immunosuppressive. Some common agents that have been loaded onto DES to inhibit restenosis are:
Sirolimus/rapamycin: these are potent immunosuppressive drugs that can also prevent migration of SMCs;
Paclitaxel: cytotoxic drug that causes inhibition of SMCs migration and proliferation;
Zotarolimus and Everolimus: bind to cytosolic FK-506-binding protein-12 and inhibit the proliferation of SMCs and T-cells;
Tacrolimus: an immunosuppressive agent;
Actinomycin D: an inhibitor of cellular proliferation like paclitaxel;
Dexamethasone: the corticosteroids are well established as anti-inflammatory drugs [10].
Several clinical trials have tested oral anti-inflammatory or immunosuppressive methods to prevent in-stent restenosis (ISR), and these studies have shown a significant decrease of angiographic ISR [11,12].
Furthermore, different specific molecular targets have also been used in treatment of restenosis. For instance, examination of the effect of PDGF receptor specific inhibitors, tyrphostin, AG1295 and AGL-2043 on neointimal formation revealed significant inhibition of restenosis [13]. Some of the important studies including in vivo studies with various drugs are reported in Table 1. Additionally, Table 2 looks into couple of clinical trials which are observed related in order to investigate physiological factors such as neointima thickness and stenosis diameter.
Table 1. . Drugs that have been tested for restenosis treatment in vivo/clinical trials.
| Drug/gene | Animal | Region of treatment | Internal elastic lamina (luminal area; mm2) | Endotheliaziation (%) | Neointima | Stenosis (%) | Inhibition of proliferation (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Imatinib mesylate |
Pig |
Coronary artery |
– |
Not affected |
50% |
– |
– |
[14] |
|
| Paclitaxel | Low dose 0.2 μg/mm2 | Pig | – | 5.331 ± 0.717 | 99.524 ± 1.289 | 1.432 ± 0.632 (mm2) | 27.648 ± 12.980 | – | [15] |
| Medium dose 0.4 μg/mm2 | Pig | – | 5.560 ± 0.780 | 99.381 ± 1.024 | 1.845 ± 0.950 (mm2) | 33.052 ± 16.428 | – | ||
| |
High dose† (0.7 μg/mm2) |
Pig |
– |
7.305 ± 0.912 |
86.708 ± 16.956 |
2.930 ± 1.144 (mm2) |
40.143 ± 15.547 |
– |
|
| Sirolimus (rapamycin) | Pig | Porcine aortic endothelial cells | – | – | – | – | 100 | [16] | |
| |
|
|
Porcine vascular smooth muscle cells |
– |
– |
– |
– |
31 |
|
| TRM-484 | Control | Rabbit | – | – | – | – | 29.5 ± 8.1 | – | [17] |
| |
1 mg/kg |
Rabbit |
– |
– |
– |
– |
22.5 ± 4.4 |
– |
|
| Adinoviral vectors containing human inducible nitric oxide synthase | Rat | Carotid | – | – | 0.248 ± 0.028 | 17.54 ± 1.8 | – | [18] | |
†Cobalt chromium stents coated with a poly-lactide and poly lactide-co-glycolide biodegradable polymer and high dose.
Table 2. . Relative physiological factors in coronary artery disease observed by optical coherence tomography in clinical therapies before and after applying drug-eluting stents (human studies).
| Drug | Region of treatment | Age (years) | Total patients (n) | Minimal lumen diameter (mm) | Diameter stenosis (%) | Neointima thickness | Evaluation method | Ref. |
|---|---|---|---|---|---|---|---|---|
| Silrolimus | Coronary artery | 63 ± 11 | 26 | In-sten: 2.22 | In-stent: 15.18 | 57.1 (μm) | OCT | [19] |
| |
|
|
|
In-segment: 1.95 |
In-segment: 22.90 |
|
|
|
| Biolimus | Coronary artery | 64.9 ± 10 | 20 | In-stent: 2.40 | In-stent: 13.13 | 67.6 (μm) | OCT | [19] |
| |
|
|
|
In-segment: 2.06 |
In-segment: 23.24 |
|
|
|
| Sirolimus |
Coronary artery |
66 ± 10 |
34 |
– |
– |
52.5 (μm) |
OCT |
[20] |
| Sirolimous |
Coronary artery |
67 ± 10 |
46 |
(Follow-up) 2.03 ± 0.42 |
(Follow-up) 23.5 ± 10.6 |
77 ± 53 (μm) |
OCT |
[21] |
| Paclitaxel | Coronary artery | 67 ± 10 | 37 | (Follow-up) 2.00 ± 0.53 | (Follow-up) 22.8 ± 15.7 | 201 ± 136 (μm) | OCT | [21] |
OCT: Optical coherence tomography.
Adhesion, activation and accumulation of platelets in injured position is one of the key factors in atherothrombosis [22]. Currently used antiplatelet agents include cyclooxygenase-1 inhibitors (aspirin, indobufen, triflusal), P2Y12 inhibitors (prasugrel and ticagrelor [23], clopidogrel, cangrelor), phosphodiesterase inhibitors (dipyridamole, cilostazol), glycoprotein (GP) IIb/IIIa blockers (abciximab, etifibatide, tirofiban), thromboxane receptor and thrombin receptor (PAR-1) antagonists [22].
Aspirin is responsible for interrupting the production of thromboxane A2, one of the influential agents on platelet activation, by blockage of cyclooxygenase-1 enzyme [24]. GP IIb/IIIa blockers attach to GP IIb/IIIa receptors instead of fibrinogen and Von Willebrand factor at the last stage of platelet accumulation and are considered as fast and effective antiplatelet agents [24]. P2Y12-receptor inhibitors are activated via a hepatic metabolism to joint the adenosine diphosphate-binding positions on P2Y12-receptor which are in charge of inducing signaling cascades in platelet accumulation process [23].
Dual antiplatelet therapy (DAPT) is the simultaneous application of aspirin and a P2Y12-receptor inhibitor [25] (e.g., ticlopidine, clopidogrel, prasugrel, ticagrelor [26]). After myocardial infraction, all the patients should follow a DAPT prescription for a year and a single agent (commonly aspirin) afterward [27]. Nevertheless, application of this method over a long time span escalates bleeding risk [28]. Mauri et al. studied the application of DAPT for 12 or 30 months after DES implantation. They reported the risk of ISR was decreased by utilizing this method follow-up to 16 months compared with merely aspirin, however, the risk of bleeding was high [29].
Plasmonic and photothermal therapies are uprising in the field of atherosclerosis therapy. They consist of a dialectric silica core covered by a metallic shell (commonly gold or silver). While these NPs irradiated with near-infrared (NIR) laser, absorbed energy leads to irreparable damage of the tissue. This approach is like excimer laser angioplasty which uses monochromatic light energy source to excite molecules within atheroma. Acoustic dissection happens when water content is superheated to produce vapor bubbles. So, acoustic energy can be applied for plaque elimination. High-energy UV rays penetrate in the tissue at small depths causes injury of internal elastic lamina, leading to exuberant response of intima and media. On the other hand, NIR laser has little impact on elastic lamina. Three groups of plasmonics are available which could be used in atherosclerosis therapy: microbubble overlapping mode, nanocluster aggregation mode and thermal explosion mode (nanobombs) [30]. Regression of plaque burden was achieved in two different approaches: first, transplantation of bioengineered on-artery patch advanced with stem cells bearing NP; second, trancatheter intravascular infusion or injection of NP loaded with iron containing stem cells or targeted microbubbles coated with protein and delivery to the specific site applying magnetic fields [31].
Nanoparticle delivery systems
Nanotechnology can be used in therapies for atherosclerosis by increasing systemic agent circulation time, lowering off-target cytotoxicity of drugs, improving drug solubility, decreasing the required dosage, combining diagnostic and therapeutic agents to form theranostics and increasing accumulation of agents at specific sites [6]. Targeted drug delivery can be categorized as either active or passive targeting. Active targeting involves the conjugation of tissue or cell-specific ligands to either the nanocarriers or to the drugs themselves. In the passive targeting strategy, therapeutic agents or drugs are coupled with macromolecules to take advantage of the EPR effect [32]. Recently, stimuli-responsive delivery systems have been emerged based on various triggers such as light irradiation, pH alteration, application of magnetic or electric fields, a change in temperature or response to redox potentials. These smart NPs could be potentially applied in DES for therapy of CAD. For example, Tang et al. tested pH-responsive delivery of antioxidants for treatment of cardiovascular disease such as atherosclerosis [33]. On the other hand, localized NP delivery via stents may be a promising strategy to combat restenosis, because it could provide a sustained drug release in the target region of the artery [32]. DES can be used to localize delivery of drugs and avoid the potential toxicity of systemic drug administration [34].
NPs used to inhibit restenosis are reviewed in the following paragraphs, and Figure 1 shows the schematic structure of important NPs.
Figure 1. . Schematic structure of important nanoparticles.
Liposomes
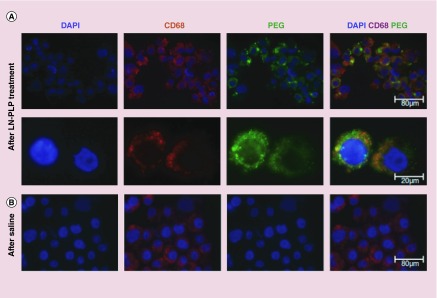
Liposomes are small vesicles, have a spherical shape and are composed of a lipid-bilayer formed from natural and nontoxic phospholipids and cholesterol [35]. The features of liposomes such as biocompatibility (because of using natural biologically safe lipids), nanometer size, the ability to tailor the hydrophobicity and hydrophilicity can provide enhanced tissue specificity for delivery of hydrophobic drugs in the lipid environment, and for hydrophilic drugs in the aqueous core [36]. The revascularization of occluded arteries in vivo was enhanced, along with a reduction in the risk of hemorrhagic side-effects. The effect of peptide-modified liposomes with good potential for vascular-targeted delivery of therapeutic and diagnostic agents has been studied. Ligands that recognize surface receptors on activated platelets (e.g., integrin GP IIb/IIIa and P-selectin) have been attached to liposomes to demonstrate the vital role of activated platelets in atherogenesis, atherosclerotic lesion progression and thrombosis in vascular diseases [37]. Phase 1 results of one study show that after 28 days of follow-up in rabbit carotid artery, liposomal alendronate can reduce ISR to 40.1% in comparison to 73.5% in empty liposomal [38]. Figures 2 & 3 demonstrate examples of liposomal delivery in CAD therapy.
Figure 2. . The liposomal nanoparticle with prednisolone phosphate stored in a macrophages of iliofemoral plaques.
(A) First row illustrates the plaque cells cured by LN-PLP marked for cell nuclei (DAPI), macrophages (CD68) and liposome-coating PEG. In the second row, magnified images of isolated cells are shown. (B) Third row shows CD68 cells from a plaque cured by saline, but there is no positivity for PEG.
LN-PLP: Liposomal nanoparticle with prednisolone phosphate.
Reproduced with permission from [39], © (2015) Nanomedicine: Nanotechnology, Biology and Medicine.
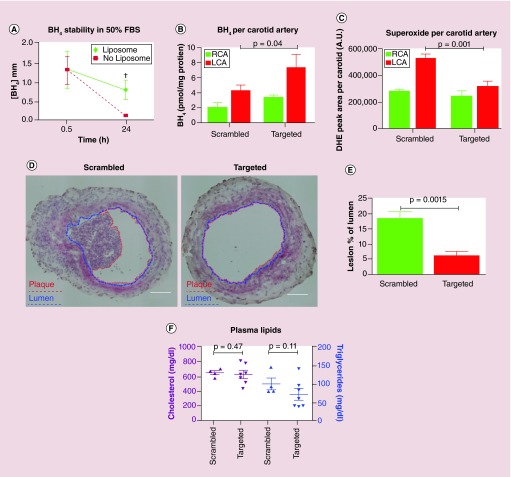
Figure 3. . Delivery of tetrahydrobiopterin (BH4) by liposome nanocarrier.
(A) Improved stability of BH4 in comparison with unencapsulated BH4 after 24 h; p = 0.017; (B) BH4 concentration in ligated artery increased by liposomal delivery; p = 0.04; (C) superoxide concentration in ligated artery with targeted liposomes decreased; (D) the plaque burden was decreased by BH4 liposomal delivery in the mice ligated left carotid artery fed by 7-day high fat diet (plaque and lumen are marked by red and blue, respectively) scale bars = 100 μm; (E) the area of plaque; p = 0.0015; (F) there is no alteration in lipid metabolism via liposome deliver; p = 0.47 and 0.11, respectively).
BH4: Tetrahydrobiopterin; DHE: Dihydroethidium; FBS: Fetal bovine serum; LCA: Left common carotid artery; RCA: Right common carotid artery.
Reproduced with permission from [40], © (2015) ACS Nano.
Micelles
Micelles are formed when amphiphilic molecules undergo self-assembly due to the energy minimization that occurs when the hydrophobic portions bunch together to form the interior. The hydrophilic shell provides the long circulation time and accounts for the relative stability in vivo. The hydrophobic core of micelles can be used for encapsulation and delivery of either bioactive therapeutic molecules, diagnostic agents or both. Attachment of moieties such as targeting ligands to the outer shell of micelles can increase active binding to disease-relevant tissues and cells [36]. There are results that suggest phospholipid-based micelles provide better antirestenotic effects (neointimal area 0.034 in comparison to 0.046 mm2 after 14 days of implantation in rat carotid arteries) than the PEGylated liposomes; probably due to the distinctly smaller size of the phospholipid-based micelles [41]. Micelles that had been surface-modified with anti-CD36 antibodies were loaded with Gd. These micelles could target macrophages in specimens from atherosclerotic human aortas [42].
Polymeric nanoparticles
Polymeric NP may be constructed in the form of solid, dense but porous structures (e.g., nanospheres and nanorods) or hollow structures (e.g., nanoshells and nanocapsules) [1,43]. Results of one study on 60-nm diameter lipid–polymeric NP functionalized by collagen IV-targeting peptides and enriched with paclitaxel demonstrate efficacious improvement. Injection of paclitaxel (0.3 mg/kg or 1 mg/kg) in a rat carotid arteries followed on days 0 and 5 resulted in lower neointima-to-media (N/M) ratio for the targeted NP group at 2 week versus the control groups. Compared with controlled groups, a 50% reduction in arterial stenosis was observed with targeted NP delivery [44]. Another study reported that PLGA NPs containing alendronate reduced neointimal formation and restenosis up to 64% for a dose of 3 mg/kg by systemic transient depletion of monocytes in a hypercholesterolemic rabbit model [45]. Delivery of imatinib (used as an inhibitor of PGDF receptor) by means of bioabsorbable polymeric NPs reduced the occurrence of ISR up to 50% compared with bare metal stents [14]. Statins have been shown to prevent the proliferation of vascular SMCs (VSMC) and also to stimulate vascular healing. Researchers formulated an NP-coated DES with 20 μg pitavastatin dosage per stent and tested it in a pig coronary artery model. This coated stent inhibited ISR as effectively as a polymer-coated sirolimus-eluting stent. There was a delay in endothelial healing with the conventional sirolimus-eluting stent, whereas no delay in re-endothelialization was observed in the pitavastatin NP-eluting stent [46].
Dendrimers
Dendrimers consist of a single molecule constructed from an original inner core with a series of macromolecular branches built up by successive additions of discrete units (generations). The ability to display multiple copies of functional groups on their surface makes them a unique structure for drug-delivery applications [47]. Dendrimers are more used in cell-labeling rather than in ISR therapy. For instance, manganese G8 dendrimers [48] have been successfully applied in atherosclerosis detection. One study described the development of ‘tadpole’ dendrimeric materials for siRNA delivery in a rat ischemia-reperfusion model. Angiotensin II (Ang II) type 1 receptor (AT1R) has been investigated since it is the major receptor that mediates most adverse effects of Ang II. Among those tadpole dendrimers evaluated, significant effective downregulation in AT1R expression in cardiomyocytes was related to the oligo-arginine-conjugated dendrimer loaded with siRNA in vitro. Delivery of the siRNA in vivo, inhibited AT1R levels to be increased, and meaningfully cardiac function recovery was improved compared with saline injection or empty dendrimer treated groups [49]. Additionally, polyamidoamine (PAMAM) dendrimers have been favored in recent years in CVD therapies. PAMAM zero generation dendrimers (G0) were tested as nanocarriers in drug delivery and conjugated G0 PAMAM dendrimers with a ZnPc photosensitizer were chosen to study their effects on the diseased and normal tissues extracted from human carotid arteries. Statistical analysis was carried out based on AFM images extracted through fractal analytical methodologies and Minkowski functionals. The affinity of the nanocarriers for healthy tissue and atheromatous tissue was different. Dissimilar aggregation behaviors between G0 and G0/ZnPc nanomaterials were observed. Larger G0/ZnPc aggregation on the atheromatous plaque were reported [50]. Photodynamic therapy with PAMAM dendrimers could have a bright future in therapy of atherosclerosis.
Gel-like nanoparticles
It was demonstrated that hydrogel nanospheres (100 nm) made of poly (N-isopropylacrylamide) were internalized by endothelial cells and VSMC to a greater degree than microspheres (1 μm), although the cellular uptake was dependent on the incubation time, nanosphere concentration and applied shear stress levels in the medium. By contrast, microspheres were rapidly taken up by phagocytes, especially at high concentrations. These findings suggest that hydrogel nanospheres were more effective as an intravascular delivery system in terms of vascular uptake and biocompatibility [51]. Since significant number of VSMC undergo rapid apoptosis following balloon angioplasty, Reddy and co-workers [52] tested the hypothesis that preventing VSMC from undergoing apoptosis could prevent intimal hyperplasia. They used rapamycin (which has antiapoptotic and antiproliferative properties) loaded into gel NPs with a mean diameter of 54 nm. When infused into a rat carotid artery model of vascular injury the authors reported significant inhibition of hyperplasia and improved re-endothelialisation of the injured artery. Furthermore, the group reported inhibition of the caspase 3/7 enzyme systems in the treated artery, thus preventing VSMC from undergoing apoptosis.
Magnetic nanoparticles
Magnetic targeting is a promising possibility for efficacious guidance of therapeutic agents to CAD diseased sites, elimination from nontargeted propagation (i.e., safety concern) and deep and long-term tissue targeting, furthermore it has suggested benefits for anti-ISR therapies, delivery of cells, gene vectors and therapeutic proteins and stented artery delivery of paclitaxel by utilization of magnetic field-guided magnetic carriers for specific-vascular delivery [53]. Functional MNP-loaded primary endothelial cells as vectors targeting vascular stents induced gene expression related to EC growth and survival, and suppressed gene-related coagulation and suggested them for re-endothelialization by the implant and decreasing neointimal hyperplasia [54].
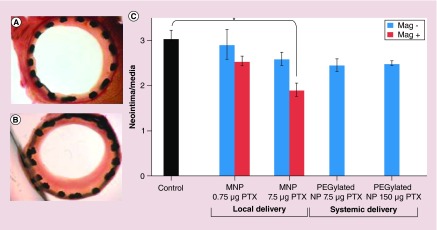
Metallic MNPs, made of iron, cobalt or nickel, are typically prepared with a core–shell structure in which gold or silica is applied as a coating material. Iron MNP composed of nanocrystalline magnetite (Fe3O4) or maghemite (γFe2O3) form a close-packed cubic lattice [55]. Furthermore, gold shell NPs (˜120 nm) have been used for both imaging and therapy applications [56]. Another study used paclitaxel-loaded magnetic NP with a uniform magnetic field that allowed the attachment of NP to the stent, and also drug release in order for it to be taken up by the target cells. In this case inhibition of ISR occurred with 7.5 μg paclitaxel, together with a noticeable reduction in the ratio of neointima/media that was only 63 ± 13% of that of the control group [57]. Figure 4 reports the results of the mentioned study.
Figure 4. . Paclitaxel-loaded magnetic nanoparticles applied to coronary stents with a uniform magnetic field.
MNPs with PTX doses of 7.5 and 0.75 μg entered into animal bodies under magnetic versus nonmagnetic conditions. The animals sacrificed and the stented carotid segments were harvested 14 days after surgery. The control group did not receive MNP but stented. Verhoeff–van Gieson-stained section of an artery lumen treated with 7.5 μg PTX under magnetic conditions (A) demonstrated versus ‘no treatment’ control (B) (p < 0.05, Dunn's Test Q statistic = 3.7). Original magnification 100×. Morphometric results as neointima/media ratios (C) pictured as a function of the magnetic field application and PTX dose (n ≥ 6). Data are presented as mean ± standard error.
MNP: Magnetic nanoparticle; NP: Nanoparticle; PTX: Paclitaxel.
Reprinted with permission from [57], © (2010) Proceedings of the National Academy of Sciences of the United States of America.
Additionally, quantum dots have been used as florescent labels to prepare traceable NP due to their tunable physicochemical features, high photostability, broad absorption spectra and narrow emission bands. Quantum dots have been proposed to monitor disease-associated events, such as macrophage cell infiltration into arterial tissues and the processes of angiogenesis and vascular remodeling [58]. Figure 5 shows using of quantum dots to monitor monocyte-macrophages in atherosclerosis plaque. More details in plaque imaging can be found in the section ’Molecular imaging and atheroslerosis detection‘.
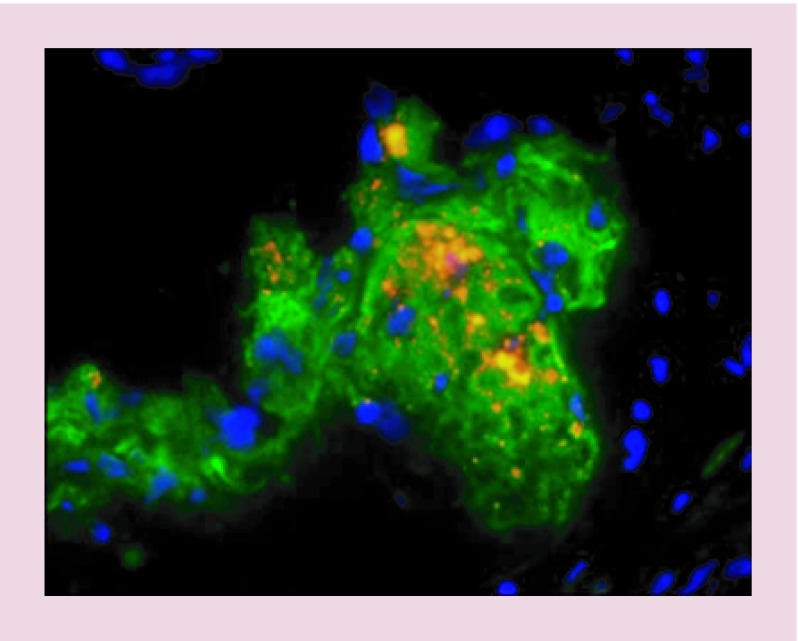
Figure 5. . Using quantum dots to image the monocyte-macrophages in atherosclerosis plaque.
The monocyte-macrophages loaded by cell penetrating quantum dots were injected to mice. Injected cells and macrophage marker CD68 are portrayed as orange and green, respectively.
Reproduced with permission from [59], © (2010) Current Atherosclerosis Reports.
Nanocoatings
Nanocoatings on DES release drugs locally at the site of plaque accumulation. Polymeric coatings are more common rather than ceramic ones. In following paragraphs, both of them are reviewed briefly.
Stent polymeric coatings
Polymer-based coatings have been used to control drug release rates from stents. However, numerous side effects could be caused by the polymer coatings themselves such as irritation, inflammation [60], blood protein adsorption, allergic reactions and in-stent thrombosis [61]. Based on bench tests and animal studies, it was found that stent implantation could cause significant problems, such as webbing and bonding, with the resulting possibility of inhomogeneous drug elution and increased thrombogenicity despite the lack of mechanical damage or deformity at the time of stent implantation [62].
Various polymers, such as PLGA, polyethylene glycol (PEG) and polycaprolactone (PCL), have been coated on stents by using the solvent evaporation technique. Improved sustained drug release profiles and a lower platelet adhesion rate were seen with stents coated with PCL matrix because of the improved surface morphology [63]. PLGA which is considered to be a biocompatible polymer has been applied widely in recent years. PLGA NP were constructed to carry paclitaxel, using an emulsion-solvent evaporation method to encapsulate drug molecules in the polymer. Moreover, the amount of paclitaxel was readily controlled by varying the preparation procedure. In vitro investigations demonstrated controlled paclitaxel release from the NP layers without any initial burst [64].
Tan et al. suggested that a nanocomposite polymer, containing polyhedral oligomeric silsesquioxane poly-(carbonate-urea) urethane (POSS-PCU), could be suitable for stent coating [65]. Antiproliferative, antithrombogenic and antiplatelet agents could be incorporated into POSS-PCU to lessen problems with BMS and DES. POSS-PCU was recently employed as the scaffold material in the first synthetic trachea transplant in a human patient [66]. Another study investigated POSS-PCU, as a stent-coating because PCU is blood compatible and antithrombogenic, particularly when POSS is added further reducing the thrombogenicity of the material. Human endothelial progenitor cells adhered and proliferated well on this nanocomposite [67].
Another strategy reported for surface modification is the preparation of nanoroughened surfaces by nitrogen ion implantation plasma deposition to promote stent performance. In addition to the deposition of nanometer sized particles, thin layers of polymers can be deposited onto metal surfaces by the IPD method. Various polymeric and metallic substrates have been modified with nanostructured Ti using the IPD technique. Rat aortic endothelial cells were used to evaluate the cytocompatibility properties [68].
Porous DES functions as a reservoir to continuously release antirestenosis agents. Nanoporous coatings may be better able to promote VSMC and endothelial cell adherence and encourage proliferation on the surface topography [69].
Table 3 summarizes some of the important advances in polymeric stent coatings in recent years.
Table 3. . Nanotextured polymeric coatings.
| Coating material | Stent body | Processing technique | Biodegradability | Type of drug | In vivo tests | Ref. |
|---|---|---|---|---|---|---|
| PLGA |
Platinum |
Sputter coating |
Yes |
Paclitaxel |
– |
[70] |
| PLGA- collagen |
316L stainless steel |
Electrophoretic deposition/dip-coating |
Yes |
N-nitro-somelatonin |
On rabbit |
[71] |
| HA-g-PLGA |
Cobalt-chromium alloy (L605) |
Layer-by-layer |
– |
Paclitaxel |
– |
[72] |
| PLGA |
316L stainless steel |
Electrodeposition |
Yes |
– |
On domestic male pigs (porcine) |
[73] |
| PLA |
– |
Solvent evaporation technique |
Yes |
Sirolimus |
– |
[74] |
| PLGA, PEG and PCL |
316L stainless steel |
Solvent evaporation technique |
Yes |
S-Nitrosoglutathione |
– |
[63] |
| Chitosan |
316L stainless steel |
Spray-coating |
Yes |
DNA |
On rabbit |
[75] |
| POSS-PCU and POSS-PCL |
– |
Layer-by-layer |
No |
No drug (anti-CD34 antibodies attached to the surface) |
– |
[65] |
| POSS-PCU | NiTi | Electrohydrodynamic spraying | No | – | – | [67] |
HA: Hyaluronic acid; PCL: Polycaprolactone; PCU: Poly (carbonate-urea) urethane; PLA: Polylactide or polylactic acid; PLGA: Poly (lactic-co-glycolic acid); POSS: Polyhedral oligomeric silsesquioxane.
Nanotextured ceramic coatings
Nanoporous aluminum oxide (Al2O3) was one of the first nanoporous ceramic coatings used by Jomed International (recently acquired by Abbott Vascular) on DES. In the process of surface manufacturing, 316L stainless steel stents were firstly coated inside and outside with a thin layer of aluminum via a physical vapor deposition process in order to ensure proper adhesion of the coating to the base metal. In the next step, the metallic layer was electrochemically converted into a nanoporous ceramic (Al2O3) using a bath of 2% oxalic acid in water. The pore diameter and pore density of the resultant surface was reported to be 5–15 nm and 109 pores/cm2, respectively. In order to deliver tacrolimus to rabbit carotid arteries in vivo, the antirestenosis drug was applied by dipping the stents into a defined solution of tacrolimus and subsequent drying steps [76]. However, the clinical performance of this system in present trials was disappointing since the coating ‘flaked off’ the stent surface and the resulting particulate debris led to a proinflammatory response producing a thicker neointima [77].
Furthermore, a hydroxyapatite-coated stent with thickness of 0.30–1 μm and porosity of 40–60% by volume has been developed by MIV Therapeutics Inc., Vancouver, British Columbia, Canada [78]. Another approach was the combination of a stainless steel platform with a nanosized microporous hydroxyapatite coating. This DES system, which was also impregnated with a small amount of sirolimus, demonstrated promising results in the first year of clinical trials [79].
Recently, it was shown that nanoporous titanium dioxide (TiO2), produced via a sol–gel synthesis process, could function as a drug-eluting surface. The main problem is loading the drugs into the nanoporous TiO2 surface. These surfaces have a large internal surface area, and the behavior of fluids is controlled by surface forces, while the ability of drugs in solution to penetrate into the surface is determined by the contact angle (surface tension) of the solution at the nanoporous surface. Modified surface methods, which enhance the hydrophilicity of the surface and the use of low viscosity solvents, can assist penetration of the solvent into the nanopores [80].
More recently, a novel bioinert paclitaxel-eluting porous carbon NP, showed excellent elastomechanical properties, and provided the possibility of tailoring drug release kinetics by adjusting the pore size, was introduced to coat stents. This surface consists of a porous composite matrix synthesized from amorphous carbon NPs embedded in glassy polymeric carbon [15]. Karagkiozaki et al. [81] prepared carbon nanocoating of stents via a manufacturing process using a radio-frequency magnetron sputtering deposition technique, with variation in surface roughness as the main design variable to control the platelet response in vivo. They showed that the surface nanotopography of the coating can influence the behavior of the platelets, and this is an important factor for controlling the thrombogenicity of the biomaterials. Among the carbon nanocoatings studied, those with a higher value of surface roughness were less thrombogenic in terms of platelet adhesion.
Rajender et al. [82] showed in a study involving pharmacological coating of stents that sustained local delivery of an antiproliferative agent provided higher tissue concentrations compared with systemic administration. Their LC/MS/MS results demonstrated consistency in the drug content on the stent surface. They also measured in-vitro release kinetics of sirolimus over 41 days from the nanoporous carbon-coated stents.
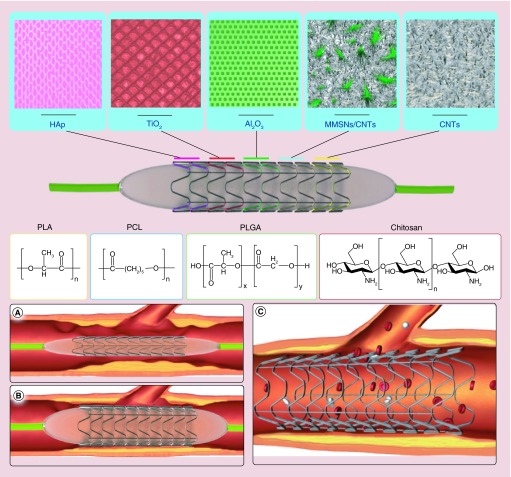
Mesoporous silica nanoparticles function as an excellent drug carrier due to their tunable pore size, high-specific surface area, large pore volume and favorable biocompatibility [83–85]. For the first time, magnetic mesoporous silica nanoparticles (MMSN) with a core−shell structure were tested as an effective rapamycin (RAPA)-loading vehicle for cardiovascular stents in vivo. This group designed a novel two-layered CNT@MMSN/CNT composite coating employing the EPD method to overcome the probable brittleness of the coatings assembled from MMSN alone. By regulating the structures composition, a crack-free two-layered coating with a remarkable network nanotopology was obtained. The thin CNT film acted as an inner buffer layer, and the MMSN/CNT composite coating acted as a functional layer. They also proposed a possible mechanism of the CO-EPD process to combine MMSN and CNT. Moreover, this polymer-free coating exhibited excellent mechanical flexibility and good blood compatibility in vitro. The RAPA-loading capability of 316L-BMS@CNT@M/C-3 was 60.10 ± 2.43 μg/mg, and the drugs could be continuously released for up to 2 weeks. Finally, an in vivo study showed that this nanostructured DES had the benefit of rapid re-endotheliazation in the early stages in comparison with the commercial P-FBII DES, in order to reduce the risk of thrombosis [86]. Figure 6 illustrates the porous structure of ceramic coatings and the chemical structure of polymers applied to DES. Furthermore, Table 4 summarizes some of the important advances in ceramic stent coatings in recent years.
Figure 6. . Stent coating structures and positioning in the coronary artery.
Top: porous structure of various ceramic coatings and chemical structures of polymers that have been applied on stents; (A) plaque accumulation in the lumen of coronary artery and delivery of nonexpanded stent; (B) expanded stent via pressure of balloon catheter; (C) expanded stent remains at the site of plaque allowing good blood flow.
CNT: Carbon nanotube; HAp: Hydroxyapatite; MMSN: Magnetic mesoporous silica nanoparticle.
Table 4. . Nanotextured ceramic coatings.
| Coating material | Stent body | Processing technique | Pore size | Type of drug | In vivo tests | Clinical trials | Ref. |
|---|---|---|---|---|---|---|---|
| Al2O3 |
316L stainless steel |
Electrochemical conversion of aluminum layer |
5–15 nm |
Tracrolimus |
On rabbit carotid arteries |
Inflammatory caused by release of particle debris |
[77] |
| HAp |
316L stainless steel |
Sol-Gel |
40–70 nm |
Sirolimus |
N/A |
Promising results in the first year of clinical trial |
[78,79,87] |
| TiO2 |
316L stainless steel |
Sol-Gel |
55 Å |
N/A |
N/A |
N/A |
[80] |
| CNTs |
Cobalt–chromium stent |
Rf magnetron sputtering deposition technique |
Nano size |
Sirolimus |
N/A |
N/A |
[81] |
| MMSNs/CNTs | 316L stainless steel | Electrophoretic deposition method | Nano size distribution | Sirolimus | On rabbit carotid arteries | N/A | [86] |
CNT: Carbon nanotube; HAp: Hydroxyapatite; MMSN: Magnetic mesoporous silica nanoparticle; N/A: Not available.
Gene therapy strategies
The use of DES can be accompanied by side effects such as in-stent stenosis, and thrombosis which could be effectively eliminated by developing new stent materials and coatings. Adverse local hypersensitivity reactions and an inflammatory response to polymeric coatings [88] or delayed or incomplete re-endothelialization might result in late stent thrombosis (associated with high mortality) due to delayed healing of the DES-induced endothelial wound. This important side effect of DES could be avoided by strategies including miRNA gene delivery that improves healing of the vessel wall injury [89], optimizing the DES design for inhibition of restenosis while not affecting the endothelial healing [90] and using fully bioabsorbable/degradable stents as well as newly described polymer-free DES with dual drug-eluting capability [91]. A variety of different gene targets have been used to treat restenosis [18,92–93]. For instance, a gene-eluting metallic stent was prepared by first laying down a robust surface layer of hyaluronic acid followed by deposition of plasmid DNA (pDNA) on the surface. The hyaluronic acid-coated surface improved deposition of pDNA/polyethylenimine polyplexes, and led to enhanced gene expression [94]. In another attempt, Paul et al. developed an endovascular stent based on a nanobiohybrid hydrogel. The hydrogel included assembled fibrin matrices built up layer-by-layer on the stent surface, with alternate layers carrying the endosomolytic Tat peptide together with DNA (NCS). Other NPs were hybridized with polyacrylic acid wrapped single-walled carbon nanotubes (NP-CNT). Six weeks after stenting in a beagle dog model, the re-endothelialization area in the NCS (+) group carrying the VEGF + angiopoetin 1 transgene was 87.2 ± 13.2%, in the null-transgene NCS (-) was 54.7 ± 9.3%, and in the bare metal stent (BMS) group was 46.44 ± 4.6%. Furthermore the degree of restenosis in the NCS (+) group was 28.5 ± 9.03%, in NCS (-) group was 39.56 ± 13.8% and in BMS group was 45.34 ± 8.3% [95]. In an injured rat artery, the use of PLGA NPs loaded with PDGF receptor-β antisense RNA was reported [43]. These NPs displayed efficient intracellular delivery and sustained intracytoplasmic release [73].
Molecular imaging & atheroslerosis detection
One of the goals in diagnosis of atherosclerosis is to distinguish the so-called ‘vulnerable plaques’ that are likely to rupture and precipitate a heart attack from the stable plaques that do not present any sudden threat. Current imaging methodologies that typically image plaque anatomy do not identify such high-risk lesions. Identification of these lesions in relevant vascular beds (coronary and carotid arteries) could alter systemic therapies (i.e., prescribing higher doses of statins or adjunctive treatments despite supposedly normal ‘target’ serum lipid levels), and also to possibly guide local therapies (e.g., intracoronary stenting of high-risk lesions) in patients at very high risk. The most commonly used targets in CVD molecular imaging are: macrophages; annexin V for phosphatidylserine (PS) in apoptosis, angiogenesis, fibrin, etc.
The metabolic activity of macrophages in inflamed lesions can be traced through uptake of glucose analogs. Cells take up fluorine-labeled 2-deoxy-D-glucose (FDG) at the same rate as glucose. After phosphorylation, FDG accumulates inside the cells. 18F-FDG imaging combined with positron emission tomography (PET) has been used in atherosclerotic patients to evaluate inflammation and macrophage load in the symptomatic carotid artery compared with the contralateral asymptomatic control vessel [96]. Cross-linked iron oxide fluorescent NPs [97] and MNP with a central iron oxide core (3–5 nm in diameter) coated with carbohydrate [98] have been used for detection of macrophage. Additionally, 19F perfluorocarbon NPs recruited in MRI spectroscopy instead of 1H signals due to better detection of 19F (since, there is no background signal from 19F in the host tissues at the final image) [99]. While monitoring cell proliferation mainly depends on ex vivo measurements, patients with atherosclerosis need to be assessed through their biopsy samples. Finding a biomarker which represents cells proliferation would be suitable to diagnose disease as soon as possible. 18F-FLT, a PET isotope-labeled thymidine have the potential to be that goal since atherosclerotic plaques in knock out mice, rabbits and humans accumulate 18F-FLT according to the report of Xiang [100]. Preventing the macrophage accumulation would be a suitable approach before atherosclerotic plaque progression and 3-hydroxy-3-methylglutaryl coenzyme A which also called statins in association with high-density lipoprotein nanoparticle showed to be effective in reducing macrophage proliferation [101]. IVUS/IVPA can be utilized to monitor the presence of systematically gold NPs within plaques in atherosclerosis as shown by Yeager et al. [102]. IVPA-assessed, selective plasmonic photothermal heating delivered through the integrated imaging catheter. Silica-coated gold nanorods tendency toward endocytosis by macrophages makes this method specific targeted [102]. Circulation half-life of prednisolone provided with a liposomal nanocarrier shown to be appropriate in atherosclerotic lesions. These liposomal NPs accumulate in macrophages. Nanomedicinal delivery of drugs to atherosclerotic lesions is feasible in humans [39].
During apoptosis, activated flippases quickly redistribute PS molecules from the inner layer into the outer layer of the cell membrane. Therefore annexin V, a protein that binds to PS, has been labeled with radionuclides (123I, 124I, 99mTc and 18F) [103] to detect apoptosis. A combination of annexin 5 labeled with 99mTc for SPECT imaging of apoptosis and together with hypericin labeled with 124I for PET imaging of necrosis was used to image plaques in apoE-/- mice [104]. Imaging of apoptotic cells in atheroma may demarcate plaques at risk for future complications [105].
Oxidized low-density lipoprotein can trigger local inflammation that is related to expression of adhesion molecules such as vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 which could be detected by superparamagnetic iron oxide and Gd particles, respectively [106].
Microvessels underlying plaques may cause intraplaque hemorrhage, and thus could identify high-risk atherosclerotic lesions. Therefore, imaging of angiogenesis can be used as a target in molecular imaging. In particular, integrin αvβ3 has been recognized as a key mediator of angiogenesis and thus may represent an important diagnostic and therapeutic target for diseases characterized by neovascularization. Recent studies have used a gadolinium coated perfluorocarbon nanomaterial (containing 90,000 individual gadolinium chelates) derivatized with an arginine-glycine-aspartic acid (RGD) peptidomimetic to target αvβ3 [107].
During the clotting process, thrombin-mediated cleavage of fibrinogen gives rise to fibrin monomers. The fibrin clot is then cross-linked via factor XIII to stabilize the clot and generate obstructive thrombi. Each of these stages contributes to subocclusive thrombosis and intraplaque hemorrhages and could represent a target for imaging [106]. One way to detect fibrin monomers in vivo is to target activated platelets via P-selectin or GP IIb/IIIa. Imaging probes targeting P-selectin can be designed using a specific antibody (VH10), peptides or polysaccharides that bind efficiently both in vitro and in vivo to platelets and thrombi and detected by MRI [108].
Another method has been studied for imaging of thrombogenesis targets factor XIII, using peptide substrates recognizing factor XIII [109]. These fluorescent-labeled peptide agents are covalently cross-linked into the clots being formed by factor XIII, and show a time-dependent increase in fluorescence signal.
Magneto-photo-acoustic imaging is helpful in assessing the delivery and determine endocytosis for magnetic nanoparticles. For this purpose, photoacoustic and magneto-motive ultrasound (MMUS) signals are linearly proportional. However, gathering NPs within cells causes to nonlinear relationship between MMUS-PA because of MMUS signal nonlinear amplification. Therefore, longitudinal magneto-photo-acoustic imaging can make it possible the delivery of NPs and identifying the endocytosis of NPs in living cells [110].
Conclusion & future perspective
The effectiveness of DES was demonstrated in numerous clinical trials, and their introduction has led to significant improvement in CAD therapy. Other effective therapies for CAD such as atherosclerosis, thrombosis, restenosis and in-stent neointimal hyperplasia have been investigated in recent years and tested in clinical trials. Nanotechnology with its superior advantages can provide innovative solutions to CAD by exploiting various nano/micro carrier platforms that can offer improved delivery of therapeutic agents to diseased sites. These sites include inflammatory cells in CAD-related diseases mediated by specific targeting and receptor-specific biomolecules. Nanotechnology can improve the function of stents coated with polymers, loaded with biomolecules (e.g., PDGF-receptor inhibitors, paclitaxel and rapamycin), leading to reduction of inflammation and angiogenesis and encouraging healing of the injured endothelium. Many antirestenosis strategies have been investigated using anti-inflammatory, antithrombogenic and antiproliferative agents. Immunosuppressive agents can be carried on DES, and a resulting reduction in ISR can be demonstrated. These novel CAD therapies have resulted in advantages such as reduced cytotoxicity, enhanced blood circulation time of agents and reduced overall dosage. Various surface modification techniques that have been applied to DES can improve the performance of intravascular stents. Stents have been coated with NP, and stent-coating materials have been formulated from polymers and ceramics and their fabrication methods have been investigated. Despite many advantages such as improved controlled/sustained release rates, enhanced re-endotheliazation, reduced platelet adhesion and the biosafety of drug-loaded NP (polymeric, nanoporous ceramic, nanoporous carbon based, etc.), concerns remain. These concerns center on allergic reactions, inflammation, increased neointimia formation. Moreover deformities and damage may be triggered by DES and some clinical catastrophes have occurred. Gene therapy approaches (e.g., pDNA, RNAi) have been used to modify gene expression, and to generate signaling molecules to inhibit growth of diseased cells and hyperplasia, and to cause apoptosis.
The diagnostic and imaging capability of nanoplatforms in CAD therapy is another area of investigation, and magnetic and metallic NP and fluorescent QD have been developed as contrast agents (besides their drug delivery capability). Combinations of diagnostic and therapeutic agents comprise the new field of theranostics. However there are still several challenges to be solved, concerning the design of balanced magnetic properties of MNP, modification of the composition of the stent material that may include magnetic responsive materials for increased targeting and loading capability. There are biocompatibility and toxicity issues due to presence of biological–NP interactions. MNP-based systems have been considered for enhanced nucleic acid transfection in cells via application of magnetic fields, in a process named ‘magnetofection’.
In future directions, MNP-based magnetic scaffold implants could be considered tissue engineering using materials containing multicellular cultures (e.g., cardiomyocytes, fibroblasts and endothelial cells) within the scaffolds.
Future approaches will undoubtedly concentrate on personalized medicine taking into account specific features of individualized patients and their particular disease site. This approach requires tailoring various parameters including the stent-design and intervention strategy, and these should be specifically designed for each patient. Moreover, cost–benefit analysis cannot be ignored in these innovative approaches. The ever-rising cost of healthcare cannot be allowed to continue its exponential rise forever. Therefore, public healthcare issues need to be kept in mind so that nanotechnology can continue to provide affordable and patient-friendly therapy accompanied by long-term effectiveness.
Executive summary.
Major problems in coronary artery disease include restenosis after angioplasty, and detection and therapy of vulnerable plaque.
Drug eluted stents are used to inhibit restenosis, prevent proliferation of SMC, enhance re-endothelialization and attenuate neointimal hyperplasia, but further advances are needed.
Antirestenosis drugs can be delivered by being encapsulated in nanoparticles (NPs) including, micelles, liposomes or polymeric NPs.
Dendrimers, gel-like NPs and gene therapy strategies have also been used to target restenosis
Sophisticated strategies using nanotextured polymer coatings and nanotextured ceramic coatings have been applied to intra-arterial stents to prevent restenosis
Metal NPs and optical fluorescence probes have been used for intra-arterial imaging
Molecular imaging approaches including positron emission tomography, single photon emission computed tomography and magnetic resonance imaging have been used to detect vulnerable plaque.
Footnotes
Financial & competing interests disclosure
MR Hamblin was supported by US NIH Grant R01AI050875. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Brito L, Amiji M. Nanoparticulate carriers for the treatment of coronary restenosis. Int. J. Nanomedicine. 2007;2:143. [PMC free article] [PubMed] [Google Scholar]
- 2.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014;276(6):618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 3.Cominacini L, Garbin U, Mozzini C, et al. The atherosclerotic plaque vulnerability: focus on the oxidative and endoplasmic reticulum stress in orchestrating the macrophage apoptosis in the formation of the necrotic core. Curr. Med. Chem. 2015;22(13):1565–1572. doi: 10.2174/0929867322666150311150829. [DOI] [PubMed] [Google Scholar]
- 4.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(14):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DR, Kamisoglu K, York AW, Moghe PV. Polymer-based therapeutics: nanoassemblies and nanoparticles for management of atherosclerosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011;3:400–420. doi: 10.1002/wnan.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasir JK, Reddy MK, Labhasetwar VD. Nanosystems in drug targeting: opportunities and challenges. Curr. Nanosci. 2005;1:47–64. [Google Scholar]
- 8.Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 2006;58:358–376. doi: 10.1016/j.addr.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Rhee J-W, Wu JC. Advances in nanotechnology for the management of coronary artery disease. Trends Cardiovasc. Med. 2013;23:39–45. doi: 10.1016/j.tcm.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan W, Farah S, Domb AJ. Drug eluting stents: developments and current status. J. Control. Release. 2012;161(2):703–712. doi: 10.1016/j.jconrel.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Ferrero V, Tomai F, Versaci F, et al. Long-term results of immunosuppressive oral prednisone after coronary angioplasty in non-diabetic patients with elevated C-reactive protein levels. EuroIntervention. 2009;5(2):250–254. doi: 10.4244/eijv5i2a39. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez AE, Granada JF, Rodriguez-Alemparte M, et al. Oral rapamycin after coronary bare-metal stent implantation to prevent restenosis: the Prospective, Randomized Oral Rapamycin in Argentina (ORAR II) Study. J. Am. College Cardiol. 2006;47(8):1522–1529. doi: 10.1016/j.jacc.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 13.Banai S, Chorny M, Gertz SD, et al. Locally delivered nanoencapsulated tyrphostin (AGL-2043) reduces neointima formation in balloon-injured rat carotid and stented porcine coronary arteries. Biomaterials. 2005;26:451–461. doi: 10.1016/j.biomaterials.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Masuda S, Nakano K, Funakoshi K, et al. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J. Atheroscl. Thromb. 2011;18:1043–1053. doi: 10.5551/jat.8730. [DOI] [PubMed] [Google Scholar]
- 15.Bhargava B, Reddy NK, Karthikeyan G, et al. A novel paclitaxel-eluting porous carbon–carbon nanoparticle coated, nonpolymeric cobalt–chromium stent: evaluation in a porcine model. Cathet. Cardiovasc. Intervent. 2006;67:698–702. doi: 10.1002/ccd.20698. [DOI] [PubMed] [Google Scholar]
- 16.Räthel T, Mannell H, Pircher J, Gleich B, Pohl U, Krötz F. Magnetic stents retain nanoparticle-bound antirestenotic drugs transported by lipid microbubbles. Pharm. Res. 2012;29:1295–1307. doi: 10.1007/s11095-011-0643-y. [DOI] [PubMed] [Google Scholar]
- 17.Joner M, Morimoto K, Kasukawa H, et al. Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler. Thromb. Vasc. Biol. 2008;28:1960–1966. doi: 10.1161/ATVBAHA.108.170662. [DOI] [PubMed] [Google Scholar]
- 18.Fishbein I, Alferiev I, Bakay M, et al. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation. 2008;117:2096–2103. doi: 10.1161/CIRCULATIONAHA.107.746412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barlis P, Regar E, Serruys PW, et al. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur. Heart J. 2009;30:ehp480. doi: 10.1093/eurheartj/ehp480. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto D, Shite J, Shinke T, et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur. Heart J. 2007;28(8):961–967. doi: 10.1093/eurheartj/ehl413. [DOI] [PubMed] [Google Scholar]
- 21.Otake H, Shite J, Ikeno F, et al. Evaluation of the peri-strut low intensity area following sirolimus-and paclitaxel-eluting stents implantation: insights from an optical coherence tomography study in humans. Int. J. Cardiol. 2012;157(1):38–42. doi: 10.1016/j.ijcard.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Patrono C, Andreotti F, Arnesen H, et al. Antiplatelet agents for the treatment and prevention of atherothrombosis. Eur. Heart J. 2011;32(23):2922–2932. doi: 10.1093/eurheartj/ehr373. [DOI] [PubMed] [Google Scholar]
- 23.Rollini F, Franchi F, Angiolillo DJ. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat. Rev. Cardiol. 2016;13:11–27. doi: 10.1038/nrcardio.2015.113. [DOI] [PubMed] [Google Scholar]
- 24.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat. Rev. Cardiol. 2015;12(1):30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 25.Tsoi M-F, Cheung C-L, Cheung TT, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: meta-analysis of large randomised controlled trials. Sci. Rep. 2015;5:13204. doi: 10.1038/srep13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA. 2013;310(2):189–198. doi: 10.1001/jama.2013.7086. [DOI] [PubMed] [Google Scholar]
- 27.Olsen A-MS, Gislason GH, Mcgettigan P, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA. 2015;313(8):805–814. doi: 10.1001/jama.2015.0809. [DOI] [PubMed] [Google Scholar]
- 28.Stefanini GG, Holmes DR., Jr Drug-eluting coronary-artery stents. N. Engl. J. Med. 2013;368(3):254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 29.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014;371(23):2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharlamov AN. Plasmonic photothermal therapy for atheroregression below Glagov threshold. Fut. Cardiol. 2013;9(3):405–425. doi: 10.2217/fca.13.16. [DOI] [PubMed] [Google Scholar]
- 31.Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica–gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7(17):8003–8015. doi: 10.1039/c5nr01050k. [DOI] [PubMed] [Google Scholar]
- 32.Patel DN, Bailey SR. Nanotechnology in cardiovascular medicine. Cathet. Cardiovasc. Intervent. 2007;69:643–654. doi: 10.1002/ccd.21060. [DOI] [PubMed] [Google Scholar]
- 33.Tang C, Amin D, Messersmith PB, Anthony JE, Prud'homme RK. Polymer directed self-assembly of pH-responsive antioxidant nanoparticles. Langmuir. 2015;31(12):3612–3620. doi: 10.1021/acs.langmuir.5b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gundogan B, Tan A, Farhatnia Y, Alavijeh MS, Cui Z, Seifalian AM. Bioabsorbable stent quo vadis: a case for nano-theranostics. Theranostics. 2014;4:514–533. doi: 10.7150/thno.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 36.Gupta AS. Nanomedicine approaches in vascular disease: a review. Nanomedicine. 2011;7:763–779. doi: 10.1016/j.nano.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan R, Marchant RE, Gupta AS. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J. Biomed. Mater. Res. Part A. 2010;93:1004–1015. doi: 10.1002/jbm.a.32549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gutman D, Golomb G. Liposomal alendronate for the treatment of restenosis. J. Control. Release. 2012;161(2):619–627. doi: 10.1016/j.jconrel.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Van Der Valk FM, Van Wijk DF, Lobatto ME, et al. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine. 2015;11(5):1039–1046. doi: 10.1016/j.nano.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmeister LH, Lee SH, Norlander AE, et al. Phage-display-guided nanocarrier targeting to atheroprone vasculature. ACS Nano. 2015;9(4):4435–4446. doi: 10.1021/acsnano.5b01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haeri A, Sadeghian S, Rabbani S, et al. Sirolimus-loaded stealth colloidal systems attenuate neointimal hyperplasia after balloon injury: a comparison of phospholipid micelles and liposomes. Int. J. Pharmaceut. 2013;455(1):320–330. doi: 10.1016/j.ijpharm.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Amirbekian V, Lipinski MJ, Frias JC, et al. MR imaging of human atherosclerosis using immunomicelles molecularly targeted to macrophages. J. Cardiovasc. Magn. Res. 2009;11:1–2. [Google Scholar]
- 43.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J. Control. Release. 2010;146:196–200. doi: 10.1016/j.jconrel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan JM, Rhee J-W, Drum CL, et al. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid–polymeric nanoparticles. Proc. Natl Acad. Sci. USA. 2011;108:19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J. Control. Release. 2009;133:90–95. doi: 10.1016/j.jconrel.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 46.Tsukie N, Nakano K, Matoba T, et al. Pitavastatin-incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model. J. Atheroscl. Thromb. 2013;20:32–45. doi: 10.5551/jat.13862. [DOI] [PubMed] [Google Scholar]
- 47.Hawker CJ, Frechet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990;112:7638–7647. [Google Scholar]
- 48.Nguyen TH, Bryant H, Shapsa A, et al. Manganese G8 dendrimers targeted to oxidation-specific epitopes: in vivo MR imaging of atherosclerosis. J. Magn. Res. Imaging. 2015;41(3):797–805. doi: 10.1002/jmri.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Gu C, Cabigas EB, et al. Functionalized dendrimer-based delivery of angiotensin type 1 receptor siRNA for preserving cardiac function following infarction. Biomaterials. 2013;34(14):3729–3736. doi: 10.1016/j.biomaterials.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spyropoulos-Antonakakis N, Sarantopoulou E, Trohopoulos PN, et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: a photodynamic therapy for atherosclerosis. Nanoscale Res. Lett. 2015;10(1):1–19. doi: 10.1186/s11671-015-0904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen KT, Shukla KP, Moctezuma M, et al. Studies of the cellular uptake of hydrogel nanospheres and microspheres by phagocytes, vascular endothelial cells, and smooth muscle cells. J. Biomed. Mater. Res. Part A. 2009;88:1020–1030. doi: 10.1002/jbm.a.31734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy MK, Vasir JK, Sahoo SK, Jain TK, Yallapu MM, Labhasetwar V. Inhibition of apoptosis through localized delivery of rapamycin-loaded nanoparticles prevented neointimal hyperplasia and reendothelialized injured artery. Circulation. 2008;1:209–216. doi: 10.1161/CIRCINTERVENTIONS.108.830018. [DOI] [PubMed] [Google Scholar]
- 53.Chorny M, Fishbein I, Forbes S, Alferiev I. Magnetic nanoparticles for targeted vascular delivery. IUBMB Life. 2011;63(8):613–620. doi: 10.1002/iub.479. [DOI] [PubMed] [Google Scholar]
- 54.Zohra FT, Medved M, Lazareva N, Polyak B. Functional behavior and gene expression of magnetic nanoparticle-loaded primary endothelial cells for targeting vascular stents. Nanomedicine. 2015;10(9):1391–1406. doi: 10.2217/nnm.15.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, Zurita AJ, Ardelt PU, Giordano RJ, Arap W, Pasqualini R. Design and validation of a bifunctional ligand display system for receptor targeting. Chem. Biol. 2004;11:1081–1091. doi: 10.1016/j.chembiol.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Chorny M, Fishbein I, Yellen BB, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl Acad. Sci. USA. 2010;107:8346–8351. doi: 10.1073/pnas.0909506107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godin B, Hu Y, La Francesca S, Ferrari M. Molecular and Translational Vascular Medicine. Springer; 2012. Cardiovascular nanomedicine: challenges and opportunities; pp. 249–281. [Google Scholar]
- 59.Jayagopal A, Linton MF, Fazio S, Haselton FR. Insights into atherosclerosis using nanotechnology. Curr. Atheroscl. Rep. 2010;12(3):209–215. doi: 10.1007/s11883-010-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Der Giessen WJ, Lincoff AM, Schwartz RS, et al. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94:1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 61.Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stentsa review of available cases from the Research on Adverse Drug events And Reports (RADAR) project. J. Am. Coll. Cardiol. 2006;47:175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 62.Finn AV, Kolodgie FD, Harnek J, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus-or paclitaxel-eluting stents. Circulation. 2005;112:270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. [DOI] [PubMed] [Google Scholar]
- 63.Acharya G, Lee CH, Lee Y. Optimization of cardiovascular stent against restenosis: factorial design-based statistical analysis of polymer coating conditions. PLoS ONE. 2012;7:e43100. doi: 10.1371/journal.pone.0043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joo JNH, Nam SH, Nam SH, Baek I, Park J-S. Thermal process for enhancing mechanical strength of PLGA nanoparticle layers on coronary stents. Bull. Korean Chem. Soc. 2009;30:1985–1988. [Google Scholar]
- 65.Tan A, Farhatnia Y, Goh D, et al. Surface modification of a polyhedral oligomeric silsesquioxane poly (carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cells. Biointerphases. 2013;8:23. doi: 10.1186/1559-4106-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 67.Bakhshi R, Darbyshire A, Evans JE, You Z, Lu J, Seifalian AM. Polymeric coating of surface modified nitinol stent with POSS-nanocomposite polymer. Colloids Surf. B Biointerfaces. 2011;86:93–105. doi: 10.1016/j.colsurfb.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 68.Pareta RA, Reising AB, Miller T, Storey D, Webster TJ. Increased endothelial cell adhesion on plasma modified nanostructured polymeric and metallic surfaces for vascular stent applications. Biotechnol. Bioeng. 2009;103:459–471. doi: 10.1002/bit.22276. [DOI] [PubMed] [Google Scholar]
- 69.Miller DC, Haberstroh KM, Webster TJ. Mechanism (s) of increased vascular cell adhesion on nanostructured poly (lactic-co-glycolic acid) films. J. Biomed. Mater. Res. Part A. 2005;73:476–484. doi: 10.1002/jbm.a.30318. [DOI] [PubMed] [Google Scholar]
- 70.Joo J-R, Nam HY, Nam SH, Baek I, Park J-S. A novel deposition method of PLGA nanoparticles on coronary stents. Bull. Korean Chem. Soc. 2009;30:1085–1087. [Google Scholar]
- 71.Liu Y, Wang W, Acharya G, Shim Y-B, Choe ES, Lee CH. Advanced stent coating for drug delivery and in vivo biocompatibility. J. Nanopart. Res. 2013;15:1–16. [Google Scholar]
- 72.Kim TG, Lee H, Jang Y, Park TG. Controlled release of paclitaxel from heparinized metal stent fabricated by layer-by-layer assembly of polylysine and hyaluronic acid-g-poly (lactic-co-glycolic acid) micelles encapsulating paclitaxel. Biomacromolecules. 2009;10:1532–1539. doi: 10.1021/bm900116r. [DOI] [PubMed] [Google Scholar]
- 73.Nakano K, Egashira K, Masuda S, et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC. 2009;2:277–283. doi: 10.1016/j.jcin.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 74.Luderer F, Sternberg K, Rohm HW, et al. World Congress on Medical Physics and Biomedical Engineering. Munich, Germany: 2010. Biodegradable sirolimus-loaded poly (lactide) nanoparticles as delivery systems for the prevention of restenosis in coronary stent application; pp. 193–196. Presented at. 7–12 September 2009. [Google Scholar]
- 75.Zhu D, Jin X, Leng X, et al. Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int. J. Nanomedicine. 2010;5:1095. doi: 10.2147/IJN.S14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieneke H, Dirsch O, Sawitowski T, et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Cathet. Cardiovasc. Intervent. 2003;60:399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
- 77.Kollum M, Farb A, Schreiber R, et al. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus-eluting stents in a porcine model of restenosis. Cathet. Cardiovasc. Intervent. 2005;64:85–90. doi: 10.1002/ccd.20213. [DOI] [PubMed] [Google Scholar]
- 78.Rajtar A, Kaluza GL, Yang Q, et al. Hydroxyapatite-coated cardiovascular stents. EuroIntervention. 2006;2:113–115. [PubMed] [Google Scholar]
- 79.Costa JR, Abizaid A, Costa R, et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC. 2009;2:422–427. doi: 10.1016/j.jcin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Ayon AA. Utilization of nanoporous titanium dioxide films on drug-eluting stents. Annual Meeting-Society For Biomaterials In Conjunction With The International Biomaterials Symposium. 2006;29:331. Presented at. [Google Scholar]
- 81.Karagkiozaki VC, Logothetidis SD, Kassavetis SN, Giannoglou GD. Nanomedicine for the reduction of the thrombogenicity of stent coatings. Int. J. Nanomedicine. 2010;5:239. doi: 10.2147/ijn.s7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajender G, Narayanan NGB. Liquid chromatography-tandem mass spectrometry method for determination of Sirolimus coated drug eluting nano porous carbon stents. Biomed. Chromatog. 2010;24:329–334. doi: 10.1002/bmc.1295. [DOI] [PubMed] [Google Scholar]
- 83.Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VSY. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 84.Popat A, Hartono SB, Stahr F, Liu J, Qiao SZ, Lu GQM. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale. 2011;3:2801–2818. doi: 10.1039/c1nr10224a. [DOI] [PubMed] [Google Scholar]
- 85.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6:1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Zhang W, Zhang J, Sun W, Zhang R, Gu H. Fabrication of a novel polymer-free nanostructured drug-eluting coating for cardiovascular stents. ACS Appl. Mater. Interfaces. 2013;5:10337–10345. doi: 10.1021/am403365j. [DOI] [PubMed] [Google Scholar]
- 87.Alviar CL, Tellez A, Wang M, et al. Low-dose sirolimus-eluting hydroxyapatite coating on stents does not increase platelet activation and adhesion ex vivo . J. Thromb. Thrombolys. 2012;34:91–98. doi: 10.1007/s11239-012-0696-8. [DOI] [PubMed] [Google Scholar]
- 88.Sun D, Zheng Y, Yin T, Tang C, Yu Q, Wang G. Coronary drug-eluting stents: from design optimization to newer strategies. J. Biomed. Mater. Res. Part A. 2014;102(5):1625–1640. doi: 10.1002/jbm.a.34806. [DOI] [PubMed] [Google Scholar]
- 89.Binder RK, Lüscher TF. Duration of dual antiplatelet therapy after coronary artery stenting: where is the sweet spot between ischaemia and bleeding? Eur. Heart J. 2015;36(20):1207–1211. doi: 10.1093/eurheartj/ehv103. [DOI] [PubMed] [Google Scholar]
- 90.Bozsak F, Gonzalez-Rodriguez D, Sternberger Z, et al. Optimization of drug delivery by drug-eluting stents. PLoS One. 2015;10(6):1–29. doi: 10.1371/journal.pone.0130182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu M, Xu B, Kandzari DE, et al. First report of a novel polymer-free dual-drug eluting stent in de novo coronary artery disease: results of the first in human BICARE trial. Cathet. Cardiovasc. Intervent. 2014;83(3):405–411. doi: 10.1002/ccd.25129. [DOI] [PubMed] [Google Scholar]
- 92.Jin X, Mei L, Song C, et al. Immobilization of plasmid DNA on an anti-DNA antibody modified coronary stent for intravascular site-specific gene therapy. J. Gene Med. 2008;10(4):421–429. doi: 10.1002/jgm.1165. [DOI] [PubMed] [Google Scholar]
- 93.Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014;4:175–200. doi: 10.7150/thno.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim TG, Lee Y, Park TG. Controlled gene-eluting metal stent fabricated by bio-inspired surface modification with hyaluronic acid and deposition of DNA/PEI polyplexes. Int. J. Pharmaceut. 2010;384:181–188. doi: 10.1016/j.ijpharm.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 95.Paul A, Shao W, Shum-Tim D, Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNA Ang1 þ Vegf nanoparticles DESIGN : NP-CNT based stent for arterial gene delivery. Biomaterials. 2012;33:7655–7664. doi: 10.1016/j.biomaterials.2012.06.096. [DOI] [PubMed] [Google Scholar]
- 96.Dweck MR, Jones C, Joshi NV, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125(1):76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 97.Aikawa E, Nahrendorf M, Figueiredo J-L, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo . Circulation. 2007;116(24):2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 98.Nguyen PK, Nag D, Wu JC. Translational Cardiology: Molecular Basis of Cardiac Metabolism, Cardiac Remodeling, Translational Therapies and Imaging Techniques. Springer: 2012. Molecular imaging of cardiovascular disease; pp. 485–531. [Google Scholar]
- 99.Flögel U, Ding Z, Hardung H, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118(2):140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye Y-X, Calcagno C, Binderup T, et al. Imaging macrophage and hematopoietic progenitor proliferation in atherosclerosis. Circulation Res. 2015;117(10):835–845. doi: 10.1161/CIRCRESAHA.115.307024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang J, Lobatto ME, Hassing L, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv. 2015;1(3):e1400223. doi: 10.1126/sciadv.1400223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeager D, Chen Y-S, Litovsky S, Emelianov S. Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: a feasibility study. Theranostics. 2014;4(1):36. doi: 10.7150/thno.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laufer E, Winkens M, Corsten M, Reutelingsperger C, Narula J, Hofstra L. PET and SPECT imaging of apoptosis in vulnerable atherosclerotic plaques with radiolabeled Annexin A5. Q. J. Nucl. Med. Mol. Imaging. 2009;53(1):26. [PubMed] [Google Scholar]
- 104.De Saint-Hubert M, Bauwens M, Deckers N, et al. In vivo molecular imaging of apoptosisand necrosis in atherosclerotic plaquesusing microSPECT-CT and microPET-CT imaging. Mol. Imaging Biol. 2014;16(2):246–254. doi: 10.1007/s11307-013-0677-0. [DOI] [PubMed] [Google Scholar]
- 105.Desai MY, Schoenhagen P. Emergence of targeted molecular imaging in atherosclerotic cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2009;7(2):197–203. doi: 10.1586/14779072.7.2.197. [DOI] [PubMed] [Google Scholar]
- 106.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ. Res. 2012;111(2):231–244. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- 108.Jacobin-Valat MJ, Deramchia K, Mornet S, et al. MRI of inducible P-selectin expression in human activated platelets involved in the early stages of atherosclerosis. NMR Biomed. 2011;24(4):413–424. doi: 10.1002/nbm.1606. [DOI] [PubMed] [Google Scholar]
- 109.Mccarthy JR, Patel P, Botnaru I, Haghayeghi P, Weissleder R, Jaffer FA. Multimodal nanoagents for the detection of intravascular thrombi. Bioconjug. Chem. 2009;20(6):1251–1255. doi: 10.1021/bc9001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qu M, Mehrmohammadi M, Emelianov S. Sensing the delivery and endocytosis of nanoparticles using magneto-photo-acoustic imaging. Photoacoustics. 2015;3(3):107–113. doi: 10.1016/j.pacs.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]