Abstract
FLAG is an affinity tag widely used for rapid and highly specific one-step protein purification. Native elution of protein from anti-FLAG antibody resins allows the identification of protein and nucleic acid binding partners and functional analysis using biochemical activity assays.
1. THEORY
3× FLAG, a small tag of only 25 amino acids, has been successfully fused with many proteins. The FLAG tag allows highly specific pull-downs that contain low nonspecific background. This protocol describes isolation of a FLAG-tagged target protein is one step and is therefore relatively quick and simple. FLAG-tags have been used to pull down recombinant proteins made in bacteria, or using a baculovirus system, as well as proteins expressed in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and mammalian cells (reviewed in Einhauer and Jungbauer, 2001).
FLAG-tagged proteins are recognized and bound by the anti-FLAG M2 antibody and efficiently pulled-down using M2-conjugated agarose beads. The pulled-down protein is then eluted from the beads by competition with a 3× FLAG peptide. Anti-FLAG antibodies specifically recognize the target protein within lysates with relatively low cross-reactivity with other cellular proteins; however, the M2 antibody can react with native protein epitopes in mammalian cells (Schäfer and Braun, 1995) as well as in S. pombe (Buker et al., 2007).
We outline a small-scale pull-down; however, the protocol can be scaled up for larger preparations of protein. The protein isolated using this method is suitable for use in a variety of techniques, such as functional assays, analysis by proteomics approaches, such as mass spectrometry to identify binding partners, or to assess associated nucleic acids (Buker et al., 2007). FLAG tags are also widely used for testing if two proteins coimmunoprecipitate (e.g., Gerace et al., 2010) and for chromatin immunoprecipitations (ChIP) (e.g., Buker et al., 2007). Please refer to Sections Co-Immunoprecipitation of proteins from yeast and Chromatin Immunoprecipitation and Multiplex Sequencing (ChIP-Seq) to identify global transcription factor binding sites in the nematode Caenorhabditis elegans for detailed protocols on coimmunoprecipitation and ChIP, respectively.
2. EQUIPMENT
Centrifuge (refrigerated)
Microcentrifuge (refrigerated)
Bead beater (e.g., BioSpec Mini-beadbeater 8)
Micropipettors
Pipettor tips
1.7-ml polypropylene microcentrifuge tubes
1.7-ml low retention microcentrifuge tubes
2-ml screw-capped microcentrifuge tubes
5-ml polypropylene round-bottom tubes
21 gauge needles
0.5-mm glass beads (BioSpec)
End-over-end rotator
3. MATERIALS
HEPES
Sodium chloride (NaCl)
Magnesium chloride (MgCl2)
EDTA
Glycerol
Dithiothreitol (DTT)
Triton X-100
Complete EDTA-free Protease Inhibitor Cocktail tablets (Roche)
Phenylmethylsulfonyl fluoride (PMSF)
Anti-FLAG M2 agarose beads (Sigma)
HA peptide (Sigma)
3× FLAG peptide (Sigma)
3.1. Solutions & buffers
Step 1 2× Buffer G
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Na-HEPES, pH 7.5 | 50 mM | 1 M | 50 ml |
| NaCl | 150 mM | 5 M | 30 ml |
| EDTA | 1 mM | 0.5 M | 2 ml |
Add water to 500 ml. Filter-sterilize and store at 4 °C
1× Lysis Buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| 2× Buffer G | 1× | 2× | 25 ml |
| Triton X-100 | 0.5% | 20% | 1.25 ml |
| Glycerol | 10% | 50% | 10 ml |
| DTT | 0.5 mM | 1 M | 25 µl |
| PMSF | 1 mM | 100 mM | 500 µl |
| Protease Inhibitor Cocktail | 1 tablet |
Add water to 50 ml
Step 3 Wash Buffer 1
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| 2× Buffer G | 1× | 2× | 25 ml |
| Triton X-100 | 0.5% | 20% | 1.25 ml |
| Glycerol | 5% | 50% | 5 ml |
| DTT | 0.5 mM | 1 M | 25 µl |
| PMSF | 1 mM | 100 mM | 500 µl |
Add water to 50 ml
Wash Buffer 2
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| 2× Buffer G | 1× | 2× | 5 ml |
| Glycerol | 5% | 50% | 1 ml |
| PMSF | 1 mM | 100 mM | 100 µl |
Add water to 10 ml
Elution Buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Na-Hepes, pH 7.5 | 25 mM | 1 M | 2.5 ml |
| NaCl | 100 mM | 5 M | 2 ml |
Add water to 100 ml. Filter-sterilize and store at 4 °C
HA buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Elution Buffer | 1 ml | ||
| HA peptide | 100 µg | 10 mg ml−1 | 10 µl |
| PMSF | 1 mM | 100 mM | 10 µl |
Step 4 FLAG buffer
| Component | Final concentration | Stock | Amount |
|---|---|---|---|
| Elution Buffer | 1 ml | ||
| 3× FLAG peptide | 200 µg | 5 mg ml−1 | 40 µl |
| PMSF | 1 mM | 100 mM | 10 µl |
4. PROTOCOL
4.1. Duration
| Preparation | About 1 day |
| Protocol | About 5–6 h |
4.2. Preparation
Grow 250 ml of yeast cells to OD600 = 1–2. Pellet cells and wash once with 1× TBS. For each strain, flash-freeze 0.5 g of cells in each of two 2-ml screw-capped tubes (total of 1 g for each strain). Tubes can be stored at −80 °C until ready to begin the experiment.
Whether the pulled-down protein will be used to find protein–protein or nucleic acid interactions or will be used in functional assays, be sure to include an isogenic untagged strain to serve as a control for background binding.
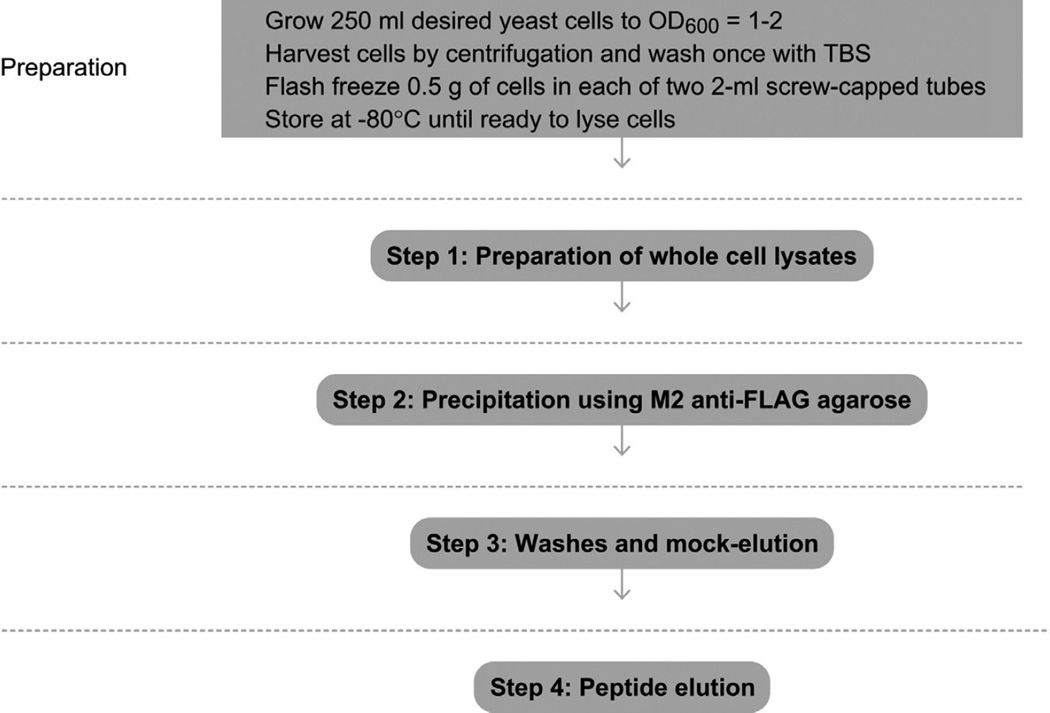
See Fig. 1 for the flowchart of the complete protocol.
Figure 1.
Flowchart of the complete protocol, including preparation.
5. STEP 1 PREPARATION OF WHOLE CELL LYSATES
5.1. Overview
Prepare cell lysates to be added to each pull-down reaction. Cell pellets are thawed, lysed by bead beating, and then cleared by centrifugation to remove cell debris.
5.2. Duration
45 min
-
1.1
To the frozen pellets, add 0.1 ml of ice-cold, freshly prepared lysis buffer for each 0.1 g of cells. Thaw the cells by gently flicking and inverting the tubes. Once completely thawed, keep all tubes on ice.
-
1.2
Add 400 µl of 0.5-mm glass beads to each tube. Pulse the tubes in a bead beating instrument (e.g., BioSpec Mini-beadbeater-8) for 45 s at homogenizing intensity. Place all tubes on ice for 5 min before a second 45-s pulse. Again, place the tubes on ice.
-
1.3
Puncture the bottom of each tube with a 21-gauge needle. Carefully place each punctured tube into an appropriately sized collection tube, such as a 5-ml polypropylene round-bottom tube.
-
1.4
Spin samples in a centrifuge (such as Eppendorf 5702R or Sorvall RC5C) at 4 °C for 5 min at 200 × g.
-
1.5
Gently resuspend the flow-through with a pipettor and transfer the entire volume to a 1.7-ml microcentrifuge tube. Combine the flow-throughs from identical strains into one microcentrifuge tube.
-
1.6
Spin the tubes in a microcentrifuge at 4 °C for 5 min at maximum speed (~20 000 × g).
-
1.7
Pipet the resulting supernatants to clean the microcentrifuge tubes. This is the whole cell lysate.
5.3. Tip
Larger cell pellets can be lysed using a BioSpec bead beating chamber. In this case, other lysis methods, which generate less heat than bead beating, may be more suitable for some protein purifications, and should be considered. These involve lysis in a coffee grinder in the presence of dry ice (Schultz et al., 1997), lysis under liquid nitrogen using a mortar and pestle, or lysis in a liquid nitrogen-cooled, steel chamber (e.g., the Retsch Cryomill).
See Fig. 2 for the flowchart of Step 1.
Figure 2.
Flowchart of Step 1.
6. STEP 2 IMMUNOPRECIPITATION USING ANTI-FLAG TAG ANTIBODY
6.1. Overview
The cell lysates will be incubated with anti-FLAG M2 agarose to pull down the FLAG-tagged target protein.
6.2. Duration
2 h 20 min
-
2.1
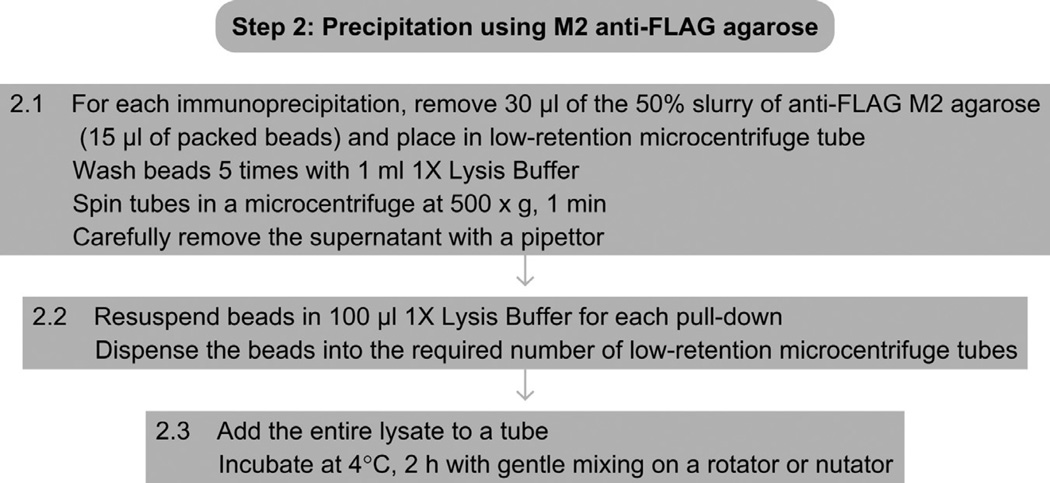
For each immunoprecipitation, remove 30 µl of the 50% slurry of anti-FLAG M2 agarose (15 µl of packed beads) and place in a low-retention microcentrifuge tube. Wash the beads 5 times in 1× Lysis Buffer. Gently resuspend the beads in 1 ml lysis buffer using a cut pipette tip. Between each wash, spin the tubes in a microcentrifuge at 500 × g for 1 min. Carefully remove the supernatant with a pipettor.
-
2.2
Resuspend the washed beads in lysis buffer, in a volume of 100 µl for each pull-down, and aliquot the beads into the required number of low-retention microcentrifuge tubes.
-
2.3
Add the entire lysate from Step 1.7 to a tube of washed M2 agarose beads. Incubate at 4 °C for 2 h on an end-over-end rotator or equivalent.
6.3. Tip
The target protein will be efficiently immunoprecipitated with 15 µl of packed bead volume for 1 g of cells.
6.4. Tip
Using a vacuum aspirator during bead washing can lead to accidental bead loss. Therefore, use a pipettor to remove the wash buffer.
6.5. Tip
When scaling up this protocol to purify from a greater number of cells, use 50 µl of packed M2 agarose beads for lysates made from 10 g of cells. These beads have a high capacity, and using more beads will not necessarily increase protein yield, but will definitely increase the background binding.
6.6. Tip
When working with agarose beads, always cut the end of the pipettor tip using a clean razor blade prior to pipetting the beads. Otherwise, the beads will clog the tip during pipetting, leading to the uptake of more buffer and fewer beads.
See Fig. 3 for the flowchart of Step 2.
Figure 3.
Flowchart of Step 2.
7. STEP 3 WASHES AND MOCK-ELUTION
7.1. Overview
After the M2 beads have incubated with the lysate for 2 h, they will be washed several times. Prior to elution, the beads will be mock-eluted using the HA peptide. This step will eliminate any proteins that would elute in the presence of any peptide not specific to 3× FLAG.
7.2. Duration
30 min
-
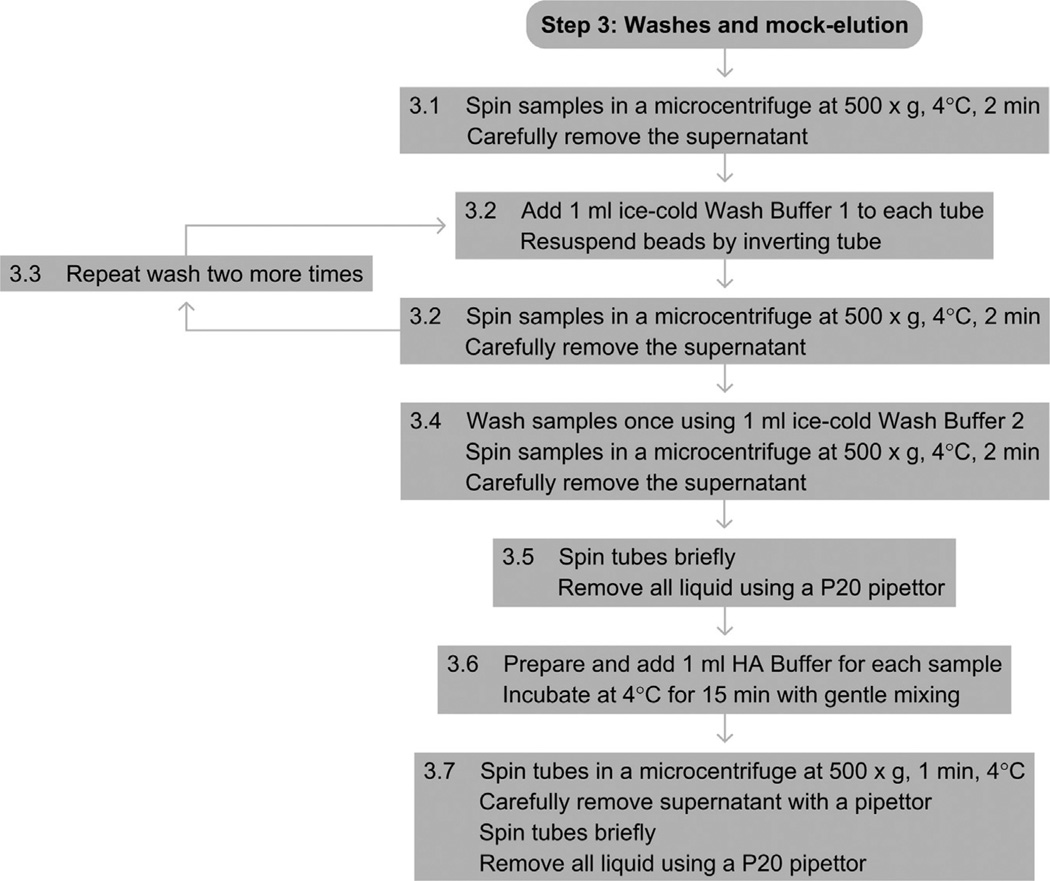
3.1
Spin samples in a microcentrifuge at 500 × g for 2 min at 4 °C. Gently remove the supernanant with a pipettor.
-
3.2
Add 1 ml of ice-cold Wash Buffer 1 to each tube, and resuspend all the beads by gently inverting each tube several times. Make sure all the beads have been completely resuspended. Spin the tubes in a microcentrifuge at 500 × g for 1 min at 4 °C. Carefully remove the supernatant with a pipettor.
-
3.3
Repeat the wash two more times.
-
3.4
Wash a final time using 1 ml of ice-cold Wash Buffer 2.
-
3.5
Spin the tubes again briefly to make sure any of the excess wash buffer does not remain on the sides of the tubes. Use a P20 pipettor to remove all of the excess wash buffer, making sure not to remove any of the beads.
-
3.6
Prepare and add 1 ml of HA Buffer for each pull-down sample. Incubate at 4 °C on an end-over-end rotator or equivalent for 15 min.
-
3.7
Spin the tubes in a microcentrifuge at 500 × g for 1 min at 4 °C. Carefully remove the supernatant with a pipettor. Spin briefly and use a P20 pipettor to remove all the excess buffer, making sure not to remove any of the beads.
See Fig. 4 for the flowchart of Step 3.
Figure 4.
Flowchart of Step 3.
8. STEP 4 PEPTIDE ELUTION
8.1. Overview
During this step, the FLAG-tagged target protein will be eluted from the beads. This is accomplished by adding an excess of 3× FLAG peptide, which will compete for the binding sites on the M2 agarose beads, releasing the protein into the eluate.
8.2. Duration
45 min
-
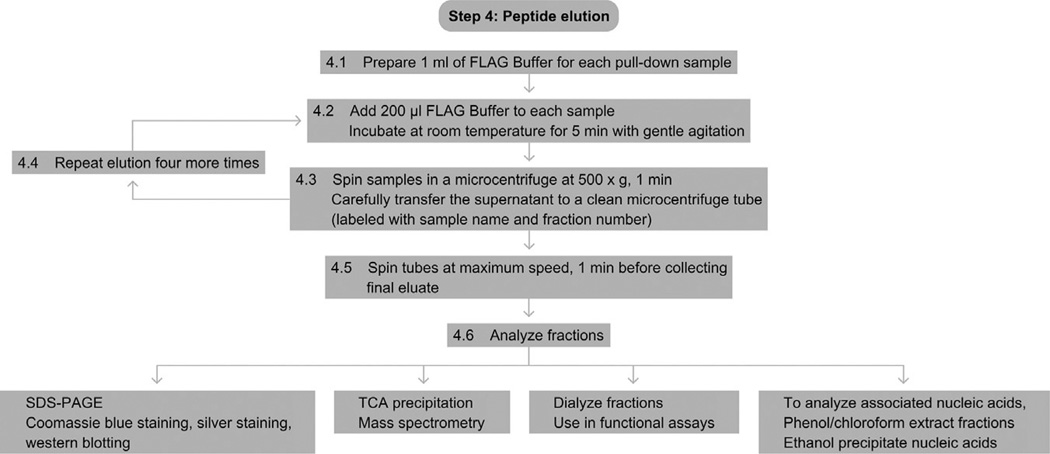
4.1
Prepare 1 ml of FLAG Buffer for each pull-down sample.
-
4.2
Add 200 µl of FLAG Buffer to each tube of washed, mock-eluted beads from Step 3.7. Incubate at room temperature on a nutator or shaking rack (such as an Eppendorf Thermomixer) for 5 min.
-
4.3
Spin the samples at 500 × g in a microcentrifuge for 1 min. Carefully collect the supernatant with a pipettor and transfer it to a clean microcentrifuge tube appropriately labeled with the sample and fraction number.
-
4.4
Repeat Steps 4.2 and 4.3 four more times.
-
4.5
After the final incubation, spin the tubes at maximum speed (~20 000 × g) before collecting the fifth fraction.
-
4.6
The fractions can now be separated by SDS-PAGE (see One-dimensional SDS-Polyacrylamide Gel Electrophoresis (1D SDS-PAGE)) and analyzed by Coomassie Blue staining (see Coomassie Blue Staining), silver staining (see Silver Staining of SDS-polyacrylamide Gel), or Western blotting (see Western Blotting using Chemiluminescent Substrates). Alternatively, the fractions can be TCA-precipitated (see TCA Precipitation) and analyzed by mass spectrometry, or dialyzed and used in functional assays.
For analysis of associated nucleic acids, a phenol/chloroform extraction followed by an ethanol precipitation can be performed on the elution fractions (Halic and Moazed, 2010).
8.3. Tip
This protocol outlines collecting five fractions of 200 µl, but this can be adjusted as necessary.
See Fig. 5 for the flowchart of Step 4.
Figure 5.
Flowchart of Step 4.
Footnotes
Referenced Protocols in Methods Navigator
Co-Immunoprecipitation of proteins from yeast.
Chromatin Immunoprecipitation and Multiplex Sequencing (ChIP-Seq) to identify global transcription factor binding sites in the nematode Caenorhabditis elegans.
One-dimensional SDS-Polyacrylamide Gel Electrophoresis (1D SDS-PAGE).
Coomassie Blue Staining.
Silver Staining of SDS-polyacrylamide Gel.
Western Blotting using Chemiluminescent Substrates.
TCA Precipitation.
REFERENCES
Referenced Literature
- Buker SM, Iida T, Bühler M, et al. Two different Argonaute complexes are required for siRNA generation and heterochromatin assembly in fission yeast. Nature Structural & Molecular Biology. 2007;14:200–207. doi: 10.1038/nsmb1211. [DOI] [PubMed] [Google Scholar]
- Einhauer A, Jungbauer A. The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. Journal of Biochemical and Biophysical Methods. 2001;49(1–3):455–465. doi: 10.1016/s0165-022x(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Gerace EL, Halic M, Moazed D. The methyltransferase activity of Clr4 (Suv39h) triggers RNAi independently of histone H3K9 methylation. Molecular Cell. 2010;39(3):360–372. doi: 10.1016/j.molcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halic M, Moazed D. Dicer-independent primal RNAs trigger RNAi and heterochromatin formation. Cell. 2010;140(4):452–454. doi: 10.1016/j.cell.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer K, Braun T. Monoclonal anti-FLAG antibodies react with a new isoform of rat Mg2+ dependent protein phosphatase beta. Biochemical and Biophysical Research Communications. 1995;207(2):708–714. doi: 10.1006/bbrc.1995.1245. [DOI] [PubMed] [Google Scholar]