Figure 1.
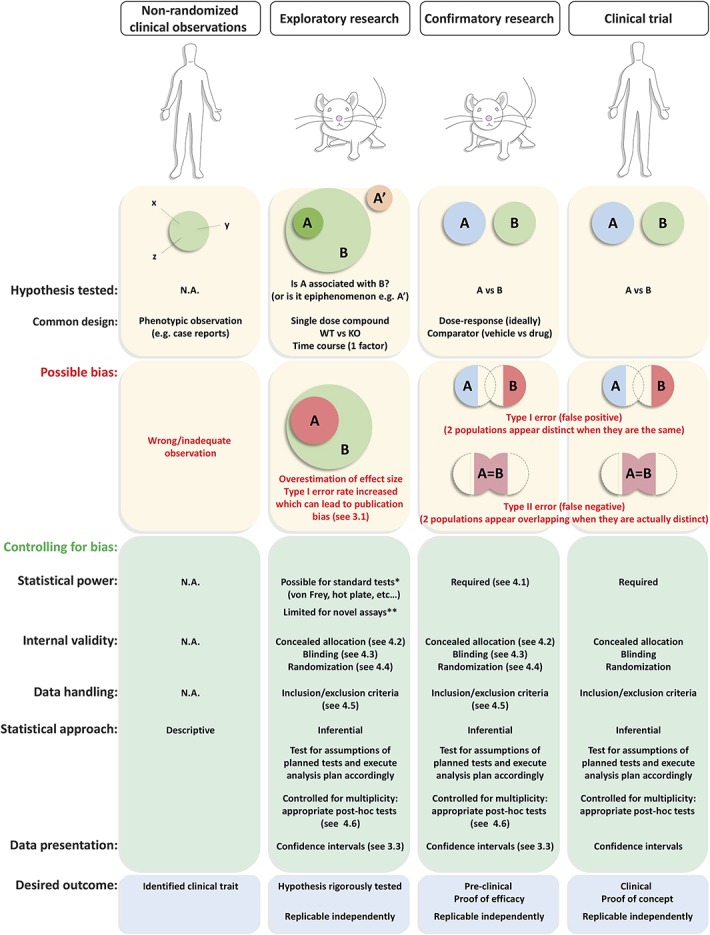
Comparison of different categories of experimental research, illustration of possible bias, and how this may be minimized with best practice to achieve a reliable outcome. Nonrandomized clinical observations, which are typically case reports, are effectively phenotypic observations often of individuals. As such, the recommended statistical approach is descriptive (summarizing the data without making further interpretations) rather than inferential (meaning, formal hypothesis testing or estimation). Such studies are most useful for hypothesis generation because inferences are not being made and so bias is not an issue. For exploratory animal research, the hypothesis being tested can be represented as “Is a mechanism (A) associated with the disease process (eg, neuropathic pain) (B) or is it an epiphenomenon (A')?”. Type 1 errors occur when the null hypothesis is incorrectly rejected; that is, the result is a “false positive” (shown by an enhanced size of A within B in the lower half of the figure). False positives commonly occur because of experimental bias and are arguably of most concern in exploratory research as it is far more likely that a false positive would be published than a false negative because of publication bias favoring the publication of “positive” findings supporting exciting new hypotheses. We propose that careful attention to internal validity (section 4) can help reduce false positives and increase the rigor by which hypotheses are tested. Clear description of inclusion/exclusion criteria, use of appropriate statistical tests after initial tests for normality, and controlling for multiplicity of statistical testing are all recommended to reduce biasing the importance of a finding. *It is possible to determine the appropriate sample size using power calculations because an effect size can be estimated from the historical data generated using standard endpoints of pain assessment in animals. **It might not be possible to determine the sample size using power calculations when both the mechanism and the endpoint are novel; however, one might want to include a known analgesic or modulator of a known mechanism in the experimental design to better define assay sensitivity. Confirmatory (preclinical) research often compares in approach with clinical trials and deals with the question of, for example, whether compound (A) is different from vehicle (B) in much the same way that clinical trials compare a test substance with placebo. This type of experiment tests existing hypotheses (eg, whether antagonism of target A results in antihyperalgesia in a particular model of neuropathic pain). We recommend that the use of power calculations to determine the appropriate sample size can and should be performed because the model and the endpoint are typically validated, and effect sizes of standard analgesics in the model and test are known. Dose responses (rather than single-dose studies) should be performed when possible and should be analysed by the appropriate statistics after tests for normality and with post hoc analysis strategies controlled for multiplicity. It is also recommended to include a comparator such as a positive control whenever possible to demonstrate assay sensitivity that should be expected from a known response.
