Abstract
Objective
Greater blood pressure reactivity to psychological stress has been associated with higher risk of developing hypertension. We hypothesised that low stress resilience based on psychological assessment early in life is associated with hypertension in adulthood.
Methods
National cohort study of 1 547 182 military conscripts in Sweden during 1969–1997 (97–98% of all 18-year-old males) without prior history of hypertension, who underwent standardised psychological assessment by trained psychologists for stress resilience (1–9 scale), and were followed up for hypertension identified from outpatient and inpatient diagnoses during 1969–2012 (maximum age 62).
Results
93 028 men were diagnosed with hypertension in 39.4 million person-years of follow-up. Adjusting for body mass index (BMI), family history and socioeconomic factors, low stress resilience at age 18 was associated with increased risk of hypertension in adulthood (lowest vs highest quintile: HR 1.43; 95% CI 1.40 to 1.46; p<0.001; incidence rates, 278.7 vs 180.0 per 100 000 person-years), including a strong linear trend across the full range of stress resilience (ptrend<0.0001). We also found a positive additive interaction between stress resilience and BMI (p<0.001), indicating that low stress resilience accounted for more hypertension cases among those with high BMI. Men with a combination of low stress resilience and high BMI had a more than threefold risk of hypertension.
Conclusions
These findings suggest that low stress resilience may contribute to etiological pathways for hypertension and accounts for more cases among those with high BMI. If confirmed, this knowledge may help inform better preventive interventions by addressing psychosocial risk factors and stress management across the lifespan.
INTRODUCTION
Hypertension currently affects one in three adults and contributes to one in seven deaths in the USA.1 Globally, hypertension is increasing in prevalence and may affect >1.5 billion people by 2025, related in part to increasing rates of obesity, unhealthy diet and sedentary lifestyle.2 Substantial evidence has shown that psychosocial factors also contribute to the development of hypertension. For example, adverse childhood family environment,3 ‘type A’ behaviour patterns such as time urgency/impatience and hostile attitudes,4 chronic financial stress,5 anxiety6 and depression7 have been associated with a higher risk of hypertension. Studies also have reported that greater blood pressure reactivity to psychological stress is associated with subsequent development of hypertension.8,9 However, to our knowledge, no studies have examined psychological assessment of stress resilience early in life in relation to hypertension risk in adulthood. Such knowledge may improve our understanding of psychosocial pathways and help inform more effective interventions to prevent hypertension across the lifespan.
We conducted a national cohort study to examine stress resilience in late adolescence in relation to hypertension risk in adulthood. Stress resilience was assessed by standardised psychological assessments of ~1.5 million 18-year-old male military conscripts in Sweden during 1969–1997, who were subsequently followed up to a maximum age of 62 years. Our aim was to determine whether low stress resilience in late adolescence is associated with subsequent development of hypertension in a large national cohort.
METHODS
Study population
We identified 1 547 478 males (age 18 years) who underwent a military conscription examination during 1969–1997. This examination was compulsory for all 18-year-old males nationwide each year except for 2–3% who either were incarcerated or had severe chronic medical conditions or disabilities documented by a physician. We excluded 296 (0.02%) individuals who had a prior diagnosis of hypertension identified from hospital discharge diagnoses. A total of 1 547 182 (>99.9% of the original cohort) remained for inclusion in the study. To ensure confidentiality, all names and national identification numbers were replaced by anonymous serial numbers in adherence to the Personal Data Act (1998:204) and the Act (1995:606) and Ordinance (1995:1060) on Certain Personal Registers.
Stress resilience ascertainment
Stress resilience assessments were obtained from the Swedish Military Conscription Registry, which contains information from a 2-day standardised physical and psychological examination administered annually to all Swedish military conscripts starting in 1969. Stress resilience was assessed using a 20–30 min semi-structured interview administered by trained psychologists.10 The overall objective of the interview was to assess the conscript’s ability to cope with the psychological requirements of military service, including stress resilience during armed combat. In the interview, the psychologist asked about adjustment problems and conflicts, as well as successes, responsibilities taken on and initiatives shown or experienced in school, work, home or in leisure activities.10 Emotional stability, social maturity and active/passive interests were rated by the psychologist, who then assigned a summary score on a ‘standardised nine’ (1–9) scale, which is constructed to have a normal distribution with a mean of 5 and an SD of 2. Consequently, this score had a consistent distribution throughout the study period. A validation study in which 30 recorded interviews from 1972 to 1973 were scored by 30 psychologists reported high inter-rater reliability (correlation 0.86).11 These data have previously been used to examine stress resilience in relation to other outcomes, including coronary heart disease.12
Hypertension ascertainment
The study cohort was followed up from the time of the military conscription examination through 31 December 2012 for essential (primary) hypertension, which was identified using International Classification of Diseases (ICD) diagnosis codes (400–401 in ICD-8, 401 in ICD-9 and I10 in ICD-10) in the Swedish Hospital Registry and Swedish Outpatient Registry. The Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964, and with nationwide coverage starting in 1987; and the Swedish Outpatient Registry contains outpatient diagnoses from all specialty clinics nationwide starting in 2001. Throughout the study period, the predominant criteria for diagnosis of hypertension were a systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg, based on WHO and European Society of Hypertension/European Society of Cardiology guidelines.13,14
Adjustment variables
Other variables that may be associated with hypertension were obtained from the Swedish Military Conscription Registry and national census data, which were linked using an anonymous personal identification number. The association between stress resilience and hypertension was adjusted for the following variables: year of the military conscription examination (modelled simultaneously as a continuous and categorical (1969–1979, 1980–1989, 1990–1997) variable to account for a potential non-linear effect); body mass index (BMI=[weight in kg]/[height in m]2; modelled simultaneously as a continuous and categorical variable to account for a potential non-linear effect, using Centers for Disease Control and Prevention (CDC) definitions for children and adolescents aged 2–19 years to facilitate comparability with US studies: ‘overweight or obese’ is defined as ≥85th percentile on the CDC’s 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥25.6 for 18-year-old males15); type 2 diabetes (identified using ICD-9 code 250 (excluding 250.X1 and 250.X3) during 1987–1996, and ICD-10 code E11 during 1997–2012, and modelled as a time-dependent variable); family history of hypertension in a parent or sibling (identified from medical diagnoses in the Swedish Hospital Registry during 1964–2012 and the Swedish Outpatient Registry during 2001–2012, using the same hypertension ICD codes as above plus ICD-7 codes 444–445 from 1964 to 1968; examined in any parent or sibling, and further stratified as hypertension in only one parent, both parents or any sibling); highest education level attained during the study period (<12, 12–14 and ≥15 years); and neighbourhood socioeconomic status at baseline (SES, included because neighbourhood SES characteristics have been associated with psychosocial stress16 and hypertension;17 comprised of an index that includes low education level, low income, unemployment and social welfare receipt, as previously described;18 and categorised as low (>1 SD below the mean), medium (within 1 SD from the mean) or high (>1 SD above the mean)).
Missing data for each variable were imputed using a standard multiple imputation procedure that included all covariates, the outcome (hypertension) and the Nelson–Aalen estimate of the cumulative hazard in the imputation model.19 Missing data were relatively infrequent for stress resilience (7.2%), BMI (7.2%), education level (0.4%) and neighbourhood SES (9.1%). Data were complete for all other variables. As an alternative to multiple imputation, sensitivity analyses were performed after restricting to individuals with complete data for all variables (N=1 327 760; 86.5%).
Statistical analysis
Cox proportional hazards regression was used to compute HRs and 95% CIs for the association between stress resilience and subsequent risk of hypertension. The Cox model time scale was elapsed time since the military conscription examination (which also corresponds to attained age because baseline age was the same (18 years) for all conscripts). Individuals were censored at emigration (n=85 131; 5.5%) or death (n=58 171; 3.8%). Stress resilience was modelled alternatively as a categorical variable (1–9) or an ordinal variable to test for trend. In the first set of models, stress resilience and each covariate was modelled separately in relation to hypertension, adjusted for year of the military conscription examination. A second (fully adjusted) model included stress resilience, year of the military conscription examination, BMI, type 2 diabetes, family history of hypertension, education level and neighbourhood SES (as defined above). The proportional hazards assumption was assessed by graphical examination of log–log plots and was met in all models.
Interactions between stress resilience and BMI were examined on either the additive or multiplicative scale in relation to hypertension risk. Additive interactions were assessed using the ‘relative excess risk due to interaction’ (RERI), which is computed for binary variables as: RERIHR=HR11 – HR10 – HR01+1.20 Multiplicative interactions were assessed using the ratio of HRs: HR11/(HR10×HR01). All statistical tests were two–sided and used an α–level of 0.05. All analyses were conducted using Stata V.14.1.
RESULTS
Among the 1 547 182 men in this cohort, 93 028 (6.0%) were subsequently diagnosed with hypertension in 39.4 million person–years of follow–up (mean follow–up 25.7 years). The median age at the end of follow–up was 47.2 years (mean 47.4, SD 7.9, range 19.0–62.0). The median age at diagnosis of hypertension was 49.8 years (mean 48.5, SD 7.2, range 18.0–62.0).
Stress resilience
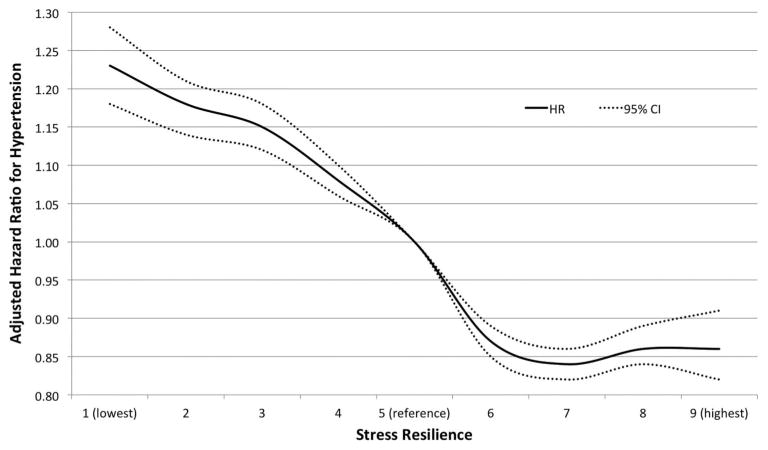
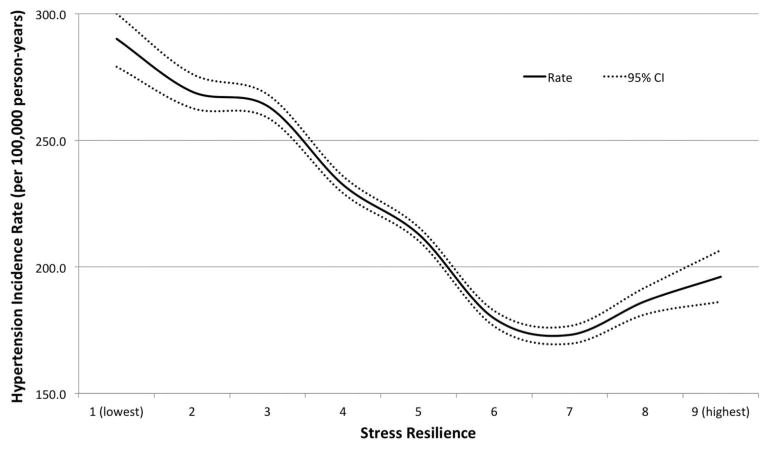
Low stress resilience was associated with subsequent increased risk of hypertension (table 1). In the fully adjusted model, men in the lowest quintile of stress resilience had more than a 40% increased risk of developing hypertension relative to those in the highest quintile (HR 1.43; 95% CI 1.40 to 1.46; p<0.001; incidence rates, 278.7 vs 180.0 per 100 000 person–years). There was a highly significant linear trend in hypertension risk across the full range of stress resilience (ptrend<0.0001). The small difference in risk estimates between adjusted models 1 and 2 was explained almost entirely by adjusting for education, whereas other adjustment variables made little difference (table 1). Figure 1 shows the fully adjusted HRs and 95% CIs for hypertension across the full range of stress resilience, modelled as a continuous variable using cubic spline curves. A relatively steep slope of increasing risk of hypertension is seen for men with below–average stress resilience relative to average. Figure 2 shows the absolute incidence rates for hypertension (per 100 000 person–years) by stress resilience level (continuous variable using cubic spline curves).
Table 1.
Adjusted HRs for associations between stress resilience or other factors among 18-year-old males and subsequent risk of hypertension
| Hypertension
|
Adjusted model 1*
|
Adjusted model 2†
|
||||||
|---|---|---|---|---|---|---|---|---|
| No (N=1 454 154) | Yes (N=93 028) | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Stress resilience | ||||||||
| 1 (lowest) | 29 170 (2.0) | 2551 (2.7) | 1.30 | 1.25 to 1.35 | <0.001 | 1.23 | 1.18 to 1.28 | <0.001 |
| 2 | 79 783 (5.5) | 6250 (6.7) | 1.22 | 1.18 to 1.25 | <0.001 | 1.18 | 1.14 to 1.21 | <0.001 |
| 3 | 160 596 (11.0) | 13 215 (14.2) | 1.21 | 1.18 to 1.23 | <0.001 | 1.15 | 1.12 to 1.18 | <0.001 |
| 4 | 266 016 (18.3) | 18 390 (19.8) | 1.10 | 1.08 to 1.12 | <0.001 | 1.08 | 1.06 to 1.10 | <0.001 |
| 5 (reference) | 373 074 (25.7) | 23 760 (23.5) | 1.00 | 1.00 | ||||
| 6 | 252 402 (17.4) | 13 303 (14.3) | 0.86 | 0.84 to 0.88 | <0.001 | 0.87 | 0.85 to 0.89 | <0.001 |
| 7 | 185 213 (12.7) | 9330 (10.0) | 0.81 | 0.79 to 0.79 | <0.001 | 0.84 | 0.82 to 0.86 | <0.001 |
| 8 | 83 763 (5.8) | 4670 (5.0) | 0.82 | 0.80 to 0.85 | <0.001 | 0.86 | 0.84 to 0.89 | <0.001 |
| 9 (highest) | 24 137 (1.7) | 1559 (1.7) | 0.84 | 0.79 to 0.88 | <0.001 | 0.86 | 0.82 to 0.91 | <0.001 |
| Per higher category (trend) | 0.92 | 0.92 to 0.93 | <0.001 | 0.94 | 0.93 to 0.94 | <0.001 | ||
| Lowest vs highest quintile | 1.53 | 1.50 to 1.57 | <0.001 | 1.43 | 1.40 to 1.46 | <0.001 | ||
| BMI‡ | ||||||||
| Normal | 1 347 242 (92.6) | 80 030 (86.0) | 1.00 | 1.00 | ||||
| Overweight or obese | 106 912 (7.4) | 12 998 (14.0) | 2.67 | 2.62 to 2.72 | <0.001 | 2.54 | 2.49 to 2.59 | <0.001 |
| Per 1 BMI unit (trend) | 1.08 | 1.07 to 1.08 | <0.001 | 1.08 | 1.07 to 1.08 | <0.001 | ||
| Type 2 diabetes | ||||||||
| No | 1 430 186 (98.4) | 82 346 (88.5) | 1.00 | 1.00 | ||||
| Yes | 23 968 (1.6) | 10 682 (11.5) | 3.33 | 2.99 to 3.72 | <0.001 | 2.76 | 2.48 to 3.08 | <0.001 |
| Family history of hypertension | ||||||||
| No | 742 697 (51.1) | 32 607 (35.1) | 1.00 | 1.00 | ||||
| Yes | 711 457 (48.9) | 60 421 (64.9) | 1.60 | 1.57 to 1.62 | <0.001 | 1.58 | 1.56 to 1.60 | <0.001 |
| Only one parent | 528 142 (36.3) | 38 939 (41.9) | 1.13 | 1.12 to 1.15 | <0.001 | 1.13 | 1.11 to 1.14 | <0.001 |
| Both parents | 108 982 (7.5) | 11 943 (12.8) | 1.52 | 1.49 to 1.55 | <0.001 | 1.50 | 1.47 to 1.53 | <0.001 |
| Sibling | 103 959 (7.1) | 14 512 (15.6) | 1.95 | 1.92 to 1.99 | <0.001 | 1.93 | 1.89 to 1.96 | <0.001 |
| Education (years) | ||||||||
| <12 | 215 583 (14.8) | 21 196 (22.8) | 1.10 | 1.08 to 1.11 | 0.04 | 1.05 | 1.03 to 1.06 | 0.001 |
| 12–14 | 642 388 (44.2) | 41 096 (44.2) | 1.00 | 1.00 | ||||
| ⪚15 | 596 183 (41.0) | 30 736 (33.0) | 0.76 | 0.75 to 0.77 | <0.001 | 0.84 | 0.83 to 0.86 | <0.001 |
| Per higher category (trend) | 0.83 | 0.82 to 0.83 | <0.001 | 0.89 | 0.89 to 0.90 | <0.001 | ||
| Neighbourhood SES | ||||||||
| Low | 222 647 (15.3) | 16 705 (18.0) | 1.06 | 1.04 to 1.08 | <0.001 | 1.02 | 1.01 to 1.04 | 0.006 |
| Medium | 957 958 (65.9) | 64 072 (68.9) | 1.00 | 1.00 | ||||
| High | 273 549 (18.8) | 12 251 (13.2) | 0.86 | 0.84 to 0.87 | <0.001 | 0.92 | 0.90 to 0.94 | <0.001 |
| Per higher category (trend) | 0.90 | 0.89 to 0.91 | <0.001 | 0.95 | 0.94 to 0.96 | <0.001 | ||
Each variable was modelled separately in relation to hypertension, adjusted for year of military conscription examination.
The model included year of military conscription examination, stress resilience, BMI, type 2 diabetes, family history of hypertension, education and neighbourhood SES. All reported risk estimates were obtained by modelling the respective variable alternatively as a categorical variable (with reference category indicated by HR of 1.00) or continuous variable (for trend tests).
BMI was categorised using CDC definitions for children and adolescents aged 2–19 years: ‘overweight or obesity’ is defined as ≥85th percentile on the CDC’s 2000 sex-specific BMI-for-age growth charts, which corresponds to BMI ≥25.6 for 18-year-old males.
BMI, body mass index; CDC, Centers for Disease Control and Prevention; SES, socioeconomic status.
Figure 1.
Adjusted HRs and 95% CIs for association between stress resilience in 18-year-old males and subsequent risk of hypertension (adjusted for year of military conscription examination, body mass index, type 2 diabetes, family history of hypertension, education and neighbourhood socioeconomic status).
Figure 2.
Hypertension incidence rates in adult men (median attained age 47 years, maximum 62) by stress resilience at age 18 years.
Other risk factors
High BMI and type 2 diabetes were independent risk factors for subsequent development of hypertension. In the fully adjusted model, overweight/obesity (≥85th percentile on the CDC’s 2000 sex–specific BMI–for–age growth chart) and type 2 diabetes were each associated with more than a 2.5–fold risk of hypertension. A first–degree family history of hypertension was associated with more than a 1.5–fold risk of developing hypertension, with a positive gradient by whether there was a history of hypertension in only one parent, both parents or a sibling (table 1). High education level and high neighbourhood SES were associated with lower risk of hypertension (ptrend<0.0001). In a sensitivity analysis that was restricted to men with no missing data, all risk estimates were very similar to the main results (data not shown).
Interaction between stress resilience and BMI
The joint effects of stress resilience and BMI in relation to hypertension risk are shown in table 2. Low stress resilience was associated with increased risk of hypertension among men with either normal or high BMI (HRs 1.38 and 1.31, respectively; table 2, right–most column). Men with a combination of low stress resilience and high BMI had the highest risk of hypertension, which was more than threefold relative to the reference group who had high stress resilience and normal BMI (HR 3.46; 95% CI 3.33 to 3.59). The interaction between low stress resilience and high BMI was non–significant on the multiplicative scale, but was highly significant on the additive scale (p<0.001), indicating that low stress resilience was associated with more hypertension cases among men with high BMI. If this association is causal, interventions to improve stress resilience would have the largest public health impact on preventing hypertension among those with high BMI.
Table 2.
Interactions between stress resilience and body mass index (BMI) among 18-year-old males in relation to subsequent risk of hypertension
| Stress resilience
|
HRs (95% CI) for medium stress resilience within strata of BMI | HRs (95% CI) for low stress resilience within strata of BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| High (levels 7–9)
|
Medium (levels 4 –6)
|
Low (levels 1– 3)
|
||||||
| No. of cases/total | HR (95% CI) | No. of cases/total | HR (95% CI) | No. of cases/total | HR (95% CI) | |||
| BMI | ||||||||
| Normal | 13 557/289 138 | 1.00 | 45 300/848 871 | 1.12 (1.10 to 1.14); p<0.001 | 21 173/289 263 | 1.38 (1.35 to 1.41); p<0.001 | 1.12 (1.10 to 1.14); p<0.001 | 1.38 (1.35 to 1.41); p<0.001 |
| Overweight or obese | 1994/17 140 | 2.64 (2.52 to 2.76); p<0.001 | 7338/71 349 | 2.85 (2.76 to 2.93); p<0.001 | 3666/29 427 | 3.46 (3.33 to 3.59); p<0.001 | 1.08 (1.03 to 1.13); p=0.004 | 1.31 (1.24 to 1.38); p<0.001 |
| HRs (95% CI) for BMI within strata of stress resilience | 2.64 (2.52 to 2.76); p<0.001 | 2.54 (2.48 to 2.60); p<0.001 | 2.51 (2.42 to 2.60); p<0.001 | |||||
| Interaction on additive scale: RERI (95% CI) | 0.09 (−0.05 to 0.22); p=0.20 | 0.44 (0.28 to 0.61); p<0.001 | ||||||
| Interaction on multiplicative scale: HR ratio (95% CI) | 0.96 (0.91 to 1.01); p=0.16 | 0.95 (0.90 to 1.01); p=0.08 | ||||||
HRs are adjusted for year of the military conscription examination, type 2 diabetes, family history of hypertension, education and neighbourhood SES.
RERI, relative excess risk due to interaction; SES, socioeconomic status.
DISCUSSION
In this large national cohort study, we found that low stress resilience in 18–year–old males was associated with increased risk of developing hypertension in adulthood, independently of BMI at baseline, diabetes, family history of hypertension and socioeconomic factors. These findings suggest that low stress resilience may play an important long–term role in etiological pathways for hypertension. If confirmed, this knowledge may help inform preventive interventions by better addressing psychosocial risk factors and stress management.
Previous smaller studies have suggested that blood pressure reactivity to various psychological stressors is associated with subsequent higher risk of hypertension.8,9 A meta–analysis of six cohort studies with a total of 34 556 adults and median follow–up of 11.5 years found that those with greater blood pressure reactivity to psychological stressor tasks had a 21% higher odds of developing hypertension or a significant increase in baseline blood pressure compared with those with low reactivity (OR 1.21; 95% CI 1.14 to 1.28; p<0.001).21 Studies that have examined chronic stress exposures rather than cardiovascular reactivity to stress also have supported a psychosocial component in the development of hypertension. For example, a US cohort study of 2739 young adults reported that adverse childhood family environment was associated with negative emotions that predicted subsequent increases in SBP.3 In an overlapping cohort of 3308 young adults, ‘type A’ behaviours including time urgency/impatience and hostile attitudes were strongly associated with increased hypertension risk in a dose–response manner.4 A UK study of 160 middle–aged adults with 3 years of follow–up reported that chronic financial stress was associated with subsequent higher baseline SBP.5 A Swedish study of 237 980 military conscripts (a subset of the present study cohort) found that low stress resilience was associated with a modestly increased risk of coronary heart disease, but did not examine hypertension or other risk factors for heart disease.12
High BMI and type 2 diabetes were the strongest risk factors for hypertension in the present study. The ~2.5–fold risk of hypertension that we observed among overweight or obese males is consistent with previously reported estimates for adult men or women.22 The ~1.5–fold risk we observed among those with a family history of hypertension and an inverse relationship between education level and hypertension risk also were in agreement with previous findings.23 In addition, we found that low neighbourhood SES was associated with higher risk of hypertension, consistent with previously reported associations between neighbourhood deprivation characteristics such as poor walkability, availability of healthy foods, safety or social cohesion and hypertension risk.17
The mechanisms by which low stress resilience may contribute to the development of hypertension are complex and likely involve both physiological and behavioural factors. According to the ‘reactivity hypothesis’, exaggerated blood pressure responses to psychological stress may cause arterial damage and dysfunction over time, leading to the development of hypertension and cardiovascular disease.24 Previous studies have found that blood pressure reactivity to psychological stress in children, adolescents or adults predicts future baseline blood pressure and cardiovascular disease risk.8,9,25 Exaggerated blood pressure responses to psychological stress may originate at the cognitive–emotional level in how events are consciously evaluated, the hypothalamic or brainstem level causing greater physiological response even with normal psychological input or the peripheral level through altered tissue function.24 Abnormal responses at any of these levels may cause exaggerated blood pressure increases and resultant shear stress, eventually altering arterial structure and function. High SBP reactivity to psychological stress tasks, for example, has been associated with increased carotid artery intima–media thickness in youth.26 In addition to these pathways, low stress resilience may contribute to unhealthy lifestyle behaviours that are known risk factors for hypertension. A Danish cohort study of 7066 adults with 10 years of follow–up found that self–reported perceived stress was associated with subsequent physical inactivity, unsuccessful smoking cessation or alcohol reduction, and use of antihypertensive medications.27 Stress–related disorders such as anxiety6 and depression7 also are associated with physical inactivity, unhealthy diet and smoking, which are established risk factors for hypertension.28
The present study provides further evidence that interventions to prevent hypertension should address psychosocial and stress–related factors. Moreover, we found that such interventions are likely to have the largest public health impact among persons with high BMI. Most stress reduction programmes have focused on treatment rather than prevention of hypertension, with mixed results. A systematic review of seven meta–analyses found that stress reduction programmes yield reliable decreases in SBP of 6–10 mm Hg and slightly larger reductions for multicomponent interventions.29 The most effective psychological treatments were reported to yield the same reductions in SBP (but not DBP) as commonly used antihypertensive medications.29 However, a later meta–analysis of 17 trials with 960 participants reported that meditation interventions but not stress management programmes resulted in significant blood pressure reductions.30 Additional studies of stress reduction for the prevention of hypertension are warranted, particularly in high–risk groups such as persons with high BMI.
Strengths of the present study include its large national cohort design with prospective assessment of stress resilience in late adolescence and sufficient follow–up to examine hypertension in adulthood. Prospective ascertainment of the exposure and outcome prevented bias that may result from self–reporting. Stress resilience was assessed using systematic interviews by trained psychologists with high inter–rater reliability. We were able to adjust for other established risk factors for hypertension, including BMI, diabetes, family history of hypertension, and individual and neighbourhood socioeconomic factors, which also were prospectively ascertained and not self–reported.
Limitations included a lack of information for certain other risk factors for hypertension, such as smoking and diet. This cohort consisted of Swedish military conscripts, and therefore included only men. However, a smaller Danish cohort study reported that perceived stress was associated with subsequent antihypertensive medication use among men and women, without gender differences.27 Outpatient diagnoses in the present study were available starting in 2001, and hence hypertension prior to this period was under–reported. This under–reporting is expected to be non–differential with respect to stress resilience level, and therefore to influence results conservatively (ie, towards the null hypothesis).
In summary, this large national cohort study examined stress resilience in 18–year–old males in relation to hypertension risk in adulthood. We found that low stress resilience was associated with higher risk of developing hypertension and accounted for more cases among those with high BMI. These findings suggest that stress resilience may play an important long–term role in etiological pathways for hypertension. If confirmed, this knowledge may help inform more effective preventive interventions by better addressing psychosocial risk factors and stress management across the lifespan.
Key messages.
What is already known on this subject?
Greater blood pressure reactivity to psychological stress has been associated with higher risk of developing hypertension. However, no studies have examined psychological assessment of stress resilience early in life in relation to hypertension risk in adulthood.
What might this study add?
In a large national cohort, we found that low stress resilience in late adolescence was associated with higher risk of developing hypertension in adulthood, independently of body mass index, family history of hypertension and socioeconomic factors.
How might this impact on clinical practice?
These findings suggest that low stress resilience may play an important long-term role in etiological pathways for hypertension. If confirmed, this knowledge may help inform more effective preventive interventions by addressing psychosocial risk factors and stress management across the lifespan.
Acknowledgments
Funding This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL116381); the Swedish Research Council; and ALF project grant, Region Skåne/Lund University, Sweden.
Footnotes
Competing interests None declared.
Patient consent Not required for this study as it used only registry-based secondary data.
Ethics approval Regional Ethics Committee of Lund University in Sweden (no. 2010/476).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data for this original research article will be shared with external scientists in accordance with the Swedish law and the agreements that exist between us and the Swedish Government agencies. Descriptions of the data sets and definitions of the variables and published manuscripts will be posted on a specially designed link at the Center for Primary Health Care Research website at Lund University.
Contributors JS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: CC, JS, MAW and KS. Acquisition of data: JS and KS. Analysis and interpretation of data: CC, JS, MAW and KS. Drafting of the manuscript: CC. Critical revision of the manuscript for important intellectual content: CC, JS, MAW and KS. Statistical analysis: CC and JS. Obtained funding: JS and KS.
References
- 1.MMWR. Vital Signs: Prevalence, Treatment, and Control of Hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–8. [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Lehman BJ, Taylor SE, Kiefe CI, et al. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol. 2009;28:338–46. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan LL, Liu K, Matthews KA, et al. Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 2003;290:2138–48. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]
- 5.Steptoe A, Brydon L, Kunz-Ebrecht S. Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosom Med. 2005;67:281–7. doi: 10.1097/01.psy.0000156932.96261.d2. [DOI] [PubMed] [Google Scholar]
- 6.Pan Y, Cai W, Cheng Q, et al. Association between anxiety and hypertension: a systematic review and meta-analysis of epidemiological studies. Neuropsychiatr Dis Treat. 2015;11:1121–30. doi: 10.2147/NDT.S77710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L, Chen D, Yang Y, et al. Depression increases the risk of hypertension incidence: a meta-analysis of prospective cohort studies. J Hypertens. 2012;30:842–51. doi: 10.1097/HJH.0b013e32835080b7. [DOI] [PubMed] [Google Scholar]
- 8.Carroll D, Smith GD, Shipley MJ, et al. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med. 2001;63:737–43. doi: 10.1097/00006842-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Katholi CR, McCreath H, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–8. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 10.Falkstedt D, Sorjonen K, Hemmingsson T, et al. Psychosocial functioning and intelligence both partly explain socioeconomic inequalities in premature death. A population-based male cohort study. PLoS ONE. 2013;8:e82031. doi: 10.1371/journal.pone.0082031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilieblad B, Stahlberg B. Reliability of the psychological assessments at conscription. Stockholm, Sweden: Armed Forces Research Department; 1977. [Google Scholar]
- 12.Bergh C, Udumyan R, Fall K, et al. Stress resilience and physical fitness in adolescence and risk of coronary heart disease in middle age. Heart. 2015;101:623–9. doi: 10.1136/heartjnl-2014-306703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.No authors listed] 1993 guidelines for the management of mild hypertension: memorandum from a World Health Organization/International Society of Hypertension meeting. Guidelines Sub-Committee. J Hypertens. 1993;11:905–18. doi: 10.1097/00004872-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 15.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Rep. 2010;(25):1–5. [PubMed] [Google Scholar]
- 16.Schulz AJ, Zenk SN, Israel BA, et al. Do neighborhood economic characteristics, racial composition, and residential stability predict perceptions of stress associated with the physical and social environment? Findings from a multilevel analysis in Detroit. J Urban Health. 2008;85:642–61. doi: 10.1007/s11524-008-9288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mujahid MS, Diez Roux AV, Morenoff JD, et al. Neighborhood characteristics and hypertension. Epidemiology. 2008;19:590–8. doi: 10.1097/EDE.0b013e3181772cb2. [DOI] [PubMed] [Google Scholar]
- 18.Crump C, Sundquist K, Sundquist J, et al. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol. 2011;21:231–7. doi: 10.1016/j.annepidem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–98. doi: 10.1002/sim.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson DB, Kaufman JS. Estimation of the relative excess risk due to interaction and associated confidence bounds. Am J Epidemiol. 2009;169:756–60. doi: 10.1093/aje/kwn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasperin D, Netuveli G, Dias-da-Costa JS, et al. Effect of psychological stress on blood pressure increase: a meta-analysis of cohort studies. Cad Saude Publica. 2009;25:715–26. doi: 10.1590/s0102-311x2009000400002. [DOI] [PubMed] [Google Scholar]
- 22.Colin Bell A, Adair LS, Popkin BM. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol. 2002;155:346–53. doi: 10.1093/aje/155.4.346. [DOI] [PubMed] [Google Scholar]
- 23.Leng B, Jin Y, Li G, et al. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–9. doi: 10.1097/HJH.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 24.Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosom Med. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- 25.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 26.Lambiase MJ, Dorn J, Roemmich JN. Metabolic and cardiovascular adjustments during psychological stress and carotid artery intima-media thickness in youth. Physiol Behav. 2012;105:1140–7. doi: 10.1016/j.physbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Rod NH, Grønbaek M, Schnohr P, et al. Perceived stress as a risk factor for changes in health behaviour and cardiac risk profile: a longitudinal study. J Intern Med. 2009;266:467–75. doi: 10.1111/j.1365-2796.2009.02124.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet F, Irving K, Terra JL, et al. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–44. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Linden W, Moseley JV. The efficacy of behavioral treatments for hypertension. Appl Psychophysiol Biofeedback. 2006;31:51–63. doi: 10.1007/s10484-006-9004-8. [DOI] [PubMed] [Google Scholar]
- 30.Rainforth MV, Schneider RH, Nidich SI, et al. Stress reduction programs in patients with elevated blood pressure: a systematic review and meta-analysis. Curr Hypertens Rep. 2007;9:520–8. doi: 10.1007/s11906-007-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]