Abstract
Rare mutations in PROC, PROS1 or SERPINC1 as well as common variants in F5, F2, F11 and SERPINC1 have been identified as risk factors for deep vein thrombosis (DVT). To identify novel genetic risk factors for DVT, we have developed and applied next-generation DNA sequencing (NGS) of the coding area of hemostatic and proinflammatory genes. Using this strategy, we previously identified a single nucleotide variant (SNV) rs6050 in the FGA gene and novel, rare SNVs in the ADAMTS13 gene associated with DVT. To identify novel coding variants in the genetic predisposition to DVT, we applied NGS analysis of the coding area of 186 hemostatic and proinflammatory genes in 94 DVT cases and 98 controls and we identified 18 variants with putative role in DVT. A group of 585 Italian idiopathic DVT patients and 550 healthy controls was used to genotype all the 18 risk-associated variants identified by NGS. Replication study in the Italian population identified the rs2232710 variant in the protein Z-dependent protease inhibitor (ZPI) gene to be associated with an increased risk of DVT (OR 2.74; 95% CI 1.33–5.65; P = 0.0045; Bonferroni P = 0.081). However, the rs2232710 SNV showed no association with DVT in two Dutch replication cohorts the LETS study (454 patients and 451 controls) and the MEGA study (3799 patients and 4399 controls), indicating that the rs2232710 variant is not a risk factor for DVT.
Introduction
Deep vein thrombosis (DVT) of the lower extremities has a strong genetic basis, with an estimated hereditary component of 60%, but established genetic risk factors for DVT explain only a fraction of disease heritability [1,2]. Genetic risk factors include rare mutations in genes encoding natural anticoagulant proteins such as antithrombin, protein C, protein S or single nucleotide polymorphisms (SNPs) in the F5 gene (rs6025 or Factor V Leiden [FVL]) that impair down-regulation of procoagulant pathway or in the F2 gene (rs1799963 or G20120A) that result in increased levels of prothrombin [3,4]. Moreover, polymorphisms in several genomic loci such as F11, SERPINC1, HIVEP1, GP6, TSPAN15 and SLC44A2 have recently been identified in genome-wide association screens (GWAS) as susceptibility loci for DVT [5–8].
The role of the protein Z (PZ) and protein Z-dependent inhibitor (ZPI) pathway in venous thromboembolism has been recently assessed in clinical studies and using murine models [9]. ZPI is a single-chain glycoprotein that together with its vitamin K-dependent glycoprotein cofactor PZ inhibits activated coagulation factors X and XI [9]. Several non-synonymous variants in the ZPI/SERPINA10 gene (henceforth called ZPI), in particular R67X and W303X mutations, have been identified in patients suffering from thrombophilia [10], even though other studies did not support these findings [11–13]. Similarly, the association of the PZ-ZPI pathway with arterial diseases, ischemic stroke, or unexplained early pregnancy loss yielded conflicting results [14–18]. In animal model, the knockout (KO) of either ZP or ZPI gene resulted in enhanced thrombotic phenotype following arterial injury [19]. However, the combination of ZPI deficiency with the homozygous FVL variant led to a more severe thrombotic phenotype than PZ KO/FVL, implying an important role for the ZPI protein in the inhibition of activated XI [19]. Recently, two missense mutations F145L and Q384R in the ZPI gene were shown to impair the inhibitory activity of the ZPI protein in vitro, even though they were not associated with DVT in humans [20].
The advent of powerful ‘next-generation’ DNA sequencing technology offers unprecedented opportunities to identify genetic risk factors for complex disorders such as deep vein thrombosis. These novel technologies enable sequencing of large proportions of the human genome (or even the entire genome) at unparalleled speed and costs. Deep sequencing of all protein-coding areas of the genome is rapidly becoming the gold standard for the identification of causal genes underlying the development of both common and rare Mendelian diseases [21–24]. To assess the potential role of rare coding variants underlying genetic predisposition to venous thrombosis, we have recently developed and successfully applied targeted next-generation DNA sequencing-based (NGS) strategy and analysis of the coding area of 186 hemostatic and proinflammatory genes in Italian idiopathic DVT cases and controls. Using this approach, we identified a non-synonymous variant rs6050 in the FGA gene [25] as well as several rare coding single nucleotide variants (SNVs) in the ADAMTS13 gene [26] as risk factors for DVT. The aim of this study was to assess the potential role of non-synonymous coding variants explaining genetic predisposition to DVT.
Materials and Methods
Participants
The details of the recruitment of DVT patients and healthy controls have been described elsewhere [25]. Briefly, DVT patients and healthy controls for this case-control study were from the DVT-Milan study. A total of 2139 unrelated Italian patients with DVT and 1938 healthy controls were recruited to the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center (Milan, Italy) between 1995 and 2010. For this study, we identified 719 unrelated idiopathic DVT cases that were diagnosed for DVT of the lower limbs. DVT cases were selected according to the following criteria: (i) objective diagnosis of DVT; (ii) Caucasian ethnicity born from Caucasian parent; (iii) absence of cancer or surgery associated with DVT; (iv) absence of natural anticoagulant deficiencies determined by natural levels of protein C, protein S and antithrombin in routine testing; (v) absence of factor V Leiden and prothrombin G20120A variants determined by sequencing; and (vi) signed informed consent. The study was approved by the Medical Ethics Committee of the Fondazione IRCCS Ca’ Granda, Hospital Maggiore and has been carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). Patients recruitment, sampling and thrombophilia screening was performed at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy. The next-generation DNA sequencing was performed on 94 idiopathic DVT cases and 98 controls (discovery phase) at the Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX, USA. Replication in the Italian patients and controls as well as in the two Dutch case-control studies were carried out at the Leiden University Medical Center, Leiden, Netherlands.
Sequencing and data analysis (discovery phase)
Data presented in this article have been sequenced and analyzed as a part of work previously described by Lotta et al. [25,26]. The protein-coding regions and intron-exon boundaries of 186 candidate hemostatic and proinflammatory genes were sequenced in 94 Italian cases of idiopathic DVT and 98 healthy controls using Applied Biosystems SOLiD 4 sequencing system at the Human Genome Sequencing Centre (HGSC) at Baylor College of Medicine, Houston, USA. A gene list is provided elsewhere [25,26]. Variant calling steps included data analysis on raw reads to produce individual binary alignment/mapping (BAM) files and PILEUP files using SAMtools [27]. Variants not meeting quality control criteria as specified by the HGSC were removed. Genetic variants were annotated on the dbSNPvs130 [28], SIFT [29] and Polyphen-2 [30] databases using ANNOVAR software [31]. Next, the Nxtgen2plink.rb software [25] was used to merge individual BAM and PILEUP files that were used for association analysis.
To carry out association analysis, we estimated the minor allele frequency (MAF) of the variants identified in our discovery cohort of 94 Italian idiopathic DVT cases and 98 matched healthy controls. Rare variants were defined as MAF ≤ 1% and common and low-frequency variants as those with MAF > 1%. The association of these coding variants with DVT was performed by individual-variant testing using two-sided Fisher’s exact test and by calculating odds ratio (OR) and 95% confidence interval (CI). In addition, we performed functional annotation of each variant identifying synonymous, non-synonymous or missense variants predicted to be damaging by SIFT [29] and Polyphen-2 [30] variant predictor databases. Genetic association testing was carried out using Plink v1.07 [32] and R programming language [33].
Replication
The Italian replication cohort consisted of 585 idiopathic DVT cases and 550 matched healthy controls. The top association of the rs2232710 variant with DVT was further tested in two Dutch studies: in 454 DVT cases and 451 controls of the Leiden Thrombophilia Study (LETS) [34] and in 3799 cases with a DVT and/or pulmonary embolism and 4399 controls of the Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Study (MEGA) [35]. Replication was performed using TaqMan genotyping at the Leiden University Medical Center, Leiden, Netherlands. The obtained results were subjected to chi-squared statistical test. To assess the association of all variants with DVT, OR and 95% CI were calculated. The P values were two-sided and P<0.05 were considered statistically significant. Bonferroni correction was used to correct for multiple testing. Genetic association testing was carried out using Plink [32] and R programming language [33]. Quantile-Quantile (QQ) plots of p-value distributions for common and low-frequency (MAF > 1%) and rare variants (MAF ≤ 1%) were generated using R programming language [33].
Results
To assess the potential role of coding variants underlying genetic predisposition to DVT, we applied targeted next-generation DNA sequencing of the coding area of 186 hemostatic and proinflammatory genes in 94 idiopathic DVT patients and 98 matched healthy controls. As previously described by Lotta et al. [26], quality control and variant filtering criteria such as consensus in the presence of mismatches, read-quality and base-quality parameters, allele balance and strand bias were used to distinguish genetic variants from sequencing errors. In this way, we identified 4366 SNVs and 187 indels in the 192 individuals that were sequenced with an average read-depth of 44 over each site. To assess the risk of DVT in individuals carrying these variants, we calculated minor allele frequencies in cases and controls and performed single variant association analysis using two-sided Fisher’s exact test. In this manner, we obtained a list of 18 genetic variants in 12 genes that showed marked differences in MAF between cases and controls, and therefore, were selected for further analysis (Table 1).
Table 1. Single nucleotide variants identified in targeted NGS pilot study in 94 DVT idiopathic patients and 98 healthy controls.
| Gene | Chr. | Genomic position | dbSNP ID | Variant type | Minor allele | Protein change | SIFT | Poly-Phen2 | Cases | Controls | OR (95% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alleles | MAF | Alleles | MAF | |||||||||||
| ZPI/ SERPINA10 | 14 | 94750486 | rs2232710 | missense | C | Q384R | Dam | Ben | 5 | 0.03 | 0 | 0 | NC | 0.03 |
| HTR3B | 11 | 113803028 | rs1176744 | missense | C | Y129S | Ben | Ben | 47 | 0.25 | 67 | 0.34 | 0.64 (0.4–1) | 0.17 |
| HTR3B | 11 | 113803666 | rs17116138 | missense | A | V183I | Ben | Ben | 1 | 0.01 | 7 | 0.04 | 0.14 (0.02–1.2) | 0.07 |
| HIF1A | 14 | 62207557 | rs11549465 | missense | T | P582S | Ben | Ben | 23 | 0.12 | 11 | 0.06 | 2.3 (1.1–5) | 0.05 |
| ADRB2 | 5 | 148206440 | rs1042713 | missense | A | G16R | Ben | Ben | 70 | 0.37 | 53 | 0.27 | 1.6 (1.04–2.5) | 0.15 |
| F13A1 | 6 | 6196141 | rs5978 | intron | T | - | - | - | 37 | 0.20 | 20 | 0.10 | 2.2 (1.2–3.8) | 0.03 |
| F13A1 | 6 | 6174856 | rs5986 | syn. | G | E/E | - | - | 30 | 0.16 | 14 | 0.07 | 2.5 (1.3–4.8) | 0.02 |
| TMEM116 | 12 | 112378768 | rs7133881 | intron | C | - | - | - | 4 | 0.02 | 0 | 0 | NC | 0.06 |
| PRKCA | 17 | 64685078 | rs2227857 | syn. | A | L/L | - | - | 55 | 0.29 | 85 | 0.43 | 0.54 (0.4–0.8) | 0.06 |
| COL4A1 | 13 | 110839550 | rs536174 | missense | A | T555P | Ben | Ben | 17 | 0.09 | 32 | 0.16 | 0.51 (0.3–0.95) | 0.07 |
| COL4A2 | 13 | 111111235 | rs7990383 | missense | A | R517K | Ben | Ben | 77 | 0.41 | 60 | 0.30 | 1.6 (1.03–2.4) | 0.16 |
| COL4A2 | 13 | 111119396 | rs3803230 | missense | C | G683A | Ben | Ben | 19 | 0.10 | 38 | 0.19 | 0.47 (0.26–0.8) | 0.03 |
| COL4A2 | 13 | 111098226 | rs4103 | syn. | T | P/P | - | - | 77 | 0.41 | 109 | 0.55 | 0.55 (0.4–0.83) | 0.11 |
| COL4A2 | 13 | 111109670 | rs9515217 | intron | C | - | - | - | 9 | 0.05 | 34 | 0.17 | 0.24 (0.1–0.51) | 0.001 |
| COL4A3 | 2 | 228111435 | rs10178458 | missense | C | L141P | Ben | Ben | 32 | 0.17 | 52 | 0.26 | 0.6 (0.4–0.93) | 0.1 |
| COL6A2 | 21 | 47542779 | rs17357592 | intron | T | - | - | - | 53 | 0.28 | 27 | 0.14 | 2.5 (1.5–4.1) | 0.01 |
| COL6A2 | 21 | 47541986 | rs9976026 | intron | C | - | - | - | 49 | 0.26 | 22 | 0.11 | 2.8 (1.6–4.8) | 0.002 |
| COL6A3 | 2 | 238262021 | rs36117715 | missense | T | P2218L | Dam | Ben | 11 | 0.06 | 2 | 0.01 | 6 (1.3–28) | 0.02 |
Chr. Chromosome; REF—reference allele sequence; ALT—alternative allele sequence; MAF—minor allele frequency; OR—odds ratio; 95% CI– 95% confidence interval. SIFT and PolyPhen2 predictions: Syn.–synonymous variant; Dam—damaging, predicted to affect protein function; Ben—benign (tolerated), predicted to have no effect on protein function; NC—not calculable.
Among these 18 genetic variants, we identified nine SNVs in genes encoding alpha chains 1, 2 and 3 of Collagen Type IV (COL4A1, COL4A2, COL4A3 genes) and alpha chains 2 and 3 of Collagen Type VI (COL6A2 and COL6A3). Furthermore, we found two missense variants rs1176744 and rs17116138 in the 5-Hydroxytryptamine (Serotonin) Receptor 3B (HTR3B) gene and an intron variant rs5978 and a synonymous variant rs5986 in the Coagulation Factor XIII, A1 Polypeptide (F13A1) gene. Three genes ZPI, HIF1A and ADRB2 carried missense substitutions and the TMEM116 and PRKCA genes carried intron and synonymous variants, respectively (Table 1).
The majority of SNVs identified were common or low-frequency variants with the minor allele frequencies above 1% (Table 1). In addition, we identified three rare variants, of which two, the rs2232710 missense variant in the ZPI gene and the rs7133881 intron variant in the TMEM116 gene were particularly interesting as we did not detect any risk allele in the controls, indicating that they are probably rare or low-frequency in the general population. The remaining rare variant was the rs36117715 missense substitution in the COL6A3 gene. Furthermore, we identified 10 variants with the risk allele frequencies higher in patients than in the healthy individuals, indicating that they could be risk factors for DVT. The remaining SNVs showed the opposite trend, suggesting a protective role in the development of this disorder (Table 1).
Ten of these 18 variants were non-synonymous missense mutations that resulted in amino acid substitutions (Table 1). The remaining eight variants were either intronic or synonymous changes. To assess the putative impact of an amino acid substitution on the structure and function of the protein, we performed an in silico analysis of all ten missense variants using SIFT and PolyPhen-2 variant predictor databases. We identified two SNVs, rs2232710 in the ZPI gene and rs36117715 in the COL6A3 gene that were predicted to be damaging by SIFT prediction (Table 1). The rs2232710 SNV encodes a missense change of Glutamine to Arginine at position p.Gln384Arg (Q384R) that has been previously shown to impair the inhibitory activity of the ZPI protein towards activated factor X (FXa) in vitro [20]. The rs36117715 SNV encodes Proline to Lysine amino acid change at position p.Pro2218Lys (P2218L) and its effect on the structure or function of the Col6a3 protein is unknown.
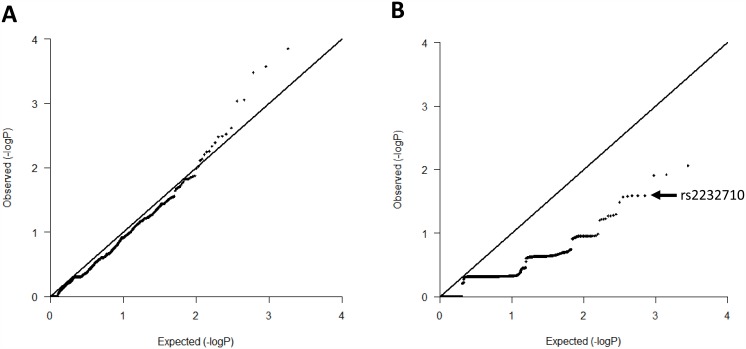
Although the advances in NGS technologies have significantly improved sequencing fidelity, the NGS data is still frequently plagued with false-positives and false-negatives [36], which often leads to signal inflation. For this reason, we generated QQ plots of p-value distributions of common and low-frequency (MAF > 1%) and rare variants (MAF ≤ 1%). As shown in Fig 1, the distribution of p-values of common and low-frequency variants showed good agreement with the expected distribution, suggesting low bias due to population stratification or technical artifacts. Rare variants however, showed a deflated p-value distribution. Since a variant can be a true or false positive in context of both inflated and deflated QQ plot, we decided to replicate all 18 variants identified by targeted sequencing. We applied TaqMan genotyping replication strategy in 585 Italian idiopathic DVT cases and 550 healthy controls. The number of DVT cases and healthy controls carrying a given risk allele in respect to an effective sample size as well as minor allele frequencies in both cases and controls are reported in Table 2. The only variant associated with DVT in the DVT-Milan replication cohort was the rs2232710 missense substitution in the ZPI gene with OR = 2.74 (95% CI 1.3–5.7; P = 0.0045; Bonferroni P = 0.081). We observed the rs2232710-C risk allele in 29 Italian idiopathic DVT cases and 10 healthy controls, corresponding to a MAF of 0.025 and 0.009 for cases and controls, respectively. For the other 17 SNVs, the risk alleles were equally distributed between cases and controls (Table 2), suggesting that these are not susceptibility loci for DVT. The discrepancy between the MAF obtained from our targeted sequencing and replication of the rs536174 SNV in the COL4A1 gene is most likely an NGS error. The raw data from our Italian replication population is presented in S1 Table.
Fig 1. Quantile-Quantile (QQ) plot of p-value distributions for A. common and low-frequency variants (MAF > 1%) and B. rare variants (MAF ≤ 1%).
An arrow indicates the rs2232710 variant.
Table 2. Association results of the 18 variants identified in the NGS and replicated in up to 585 idiopathic DVT patients and 550 healthy controls from the DVT-Milan study.
Analysis was performed using chi-squared test.
| Gene | Chr. | dbSNP ID | Cases | Controls | Effective sample size | OR (95% CI) | P-value | Bonferroni P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Alleles | MAF | Alleles | MAF | |||||||
| ZPI/ SERPINA10 | 14 | rs2232710 | 29 | 0.025 | 10 | 0.009 | 1119 | 2.74 (1.3–5.7) | 0.0045 | 0.081 |
| HTR3B | 11 | rs1176744 | 317 | 0.324 | 311 | 0.343 | 1118 | 0.9 (0.8–1.1) | 0.34 | 1 |
| HTR3B | 11 | rs17116138 | 24 | 0.021 | 26 | 0.024 | 1119 | 0.9 (0.5–1.5) | 0.58 | 1 |
| HIF1A | 14 | rs11549465 | 143 | 0.134 | 132 | 0.132 | 1118 | 1 (0.8–1.3) | 0.88 | 1 |
| ADRB2 | 5 | rs1042713 | 339 | 0.374 | 321 | 0.365 | 1113 | 1 (0.9–1.2) | 0.65 | 1 |
| F13A1 | 6 | rs5978 | 293 | 0.301 | 274 | 0.300 | 1117 | 1 (0.8–1.2) | 0.98 | 1 |
| F13A1 | 6 | rs5986 | 125 | 0.116 | 117 | 0.116 | 1118 | 1 (0.8–1.3) | 0.96 | 1 |
| TMEM116 | 12 | rs7133881 | 162 | 0.154 | 164 | 0.163 | 1112 | 0.9 (0.7–1.2) | 0.56 | 1 |
| PRKCA | 17 | rs2227857 | 322 | 0.338 | 314 | 0.352 | 1116 | 0.9 (0.8–1.1) | 0.48 | 1 |
| COL4A1 | 13 | rs536174 | 0 | 0 | 1 | 0.002 | 1104 | NC | 0.14 | 1 |
| COL4A2 | 13 | rs7990383 | 347 | 0.389 | 326 | 0.373 | 1118 | 1.1 (0.9–1.3) | 0.44 | 1 |
| COL4A2 | 13 | rs3803230 | 153 | 0.141 | 156 | 0.153 | 1119 | 0.9 (0.7–1.2) | 0.43 | 1 |
| COL4A2 | 13 | rs4103 | 453 | 0.522 | 416 | 0.521 | 1113 | 1 (0.9–1.2) | 0.96 | 1 |
| COL4A2 | 13 | rs9515217 | 121 | 0.108 | 128 | 0.122 | 1118 | 0.9 (0.7–1.1) | 0.3 | 1 |
| COL4A3 | 2 | rs10178458 | 208 | 0.203 | 206 | 0.207 | 1111 | 1 (0.8–1.2) | 0.83 | 1 |
| COL6A2 | 21 | rs17357592 | 166 | 0.154 | 171 | 0.169 | 1044 | 0.9 (0.7–1.1) | 0.21 | 1 |
| COL6A2 | 21 | rs9976026 | 159 | 0.177 | 171 | 0.194 | 1015 | 0.9 (0.7–1.1) | 0.32 | 1 |
| COL6A3 | 2 | rs36117715 | 65 | 0.058 | 49 | 0.046 | 1117 | 1.3 (0.9–1.9) | 0.2 | 1 |
Chr. Chromosome; MAF—minor allele frequency; OR—odds ratio; 95% CI– 95% confidence interval; NC—not calculable.
To confirm the association of the rs2232710 missense variant with DVT, we decided to replicate this variant in two independent Dutch studies: in 454 cases and 451 controls in the LETS study [34] and in 3799 cases and 4399 controls in the MEGA study [35]. Neither of the Dutch cohorts confirmed our previously obtained association with an OR = 0.71 (95% CI 0.36–1.39) and OR = 0.92 (95% CI 0.74–1.16) for LETS and MEGA, respectively (Table 3). Indeed, we observed the rs2232710-C risk allele equally distributed between cases and controls in both LETs (controls 0.023, cases 0.016) and MEGA (controls 0.019, cases 0.018) (Table 3).
Table 3. Replication analysis of the rs2232710 (SERPINA10/ZPI gene) single nucleotide variant in patients with idiopathic deep vein thrombosis of lower extremities and heathy controls belonging to three independent cohorts: DVT-Milan, LETS and MEGA.
Analysis was performed using chi-squared test.
| rs2232710 genotype | ||||||
|---|---|---|---|---|---|---|
| TT | TC | CC | MAF | OR (95% CI) | P value | |
| DVT-Milan | ||||||
| Controls | 529 | 10 | 0 | 0.009 | 1 (ref) | |
| Cases | 551 | 29 | 0 | 0.025 | 2.74 (1.3–5.7) | 0.0045 |
| LETS | ||||||
| Controls | 451 | 19 | 1 | 0.023 | 1 (ref) | |
| Cases | 454 | 13 | 1 | 0.016 | 0.7 (0.4–1.4) | 0.32 |
| MEGA | ||||||
| Controls | 4399 | 144 | 14 | 0.019 | 1 (ref) | |
| Cases | 3799 | 127 | 5 | 0.018 | 0.9 (0.7–1.2) | 0.48 |
| TOTAL | ||||||
| Controls | 5379 | 173 | 15 | 0.018 | 1 (ref) | |
| Cases | 4804 | 169 | 6 | 0.018 | 1 (0.8–1.2) | 0.98 |
DVT-Milan—Deep Vein Thrombosis Milan Study; LETS—Leiden Thrombophilia Study; MEGA—Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis Study; TT—reference genotype; TC—heterozygote for rs2232710-C risk allele; CC—homozygote for rs2232710-C risk allele; MAF—minor allele frequency; OR—odds ratio; CI—confidence interval.
Discussion
In this manuscript, we report the results of targeted next-generation DNA sequencing of the coding area of 186 hemostatic and proinflammatory genes in 94 idiopathic DVT cases and 98 matched healthy controls employed to assess the potential role of coding variants underlying genetic predisposition to DVT. Using this approach we identified 18 single nucleotide variants in 12 genes with putative role in DVT. Replication of these variants in 585 Italian idiopathic DVT cases and 550 healthy controls from the DVT-Milan cohort revealed the rs2232710 variant in the ZPI gene as putative risk factor for DVT. However, independent replication in the two Dutch studies LETS and MEGA did not reproduce this finding. These results confirmed in a large replication cohort the lack of association of the rs2232710 variant with DVT that was previously observed by Young et al. [20].
The reasons for the different prevalence of the rs2232710-C risk allele among controls and cases in DVT-Milan replication population and in the two independent Dutch cohorts LETS and MEGA as well as that of Young et al. [20] may be the different recruitment criteria for patients and controls, different ancestral origin or a random effect due to the relatively small Italian replication cohort. Since other genetic risk factors such as FVL and prothrombin G20120A variants or deficiencies in natural anticoagulants (protein C, protein S and antithrombin) can influence the predisposition to develop venous thrombosis, patients carrying these defects were excluded from our analysis. In the study conducted by Young et al. [20], only the presence of the FVL variant was reported leaving all the other thrombophilia markers unchecked. The different patient recruitment criteria may also be a reason for the negative replication of the rs2232710 in the Dutch population where patients with provoked and unprovoked first events of deep vein thrombosis or pulmonary embolism have been included [34,35].
Another explanation might be the different ancestral origin of the replication cohorts. Our discovery and first replication cohorts consisted mainly of the Southern European Italian ancestry, whereas, that of LETS and MEGA were mainly of the Dutch origin and that of Young et al. [20] of the white British ancestry of New Zealand. Indeed, comparing our study with that of Young et al. [20] we noticed that with the similar size of the replication cohorts, we observed marked differences in the prevalence of the rs2232710-C risk allele in DVT patients compared to controls (5% and 1.9%, respectively), while the previous study did not. We also found differences in MAF in controls coming from our DVT-Milan study and those from LETS and MEGA studies. In comparison to the frequency of the rs2232710-C risk allele in controls from the DVT-Milan, we observed a 2.5-fold and 2.1-fold increase in MAF of controls from LETS and MEGA, respectively. Moreover, we found the rs2232710-C risk allele present in homozygous state (rs2232710-C/C) in both cases and controls in the LETS and MEGA studies but none in our DVT-Milan study (Table 3). To verify this result, we analyzed the prevalence of the rs2232710-C risk allele in healthy European individuals in the 1000 Genomes project phase 3 [37] and the HapMap project phase 3 [38]. We found that in the 1000 Genomes project, the MAF for Italian (Population of Tuscany; TSI) and Iberian populations were identical (0.9%) to that obtained from our replication in the heathy Italian individuals. In comparison, the allele frequency found in the British population from England and Scotland was 1.6%. Similar estimates were obtained when looking at the HapMap project with the MAF of 1.1% and 1.8% for the Italian Tuscany population and general European population, respectively. Since the results of both the 1000 Genomes and the HapMap projects were based on relatively small number of alleles sequenced, we decided to check the estimates for the rs2232710-C risk allele in the Exome Variant Server (URL: http://evs.gs.washington.edu/EVS/; accessed on 18 September 2015) and we found the minor allele frequency for our variant to be 1.1% (with sequences from 8502 alleles from 4251 European Americans). Based on these observations, especially when considering that the healthy Italian and the Iberian individuals harbor similar allele frequencies, one is tempted to speculate about a potential prothrombotic role of the rs2232710-C risk allele solely in the Southern European populations. However, a large-scale genotyping studies in the Southern European ancestries would have to be conducted to confirm the rs2232710 variant as a risk factor for DVT in the Southern European populations.
Similar, however opposite effects have been observed for a nonsense mutation, Trp303Stop (W303X), in the ZPI gene. The W303X has been identified as risk factor for DVT in the New Zealand white British ancestry [10]. In the Southern European Spanish and Italian ancestries however, the presence of the W303X mutation is rare and is not associated with DVT [11–13], suggesting that it may have a thrombotic relevance only in the Northern European ancestries. However, sampling error due to the limited replication cohorts may have influenced the results obtained for the rs2232710 SNV in our Italian population as well as those obtained by Van der Water et al. [10] for the W303X mutation. In fact, correcting the results of our Italian replication analysis for multiple testing using the Bonferroni correction revealed borderline significance for the rs2232710 variant (Table 2). In addition, QQ plot analysis of the p-value distribution of rare variants showed a greatly deflated signal (Fig 1). Although this kind of behavior is frequently observed in p-value distributions of rare variants where large P values are obtained due to the rarity of their allele counts, it may also reflect bias based on population stratification, differences between cases and controls, lack of statistical power or sequencing errors.
In conclusion, our NGS analysis revealed 18 variants with putative role in DVT. One of them, a missense variant rs2232710 (Q384R) in the ZPI gene, was associated with DVT in the Southern European, Italian replication cohort. An independent, large-scale replication in two Dutch cohorts did not confirm this association, implying that the presence of this variant does not increase the risk for venous thrombosis.
Supporting Information
(XLSX)
Acknowledgments
The authors would like to thank the following colleges from Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Cà Granda Hospital Maggiore: A. Cairo for help with experiments and sample shipment, P. Bucciarelli for help in recruiting patients and M. Boscarino for help in the preparation of the figures. We thank F.R. Rosendaal from the Departments of Clinical Epidemiology, Thrombosis and Hemostasis, Leiden University Medical Center for critical reading of the manuscript.
Data Availability
All relevant data are available from previously published articles: Lotta LA et al. BMC Med Genomics. 2012 (doi: 10.1186/1755-8794-5-72012) and Lotta LA et al. J Thromb Haemost. 2013 (doi: 10.1111/jth.12291).
Funding Statement
This work was supported by the Italian Ministry of Health Grant No. RF-2009-1530493 and the Fondazione Cariplo Grant No. 2011-0524.
References
- 1.Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009. March 23;169(6):610–5. 10.1001/archinternmed.2008.589 [DOI] [PubMed] [Google Scholar]
- 2.Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost 2009. July;7 Suppl 1:301–4. 10.1111/j.1538-7836.2009.03394.x [DOI] [PubMed] [Google Scholar]
- 3.Dahlback B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood 2008. July 1;112(1):19–27. 10.1182/blood-2008-01-077909 [DOI] [PubMed] [Google Scholar]
- 4.Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nat Rev Cardiol 2014. March;11(3):140–56. 10.1038/nrcardio.2013.211 [DOI] [PubMed] [Google Scholar]
- 5.Bezemer ID, Bare LA, Doggen CJ, Arellano AR, Tong C, Rowland CM, et al. Gene variants associated with deep vein thrombosis. JAMA 2008. March 19;299(11):1306–14. 10.1001/jama.299.11.1306 [DOI] [PubMed] [Google Scholar]
- 6.Tregouet DA, Heath S, Saut N, Biron-Andreani C, Schved JF, Pernod G, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood 2009. May 21;113(21):5298–303. 10.1182/blood-2008-11-190389 [DOI] [PubMed] [Google Scholar]
- 7.Morange PE, Bezemer I, Saut N, Bare L, Burgos G, Brocheton J, et al. A follow-up study of a genome-wide association scan identifies a susceptibility locus for venous thrombosis on chromosome 6p24.1. Am J Hum Genet 2010. April 9;86(4):592–5. 10.1016/j.ajhg.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Germain M, Chasman DI, de Haan H, Tang W, Lindstrom S, Weng LC, et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet 2015. April 2;96(4):532–42. 10.1016/j.ajhg.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corral J, Gonzalez-Conejero R, Hernandez-Espinosa D, Vicente V. Protein Z/Z-dependent protease inhibitor (PZ/ZPI) anticoagulant system and thrombosis. Br J Haematol 2007. April;137(2):99–108. [DOI] [PubMed] [Google Scholar]
- 10.Van de Water N, Tan T, Ashton F, O'Grady A, Day T, Browett P, et al. Mutations within the protein Z-dependent protease inhibitor gene are associated with venous thromboembolic disease: a new form of thrombophilia. Br J Haematol 2004. October;127(2):190–4. [DOI] [PubMed] [Google Scholar]
- 11.Corral J, Gonzalez-Conejero R, Soria JM, Gonzalez-Porras JR, Perez-Ceballos E, Lecumberri R, et al. A nonsense polymorphism in the protein Z-dependent protease inhibitor increases the risk for venous thrombosis. Blood 2006. July 1;108(1):177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzari C, Martinelli I, Bucciarelli P, Viscardi Y, Biguzzi E. Polymorphisms of the protein Z-dependent protease inhibitor (ZPI) gene and the risk of venous thromboembolism. Thromb Haemost 2006. May;95(5):909–10. [PubMed] [Google Scholar]
- 13.Fabbro D, Barillari G, Damante G. Mutations R67X and W303X of the protein Z-dependent protease inhibitor gene and venous thromboembolic disease: a case-control study in Italian subjects. J Thromb Thrombolysis 2007. February;23(1):77–8. [DOI] [PubMed] [Google Scholar]
- 14.Sofi F, Cesari F, Tu Y, Pratesi G, Pulli R, Pratesi C, et al. Protein Z-dependent protease inhibitor and protein Z in peripheral arterial disease patients. J Thromb Haemost 2009. May;7(5):731–5. 10.1111/j.1538-7836.2009.03325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gris JC, Quere I, Dechaud H, Mercier E, Pincon C, Hoffet M, et al. High frequency of protein Z deficiency in patients with unexplained early fetal loss. Blood 2002. April 1;99(7):2606–8. [DOI] [PubMed] [Google Scholar]
- 16.Kobelt K, Biasiutti FD, Mattle HP, Lammle B, Wuillemin WA. Protein Z in ischaemic stroke. Br J Haematol 2001. July;114(1):169–73. [DOI] [PubMed] [Google Scholar]
- 17.McQuillan AM, Eikelboom JW, Hankey GJ, Baker R, Thom J, Staton J, et al. Protein Z in ischemic stroke and its etiologic subtypes. Stroke 2003. October;34(10):2415–9. [DOI] [PubMed] [Google Scholar]
- 18.Refaai MA, Ahn C, Lu L, Wu K, Broze GJ Jr. Protein Z and ZPI levels and cardiovascular events. J Thromb Haemost 2006. July;4(7):1628–9. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Tu Y, Lu L, Lasky N, Broze GJ Jr. Protein Z-dependent protease inhibitor deficiency produces a more severe murine phenotype than protein Z deficiency. Blood 2008. May 15;111(10):4973–8. 10.1182/blood-2007-12-126391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young LK, Birch NP, Browett PJ, Coughlin PB, Horvath AJ, Van de Water NS, et al. Two missense mutations identified in venous thrombosis patients impair the inhibitory function of the protein Z dependent protease inhibitor. Thromb Haemost 2012. May;107(5):854–63. 10.1160/TH11-10-0708 [DOI] [PubMed] [Google Scholar]
- 21.Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature 2009. September 10;461(7261):272–6. 10.1038/nature08250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 2010. January;42(1):30–5. 10.1038/ng.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet 2010. September;42(9):790–3. 10.1038/ng.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foo JN, Liu JJ, Tan EK. Whole-genome and whole-exome sequencing in neurological diseases. Nat Rev Neurol 2012. September;8(9):508–17. 10.1038/nrneurol.2012.148 [DOI] [PubMed] [Google Scholar]
- 25.Lotta LA, Wang M, Yu J, Martinelli I, Yu F, Passamonti SM, et al. Identification of genetic risk variants for deep vein thrombosis by multiplexed next-generation sequencing of 186 hemostatic/pro-inflammatory genes. BMC Med Genomics 2012;5:7 10.1186/1755-8794-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotta LA, Tuana G, Yu J, Martinelli I, Wang M, Yu F, et al. Next-generation sequencing study finds an excess of rare, coding single-nucleotide variants of ADAMTS13 in patients with deep vein thrombosis. J Thromb Haemost 2013. July;11(7):1228–39. 10.1111/jth.12291 [DOI] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009. August 15;25(16):2078–9. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherry ST, Ward M, Sirotkin K. dbSNP-database for single nucleotide polymorphisms and other classes of minor genetic variation. Genome Res 1999. August;9(8):677–9. [PubMed] [Google Scholar]
- 29.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 2003. July 1;31(13):3812–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res 2002. September 1;30(17):3894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010. September;38(16):e164 10.1093/nar/gkq603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007. September;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clayton D, Leung HT. An R package for analysis of whole-genome association studies. Hum Hered 2007;64(1):45–51. [DOI] [PubMed] [Google Scholar]
- 34.van der Meer FJ, Koster T, Vandenbroucke JP, Briet E, Rosendaal FR. The Leiden Thrombophilia Study (LETS). Thromb Haemost 1997. July;78(1):631–5. [PubMed] [Google Scholar]
- 35.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005. February 9;293(6):715–22. [DOI] [PubMed] [Google Scholar]
- 36.Robasky K, Lewis NE, Church GM. The role of replicates for error mitigation in next-generation sequencing. Nat Rev Genet 2014. January;15(1):56–62. 10.1038/nrg3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012. November 1;491(7422):56–65. 10.1038/nature11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature 2010. September 2;467(7311):52–8. 10.1038/nature09298 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are available from previously published articles: Lotta LA et al. BMC Med Genomics. 2012 (doi: 10.1186/1755-8794-5-72012) and Lotta LA et al. J Thromb Haemost. 2013 (doi: 10.1111/jth.12291).