Abstract
Favism is a life-threatening hemolytic anemia resulting from the intake of fava beans by susceptible individuals with low erythrocytic glucose 6-phosphate dehydrogenase (G6PD) activity. However, little is known about the metabolomic changes in plasma and liver after the intake of fava beans in G6PD normal and deficient states. In this study, gas chromatography/mass spectrometry was used to analyze the plasma and liver metabolic alterations underlying the effects of fava beans in C3H- and G6PD-deficient (G6PDx) mice, and to find potential biomarkers and metabolic changes associated with favism. Our results showed that fava beans induced oxidative stress in both C3H and G6PDx mice. Significantly, metabolomic differences were observed in plasma and liver between the control and fava bean treated groups of both C3H and G6PDx mice. The levels of 7 and 21 metabolites in plasma showed significant differences between C3H-control (C3H-C)- and C3H fava beans-treated (C3H-FB) mice, and G6PDx-control (G6PDx-C)- and G6PDx fava beans-treated (G6PDx-FB) mice, respectively. Similarly, the levels of 7 and 25 metabolites in the liver showed significant differences between C3H and C3H-FB, and G6PDx and G6PDx-FB, respectively. The levels of oleic acid, linoleic acid, and creatinine were significantly increased in the plasma of both C3H-FB and G6PDx-FB mice. In the liver, more metabolic alterations were observed in G6PDx-FB mice than in C3H-FB mice, and were involved in a sugar, fatty acids, amino acids, cholesterol biosynthesis, the urea cycle, and the nucleotide metabolic pathway. These findings suggest that oleic acid, linoleic acid, and creatinine may be potential biomarkers of the response to fava beans in C3H and G6PDx mice and therefore that oleic acid and linoleic acid may be involved in oxidative stress induced by fava beans. This study demonstrates that G6PD activity in mice can affect their metabolic pathways in response to fava beans.
Introduction
Glucose-6-phosphate dehydrogenase (G6PD, D-glucose-6-phosphate: NADP+ 1-oxidoreductase, E.C.1.1.1.49) is the enzyme that catalyzes the first reaction in the pentose phosphate pathway that converts glucose 6-phosphate (G6P) to 6-phosphogluconolactone, concomitantly producing the reduced form of nicotinamide dinucleotide hydrogen phosphate (NADPH) for reductive biosynthesis and oxidation reduction in the cell. NADPH enables cells to keep glutathione in the reduced form (GSH) and to detoxify hydrogen peroxide (H2O2) produced from oxygen radicals whenever cells are subjected to oxidative stress. Because the pentose phosphate pathway is the only source of NADPH in red blood cells (RBCs), G6PD activity is particularly crucial to prevent oxidative damage to hemoglobin and other essential molecules. Reactive oxygen species (ROS) play an important role in the pathophysiology of acute hemolytic anemia in G6PD-deficient patients[1–3]. G6PD deficiency leads to RBCs being highly vulnerable to oxidative damage owing to lack of NADPH, with limited capacity for regenerating GSH and displaying depletion of GSH. The enzyme deficiency can clinically cause neonatal jaundice, favism, drug- or infection-induced hemolytic crisis, and non-spherocytic hemolytic anemia[1–3].
Fava beans (Vicia fava), also called broad beans, are a valuable food resource because of their substantial and fairly well balanced protein content. Its high lysine content makes it a valuable nutritional supplement to cereals, although it contains less methionine[4]. The major drawback of fava beans comes from its hemotoxicity, which can give rise to favism in individuals with G6PD deficiency [5]. Fava beans contain high concentrations of the β-glycosides vicine and convicine, which can be converted by β-glycosidase to their respective aglycones divicine and isouramil. Hemotoxicity assays in vitro have demonstrated that divicine and isouramil are the components responsible for the oxidative damage to hemoglobin and the cell membrane [6–8]. It had been reported that divicine can induce favic response in G6PD-normal rats and produced hemolytic activity in rat RBCs [9–10]. Although the glycosides divicine and isouramil from fava beans have been proposed to be the components associated with hemolysis in favism, the metabolites of fava beans involved in triggering hemolysis in vivo are not entirely defined.
Metabolomics is the study of small biological molecules (i.e., metabolites) found within cells, tissues, and body fluids in response to environmental, pathogenic, and dietary changes or a genetic alteration. Endogenous metabolites are the most proximal reporters of alteration in the cells, tissue, and body in metabolic processes that respond to physiological or pathophysiological stimuli[11–12]. Therefore, measurement of metabolic changes in animal models in response to physiological or pathophysiological stimuli can be used to define the varying phenotypes observed. Many studies have employed high-throughput metabolite profiling to elucidate the biological pathways responsible for metabolic syndromes, nutrition, cancer, disease diagnosis, and drug metabolism[13–17].
To better understand the metabolic perturbations caused by fava beans possibly associated with favism, a comprehensive investigation in an animal model using high-throughput analysis such as metabolomics is necessary to identify the metabolites response to fava beans in liver and plasma samples. In this study, we employed a gas chromatography/mass spectrometry (GC/MS)-based metabolic profiling approach to compare the metabolites in plasma and liver tissue from G6PD-normal C3H mice and G6PD-deficient (G6PDx) mice, and to reveal alterations in metabolites as a result of the favic response.
Materials and Methods
Animal experiments
Animal experiments were carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All the protocols and studies were approved by the Ethics Committee of Hainan Medical College for Animal Care and Use. For the care and use of animals utilized in this research, we monitored the animals twice per week and none of animals showed severe ill, died or moribund required for humane endpoints during the whole experiments. A protocol for early euthanasia/humane endpoints is performed if one of the following criteria is met: the loss of body weight more than 20%, a wound that cannot be improved after medication or animals developing neurological symptoms and unable to feed by themselves. For anesthesia and euthanasia/ humane endpoints, mice were treated with 2–3% of isoflurane and 3% of CO2 inhalation, respectively.
A total of 32 5- to 6-week-old male mice, including 16 C3H/HeJ (C3H) mice and 16 G6PD-deficient (G6PDx) mice on a C3H background[18], were obtained from the Model Animal Research Center of Nanjing University, China. All mice were housed in an environmentally controlled room at 22 ± 1°C with a relative humidity of 50 ± 5% under a light cycle of 12 h light/12 h dark. Food and tap water were provided ad libitum. Both C3H and G6PDx mice were randomly divided into a control group and a FB-treated group (n = 8 per group). The FB-treated mice underwent intragastric administration of fava bean flesh homogenate (20% (w/v)) at a dose of 10 g/kg body weight and the control mice received the same volume of water at intervals of 4 h for 32 h. At time points 0 (no fava beans or water ingestion), 1, 2, 4, 8, 12, 16, 20, 24, 28, and 32 h after the fava beans or water ingestion, blood samples were collected from the mouse tail for detecting the ROS, GSH, and malondialdehyde (MDA) content of RBCs. The mice were euthanized under chloral hydrate anesthesia when the RBC content of ROS plateaued. Plasma was separated by centrifugation at 3000xg at 4°C for 15 min and stored at −80°C for metabolomic analysis. Approximately 50-mg liver samples from the left lateral liver lobe were frozen for metabolomic analysis.
Flow Cytometry
The level of intracellular ROS and GSH was determined by the alterations of fluorescence resulting from oxidation of dihydroethidium (DHE)[19] and Thiolite green (AAT Bioquest®, Inc., Sunnyvale, CA, USA). To determine the levels of ROS and GSH in the red blood cells, the RBCs from the mice with and without fava bean ingestion were diluted 10,000 times and incubated with 250 μM DHE and 50 μM Thiolite green at 37°C for 30 min. The excess fluorescent dyes were washed off with PBS. The levels of ROS and GSH in the RBCs were determined by a fluorescence-activated cell sorter (FACS Calibur, Becton Dickinson, Immunofluorometry systems, Mountain View, CA, USA). DHE or Thiolite green fluorescence was detected with excitation/emission at 518/605 nm (for DHE) or 490/520 nm (for Thiolite green). The mean fluorescence channel (MFC) of the entire RBC population was calculated for DHE and Thiolite Green by the FACS-equipped Cell Quest software.
MDA assay
RBCs from citrated whole blood were washed three times with saline, and the MDA content of the red blood cell membrane measured using a MDA test kit (Nan jing Jian cheng Bioengineering Institute, Nangjing, China) according to the manufacturer’s instructions. The MDA content was calculated and presented as μmol/ g hemoglobin.
Plasma sample pretreatment
Plasma samples were thawed at room temperature and vortex-mixed for 5 s. Then, 150 μL of methanol-chloroform solution (3:1, v/v) and 10 μL of L-2-chlorophenylalanine were added to 40 μL plasma. The mixture was vortexed for 30 s, then placed at −20°C for 20 min. The mixture was centrifuged at 14,000 g at 4°C for 15 min, then 160 μL of the supernatant was transferred to a GC vial and evaporated to dryness under a stream of nitrogen gas. Then, 30 μL of 20 mg/mL methoxyamine pyridine solution was added and reacted at 37°C for 90 min. Finally, 30 μL BSTFA reagent (containing 1% TMCS) was added into the mixture and reacted at 70°C for 60 min. After cooling to room temperature, 1 μL of the solution was injected into the GC/MS for analysis.
Liver tissue sample pretreatment
The procedure for liver tissue sample pretreatment was performed as previously reported [20]. Briefly, for GC/MS analysis, 40 mg mouse liver tissue sample was submerged in 1.0 mL of water–methanol–chloroform solution (2:5:2, v/v/v), then 100 μL L-2-chlorophenylalanine added as an internal control. The mixture was then ultrasonicated in an ice bath for 60 min and then vortex mixed for another 2 min to extract metabolites and precipitate proteins. The precipitated proteins were separated by centrifugation at 14,000 g at 4°C for 15 min and 600 μL supernatant was collected separately from each sample into a vial (4 mL) with PTFE-lined screw cap. To concentrate and derivatize the metabolites in the solution, the supernatant was evaporated under nitrogen gas at 50°C to dryness. After the vial was dry, 30 μL of 20 mg/mL methoxyamine pyridine solution was added and reacted at 37°C for 90 min. Finally, 30 μL BSTFA reagent (containing 1% TMCS) was added into the mixture and reacted at 70°C for 60 min. After cooling to room temperature, 1 μL solution was injected into the GC-MS for analysis.
Gas chromatography/mass spectrometry (GC/MS)
A HP 6890/5973 system coupled with a HP-5MS fused silica capillary column (30 m × 250 μm, 0.25 μm) was employed to profile metabolites. Helium (99.999%) was used as carrier gas with a flow rate of 1.0 mL per min, and 1 μL of sample was injected into the column with a splitless mode. The temperature of injection was set to 250°C. The column temperature was initially kept at 40°C for 3 min, then increased to 280°C at a rate of 8°C per min and maintained at 280°C for 3 min. The detector was a quadrupole mass spectrometer and the temperature of quadrupole and ion source were 150°C and 230°C, respectively. The data were acquired in full scan mode from m/z 45 to 800 and the acceleration voltage was turned on after a solvent delay of 3.0 min. Total ion chromatograms (TICs) and fragmentation patterns were acquired by GC/MSD ChemStation Software (Agilent, Shanghai, China). The software was used for metabolite identification by comparing the mass-to-charge ratios and the abundance of each compound detected against a standard mass chromatogram in the NIST (National Institute of Standards and Technology) mass spectral library. Peaks with a similarity index greater than 70% were tentatively identified as metabolites, whereas those having less than 70% similarity index were considered as unknown metabolites.
Data processing and pattern recognition
All GC/MS raw files were processed by the R platform. The resulting tables were exported into Microsoft Excel, where normalization was performed prior to multivariate analyses. The resulting three-dimensional data matrix involving peak index (RT–m/z pair), sample names (observations), and normalized peak area percentage were submitted to the SIMCA-P software package (version 11.0, Umetrics, Umea, Sweden) for Principle component analysis (PCA), Partial Least Squares-Discriminant Analysis (PLS-DA), and Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA). Unsupervised PCA was initially performed to explore dataset variations. PLS-DA and OPLS-DA were subsequently used to distinguish the differences between FB-treated groups and the control groups. The variable importance in the projection (VIP) values of all the metabolites from OPLS-DA model were taken as a criterion to find the variable importance of differential metabolites. Those variables with a VIP > 1.0 and difference with a p-value <0.05 were considered relevant for group discrimination. The statistical significance between two groups was evaluated by a univariate Student’s t-test. Statistical differences at p < 0.05 were considered significant. Two sample t-test statistics were used for the comparison of metabolite levels to determine the differences between the FB-treated and control groups.
Results
Fava beans induce oxidative stress in mice
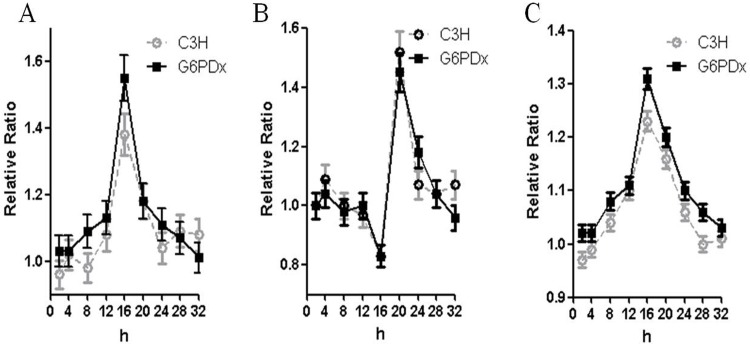
To determine whether the fava beans would induce biochemical alterations in mouse RBCs, we performed time-course analyses to evaluate the ROS, GSH, and MDA level of RBCs in G6PDx and C3H mice after intragastric administration of fava beans. Fig 1A clearly show that fava beans induced oxidative stress in both C3H and G6PDx mice (p < 0.05). The levels of ROS were significantly elevated, approximately 37% and 52% at 16 h in C3H-FB and G6PDx-FB mice, respectively, compared with the control and then decreased at 20 h. The production of ROS was also significantly higher at 16 h than that at the other time points in both C3H and G6PDx mice (p < 0.05). In contrast, the levels of GSH were significantly decreased at 16 h, then increased by about 46% and 43% at 20 h and then decreased at 24 h, respectively, in C3H and G6PDx mice (Fig 1B), correlating well with the ROS increase. Moreover, the levels of MDA were significantly elevated by about 23% and 31% at 16 h and then decreased at 20 h in C3H and G6PDx mice respectively (Fig 1C). The results suggest that ingestion of fava beans can trigger oxidative stress and lead to oxidative damage of lipids in mice, although a significant difference was not observed between C3H mice and G6PDx mice.
Fig 1. Kinetics of the changes of ROS, GSH, and MDA in response to fava beans in red blood cells of C3H and G6PDx mice.
Results are presented as the relative ratios of ROS, GSH, and MDA in red blood cells of C3H-FB mice and G6PDx-FB mice to those in red blood cells of C3H control mice and G6PDx control mice, respectively. A. The relative ratio changes of ROS. B. The relative ratio changes of GSH. C. The relative ratio changes of MDA.
Fava beans induce metabolic changes in the plasma
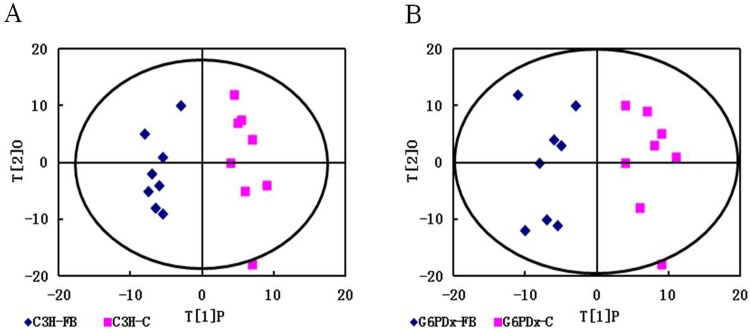
Representative GC/MS TIC chromatograms of plasma samples from the FB-treated and control groups in both G6PDx mice and C3H mice are shown in S1 Fig. After mean-centering, UV-scaling, and deconvolution, a total of 403 metabolites were detected in mouse plasma extracts by GC/MS. PCA results of the GC/MS data from the FB-treated and control groups are shown in S2 Fig. PCA indicated a clear distinction between the FB-treated and control groups in both C3H mice (R2X = 0.658) and G6PDx mice (R2X = 0.55). A PLS-DA scores plot showed the considerable separation achieved between FB-treated group and control group in both C3H mice (R2X = 0.854, Q2 = 0.328) and G6PDx mice (R2X = 0.913, Q2 = 0.68) (S3 Fig). Further analysis by the OPLS-DA model showed distinct discrimination between the metabolite profiles of the FB-treated and control groups in both C3H mice (R2X = 0.941, Q2 = 0.378) and G6PDx mice (R2X = 0.915, Q2 = 0.696) (Fig 2). The FB-treated group fell into the same region in both PLS-DA and OPLS-DA models with high R2Y values and Q2 values. These results indicate that there were significant alterations of the plasma metabolic profile among the FB-treated mice in comparison with the controls.
Fig 2. OPLS-DA scatter plots of GC/MS data of extracts from plasma of mice.
Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots (t(1)P/ t(2)O) of the plasma of C3H group (A) and G6PDx group (B).
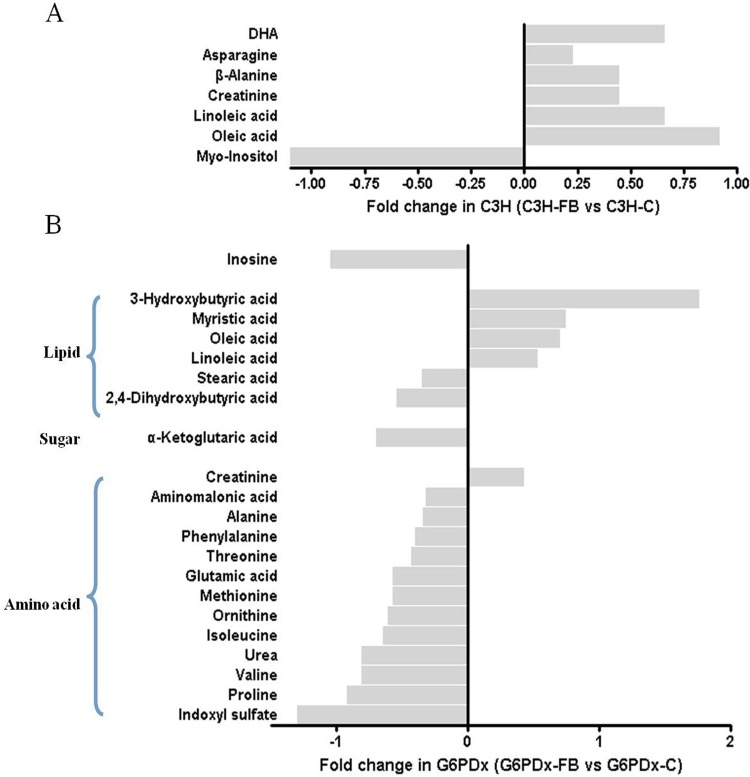
The metabolic profiles of plasma showed that the differential metabolites in C3H-FB mice and G6PDx-FB mice were involved in the metabolism of carbohydrates, lipids, proteins, and purine (Tables 1 and 2 and Fig 3). Seven differential metabolites were found in C3H-FB mice compared with C3H control mice, including elevated β-alanine, asparagine, creatinine, DHA, oleic acid, linoleic acid, and decreased myo-inositol (Table 1; Fig 3A). However, 21 differential metabolites were significantly altered in G6PDx-FB mice compared with G6PDx control mice, including decreased indoxyl sulfate, inosine, α-ketoglutaric acid, alanine, ornithine, threonine, proline, phenylalanine, glutamic acid, isoleucine, valine, methionine, aminomalonic acid, 2,4-dihydroxybutyric acid, urea, and stearic acid, as well as increased creatinine, linoleic acid, oleic acid, myristic acid and 3-hydroxybutyric acid (Table 2 and Fig 3B). Among the differential metabolites, elevation of creatinine, oleic acid, and linoleic acid occurred in both C3H and G6PDx mice. Compared with C3H-FB mice, more striking alterations in amino acid metabolism were observed in the plasma of G6PDx-FB mice. The metabolites of protein, indoxyl sulfate, ornithine, urea, ketogenic and glucogenic amino acids (threonine, phenylalanine, and isoleucine), glucogenic amino acids (alanine, glutamic acid, proline, valine, and methionine) and aminomalonic acid, were significantly decreased in G6PDx mice (Table 2). The results showed clear different metabolic alterations in response to fava beans in plasma between G6PDx-FB mice and C3H-FB mice.
Table 1. The differential metabolites in the plasma between C3H-FB and C3H control mice.
| No. | Compounds | NIST | VIP-Value | p-Value | Fold |
|---|---|---|---|---|---|
| probability match(%) | (OPLS-DA) | (t-test) | Change | ||
| 1 | Myo-inositol | 83.3 | 2.2 | 4.35E-03 | −1.10 |
| 2 | Oleic acid | 87.5 | 1.98 | 1.28E-02 | 0.92 |
| 3 | Linoleic acid | 90.2 | 1.84 | 2.38E-02 | 0.66 |
| 4 | Creatinine | 89.6 | 1.76 | 3.28E-02 | 0.45 |
| 5 | β-Alanine | 79.5 | 1.81 | 2.63E-02 | 0.45 |
| 6 | Asparagine | 80.7 | 1.98 | 1.35E-02 | 0.23 |
| 7 | DHA | 88.3 | 1.97 | 1.40E-02 | 0.66 |
Table 2. The differential metabolites in the plasma between G6PDx-FB and G6PDx control mice.
| No. | Compounds | NIST | VIP-Value | p-Value | Fold |
|---|---|---|---|---|---|
| probability match(%) | (OPLS-DA) | (t-test) | Change | ||
| 1 | Indoxyl sulfate | 82.3 | 2 | 9.98E-04 | −1.30 |
| 2 | Inosine | 84.5 | 1.82 | 4.06E-03 | −1.05 |
| 3 | ProlineΔ | 81.7 | 2.1 | 3.36E-04 | −0.92 |
| 4 | ValineΔ | 90.8 | 2.2 | 1.11E-04 | −0.81 |
| 5 | Urea | 91.6 | 1.92 | 1.97E-03 | −0.81 |
| 6 | α-Ketoglutaric acid | 87.4 | 1.42 | 3.60E-02 | −0.70 |
| 7 | Isoleucine* | 91.5 | 2.02 | 8.19E-04 | −0.65 |
| 8 | Ornithine | 86.1 | 1.77 | 5.64E-03 | −0.61 |
| 9 | MethionineΔ | 93.2 | 2.06 | 5.37E-04 | −0.57 |
| 10 | Glutamic acid | 89.5 | 1.64 | 1.23E-02 | −0.57 |
| 11 | 2,4-Dihydroxybutyric acid | 79.8 | 1.92 | 1.89E-03 | −0.54 |
| 12 | ThreonineΔ | 90.7 | 2.07 | 5.10E-04 | −0.43 |
| 13 | Phenylalanine* | 88.6 | 2.1 | 3.52E-04 | −0.40 |
| 14 | Stearic acid | 81.6 | 1.7 | 8.70E-03 | −0.35 |
| 15 | AlanineΔ | 93.2 | 1.51 | 2.34E-02 | −0.34 |
| 16 | Aminomalonic acid | 87.3 | 1.56 | 1.84E-02 | −0.32 |
| 17 | Creatinine | 88.4 | 1.74 | 6.71E-03 | 0.43 |
| 18 | Linoleic acid | 89.7 | 1.47 | 2.83E-02 | 0.53 |
| 19 | Oleic acid | 92.4 | 1.6 | 1.45E-02 | 0.7 |
| 20 | Myristic acid | 87.3 | 1.5 | 2.48E-02 | 0.75 |
| 21 | 3-Hydroxybutyric acid | 80.2 | 1.62 | 1.31E-02 | 1.76 |
Δ glucogenic amino acids
* glucogenic and ketogenic amino acids
Fig 3. Relative levels of metabolites detected with significant changes in the plasma of the C3H-FB vs.
C3H group and the G6PDx-FB vs. G6PDx group. Seven and 21 differential metabolites were significantly altered in C3H-FB mice compared with C3H control mice (A) and G6PDx-FB mice compared with G6PDx control mice (B) respectively.
Fava beans induce metabolic changes in the liver
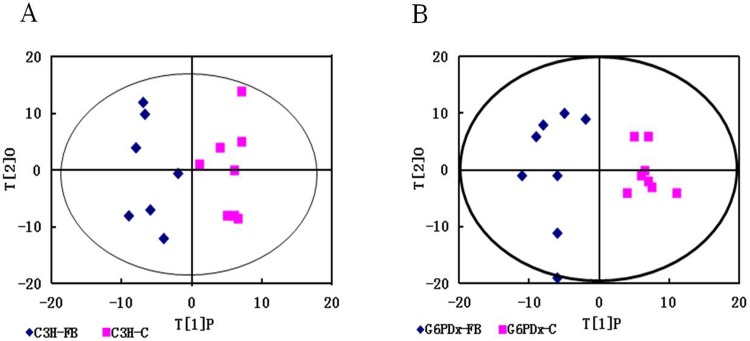
Representative GC/MS TIC chromatograms of liver samples from the FB-treated and control groups in both C3H- and G6PD-deficient mice are shown in S4 Fig. After mean-centering, UV-scaling, and deconvolution, a total of 316 metabolites were detected in mouse liver extracts by GC-MS. PCA was first performed to identify the differences between the sample profiles. PCA indicated a clear distinction between the FB-treated group and control group in both C3H mice (R2X = 0.635) and G6PDx mice (R2X = 0.575). PLS-DA was carried out for class discrimination and biomarker identification. The PLS-DA scores plot showed the considerable separation achieved between the FB-treated group and control group in both C3H mice (R2X = 0.886, Q2 = 0.34) and G6PDx mice (R2X = 0.966, Q2 = 0.669). PCA and PLS-DA results are shown in S5 and S6 Figs. The OPLS-DA model was further performed to remove the structured noise and get more reliable metabolite information. The OPLS-DA models showed distinct discrimination between the metabolite profiles of the FB-treated and control groups in both C3H mice (R2X = 0.884, Q2 = 0.27) and G6PDx mice (R2X = 0.897, Q2 = 0.635) (Fig 4). The FB-treated group fell into the same region in both PLS-DA and OPLS-DA models with high R2Y values and Q2 values. These results indicate that there were significant variations in liver metabolites among the FB-treated mice compared with controls.
Fig 4. OPLS-DA scatter plots of GC/MS data of extracts from the liver of mice.
Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots (t(1)P/ t(2)O) of the liver of C3H group (A) and G6PDx group (B).
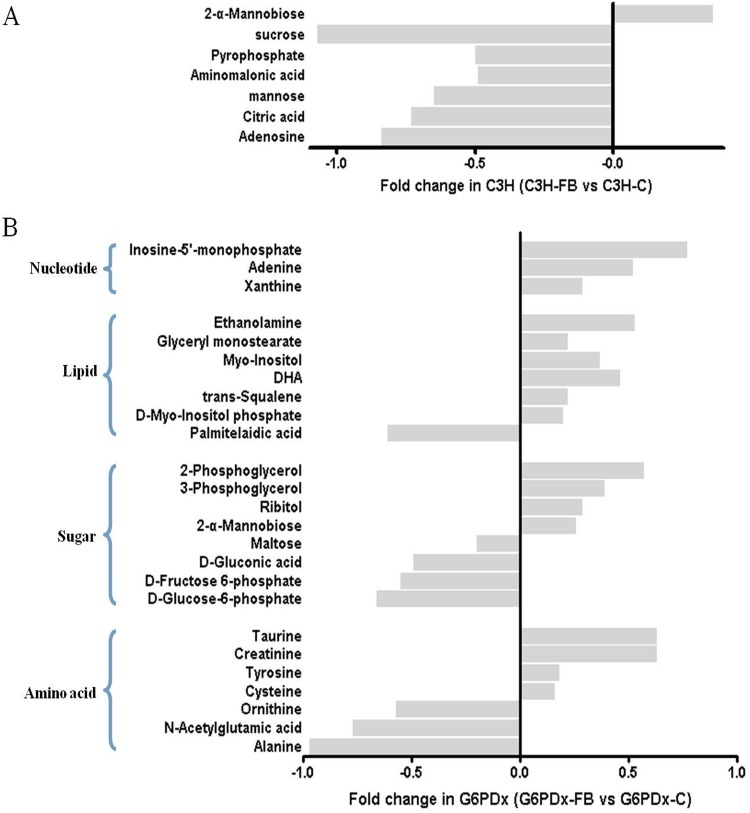
Table 3 and Fig 5A show the metabolic profiles of liver in FB-treated mice compared with the controls. The differential metabolites were involved in the metabolism of carbohydrate, lipids, nucleic acid, energy, and protein. Seven differential metabolites were found with significant differences in C3H-FB mice in comparison with the C3H controls, including decreased adenosine, citric acid, mannose, amino malonic acid, pyrophosphate, sucrose, and increased 2-α-mannobiose. In G6PDx-FB mice, 25 differential metabolites were detected with significant differences in comparison with the G6PDx controls, including increased D-myo-inositol phosphate, creatinine, cis-4,7,10,13,16,19-docosahexaenoic acid, glyceryl monostearate, ethanolamine, 2-phosphoglycerol, 3-phosphoglycerol, cysteine, tyrosine, taurine, trans-squalene, adenine, rititol, xanthine, inosine-5’-monophosphate, 2-α-mannobiose and decreased maltose, D-gluconic acid, D-fructose 6-phosphate, D-glucose-6-phosphate, alanine, ornithine, N-acetylglutamic acid, and palmitelaidic acid (Table 4 and Fig 5B). These findings suggest that more metabolic pathways were affected in response to fava beans in the liver of G6PDx-FB mice compared with C3H-FB mice.
Table 3. The differential metabolites in liver between C3H-FB and C3H control mice.
| No. | Compounds | NIST | VIP-Value | p-Value | Fold |
|---|---|---|---|---|---|
| probability match(%) | (OPLS-DA) | (t-test) | Change | ||
| 1 | Adenosine | 91.5 | 1.83 | 2.91E-02 | -0.84 |
| 2 | Citric acid | 88.5 | 1.95 | 1.83E-02 | -0.73 |
| 3 | Mannose | 84.3 | 2.24 | 4.58E-03 | -0.65 |
| 4 | Aminomalonic acid | 79.6 | 2.2 | 5.53E-03 | -0.49 |
| 5 | Pyrophosphate | 80.1 | 2.18 | 6.43E-03 | -0.5 |
| 6 | Sucrose | 90.5 | 1.89 | 2.33E-02 | -1.07 |
| 7 | 2-α-Mannobiose | 83.6 | 1.72 | 4.32E-02 | 0.36 |
Fig 5. Relative levels of metabolites detected with significant changes in the liver of the C3H-FB vs.
C3H group and the G6PDx-FB vs. G6PDx group. Seven and 25 differential metabolites were significantly altered in C3H-FB mice compared with C3H control mice (A) and G6PDx-FB mice compared with G6PDx control mice (B) respectively.
Table 4. The differential metabolites in liver between G6PDx-FB and G6PDx control mice.
| No. | Compounds | NIST | VIP-Value | p-Value | Fold |
|---|---|---|---|---|---|
| probability match(%) | (OPLS-DA) | (t-test) | Change | ||
| 1 | AlanineΔ | 89.5 | 1.75 | 2.54E-02 | -0.97 |
| 2 | D-Glucose-6-phosphate | 92.7 | 1.34 | 7.49E-03 | -0.66 |
| 3 | Ornithine | 90.1 | 1.73 | 3.87E-03 | -0.57 |
| 4 | D-Fructose 6-phosphate | 91.3 | 1.25 | 1.42E-02 | -0.55 |
| 5 | D-Gluconic acid | 85.6 | 1.84 | 4.21E-02 | -0.49 |
| 6 | N-Acetylglutamic acid | 84.1 | 1.68 | 3.66E-03 | -0.77 |
| 7 | Palmitelaidic acid | 79.6 | 1.32 | 2.20E-02 | -0.61 |
| 8 | Maltose | 85.3 | 1.09 | 3.24E-02 | -0.2 |
| 9 | CysteineΔ | 90.8 | 1.25 | 3.90E-02 | 0.16 |
| 10 | Tyrosine* | 92.3 | 1.19 | 8.93E-03 | 0.18 |
| 11 | D-Myo-Inositol phosphate | 86.5 | 1.07 | 4.29E-03 | 0.2 |
| 12 | Ribitol | 90.7 | 1.12 | 4.19E-02 | 0.29 |
| 13 | 2-α-Mannobiose | 83.7 | 1.26 | 2.33E-03 | 0.26 |
| 14 | Inosine-5'-monophosphate | 90.2 | 1.96 | 5.62E-06 | 0.77 |
| 15 | Xanthine | 85.4 | 1.8 | 4.99E-02 | 0.29 |
| 16 | Adenine | 87.3 | 1.65 | 2.51E-04 | 0.52 |
| 17 | Myo-Inositol | 91.8 | 2.02 | 5.72E-06 | 0.37 |
| 18 | DHA | 90.8 | 1.47 | 3.91E-02 | 0.46 |
| cis-4,7,10,13,16,19-Docosahexaenoic acid | 79.5 | ||||
| 19 | trans-Squalene | 77.6 | 1.47 | 1.57E-03 | 0.33 |
| 20 | Glyceryl monostearate | 78.3 | 1.25 | 6.37E-03 | 0.22 |
| 21 | 2-Phosphoglycerol | 85.6 | 1.48 | 6.42E-04 | 0.39 |
| 22 | 3-Phosphoglycerol | 83.4 | 1.53 | 8.33E-04 | 0.57 |
| 23 | Ethanolamine | 70.8 | 1.46 | 1.93E-03 | 0.53 |
| 24 | Creatinine | 89.3 | 2.17 | 1.03E-05 | 0.63 |
| 25 | Taurine | 90.7 | 1.7 | 5.18E-03 | 0.63 |
Δ glucogenic amino acids
* glucogenic and ketogenic amino acids
Discussion
We studied the kinetics of changes in the levels of ROS, GSH, and MDA in both C3H mice and G6PDx mice in response to fava beans. Our results showed that the contents of ROS and MDA were increased and that the depletion of GSH was well correlated with production of ROS during the favic response in both C3H and G6PDx mice. The results suggest that ingestion of fava beans can trigger oxidative stress and lead to oxidative damage of lipids in mice. However, we did not find any significant difference in the levels of ROS, GSH, and MDA between the C3H mice and G6PDx mice in response to fava beans. This may be owing to the G6PD activity in G6PDx mice not being low enough to produce an oxidative phenotype different from C3H mice, because the G6PD activity of erythrocytes in G6PDx mice is about 20% normal activity [18]. In human, it had been observed that only a few of G6PD deficient-individuals have hemolytic episode in response to fava beans though favism is associated with class Ⅱ(1%-10% of normal) and Ⅲ(10%-50% of normal) of G6PD deficiency, suggesting that the deficiency is a necessary but not sufficient cause of hemolysis [1–2,5].
One of the most important changes observed in this study is the increased levels of the unsaturated fatty acids, oleic acid, and linoleic acid, in the plasma of both G6PDx mice and C3H mice, suggesting that the lipid metabolic pathway was perturbed. Oleic and linoleic acids are the major components of lipoproteins and phospholipids in the cell membrane. The elevation of oleic acid and linoleic acid might indicate enhanced lipolysis or damage of the membrane, which may be associated with oxidative stress. It has been suggested that linoleic and oleic acids have antioxidant effects, protecting cells from oxidative stress[21]. However, the overall evidence of oleic acid and linoleic acid as antioxidants remains controversial. Oleic acid and linoleic acid might also exert pro-oxidant effects mediating peroxidation and ROS production. It had been shown that oleic acid and linoleic acid are more susceptible to lipid peroxidation and are considered to be the predominant substrate for lipid peroxidation processes [21–22]. Furthermore, oleic acid and linoleic acid may act as inducers of ROS production and enhance peroxidation and oxidative stress [23–30]. Linoleic acid can also decrease glutathione levels [31]. Our results show that the levels of oleic acid and linoleic acid were significantly elevated in plasma concomitantly with increased levels of ROS and MDA and depletion of GSH, which may be indicative of enhanced peroxidation and oxidative stress. These results indicate that oleic acid and linoleic acid may be potential metabolic biomarkers of the response to fava bean-induced oxidative stress in C3H and G6PDx mice.
In the liver, we observed significant changes of lipid metabolites in G6PDx-FB mice but not in C3H-FB mice, including myo-inositol, trans-squalene, fatty acids (glyceryl monostearate, DHA, and palmitelaidic acid), and ethanolamine. The changes in these lipids presumably reflect the dysregulation of lipid metabolism in the liver of G6PDx-FB mice. These findings indicate that the metabolism of lipids in the liver is different between C3H mice and G6PDx mice in response to fava beans.
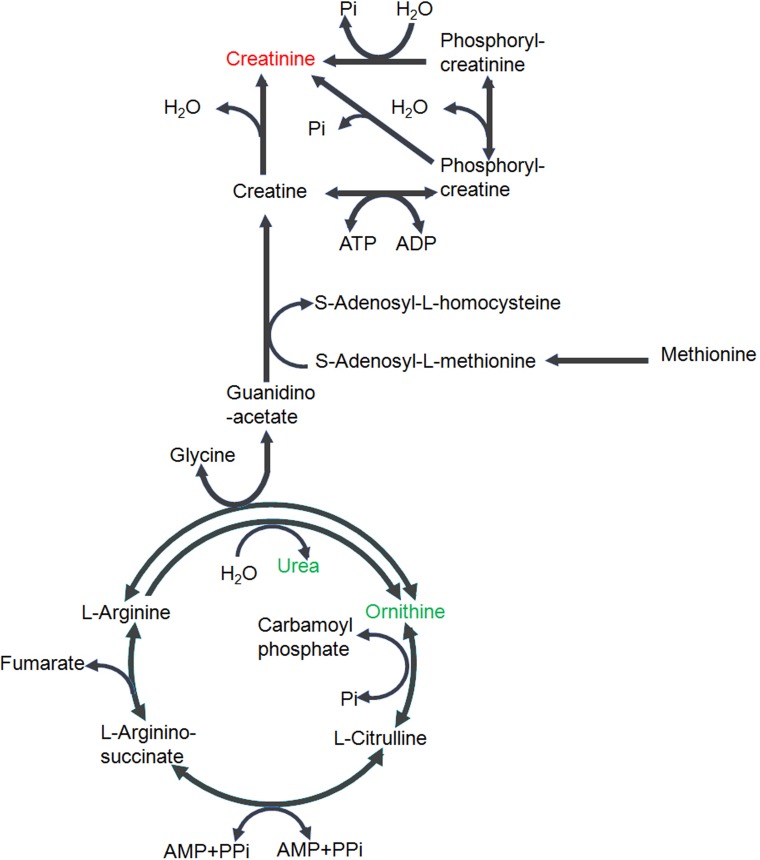
It can also be noted that significant alterations in creatine metabolism and the urea cycle were observed in the plasma of G6PDx mice in comparison with C3H mice. In G6PDx mice, we observed a significant increase in creatinine and decrease in ornithine and urea. Creatinine, ornithine, and urea are the products of L-arginine via different metabolic shunts[32] (Fig 6). Creatinine is the non-enzymatic degradation product of creatine and phosphocreatine, which play a fundamental role in the transfer of energy from mitochondria to cytosol, while ornithine and urea are the metabolites involved in urea cycle. The changes in creatinine, ornithine, and urea indicated that creatinine synthesis from L-arginine via creatine was up-regulated or more phosphocreatine was hydrolyzed to release energy, and the urea cycle was down-regulated in G6PDx mice in response to fava beans. Of interest, we also observed that creatinine was increased and ornithine was significantly decreased in the liver of G6PDx-FB mice although urea was not changed, consistent with the changes in plasma, confirming that the metabolism of creatine or phosphocreatine and urea cycle were affected in G6PDx-FB mice.
Fig 6. Effects of fava beans on metabolic pathways of the urea cycle and creatine according to the KEGG PATHWAY database.
Putative metabolic pathways were inferred from changes in the plasma and liver levels of intermediates in the G6PDx-FB mice. Red indicates the significantly elevated metabolites relative to those in the controls. Green indicates significantly reduced metabolites relative to those of the controls.
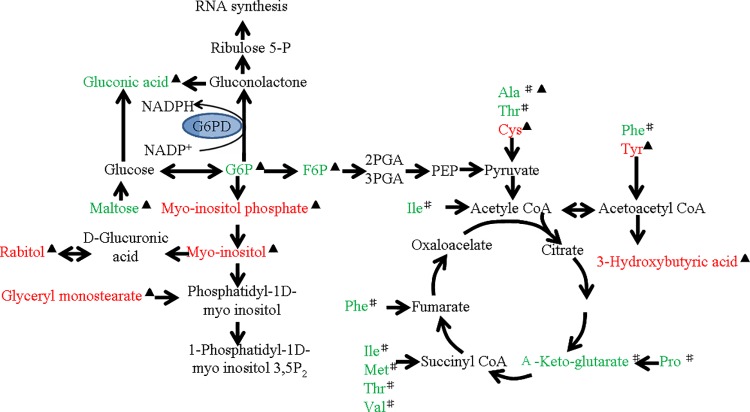
It had been reported that pentose phosphate pathway flux increased in human G6PD normal RBCs and the glycolysis pathway was enhanced in G6PD-deficient RBCs under diamide-induced oxidative stress [33]. The glycolysis pathway is also enhanced in G6PD-deficient hepatoma cells under diamide-induced oxidative stress[34]. We found that some differential metabolites of energy metabolism in the liver were different between C3H mice and G6PDx mice (Table 3 and Fig 5A). Decreased levels of citric acid and pyrophosphate were observed in C3H-FB mice, whereas decreased levels of D-glucose-6-phosphate and D-fructose-6-phosphate and elevated levels of 2-phosphoglycerol and 3-phosphoglycerol were found in G6PDx-FB mice, indicating that the affected pathways in C3H-FB mice are different from those in the G6PDx-FB mice in response to fava beans. Citric acid, adenosine, and pyrophosphate are involved respectively in tricarboxylic acid cycle (TCA cycle) and ATP hydrolysis. The significant declines of citric acid, adenosine, and pyrophosphate in C3H mice suggest that the TCA cycle and ATP hydrolysis are down-regulated and that probably more glucose enters the pentose phosphate pathway under fava bean-induced oxidative stress, although we did not find the changed metabolites such as ribose-5-phosphate and sedoheptulose-7-phosphate in the pentose phosphate pathway as described in human red blood cells[33]. In G6PDx-FB mice, the changes of glycolytic pathway metabolites, decreased levels in glucose-6-phosphate and fructose-6-phosphate suggest that the entry of glucose into glycolysis may be enhanced (Fig 7). These changes of metabolic pathways in energy metabolism could be related to the G6PD deficiency, which results in reduced flux of glucose into pentose phosphate pathway. The findings are to a some extent in agreement with the recent findings on the metabolic changes associated with enhanced glucolysis in G6PD-deficient hepatoma cells [34] and human G6PD-deficient RBCs in response to diamide [33]. In addition, the levels of inosine-5'-monophosphate (IMP), adenine, and xanthine were increased, which corresponds well with the increased energy metabolism induced by oxidative stress found in human erythrocytes [33, 35], suggesting that excessive ATP use occurred in the liver in response to fava bean-induced oxidative stress. Our findings indicate that metabolic pathways in energy metabolism in the liver were differentially perturbed in response to fava beans in C3H mice and G6PDx mice.
Fig 7. Effects of fava beans on metabolic pathways according to the KEGG PATHWAY database.
Putative metabolic pathways were inferred from changes of the intermediates levels in the liver and plasma of the G6PDx-FB mice. Red indicates the significantly elevated metabolites relative to those in the controls. Green shows significantly reduced metabolites relative to those in the controls.
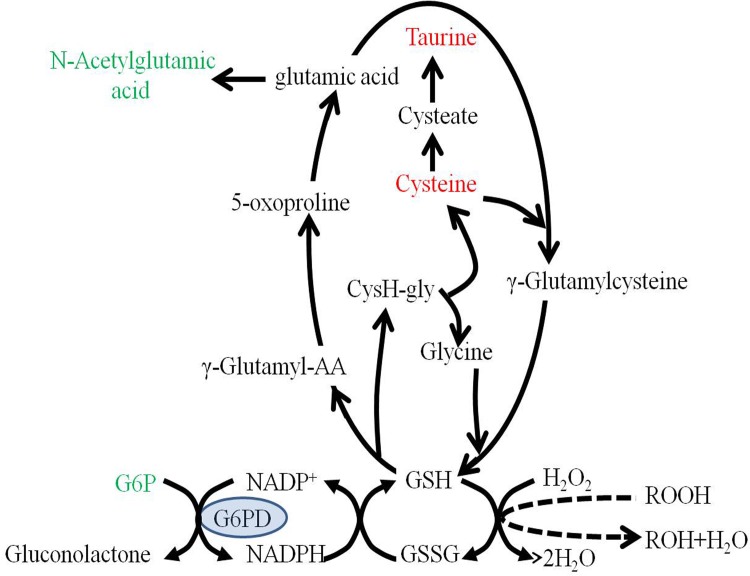
Differential significant changes in the metabolites of amino acids were found in plasma and liver in both C3H-FB mice and G6PDx-FB mice in response to fava beans. In plasma, more striking alterations in amino acid metabolism were observed in G6PDx mice compared with C3H mice. We observed a significant decrease in glucogenic amino acids and glucogenic and ketogenic amino acids along with an increase in 3-hydroxybutyratethe, one compound of the ketone bodies, suggesting that these amino acids may be transformed to ketone bodies via the ketone bodies pathway or to glucose via the gluconeogenesis pathway (Fig 7). These results implied that the cellular pathways associated with some glucogenic amino acids, and glucogenic and ketogenic amino acids could be perturbed and the differential amino acids may be potential biomarkers in plasma in response to fava beans in G6PDx mice. In the liver, the differential amino acids involved in cysteine metabolism were found in both C3H-FB mice and G6PDx-FB mice, which were different from those in the plasma of both C3H mice and G6PDx mice. Cysteine and taurine were increased in the liver of G6PDx-FB mice, whereas aminomalonic acid was decreased in the liver of C3H-FB mice. Cysteine can come from the degradation of protein and GSH or from methionine metabolism[36], and taurine is the product of cysteine metabolism[37–38]. It had been reported that the elevated level of cysteine is related to depletion of glutathione metabolism and oxidative stress induced by diamide in G6PD-deficient hepatoma cells [34].The elevation of cysteine and taurine may reflect the dysregulation of GSH metabolism in G6PDx-FB mice in response to fava bean-induced oxidative stress (Fig 8). Aminomalonic acid is a dicarboxylic acid that could be derived via β-elimination of sulfur from cysteine and the product of oxidative damage to amino acid residues in proteins[39]. The decrease of aminomalonic acid indicates that the flux of cysteine into the GSH synthesis pathway may be enhanced in the liver of C3H mice with normal G6PD activity compared with G6PDx mice.
Fig 8. The alteration of cysteine and its relevant metabolic pathways according to the KEGG PATHWAY database.
Putative metabolic pathways were inferred from changes in the intermediates levels in the liver of the G6PDx-FB mice. Red indicates the significantly elevated metabolites relative to those in the controls. Green shows significantly reduced metabolites relative to those in the controls.
In this study, we found that the changes in many metabolites in the liver were inconsistent with their levels in plasma in both C3H and G6PDx mice. This may be due to the plasma metabolic profile reflecting systemic metabolic effects in response to fava beans from all cells in the mice, while the liver metabolic profile only demonstrates metabolism related to the response to fava beans in the liver.
In summary, our results demonstrate that fava beans induced oxidative stress in both C3H and G6PDx mice. Significant metabolomic differences in plasma and liver were observed among the C3H-FB mice, G6PDx-FB mice, and their controls. The results also indicate that some significantly changed metabolites may be potential biomarkers of the response to fava beans, and moreover, may be associated with oxidative stress. Further studies on the plasma metabolic profile of favic patients might better elucidate the precise metabolic changes occurring in favism.
Supporting Information
X-axis is retention time (min); Y-axis is intensity of MS (%).
(TIF)
Principal Component Analysis (PCA) score plots (t(1)P/ t(2)O) of the plasma of C3H (A) group and G6PDx group (B).
(TIF)
Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots (t(1)P/ t(2)O) of the plasma of C3H (A) group and G6PDx group (B).
(TIF)
X-axis is retention time (min); Y-axis is intensity of MS (%).
(TIF)
Principal Component Analysis (PCA) score plots (t(1)P/ t(2)O) of the liver of C3H (A) group and G6PDx group (B).
(TIF)
Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots (t(1)P/ t(2)O) of the liver of C3H (A) group and G6PDx group (B).
(TIF)
(A) C3H group and (B) G6PDx group.
(TIF)
(A) C3H group and (B) G6PDx group.
(TIF)
Abbreviations
- G6PD
glucose 6-phosphate dehydrogenase
- G6PDx
G6PD-deficient
- NADPH
nicotinamide dinucleotide hydrogen phosphate
- GSH
reduced glutathione
- H2O2
hydrogen peroxide
- RBCs
red blood cells
- ROS
Reactive oxygen species
- GC/MS
gas chromatography/mass spectrometry
- MDA
malondialdehyde
- DHE
dihydroethidium
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The National Key Basic Research Program of China (2012CB526600) (WWC), National Natural Science Foundation of China (No. 30960171, No. 81260092) (WWC) and Natural Science Foundation of Hainan Province (No. 812195) (MX) had role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Shanghai Sensichip Infotech Co., Ltd. provided support in the form of salaries for author HZ, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008; 371:64–74. 10.1016/S0140-6736(08)60073-2 [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood 2008; 111: 16–24. [DOI] [PubMed] [Google Scholar]
- 3.Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. G6PD deficiency: global distribution,genetic variants and primaquine therapy. Adv Parasitol 2013; 81: 133–201. 10.1016/B978-0-12-407826-0.00004-7 [DOI] [PubMed] [Google Scholar]
- 4.Aydroyd WR, Doughty J. Legumes in Human Nutrition. FAO Nutr Stud 1964; 19: 1–138. [PubMed] [Google Scholar]
- 5.Belsey MA. The epidemiology of favism. Bull World Health Organ 1973; 8:1–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Mager J, Glaser G, Razin A, Izak G, Bien S, Noam M. Metabolic effects of pyrimidines derived from fava bean glycosides on human erythrocytes deficient in glucose-6-phosphate dehydrogenase. Biochem Biophys Res Commun 1965; 20:235–240. [DOI] [PubMed] [Google Scholar]
- 7.Chevion M, Navok T, Glaser G, Mager J. The chemistry of favism-inducing compounds. The properties of isouramil and divicine and their reaction with glutathione. Eur J Biochem 1982; 127: 405–409. [DOI] [PubMed] [Google Scholar]
- 8.Winterbourn CC, Cowden WB, Sutton HC. Auto-oxidation of dialuric acid, divicine and isouramil. Superoxide dependent and independent mechanisms. Biochem Pharmacol 1989; 38: 611–618. [DOI] [PubMed] [Google Scholar]
- 9.McMillan DC, Bolchoz LJ, Jollow DJ. Favism: effect of divicine on rat erythrocyte sulfhydryl status, hexose monophosphate shunt activity, morphology, and membrane skeletal proteins. Toxicol Sci 2001; 62:353–359. [DOI] [PubMed] [Google Scholar]
- 10.McMillan DC, Jollow DJ. Favism: divicine hemotoxicity in the rat. Toxicol Sci 1999; 51:310–316. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson JK, Holmes E, Kinross JM, Darzi AW, Takats Z, Lindon JC. Metabolic phenotyping in clinical and surgical environments. Nature 2012; 91:384–392. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CH, Gonzalez FJ. Challenges and opportunities of metabolomics. J Cell Physiol 2012; 227:2975–2981. 10.1002/jcp.24002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putri SP, Nakayama Y, Matsuda F, Uchikata T, Kobayashi S, Matsubara A, et al. Current metabolomics: practical applications. J Biosci Bioeng 2013; 115: 579–589. 10.1016/j.jbiosc.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 14.Astarita G, Langridge J. An emerging role for metabolomics in nutrition science. J Nutrigenet Nutrigenomics 2013; 6:181–200. 10.1159/000354403 [DOI] [PubMed] [Google Scholar]
- 15.Madsen R, Lundstedt T, Trygg J. Chemometrics in metabolomics—a review in human disease diagnosis. Anal Chim Acta 2010; 659:23–33. 10.1016/j.aca.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 16.Xu EY, Schaefer WH, Xu Q. Metabolomics in pharmaceutical research and development: metabolites, mechanisms and pathways. Curr Opin Drug Discov Devel 2009; 12:40–52. [PubMed] [Google Scholar]
- 17.Beger RD, Sun J, Schnackenberg LK. Metabolomics approaches for discovering biomarkers of drug-induced hepatotoxicity and nephrotoxicity. Toxicol Appl Pharmacol 2010; 243:154–66. 10.1016/j.taap.2009.11.019 [DOI] [PubMed] [Google Scholar]
- 18.Sanders S, Smith DP, Thomas GA, Williams ED. A glucose-6-phosphate dehydrogenase (G6PD) splice site consensus sequence mutation associated with G6PD enzyme deficiency. Mutat Res 1997; 374:79–87. [DOI] [PubMed] [Google Scholar]
- 19.Takada E, Hata K, Mizuguchi J. c-Jun-NH2-terminal kinase potentiates apoptotic cell death in response to carboplatin in B lymphoma cells. Cancer Chemother Pharmacol 2008; 62:569–76. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Xue R, Lu C, Deng C, Liu T, Zeng H, et al. Metabolomic study for diagnostic model of oesophageal cancer using gas chromatography/mass spectrometry. Journal of Chromatography B 2009; 877:3111–3117. [DOI] [PubMed] [Google Scholar]
- 21.Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacological Research 2008; 57:451–455. 10.1016/j.phrs.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Healy DA, Watson RW, Newsholme P. Polyunsaturated and monounsaturated fatty acids increase neutral lipid accumulation, caspase activation and apoptosis in a neutrophil-like, differentiated HL-60 cell line. Clin Sci (Lond) 2003; 104:171–179. [DOI] [PubMed] [Google Scholar]
- 23.Spiteller G. Linoleic acid peroxidation—the dominant lipid peroxidation process in low density lipoprotein—and its relationship to chronic diseases. Chem Phys Lipids 1998; 95:105–162. [DOI] [PubMed] [Google Scholar]
- 24.Reiterer G, Toborek M, Hennig B. Quercetin protects against linoleic acid-induced porcine endothelial cell dysfunction. J Nutr 2004; 134:771–775. [DOI] [PubMed] [Google Scholar]
- 25.Innis SM. Dietary lipids in early development: relevance to obesity, immune and inflammatory disorders. Curr Opin Endocrinol Diabetes Obes 2007; 14:359–364. [DOI] [PubMed] [Google Scholar]
- 26.Hatanaka E, Levada-Pires AC, Pithon-Curi TC, Curi R. Systematic study on ROS production induced by oleic, linoleic, and gamma-linolenic acids in human and rat neutrophils. Free Radic Biol Med 2006; 41:1124–1132. [DOI] [PubMed] [Google Scholar]
- 27.Cury-Boaventura MF, Gorjao R, de Lima TM, Newsholme P, Curi R. Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci 2006; 78:1448–1456. [DOI] [PubMed] [Google Scholar]
- 28.Cury-Boaventura MF, Pompeia C, Curi R. Comparative toxicity of oleic acid and linoleic acid on Raji cells. Nutrition 2005; 21:395–405. [DOI] [PubMed] [Google Scholar]
- 29.Hatanaka E, Dermargos A, Hirata AE, Vinolo MA, Carpinelli AR, Newsholme P, et al. Oleic, linoleic and linolenic acids increase ros production by fibroblasts via NADPH oxidase activation. PLoS One 2013; 8: e58626 10.1371/journal.pone.0058626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koppers AJ, Garg ML, Aitken RJ. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic Biol Med 2010; 48: 112–119. 10.1016/j.freeradbiomed.2009.10.033 [DOI] [PubMed] [Google Scholar]
- 31.Toborek M, Hennig B. Fatty acid-mediated effects on the glutathione redox cycle in cultured endothelial cells. Am J Clin Nutr 1994; 59:60–65. [DOI] [PubMed] [Google Scholar]
- 32.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000; 80:1107–1213. [DOI] [PubMed] [Google Scholar]
- 33.Tang H-Y, Ho H-Y, Wu P-r, Chen S-h, Kuypers FA, Cheng M-L, et al. Inability to Maintain GSH Pool in G6PD-Deficient Red Cells Causes Futile AMPK Activation and Irreversible Metabolic Disturbance. Antioxidants and Redox Signaling 2015; 22:744–759. 10.1089/ars.2014.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho H-Y, Cheng M-L, Shiao M-S, Chiu DT-Y. Characterization of global metabolic responses of glucose-6-phosphate dehydrogenase-deficient hepatoma cells to diamide-induced oxidative stress. Free Radical Biology and Medicine 2013; 54:71–84. 10.1016/j.freeradbiomed.2012.10.557 [DOI] [PubMed] [Google Scholar]
- 35.Tavazzi B, Di Pierro D, Amorini AM, Fazzina G, Tuttobene M, Giardina B, et al. Energy metabolism and lipid peroxidation of human erythrocytes as a function of increased oxidative stress. Eur J Biochem 2000; 267:684–689. [DOI] [PubMed] [Google Scholar]
- 36.Stipanuk MH, Dominy JE, Lee J-I, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. The Journal of nutrition 2006; 136:1652S–9S. [DOI] [PubMed] [Google Scholar]
- 37.Birdsall TC. Therapeutic applications of taurine. Altern Med Rev 1998; 3:128–136. [PubMed] [Google Scholar]
- 38.Satoh H. Cardioprotective actions of taurine against intracellular and extracellular calcium-induced effects. Adv Exp Med Biol 1994; 359:181–196. [DOI] [PubMed] [Google Scholar]
- 39.Copley SD, Frank E, Kirsch WM, Koch TH. Detection and possible origins of aminomalonic acid in protein hydrolysates. Anal Biochem 1992; 201:152–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
X-axis is retention time (min); Y-axis is intensity of MS (%).
(TIF)
Principal Component Analysis (PCA) score plots (t(1)P/ t(2)O) of the plasma of C3H (A) group and G6PDx group (B).
(TIF)
Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots (t(1)P/ t(2)O) of the plasma of C3H (A) group and G6PDx group (B).
(TIF)
X-axis is retention time (min); Y-axis is intensity of MS (%).
(TIF)
Principal Component Analysis (PCA) score plots (t(1)P/ t(2)O) of the liver of C3H (A) group and G6PDx group (B).
(TIF)
Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots (t(1)P/ t(2)O) of the liver of C3H (A) group and G6PDx group (B).
(TIF)
(A) C3H group and (B) G6PDx group.
(TIF)
(A) C3H group and (B) G6PDx group.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.