Abstract
The role that meal pattern plays in weight regulation is a popular topic of scientific and common debate. The goal of this study was to evaluate the relationship between meal timing with caloric intake and body mass index (BMI). We hypothesized that latemeal timing and eating closer to sleep onset time would be associated with greater energy intake and higher BMI. Participants included 59 individuals recruited from the community. Rest/activity patterns were assessed using seven days of wrist actigraphy, and caloric intake was evaluated using seven days of diet logs. Results demonstrated that the timing of meals was associated with overall energy intake but not with BMI. In multivariate analyses controlling for age, gender, sleep duration, and timing; eating more frequently, later timing of the last meal, and a shorter duration between last meal and sleep onset predicted higher total caloric intake. In a mediational model, eating frequency explained the relationship between eating closer to sleep onset and total caloric intake. Results suggest that later relative timing of meals, particularly eating close to sleep, could lead to weight gain due to a greater number of eating occasions and higher total daily caloric intake. These findings have important implications for the development of novel, time-based interventions for weight management.
Keywords: Eating late, caloric intake, sleep, meal timing, sleep onset, human
1. Introduction
The role of meal pattern and timing in weight management is not well understood, with most weight loss interventions focusing primarily on total energy intake and less on timing of meals. However, recent evidence from several animal studies suggest that alterations in the timing of feeding impacts weight gain [1–3]. Arble and colleagues [1] observed a greater weight gain in mice fed only during the light phase (normal rest period), compared to mice fed only during the dark phase (normal active period). This finding was replicated by Fonken and colleagues [2] who found when mice were kept in constant light, they gained more weight than mice under a light/dark cycle; however, when feeding was restricted to the normal feeding time (biological night), the effects of the constant light were no longer observed. In addition, a more recent study, using a restricted feeding regime with similar caloric intake in mice, suggests that the duration of fasting countered the adverse effects of a high fat diet [3].
There are few published studies that have examined meal timing specifically in humans, and the majority have only focused on breakfast consumption and regularity of eating [4]. In the few human meal timing studies, [5–8] the findings are mixed. Obese men had double the energy intake between 10 pm and 4 am than normal weight men (8% vs. 4%) [5]; and in a more recent study of women, the only difference in meal timing reported between obese and normal weight women was that normal weight women to eat dinner later on the weekends [6]. Night eating or eating the majority of calories either in the evening or after sleep onset is also associated with a higher BMI in obese individuals [9]. These studies highlight the need for additional investigation into the role played by the timing of meals in human weight regulation.
Recent research by our group indicated that eating after 8pm was associated with a higher body mass index (BMI), even after controlling for sleep timing and duration [10]. The goal of the current study is to evaluate the relationship between the timing of meals with caloric intake and BMI while controlling for the timing and duration of sleep. Typical meal timing and caloric intake was determined using seven-day food diaries. We hypothesize that later meal timing and eating closer to sleep onset time will be associated with greater energy intake and higher BMI.
2. Methods and Materials
2.1 Participants
Via advertisements, participants (males and females) were recruited from the community for a larger study of circadian rhythms targeting “healthy sleepers” and “night owls, or larks”. The study was approved by the Northwestern University Institutional Review Board, and all participants gave written informed consent prior to enrollment. Exclusionary criteria included elevated depressive symptoms, as indicated by a score of > 20 on the Center for Epidemiologic Studies Depression Scale (CESD) [11]. None of the participants reported employment involving shift work.
2.2 Procedure
Participants underwent preliminary screening via telephone or email to determine eligibility and willingness to participate in the study. Once informed consent was obtained, participants were provided with seven days of diet logs, sleep logs, and a wrist actigraph (AW-L Actiwatch, Mini Mitter Co. Inc., Bend, OR), which was worn for at least seven days [12]. In the daily diet logs, the participants were asked to list a description of each food (quantity, preparation, name brand, etc.), as well as the time and location in which the meal or snack was consumed. In the sleep logs, participants were asked to report sleep and wake times, which were used in combination with the actigraphy (Actiware-Sleep 3.4 software) to determine sleep duration and sleep quality [13].
2.3 Measures
Participants were screened for depression with the Center for Epidemiologic Studies-Depression Scale (CES-D); this widely-used 20 item questionnaire reports adequate internal consistency [11]. As based upon self-reported height and weight, BMI was calculated as kg/m2. The BMI was calculated in this manner since objective height and weight were not obtained, as theassessments were conducted exclusively via mail and telephone.
2.4 Dietary Assessment
Dietary intake was assessed using a diet log in which participants recorded all food and drinks for a seven day period [10]. We asked participants to record the time the food or drink was consumed; type of meal (breakfast, lunch, dinner, or snack); type of food (with brand name, if possible); the location (i.e., home or restaurant) where the meal or snack was consumed; portion size; and whether it was a day they consumed less than a typical diet, more than a typical diet, or a typical diet. Along with the diet logs, participants were provided with two pages of instructions for completing the diet logs. The first page of the instructions indicated for the participants to include portion size (cups, ounces, and pieces), brand, information on the preparation method (e.g. boiled, fried in oil, eaten with refuse), and condiment usage, as well as break up the foods into component parts (e.g. sandwich is two pieces of wheat bread, 2 oz of turkey breast). The second sheet was a portion size guide, and it provided suggestions for how to judge portions without measuring (e.g. the size of a deck of cards, ping pong ball, your fist).
Diet logs were analyzed using publicly available nutrition information (www.sparkpeople.com), as well as restaurant and manufacturer websites. Daily, caloric intakes for each meal and for each entire day were computed and then the mean was computed for the seven day period. Meals were classified as breakfast, lunch, or dinner, based upon the designation the participant indicated in the diet log. Meals listed as “brunch” were considered neither, and included only as meal one (where appropriate) and in the total nutrition analyses for the day, but not as a meal type (breakfast, lunch, dinner, snack), in order to avoid redundancy. Logs were considered valid if there were at least two weekdays and one weekend day completed, in order to provide a representative average of a typical week. Dietary logs were excluded if total calories per day were <500. If participants had fewer than seven days recorded, all of the available data was used; alternatively, if an excess of seven days were completed, the investigators used the first seven consecutive days that best coincided with the actigraphy recordings. Timing of a meal was listed at the start time in the log. In case of skipping meals, meal times and caloric content were only calculated if at least three of that particular meals episode (that day's meal episodes) were consumed. Meal episodes were calculated as foods consumed within a 30 min duration. For example, if a snack was consumed at 1:00 and another at 1:45, the latter would be considered a separate meal. Since an eating occasion designated by the participant as “breakfast” or “dinner” may not have been the first or last meal consumed in a given day, two additional variables were calculated to represent the first (“first meal”) and last (“last meal”) intake of food or drink (other than water) of each day. In some cases, first and last meal could be the same as a designated meal (i.e. breakfast, lunch, dinner) or a snack. In the case of conflicting data, such as a breakfast time listed prior to a wake time, calorie information was utilized but meal time was omitted.
2.5 Sleep Timing and Duration
Sleep timing and duration were assessed using sleep logs and wrist actigraphy. The following variables were determined: sleep start (sleep onset), sleep end (final wake time), and sleep duration (total sleep time). Rest intervals (inclusive of bedtime and wake time) were set by the investigators, using the sleep logs as a guide [13]. Sleep variables were calculated by the Actiware 3.4 software (Philips/Respironics), using default settings. Sleep start was defined as the first ten minute period in which no more than one epoch was scored as mobile. Sleep end was defined as the last ten minute period in which no more than one epoch was scored as immobile (wake threshold set at medium). Sleep duration was defined as the amount of time between sleep start and sleep end. We calculated midpoint of sleep based on the average of the sleep onset and sleep offset for the seven day period.
In all but three participants, sleep logs and actigraphy were conducted during the same seven days as the diet logs. For these three participants, we used actigraphy data from within the same month as the diet logs to calculate sleep times, but we did not calculate relationships between sleep times and meal times since actigraphy was conducted at a different time.
2.6 Statistical analyses
Data were analyzed using SPSS (v17.0). The number, mean, and standard deviation for all variables were determined. Univariate analyses were first conducted using Pearson correlations. Significant correlations were followed up by multivariable analyses, controlling for relevant covariates (age, gender, sleep duration, and sleep timing). BMI was not used as a covariate, as it was not significantly associated with sleep, total calories, meal timing, or eating frequency. We also conducted mediation analysis using multiple regression analyses [14]. Statistical significance was determined as <0.05 on two tailed tests.
3. Results
The characteristics of the participant are listed in Table 1. Average age of the participants was 31.7 ± 11.8 years, and half of the participants were female. Average BMI was normal at 24.1 ± 4.2 (range 19–35). Average meal times are listed in Table 1. Average breakfast time was 9:37 AM ± 1:50, and average last meal was 9:02 PM ± 1:47. Last meal was approximately four hours before sleep onset (4:16 ± 1:41). The average number of meals and snacks was 4.5 ± 1.4 (range 2–9).
Table 1.
Demographics and sleep, meal, and calorie characteristics of participants
| Variable | Means ± SD |
|---|---|
| Sample characteristics | |
| Age (years) | 31 ± 11.8 |
| BMI (kg/m2) | 24.1 ± 4.2 |
| Actigraphy Variables | |
| Sleep start (hh:mm) | 01:17 ± 2:00 |
| Sleep end (hh:mm) | 8:34 ± 2:00 |
| Sleep duration (hours) | 6:11 ± 1:02 |
| Midpoint (hours) | 4:52 ± 1:57 |
| Meal Times/Calories * | |
| Breakfast Time (hh:mm)a | 9:37 ± 1:50 |
| Calories | 330 ± 151 |
| First Meal (hh:mm) | 10:02 ± 2:02 |
| Calories | 431 ± 1374 |
| Lunch Time (hh:mm)b | 13:32 ± 1:08 |
| Calories | 471 ± 267 |
| Dinner Time(hh:mm)c | 19:10 ± 2:05 |
| Calories | 682 ± 305 |
| Last Meal (hh:mm) | 21:02 ± 1:46 |
| Calories | 564 ± 292 |
| Total Calories | 1992 ± 539 |
| Calories from snacks | 503 ± 375 |
| Calories after dinner | 161 ± 153 |
| Eating Frequency (n) | 4.5 ± 1.5 |
| Meal – Sleep Relationships ** | |
| Sleep end- First Meald | 1:25 ± 0:58 |
| Breakfast-lunche | 4:06 ± 1:03 |
| Lunch-Dinnerf | 5:51 ± 1:04 |
| Dinner- Last Meal | 1:46 ± 1:22 |
| Last Meal – sleep onsetg | 4:17 ± 1:41 |
Data is presented as mean ± SD
N=59 unless otherwise indicated
Uneven n for meal times is due to participants skipping some meals. Meal times were not calculated if the meal was consumed less than 3 out of 7 days.
N=54
N=52
N=56
Missing associations between sleep times and meal times were due to actigraphy not worn at same time as diet log n=3 or meal skipping (see above). All values hours:minutes.
N=56
N=49
N=52
N=56
3.1 Correlations between meal timing and caloric intake
Correlations between calories, body mass index, meal timing, meal frequency, and measures of sleep are listed in Table 2. Eating frequency (r= .44 p=.001), time of last meal (r= .39, p=.002), duration between lunch and dinner (r= .34 p=.01), duration between dinner and last meal (r=.32 p=.02), and duration between sleep onset and the last meal (r= −.36 p=.007) were associated with daily calories (Figure 1). Timing of breakfast, lunch, or dinner was not associated with caloric intake, and meal timing variables were not associated with BMI.
Table 2.
Associations between total calories, body mass index, meal timing, meal frequency, and measures of sleep
| Total Calories | BMI | Last Meal | Eating Frequency | Dinner-Last Meal | Last Meal – Sleep onset | Sleep Duration | Sleep Midpoint | |
|---|---|---|---|---|---|---|---|---|
| Total Calories | - | 0.21 | 0.39 ** | 0.44 ** | 0.32 * | − 0.36 ** | − 0.29 * | −0.09 |
| BMI | - | 0.20 | 0.04 | 0.27 | −0.01 | −0.14 | 0.16 | |
| Last Meal | - | − 0.44 ** | 0.75 ** | − 0.32 * | − 0.37 ** | 0.53 ** | ||
| Eating Frequency | - | 0.38 ** | − 0.57 ** | −0.06 | −0.18 | |||
| Dinner- Last Meal | - | −0.24# | − 0.28 * | 0.36 ** | ||||
| Last Meal – Sleep onset | - | 0.11 | 0.58 ** | |||||
| Sleep Duration | - | −0.12 | ||||||
| Sleep Midpoint | - |
Values indicate r values, body mass index (BMI)
p= <0.05
p<0.001
p=0.08
Figure 1.
Association between measures of meal timing and frequency and total calories.
3.2 Multivariable Analyses
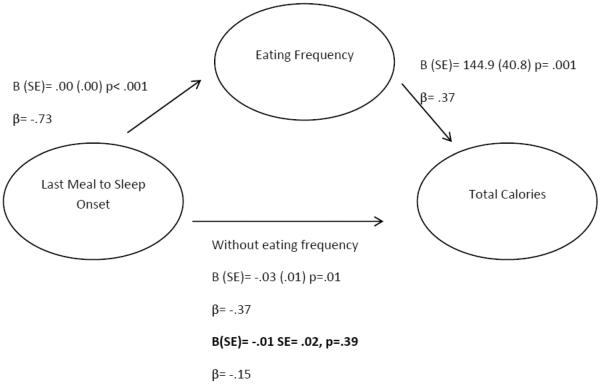
In multivariable regression analyses, which controlled for age, gender, sleep duration, and sleep timing, the eating frequency (B(SE) = 144.90 (40.83), p=.002, r2Δ = .15), time of last meal (B(SE)= 0.4 (.01), p=.001, r2Δ = .16), duration between last meal to sleep onset (B(SE)=0−.03 (.01), p=.02, r2Δ = .09), and duration between dinner and last meal (B(SE)= 0.04 (0.01), p=.007, r2Δ = .11) were associated with total caloric intake. We conducted mediational analyses to determine if the relationship between last meal and sleep onset was mediated by eating frequency (Figure 2). When both eating frequency and duration between last meal and sleep onset were entered in the same model, only eating frequency remained significant (B(SE)= 108.58 (52.4) p=.044).
Figure 2.
Mediation Model between last meal to sleep onset, eating frequency, and total calories.
Bold= estimate with the mediator in the model. The relationship between last meal to sleep onset and total calories is not significant after controlling for eating frequency. Model includes age, gender, sleep duration, and sleep timing.
4. Discussion
The primary hypothesis for the current study was that later meal timing would be associated with greater energy intake and a higher BMI, independent of sleep timing and duration. Our findings demonstrate that after controlling for demographic factors and sleep, two measures of eating late - eating a later last meal and eating closer to sleep onset - were associated with greater caloric intake. In addition, eating frequency was associated with caloric intake. Interestingly, the timing of the first meal consumed (breakfast/meal one) was not associated with total caloric intake, and none of the meal timing variables examined were associated with BMI.
To date, research on the timing of feeding has primarily focused on early in the day eating patterns [4, 15, 16]. In this study, we examined the timing of all meals, including the meal that participants designated as “breakfast” and/or first meal, and found no association between eating earlier in the day and overall caloric intake or BMI. This lack of association between “breakfast” time and caloric intake may be the result of 85% of the sample consuming breakfast and having this first meal within an average of 85 minutes of waking up. In contrast, eating late and eating closer to sleep onset was associated with a greater daily caloric intake. We also noted that there was considerable variability among participants in the timing of the last meal and how close that meal was to sleep onset. The average last meal was at 09:02 pm but ranged from as early as 05:00 pm to as late as 02:11 am, and was anywhere from 52 minutes to approximately 9 hours prior to sleep onset. The considerable difference in the average duration between sleep end and first meal and last meal and sleep start suggests that the first meal is being eaten at a more appropriate biological time in relation to sleep than the last meal.
Since many of these variables are interrelated, we used meditational analysis to test a theoretical pathway between variables. We found that eating frequency explained the relationship between eating close to sleep onset and higher caloric intake. Perhaps, eating later, provided a longer “window” to eat, and led to more total calories per day. This finding brings up the hotly debated topic of the “optimal” number of meals; many debate on if it is better to consume three “standard” meals or six smaller meals each day [4, 8, 15, 17]. In our sample, eating frequency ranged from as few as two up to as many as nine eating occasions per day. Our results appear to contrast with research that suggests frequency of eating may be beneficial. For example, in experimental weight reduction studies that usually regulate caloric intake, increasing meal frequency may reduce ratings of hunger while still helping participants meet their weight loss goals [18, 19]. However, in individuals who are not regulating caloric intake for weight reduction, such as those in our study and that by Mills and colleagues [8], increased frequency may lead to increased calories.
In addition to providing a greater opportunity to eat, eating relatively late may have direct effects on metabolism by causing “misalignment” between caloric intake and the internal circadian rhythm. Animal studies have shown that eating during the biological night (light phase in nocturnal animals) is associated with greater weight gain, even with similar calorie consumption [2]. Although biological markers of circadian timing such as melatonin were not measured in this study, our data show that eating closer to sleep onset (i.e. closer to the biological night) was associated with greater caloric intake. Eating later and closer to sleep onset is of particular interest in relation to metabolic dysfunction since insulin response to evening meals is lower, and thus, glucose levels remain high over a longer period of time [20, 21]. This may also be important in relation to meal content since a recent study suggests that greater high-glycaemic calories ingested in the evening compared to the morning leads to an increase in postprandial glucose and insulin response [22]. A recent laboratory study also demonstrated that circadian misalignment further exacerbates the metabolic effects of short sleep duration. In this study, sleep and eating times were scheduled during typical 24-hour periods and then progressively delayed by implementing 28 hour “days”. Results demonstrated that there was higher postprandial glucose due to lower insulin response in the misaligned condition [23]. In combination, these studies provide evidence that suggests that eating close to sleep onset (biological night) may increase obesity risk over time.
The findings reported in the literature vary on whether eating late is associated with a higher BMI and whether sleep plays a role in this association. In our sample, late eating and sleep duration, but not sleep timing, were associated with the amount of caloric intake; however, none of these factors were related to BMI. In contrast, a recent study in middle-age, obese Japanese men indicated that a late dinner time (after 9 pm) was associated with short sleep duration and higher BMI [7]. Additionally, a study of middle-age women found no association between eating after 10 pm and BMI [8], although there was no adjustment for sleep duration in this study. Taken together, these findings suggest that gender, age, and preexisting obesity may be important factors in explaining these inconsistencies. Although we controlled for age, gender, and sleep factors, our results did not show an association between meal timing and BMI. However, our sample is relatively younger and has a lower BMI than in the middle-age studies, and it is possible that higher caloric intake over time may lead to weight gain.
Our study has several limitations. The small sample size may limit our statistical power to observe associations between variables of interest. Also, the current analyses did not control for race or socioeconomic factors. While we did control for gender, we were underpowered to fully evaluate gender differences, which have been shown to have a relationship with both sleep and eating patterns [24–26]. Participants were recruited for their sleep timing preferences and were not randomly selected. Some of the differences between this and our previous work may be explained by the inclusion in this sample of those with earlier sleep timing. Given the self-report nature of this study, for both dietary assessment and BMI, it should be noted that approximately 30% of participants who complete any type of dietary assessment tend to underreport via forgetfulness, inaccurate measurements, and underreporting [27]. Although respondents tend to overestimate BMI at the lower range and underestimate BMI at the higher range, young and normal weight adults, such as those included in this study, show relatively less bias [28]. Also, since this was an observational study, we could not test the causal nature of the relationship between meal timing and caloric intake.
In conclusion, we were only able to accept our research hypothesis that later meal timing and eating closer to sleep onset time are associated with greater total calories but not BMI. This study also demonstrates that the link between eating closer to sleep onset and total calories may be via eating frequency. These results support the growing body of research demonstrating that the timing of eating is likely to play an important role in weight regulation. Thus, interventions which control the number of meals and/or when the last meal is consumed may potentially enhance the effectiveness of standard weight management programs. Future directions in meal timing research should investigate how the timing of meals can best be aligned with an individual's internal circadian rhythms, as well as the role that gender and age play in these relationships. Strategies aimed at optimizing the timing of meals relative to a person's own internal circadian rhythms represent a novel approach to personalize weight regulation.
Acknowledgment
The authors would like to acknowledge Gregory Kodesh, Ashely Jaksa, Tiffany St James, Brandon Lu, Andrew Kern, Brittany Fondel, and Erin McGorry for their assistance with data collection and entry. The work on this project was funded through Grants R01 HL069988, 1K23HL109110, and 5K12 HD055884.
Abbreviations
- BMI
Body Mass Index
- CESD
Center for Epidemiologic Studies Depression Scale
- SPSS
Statistical Package for the Social Sciences
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].D.M. Arble, J. Bass, A.D. Laposky, M.H. Vitaterna, F.W. Turek. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].L.K. Fonken, J.L. Workman, J.C. Walton, Z.M. Weil, J.S. Morris, A. Haim, R.J. Nelson. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].M. Hatori, C. Vollmers, A. Zarrinpar, L. DiTacchio, E.A. Bushong, S. Gill, M. Leblanc, A. Chaix, M. Joens, J.A. Fitzpatrick, M.H. Ellisman, S. Panda. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].A.E. Mesas, M. Munoz-Pareja, E. Lopez-Garcia, F. Rodriguez-Artalejo. Selected eating behaviours and excess body weight: a systematic review. Obes Rev. 2012;13:106–135. doi: 10.1111/j.1467-789X.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- [5].I. Andersson, S. Rossner. Meal patterns in obese and normal weight men: the `Gustaf' study. Eur J Clin Nutr. 1996;50:639–646. [PubMed] [Google Scholar]
- [6].M.D. Corbalan-Tutau, J.A. Madrid, M. Garaulet. Timing and duration of sleep and meals in obese and normal weight women. Association with increase blood pressure. Appetite. 2012;59:9–16. doi: 10.1016/j.appet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- [7].S.D. Hsieh, T. Muto, T. Murase, H. Tsuji, Y. Arase. Association of short sleep duration with obesity, diabetes, fatty liver and behavioral factors in Japanese men. Intern Med. 2011;50:2499–2502. doi: 10.2169/internalmedicine.50.5844. [DOI] [PubMed] [Google Scholar]
- [8].J.P. Mills, C.D. Perry, M. Reicks. Eating frequency is associated with energy intake but not obesity in midlife women. Obesity (Silver Spring) 2011;19:552–559. doi: 10.1038/oby.2010.265. [DOI] [PubMed] [Google Scholar]
- [9].N.J. Aronoff, A. Geliebter, G. Zammit. Gender and body mass index as related to the night-eating syndrome in obese outpatients. Journal of the American Dietetic Association. 2001;101:102–104. doi: 10.1016/S0002-8223(01)00022-0. [DOI] [PubMed] [Google Scholar]
- [10].K.G. Baron, K.J. Reid, A.S. Kern, P.C. Zee. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- [11].L.S. Radloff. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;Vol. 1:385–401. [Google Scholar]
- [12].S. Briscoe, E. Hardy, M.F. Pengo, C. Kosky, A.J. Williams, N. Hart, J. Steier. Comparison of 7 versus 14 days wrist actigraphy monitoring in a sleep disorders clinic population. Chronobiology international. 2014;31:356–362. doi: 10.3109/07420528.2013.858163. [DOI] [PubMed] [Google Scholar]
- [13].M. Littner, C.A. Kushida, W.M. Anderson, D. Bailey, R.B. Berry, D.G. Davila, M. Hirshkowitz, S. Kapen, M. Kramer, D. Loube, M. Wise, S.F. Johnson, M. Standards of Practice Committee of the American Academy of Sleep Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- [14].R.M. Baron, D.A. Kenny. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- [15].A.A. van der Heijden, F.B. Hu, E.B. Rimm, R.M. van Dam. A prospective study of breakfast consumption and weight gain among U.S. men. Obesity (Silver Spring) 2007;15:2463–2469. doi: 10.1038/oby.2007.292. [DOI] [PubMed] [Google Scholar]
- [16].M.A. McCrory, W.W. Campbell. Effects of eating frequency, snacking, and breakfast skipping on energy regulation: symposium overview. J Nutr. 2011;141:144–147. doi: 10.3945/jn.109.114918. [DOI] [PubMed] [Google Scholar]
- [17].I. Holmback, U. Ericson, B. Gullberg, E. Wirfalt. A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br J Nutr. 2010;104:1065–1073. doi: 10.1017/S0007114510001753. [DOI] [PubMed] [Google Scholar]
- [18].J.L. Bachman, H.A. Raynor. Effects of manipulating eating frequency during a behavioral weight loss intervention: a pilot randomized controlled trial. Obesity (Silver Spring) 20(2012):985–992. doi: 10.1038/oby.2011.360. [DOI] [PubMed] [Google Scholar]
- [19].H.J. Leidy, W.W. Campbell. The effect of eating frequency on appetite control and food intake: brief synopsis of controlled feeding studies. J Nutr. 2011;141:154–157. doi: 10.3945/jn.109.114389. [DOI] [PubMed] [Google Scholar]
- [20].E. Van Cauter, K.S. Polonsky, A.J. Scheen. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- [21].E. Van Cauter, E.T. Shapiro, H. Tillil, K.S. Polonsky. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- [22].L.M. Morgan, J.W. Shi, S.M. Hampton, G. Frost. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br J Nutr. 2012;108:1286–1291. doi: 10.1017/S0007114511006507. [DOI] [PubMed] [Google Scholar]
- [23].O.M. Buxton, S.W. Cain, S.P. O'Connor, J.H. Porter, J.F. Duffy, W. Wang, C.A. Czeisler, S.A. Shea. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra143. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].M. Brandhagen, H.B. Forslund, L. Lissner, A. Winkvist, A.K. Lindroos, L.M. Carlsson, L. Sjostrom, I. Larsson. Alcohol and macronutrient intake patterns are related to general and central adiposity. Eur J Clin Nutr. 2012;66:305–313. doi: 10.1038/ejcn.2011.189. [DOI] [PubMed] [Google Scholar]
- [25].M.A. Cornier, A.K. Salzberg, D.C. Endly, D.H. Bessesen, J.R. Tregellas. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].D.S. Lauderdale, K.L. Knutson, L.L. Yan, P.J. Rathouz, S.B. Hulley, S. Sidney, K. Liu. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- [27].K. Poslusna, J. Ruprich, J.H. de Vries, M. Jakubikova, P. van't Veer. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009;101(Suppl 2):S73–85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- [28].M. Stommel, C.A. Schoenborn. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001–2006. BMC Public Health. 2009;9:421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]