Abstract
Therapy-related myeloid neoplasms (tMN) are serious late effects of the treatment of cancer with poor response to conventional treatment. Azacitidine (AZA) has been used to treat patients with tMN but current data are retrospective. We present here 47 tMN patients prospectively enrolled as a specific cohort in the E1905 study. TheE1905 study was a randomized phase 2 study (NCT00313586) testing 10 d of AZA (50 mg/m2/d) +/− the histone deacetylase inhibitor entinostat (4 mg/m2/d PO day-3 and day-10). A total of 47 patients [29 therapy-related myelosyspastic syndrome (t-MDS) and 18 therapy-related acute myeloid leukaemia (t-AML)] were recruited to the study. 24 patients were treated with AZA monotherapy and 23 with AZA+entinostat. The median number of administered cycles was 4, significantly higher in patients treated with AZA (6 cycles vs. 3 cycles, P = 0·008). Haematological normalization rates were 46% in monotherapy and 17% in the combination arm. Median overall survivals were 13 and 6 months, respectively. The novel 50 * 10 schedule of azacitidine appears effective, with response rates, when given as single agent, comparable to those for patients with de novo MDS/AML treated on the same protocol. However, the combination of AZA and entinostat was associated with increased toxicity and could not be recommended for treatment of tMN.
Keywords: myelodysplasia, acute myeloid leukaemia, azacitidine, histone deacetylase inhibitor, therapy related
Among the new entities of the 2008 World Health Organization (WHO) classification (Vardiman et al, 2009), one subtype was not defined either by pathological or cytogenetic/molecular features but by its causality: therapy-related myeloid neoplasms (tMN), which are defined as myelodysplastic syndrome (MDS) or acute myeloid leukaemia (AML) arising after exposure to chemotherapy or radiation therapy. As cancer care has improved, more patients are exposed to the risk of developing tMN. Depending on the causal agents, tMN displays recurrent genetic lesions, such as translocations (implicating KMT2A [MLL], RUNX1, CBFB, or RARA) or multiple aberrations including chromosome 5 or 7 deletions within complex karyotypes (Rowley & Olney, 2002; Stone, 2009; Dohner et al, 2010). The prognosis of tMN treated with conventional therapy is usually considered to be poor and is strongly influenced by cytogenetics and performance status, potentially impaired by prior cancer and treatments.
In this context, the use of alternative treatments is warranted and azacitidine (AZA) may represent a suitable option. Azacitidine is the standard of care of treatment for high-risk MDS (Silverman et al, 2006; Fenaux et al, 2009) and is also effective in AML (Fenaux et al, 2010). Several studies have shown that AZA could be safe and effective in patients with poor general condition performance status and/or comorbidities (Garcia-Manero et al, 2014) and may be associated with significant response rate in patients with high risk cytogenetics. Three retrospective studies (Fianchi et al, 2012; Bally et al, 2013; Duong et al, 2013) suggested significant activity of AZA in patients with tMN with ORR ranging from 39% to 43% and median overall survival from 10 to 14 months. However, no prospective data have been presented so far.
More importantly, AZA results still need to be improved. Histone deacetylase inhibitors (HDACi) and DNA methyltransferase inhibitors (DNMTi) combine synergistically in their interactive epigenetic effects when appropriately sequenced (Cameron et al, 1999; Gore, 2006). The orally bioavailable benzamide HDACi entinostat inhibits the Class I HDAC enzymes and had shown activity in a monotherapy phase I trial (Gojo et al, 2007). In a previous phase I pilot study (J0443 study, ClinicalTrials.gov Identifier NCT00101179), we found that the combination of AZA and entinostat was effective and tolerable for patients with MDS and AML. This trial, built on a 10 d schedule of AZA that had been developed to optimize DNA methylation through prolonged administration of lower daily dose AZA, was designed to cause less cell cycle inhibition (Fandy et al, 2009). The recommended phase II schedule was AZA 50 mg/m2/d s.c. for 10 d (500 mg/m2/cycle) and entinostat 4 mg/m2/d orally on day 3 and day 10 of AZA each 28 d. The Eastern Cooperative Oncology Group (ECOG) and North American Leukemia Intergroup subsequently conducted a randomized Phase II trial, E1905 (ClinicalTrials.gov Identifier NCT00313586), aiming to improve the response rate of AZA through administration of the 10 d schedule with or without addition of entinostat. Results for de novo MDS and AML with myelodysplasia-related changes (MRC) have already been published (Prebet et al, 2014). A dedicated tMN cohort was accrued following an amendment of the original protocol. Results for this specific cohort are described in the present report.
Patients and methods
Patients
All patients included fulfilled the following criteria: (i) diagnosis of t-MDS or t-AML according to the 2008 WHO classification (Vardiman et al, 2009); (ii) patients with MDS could have any international prognostic scoring system (IPSS) classification (Greenberg et al, 1997). However, a required platelet count <50 × 109/l and/or an absolute neutrophil count <0·5 × 109/l was necessary for patients with low or intermediate-1 MDS; (iii) patients with AML could have any degree of bone marrow blast involvement (i.e. not limited to 20–30% blasts) but should not have signs of rapidly progressive disease [white blood cell count (WBC) >30 × 109/l or doubling time below 4 weeks and WBC >20 × 109/l]. Patients with prior exposure to DNMTi, entinostat, induction chemotherapy or stem cell transplantation were not eligible. Patients with any sign of activity of their original cancer were also not eligible. The study was approved by the institutional review board of each participating centre. All patients gave their signed informed consent for the use of the clinical and biological data. The cytogenetic risk group assessment used the IPSS stratification for all patients.
Protocol design
The E1905 trial is an ECOG-led joint trial from the North American Leukemia Intergroup, which includes Cancer and Leukemia Group B (CALGB), Eastern Collaborative Oncology Group (ECOG), South Western Oncology group (SWOG) and Cancer Trials Support Unit (CTSU). This study was a phase II 1:1 randomized trial evaluating the efficacy of AZA alone 50 mg/m2/d s.c. for 10 d (days 1–10, Arm A) and AZA with the addition of entinostat 4 mg/m2/d orally on day 3 and day 10 (Arm B). Each cycle was of 28 d duration. Following six cycles of treatment, patients with documented clinical response continued for the lesser of a total of 24 cycles or disease progression. Bone marrow was evaluated after cycle 6, 12, 18 and 24 (end of treatment).
Protocol evaluation
In the protocol, haematological normalization (HN) was defined by achieving complete remission (CR), partial remission (PR) or major trilineage haematological improvement (TL). The primary objective was to evaluate the rate of HN in each arm.
The clinical response and cytogenetic response assessment used International working group (IWG) 2000 criteria (Cheson et al, 2000) because the protocol was designed in 2005 and the first patients of the de novo cohort were included in Q4 2006. Use of HN as a primary endpoint was decided based on some of the criticisms of IWG 2000 (clinical meaning of minor haematological improvement, etc.). Clinical data, biological data (bone marrow smears, biopsy sections and cytogenetics) and response assessment were centrally reviewed. Other types of major haematological improvements (in one or two lineages) were also registered but were not included in response as defined per protocol objectives. Toxicities were assessed using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3 definitions (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Statistical analysis
One-stage designs were employed for each arm. The primary objective of the study was to achieve a HN rate of 25% or higher. It was considered evidence that the treatment arm merited further study, while 5% or less would be of no clinical interest. Twenty eligible patients per arm were planned for this study to achieve power of 90% with one-sided type I error of 0·1. Allowing for a 10% rate of ineligibility, the total accrual for both arms was targeted at 44 patients.
Patient baseline characteristics were compared using Fisher’s exact test for categorical and Wilcoxon rank sum test for continuous variables. P values <0·05 were considered as significant. Adverse events were compared using Fisher’s exact test. The confidence intervals of response rates on each treatment arm were calculated based on exact binomial distribution. Overall survival (OS) was defined as time from study randomization/registration to death from any cause, with follow-up censored at the date of last contact. Duration of response was defined by the time interval between the date of first response and the date of disease progression. Patients without progression were censored at the date of the last follow-up. Kaplan–Meier estimates were used to estimate the event-time distributions. For survival analysis, log-rank tests stratified by disease classification at randomization were used. Logistic and Cox proportional hazards models were used to compare HN rates and OS respectively between tMN patients and de novo MDS/AML patients, controlling for other risk factors. All p values were based on 2-sided tests.
Results
Patients’ characteristics
Between September 2009 and May 2011, a total of 47 tMN patients were enrolled on the E1905 trial. All patients were deemed eligible and were included in this analysis. Median age was 69 years (range, 39–83), 45% were male and 94% of patients had ECOG performance score (PS) 0–1. Lymphoid malignancies and invasive breast cancer were the most common reasons of exposure to cytotoxic agents or radiotherapy (see Table SI for details). Twenty-nine patients could be sub-classified as t-MDS and 18 as t-AML. At inclusion, median peripheral blood counts were: neutrophils 1·0 × 109/l, platelets 35 × 109/l, haemoglobin 92 g/l, peripheral blood blasts 0%. Sixty eight per cent of patients were red blood cell (RBC) transfusion dependent and 40% were platelet transfusion dependent. The median bone marrow blast count was 14·0%. As expected, the cytogenetic evaluation showed a high frequency of unfavourable risk cytogenetics (74%) as compared to normal or intermediate or low risk cytogenetics (26%). Baseline characteristics were not statistically different between the 2 arms (Table I).
Table I.
Patient characteristics.
| Whole population | AZA alone (Arm A) | AZA+entinostat (Arm B) | P value | |
|---|---|---|---|---|
| n | 47 | 24 | 23 | |
| Median age (years) | 69 (39–83) | 68 (54–83) | 71 (39–81) | 0·81 |
| Sex ratio (Male/Female) | 21/26 | 9/15 | 12/11 | 0·39 |
| ECOG PS 0–1/2 | 44/3 | 22/2 | 22/1 | |
| Disease classification | ||||
| IPSS Low/Int-1 MDS | 2 (4%) | 1 (4%) | 1 (4%) | 0·88 |
| IPSS Int-2/High MDS | 27 (57%) | 13 (54%) | 14 (61%) | |
| AML | 18 (38%) | 10 (42%) | 8 (35%) | |
| Median bone marrow blast count (%) | 14% (0–65) | 18% (0–60) | 11% (3–65) | 0·87 |
| IPSS cytogenetic risk stratification | ||||
| Favourable | 4 (8%) | 2 (8%) | 2 (8%) | 1·00 |
| Intermediate | 6 (15%) | 3 (13%) | 3 (13%) | |
| High risk | 29 (62%) | 16 (67%) | 13 (57%) | |
| Missing | 8 (13%) | 3 (14%) | 5 (12%) | |
| RBC transfusion dependency | 32 (68%) | 16 (67%) | 16 (70%) | 1·00 |
| Platelet transfusion dependency | 19 (40%) | 9 (38%) | 10 (44%) | 0·77 |
ECOG PS, Eastern Cooperative Oncology Group performance score IPSS, international Prognostic Scoring System; Int, intermediate; MDS, myelodysplastic syndrome; AML, acute myeloid leukaemia; RBC, red blood cell*: IPSS stratification was assessed only for patient with MDS and excluding AML.
Treatment administration and toxicities
Twenty-four patients were treated with AZA monotherapy and 23 with AZA+entinostat. The median duration of each cycle was 28 d. The median number of administered cycles was 4 and was significantly higher in patients treated with AZA monotherapy (6 cycles vs. 3 cycles, P = 0·008). Details of severe adverse events are presented in Table II. Grade 3 and 4 treatment-related non-haematological adverse events were reported in 57% of the patients (54% in Arm A, 61% in Arm B) and virtually all patients experienced some degree of haematological toxicity during treatment (100% in Arm A and 87% in Arm B). The most frequent reasons for stopping treatment were disease progression (n = 13, 10 in Arm A, 3 in Arm B) or treatment-related toxicity (n = 13, 3 in Arm A, 10 in Arm B). A total of 8 patients died while on study (infections: 1 in Arm A, 6 in Arm B, ischaemia: 1 in Arm B). Taken together, these data showed a significant excess of treatment discontinuation related either to treatment-related intolerance or to death on study in Arm B (17% vs. 74%, P < 0·001).
Table II.
Report of the most common grade 3–4 CTCAE drug-related adverse events and death on study.
| Arm A grade 3 |
Arm A grade 4 |
Arm A death on study |
Arm B grade 3 |
Arm B grade 4 |
Arm-B death on study |
|
|---|---|---|---|---|---|---|
| Haematological toxicities | ||||||
| Anaemia | 12 (50%) | 4 (17%) | 15 (65%) | 2 (9%) | ||
| Thrombocytopenia | 8 (33%) | 15 (63%) | 3 (13%) | 14 (61%) | ||
| Neutropenia | 2 (8%) | 22 (92%) | 2 (9%) | 16 (70%) | ||
| Non-haematological toxicities | 11 (48%) | 2 (8%) | 11 (48%) | 3 (13%) | ||
| Fatigue/asthenia | 1 (4%) | 3 (13%) | 1 (4%) | |||
| Joint/muscle pain/weakness | 1 (4%) | 4 (18%) | 1 (4%) | |||
| Nausea/vomiting | 3 (12%) | 2 (9%) | ||||
| Infections including aplastic fever | 10 (42%) | 1 (4%) | 1 (4%) | 7 (30%) | 4 (18%) | 6 (26%) |
| Hyponatraemia | 0 | 3 (13%) | ||||
| Hypokalaemia | 2 (8%) | 1 (4%) | ||||
| Ischaemia | 1 (4%) |
Results are presented as number (percentage). Patients may have had more than one adverse event. All adverse events were evaluated according to CTCAE (Common Terminology Criteria for Adverse Events) v3 definitions (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). All adverse events with a frequency of 5% or more in at least one arm are listed.
We further analysed the potential predictors of early death and did not find notable differences in age, ECOG PS, incidence of grade 3–4 neutropenia at inclusion, or disease subtype (MDS versus AML) (data not shown). The only significant difference was an over representation of prior B-cell malignancies (39 patients documented, 5 patients (71%) with B-cell malignancies in early death group versus 11 patients (33%) with B-cell malignancies in surviving patients, P = 0·09).
Response and survival
HN was achieved in 31% including 9% CR + PR, and 23% TL. The HN rates were 46% in Arm A {[95% confidence interval (CI): 26–67%], including 17% CR + PR and 29% TL} and 17% Arm B [(95% CI: 5–39%), including 0% CR + PR and 17% TL] (P = 0·06). Non-trilineage HI was achieved in an additional patient in Arm A (4%) and no patients in Arm B. Table III shows the details of the response evaluation. Overall response rate (ORR) was 50% and 17% in Arms A and B, respectively. The median time to first response was 3 months in both arms. Median duration of response was 8 months and 5 months in Arm A and Arm B, respectively. Cytogenetic response was evaluable for 13 patients over 35 with baseline cytogenetic abnormalities. No complete cytogenetic response and 4 partial cytogenetic responses were observed.
Table III.
Response evaluation.
| Azacitidine | Azacitidine +Entinostat |
|||
|---|---|---|---|---|
| N | % | N | % | |
| CR | 3 | 12·5 | 0 | 0 |
| PR | 1 | 4·2 | 0 | 0 |
| No change/stable | 8 | 33·3 | 17 | 73·9 |
| Progression/relapse | 4 | 16·7 | 2 | 8·7 |
| Trilineage HI without CR or PR | 7 | 29·2 | 4 | 17·4 |
| HI -not trilineage | 1 | 4·2 | 0 | 0 |
| Platelet HI | 1 | |||
| Haematological Normalization (CR/PR/trilineage HI) | 11 | 46% | 4 | 17% |
CR, complete response; PR, partial response; HI, haematological improvement.
Response was evaluated according to International Working Group 2000 criteria (Cheson et al, 2000).
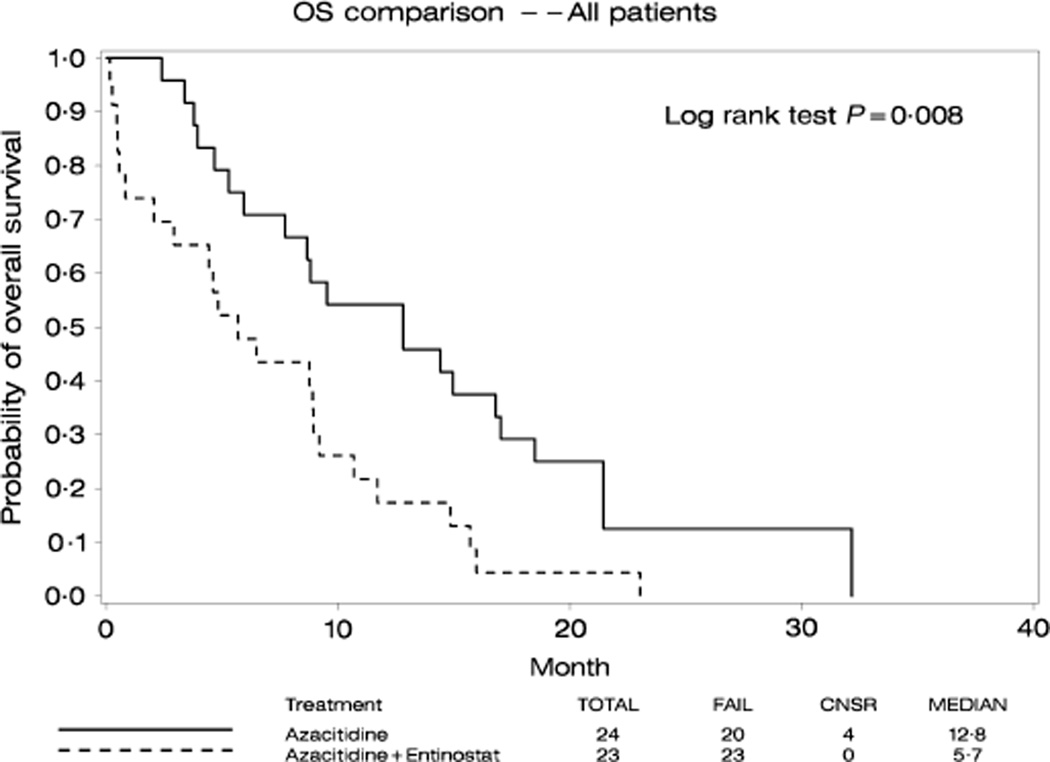
With a median follow-up of 21 months, 4 patients were alive and 43 had died. The median OS was 13 months in Arm A and 6 months in Arm B (Fig 1). Among those who survived to day 60 after study registration, a landmark analysis at day 60 (Figure S1) showed a median survival of 13 months in Arm A and 9 months in Arm B. For responding patients, median survival was 17 months in Arm A and 13 months in Arm B. Long-term survival was limited in both arms with a 2-year probability of OS below 15%. Only one patient in Arm A bridged to transplantation and was alive in CR at last follow-up.
Fig 1.
Kaplan Meier Estimates of overall survival for the patients treated with azacitidine (solid line) and azacitidine+entinostat (broken line). Survival is defined from the initiation of therapy to death of any causes. Time interval is in months.
Comparison with de novo MDS/AML patients
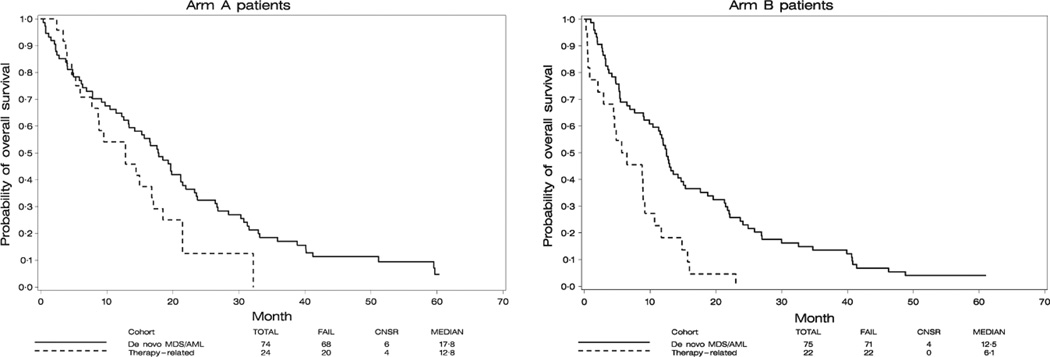
We performed multivariate analyses comparing the tMN patients with de novo MDS/AML patients treated in the previous cohort of the same protocol (Prebet et al, 2014) (149 patients analysed in the de novo cohort and 47 in the tMN cohort). All analyses were adjusted for age (>70 vs. ≤70 years), haemoglobin counts (>100 vs. ≤100 g/l), disease classification (IPSS low/int-1 MDS versus Int-2/high MDS versus AML versus chronic myelomonocytic leukaemia) and IPSS cytogenetic risk classification (favourable versus intermediate versus unfavourable). Adjusted for those factors, no significant difference in HN rate was observed between tMN and de novo MDS for either Arm A [odds ratio (OR): 1·05, 95% CI: (0·70, 1·57), P = 0·82] or Arm B [OR: 1·32, 95% CI: (0·70, 2·46), P = 0·39; interaction of treatment arms and patient cohorts: P = 0·25]. Similarly, no significant differences in overall response rate were observed between tMN and de novo MDS for Arm A [OR: 1·30, 95% CI: (0·88, 1·94), P = 0·19] but, for Arm B, de novo MDS/AML patients had marginally significantly higher overall response rate compared to tMN patients [44% vs. 18%; OR: 1·80, 95% CI: (0·98, 3·32), P = 0·06; interaction of treatment arms and patient cohorts: P = 0·10]. The Kaplan–Meier curves of OS for the two cohorts of patients according to treatment arm are shown in Fig 2. In Arm A, no statistical difference in OS was observed in the multivariate analysis between the two cohorts of patients [hazard ratio (HR) of de novo versus tMN: 0·78, 95% CI: (0·46, 1·33), P = 0·36]. In Arm B, de novo MDS/AML patients had significantly better OS compared to tMN patients in the multivariate analysis [HR de novo versus tMN: 0·42, 95% CI: (0·25, 0·70), P = 0·001; interaction of treatment arms and patient cohorts: P = 0·09].
Fig 2.
Comparison of overall survival in patients treated in the E1905 trials for de novo (solid line) or therapy-related (dash line) myeloid neoplasms. (A) Patients treated with azacitidine single agent (B) Patients treated with azacitidine+entinostat. Survival is defined from the initiation of therapy to death of any causes. Time interval is in months. MDS, myelodysplastic syndrome; AML, acute myeloid leukaemia.
Discussion
This study is the first prospective study specifically addressing the question of the treatment of tMN with DNMTi. Azacitidine 50 mg/m2 given for 10 d allowed half of the patients to experience response. Addition of Entinostat did not lead to an improvement in results.
The results presented here are supported by the retrospective series already published. Patients included in the North American (Duong et al, 2013) and European (Fianchi et al, 2012; Bally et al, 2013) multicentre cohorts had remarkably similar baseline characteristics with a younger age as compared to de novo MDS/AML, an increased frequency of female patients (underlining the prevalence in breast cancer patients) and 70–80% of adverse cytogenetics. Observed response and survival results in patients treated with AZA monotherapy also seem comparable, however with a trend to a higher response rate with the 50 mg/m2 for 10 d schedule: 38–43% in the retrospective studies as compared to 50% in our study. This was already noted in the initial E1905 report and we can speculate that it may be related to a more prolonged demethylation of DNA. These short response duration and survival may be related to multiple factors related either to the patient condition or to the disease characteristics. Based on the recent works on TP53 (Bejar et al, 2011; Ramsingh et al, 2013), an increased frequency of adverse TP53 mutation may be expected in our cohort and impact the prognosis. Unfortunately, we were not able to evaluate mutational patterns of these patients. Allogeneic stem cell transplantation is commonly recommended for this group of patients; dedicated prospective trials of this modality are not available but DNMTi based regimens may be effective strategies to bridge to transplantation, keeping in mind the short duration of response observed in this group of patients (12 months in the de novo MDS/AML cohort of E1905).
The comparison with patients treated for de novo AML/MDS was made possible by the availability of a prior cohort of patients prospectively treated with the same schedules. The response evaluation was performed according to IWG 2000 criteria (Cheson et al, 2000) and by using our composite HN criteria in order to use the same methodology that was used in 2005 when E1905 trial was initially designed and used for the de novo cohort of patients. Interestingly, after adjusting for the most significant variables, including cytogenetic clustering, there was no difference in survival or response between tMN and de novo diseases in patients with AZA monotherapy. This highlights the question of more broadly opening the inclusion in clinical trials for tMN patients, a population of patients with poor expectations when conventional treatments are used. We also investigated the impact of the addition of entinostat. In our prior study, we were not able to demonstrate any additive effect of combining entinostat to AZA (Prebet et al, 2014), most probably resulting from an excess of toxicity and, based on our prior correlative studies, from a potential pharmacodynamic antagonism between the two drugs when administered in an overlapping schedule. In this cohort of treatment-related patients, this trend tends to be confirmed with an increased frequency of early treatment discontinuation for toxicities in the combination arm. While the combination arm was not excessively toxic in the de novo cohort (Prebet et al, 2014), it seems likely that these previously treated patients may have reduced biological reserves, amplifying the additional toxicity in the combination arm even if there was only a limited number of patients with impaired PS (less than 10% in each cohort). Another point is the increased incidence of prior treated B-cell malignancies in the patients that died early: It may partially explain a more severe immunosuppression and an increased severity of infections. Our data underline the importance of close clinical and biological monitoring of these patients during the first cycles of treatment, particularly for patients treated with combination therapies given the potential additional toxicities. A more careful selection of patients before treatment may also be warranted: Application of prognosis scoring systems such as the revised IPSS (Greenberg et al, 2012) or the French prognostic score [FPS (Itzykson et al, 2011)] may predict outcome: In our cohort, patients with FPS low or intermediate had an OS of 9·4 months as compared to 5 months for patients with a score ranked high (P = 0·03). Interestingly, Garcia-Manero et al (2014) recently updated a phase I–II combination study of AZA and vorinostat, another HDACi, for patients not eligible for ‘conventional’ clinical trials. In this study, a majority of patients had previously treated cancer (either slowly progressive or recently treated) but no excess of toxicity was noted.
Taken together, our data showed that AZA, at 50 mg/m2/d for 10 d, is safe and feasible for patients with tMN. Response rate as well as survival seems similar to that observed for de novo patients treated with the same schedule (Prebet et al, 2014). Superiority of the 10 d schedule to the conventional 75 mg/m2/d for 7 d schedule cannot be confirmed without a randomized trial; however, response rate and survival to the newer schedule are promising. This schedule is potentially pharmacodynamically superior due to lesser cell cycle inhibition and longer exposure to drug enabling more cells to replicate in the presence of 5AC. Promising results have also been reported using prolonged exposure to low dose decitabine (Blum et al, 2010; Phillips et al, 2013). The addition of entinostat to this specific schedule does not add any supplemental benefit and cannot be recommended considering the excess of toxicity observed. Patients should be systematically considered for allogeneic transplantation and further studies will be necessary to explore alternative schedules and comparison in order to improve survival of this group of patients.
Supplementary Material
Acknowledgments
The authors would like to thank all the participating centres from ECOG-ACRIN, SWOG, CALGB and CTSU. The authors would also like to thank Elene Assefa from the ECOG data centre for her work on the data management of the study, and Xerxes Vevai from the ECOG Leukemia Translational Research Laboratory for collecting bone marrow smears from submitting institutions.
Funding
Supported by R01 CA125563501 (SG), R6034-08 from the Leukemia and Lymphoma Society of America (SG), K24 CA111717 (SG) and a grant from the Fulbright Franco-American commission/Foundation Monahan, Paris, France (TP). CA32102 and CA27057 (HPE).
Disclosures
SG: consultancy, research support and stock options from Celgene, Consultancy by Syndax. HPE: consultancy and speakers bureau member for Celgene.
Footnotes
Presented in part at the 2013 meeting of the American Society of Hematology.
Author contributions
SG designed the research, treated patients, analysed data and wrote the manuscript. TP, ZS analysed data and wrote the manuscript. LM, MS, MF analysed data. RK, EP, MC collected biological materials and analysed samples. PG helped design the study, treated patients on study and analysed data. MJ AZ treated patients on study. ML helped design the study, treated patients on the study and is the chair of the ECOG-ACRIN Leukemia Committee. JG and HPE chaired the study for CALGB and SWOG, respectively. MT was the former chair of the ECOG Leukemia Committee. All authors reviewed and agreed the present manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table SI. Prior exposures defining therapy related Myeloid Neoplasm in the cohort of patients included in E1905 trial.
Fig S1.
Landmark analysis at day 60 comparing azacitidine single agent and azacitidine+entinostat arms of treatment. Only patients surviving at day 60 were included in the analysis and survival is expressed on this graph from day 60.
References
- Bally C, Thepot S, Quesnel B, Vey N, Dreyfus F, Fadlallah J, Turlure P, de Botton S, Dartigeas C, de Renzis B, Itzykson R, Fenaux P, Ades L. Azacitidine in the treatment of therapy related myelodysplastic syndrome and acute myeloid leukemia (tMDS/AML): a report on 54 patients by the Groupe Francophone Des Myelodysplasies (GFM) Leukemia Research. 2013;37:637–640. doi: 10.1016/j.leukres.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, Kantarjian H, Raza A, Levine RL, Neuberg D, Ebert BL. Clinical effect of point mutations in myelodysplastic syndromes. New England Journal of Medicine. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KA, Liu Z, Lucas DM, Chen P, Xie Z, Baiocchi R, Benson DM, Devine SM, Jones J, Andritsos L, Flynn J, Plass C, Marcucci G, Chan KK, Grever MR, Byrd JC. Phase I trial of low dose decitabine targeting DNA hypermethylation in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: dose-limiting myelosuppression without evidence of DNA hypomethylation. British Journal of Haematology. 2010;150:189–195. doi: 10.1111/j.1365-2141.2010.08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nature Genetics. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, Lowenberg B, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Wijermans PW, Gore S, Greenberg PL. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Lowenberg B, Bloomfield CD. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- Duong VH, Lancet JE, Alrawi E, Al-Ali NH, Perkins J, Field T, Epling-Burnette PK, Zhang L, List AF, Komrokji RS. Outcome of azacitidine treatment in patients with therapy-related myeloid neoplasms with assessment of prognostic risk stratification models. Leukemia Research. 2013;37:510–515. doi: 10.1016/j.leukres.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Fandy TE, Herman JG, Kerns P, Jiemjit A, Sugar EA, Choi SH, Yang AS, Aucott T, Dauses T, Odchimar-Reissig R, Licht J, McConnell MJ, Nasrallah C, Kim MK, Zhang W, Sun Y, Murgo A, Espinoza-Delgado I, Oteiza K, Owoeye I, Silverman LR, Gore SD, Carraway HE. Early epigenetic changes and DNA damage do not predict clinical response in an overlapping schedule of 5-azacytidine and entinostat in patients with myeloid malignancies. Blood. 2009;114:2764–2773. doi: 10.1182/blood-2009-02-203547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncology. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of Clinical Oncology. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- Fianchi L, Criscuolo M, Lunghi M, Gaidano G, Breccia M, Levis A, Finelli C, Santini V, Musto P, Oliva EN, Leoni P, Aloe Spiriti A, D’Alo F, Hohaus S, Pagano L, Leone G, Voso MT. Outcome of therapy-related myeloid neoplasms treated with azacitidine. Journal of Hematology and Oncology. 2012;5:44. doi: 10.1186/1756-8722-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Manero G, Huang X, Cabrero M, DiNardo C, Pemmaraju N, Daver NG, Borthakur G, Wierda W, Kadia T, Alvarado Y, Cortes J, Jain N, Ravandi F, Jabbour E, Brandt M, Sneed T, Sukholutsky V, Pierce S, Bohannan Z, Kantarjian H. A bayesian phase II randomized trial of azacitidine versus Azacitidine + Vorinostat in patients with newly diagnosed AML or high-risk MDS with poor performance status, organ dysfunction, or other comorbidities. Blood (ASH Annual Meeting Abstracts) 2014;124:3277. [Google Scholar]
- Gojo I, Jiemjit A, Trepel JB, Sparreboom A, Figg WD, Rollins S, Tidwell ML, Greer J, Chung EJ, Lee MJ, Gore SD, Sausville EA, Zwiebel J, Karp JE. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore SD. Six (or more) drugs in search of a mechanism: DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Journal of the National Comprehensive Cancer Network. 2006;4:83–90. doi: 10.6004/jnccn.2006.0009. [DOI] [PubMed] [Google Scholar]
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstocker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, de Botton S, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Ades L, Fenaux P. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- Phillips CL, Davies SM, McMasters R, Absalon M, O’Brien M, Mo J, Broun R, Moscow JA, Smolarek T, Garzon R, Blum W, Schwind S, Marcucci G, Perentesis JP. Low dose decitabine in very high risk relapsed or refractory acute myeloid leukaemia in children and young adults. British Journal of Haematology. 2013;161:406–410. doi: 10.1111/bjh.12268. [DOI] [PubMed] [Google Scholar]
- Prebet T, Sun Z, Figueroa ME, Ketterling R, Melnick A, Greenberg PL, Herman JG, Juckett M, Smith MR, Malick L, Paietta E, Czader M, Litzow MR, Gabrilove J, Erba HP, Gore SD, Tallman MS. Prolonged administration of Azacitidine with or without Entinostat increases rate of hematologic normalization for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905. Journal of Clinical Oncology. 2014;32:1242–1248. doi: 10.1200/JCO.2013.50.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsingh G, Young A, Shen D, Miller C, Lamprecht T, Heath S, Fulton RS, Mardis ER, Ding L, Westervelt P, Welch J, Walter MJ, Graubert T, DiPersio JF, Ley TJ, Druley TE, Wilson RK, Link DC. The role of early TP53 mutations on the evolution of therapy-related AML. Blood. 2013;122:5. [Google Scholar]
- Rowley JD, Olney HJ. International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: overview report. Genes Chromosomes Cancer. 2002;33:331–345. doi: 10.1002/gcc.10040. [DOI] [PubMed] [Google Scholar]
- Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, Larson RA. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. Journal of Clinical Oncology. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- Stone RM. How I treat patients with Myelodysplastic syndroms. Blood. 2009;113:6296–6303. doi: 10.1182/blood-2008-09-038935. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.