Abstract
Acute leukemia (AL) and myelodysplastic syndrome (MDS) are uncommon in CLL. We retrospectively identified 95 patients with CLL also diagnosed with AL (n=38) or MDS (n=57), either concurrently (n=5) or subsequent (n=90) to CLL diagnosis and report their outcomes. Median number of CLL treatments prior to AL and MDS was 2(0–9) and 1(0–8), respectively; the most common regimen was purine analogue combined with alkylating agent±CD20 mAb. Twelve had no prior CLL treatment. Among 38 with AL, 33 had AML, 3 had ALL (1Ph+), 1 had biphenotypic, and 1 had extramedullary (bladder) AML. Unfavorable AML karyotype was noted in 26, intermediate-risk in 7. There was no association between survival from AL and number of prior CLL regimens or karyotype. Expression of CD7 on blasts was associated with shorter survival. Among MDS cases, all IPSS were represented; karyotype was unfavorable in 36, intermediate in 6, and favorable in 12 patients; 10 experienced transformation to AML. Shorter survival from MDS correlated with higher-risk IPSS, poor-risk karyotype, and increased number of prior CLL treatments. Overall, outcomes for patients with CLL subsequently diagnosed with AL or MDS were poor; AL/MDS occurred without prior CLL treatment. Effective therapies for these patients are desperately needed.
Keywords: acute leukemia, chronic lymphocytic leukemia, chemotherapy, chemoimmunotherapy, myelodysplastic syndrome
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is a malignancy of well-differentiated CD5+CD19+ monoclonal B cells; the incidence increases with age. There is a very broad spectrum of clinical course for CLL. No standard-dose chemotherapy has been shown to be curative, allogeneic hematopoietic stem cell transplant (allo-HSCT) may represent treatment rendering long-term remission and disease control, however, it is associated with significant morbidity and mortality.
CLL-associated complications include infection, autoimmune disease, transformation to a more aggressive lymphoid malignancy (Richter’s transformation), second solid tumors, including melanoma and non-melanoma skin cancers, and second hematologic malignancies such as acute leukemia (AL) and myelodysplastic syndrome (MDS)[reviewed in1]. Data and insights into the incidence, risk factors, and predisposing conditions for second hematologic malignancies are limited and to date there has been very limited data reporting characteristics and outcomes of second hematologic malignancies in patients with CLL2–15. We report the characteristics and outcomes for a series of 95 patients with CLL seen at MD Anderson Cancer Center (MDACC) who developed a second hematologic malignancy.
PATIENTS AND METHODS
Patients seen at MDACC between August 1981 and August 2011 with a diagnosis of CLL and AL or MDS were identified in the leukemia patient database. Patients provided informed consent at initial presentation and according to MDACC institutional guidelines and had workup and data collected on characteristics of their CLL, AL, and MDS, based on current standard evaluation. Because standard workup evolved over the years, with many new prognostic factors for CLL identified more recently, data were limited to available tests at the time of diagnosis or follow up. We reviewed date of CLL diagnosis, characteristics of CLL at presentation to MDACC, prior CLL treatment, time and characteristics of AL or MDS diagnosis, treatment for AL or MDS, response, and survival. All patients had bone marrow evaluation to confirm diagnosis of CLL, MDS, and AL, and diagnosis was described according to WHO and FAB classifications. Indications for performing bone marrow biopsy included initial workup for CLL, pretreatment evaluation for CLL, during treatment or response assessment for CLL, post-treatment monitoring for CLL, and based on clinical suspicion, most commonly in patients with CLL who do not otherwise have a clinical explanation for cytopenia(s) or who have circulating blasts.
Patients included in this summary may have been diagnosed with AL/MDS during follow up at MDACC and included in previous reports2, 8, 13, 15, 16. Patients were treated and followed at MDACC for their diagnosis of AL/MDS. Overall survival was updated through follow up at MDACC, with the referring physician, direct contact with patients, and by review of Social Security Death Index.
Descriptive statistics were used to summarize patient characteristics. Progression-free (PFS) and overall survival (OS) were calculated from the referenced date of CLL, AL, or MDS diagnosis, to the date of progression or death, respectively. Survival distributions were calculated using the method of Kaplan and Meier. Univariable comparisons were made using the log-rank test. Categorical and continuous variables were compared using the χ2, Fisher exact, or Mann-Whitney test, as appropriate. All P-values were 2-sided and considered significant if ≤0.05.
RESULTS
Patient Characteristics
We identified 95 patients with CLL who were diagnosed with AL (n=38) or MDS (n=57) (Table 1) between August 1981 and August 2011. The median time from CLL to AL and MDS diagnosis was 57 (range 0–182) and 67 (range 3–318) months, respectively. The median age at diagnosis was 65 years for AL (range: 41–78) and 68 (range: 42–85) for MDS.
Table 1.
Patient Characteristics (N=95)
| Characteristic | Number
|
|
|---|---|---|
| AL (n=38) | MDS (n=57) | |
| Median age @ AL or MDS diagnosis, yrs | 65 | 68 |
|
| ||
| Male, % | 82 | 72 |
|
| ||
| Median number prior CLL treatments, (range) | 2 (0–9) | 1 (0–8) |
|
| ||
| Number prior treatments for CLL, % of patients | ||
| 0 (de novo) | 11 | 14 |
| 1 | 34 | 44 |
| 2 | 26 | 16 |
| ≥ 3 | 29 | 26 |
|
| ||
| Median time from CLL to 2nd AL/MDS Dx, months | 57 | 67 |
|
| ||
| CLL treatment, % of patients | ||
| None | 11 | 14 |
| Chlorambucil | 21 | 14 |
| Fludarabine-based | 82 | 79 |
| CHOP-like | 8 | 12 |
| Monoclonal antibody (CD20, CD52) | 50 | 65 |
| HCVAD | 3 | 50 |
| ASCT | 0 | 9 |
| Allo-HSCT | 3 | 2 |
| Other | 6 | 16 |
|
| ||
| Complex karyotype, % of patients | 45 | 51 |
|
| ||
| Involved chromosome on metaphase karyotype, % of patients | ||
| 5 | 37 | 40 |
| 7 | 37 | 46 |
| 8 | 29 | 23 |
| 1 | 26 | 16 |
| 17 | 18 | 9 |
| 20 | 5 | 19 |
| 21 | 11 | 2 |
| No data | 13 | 9 |
AL, acute leukemia; MDS, myelodysplastic syndrome; n, number; yrs, years; Dx, diagnosis; Rx, treatments
Allo-HSCT, allogeneic hematopoietic stem cell transplant; ASCT, autologous hematopoietic stem cell transplant; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; HVCAD, hyper-fractionated cyclophosphamide, adriamycin, vincristine, dexamethasone; HSCT, hematopoietic stem cell transplant
The median follow up time from presentation to MDACC was 58 months (range: 7–191). Five patients presented to MDACC concurrently with CLL and AL or MDS, for the remainder, AL or MDS was diagnosed after CLL. The median overall survival (OS) from time of CLL diagnosis was 65 months for patients who developed AL and 80 months for patients who developed MDS. The median OS from time of AL and MDS diagnosis was 4.8 months and 9 months, respectively (Figure 1A). At the time of this analysis, there were 11 living patients, (n=4 AL; n=7 MDS); their overall median survival was 95.6 months from CLL diagnosis (60 for MDS and 156 months for AL) and 26.8 months from AL or MDS diagnosis (26.8 for MDS and 54.4 months for AL).
Figure 1. Overall Survival.
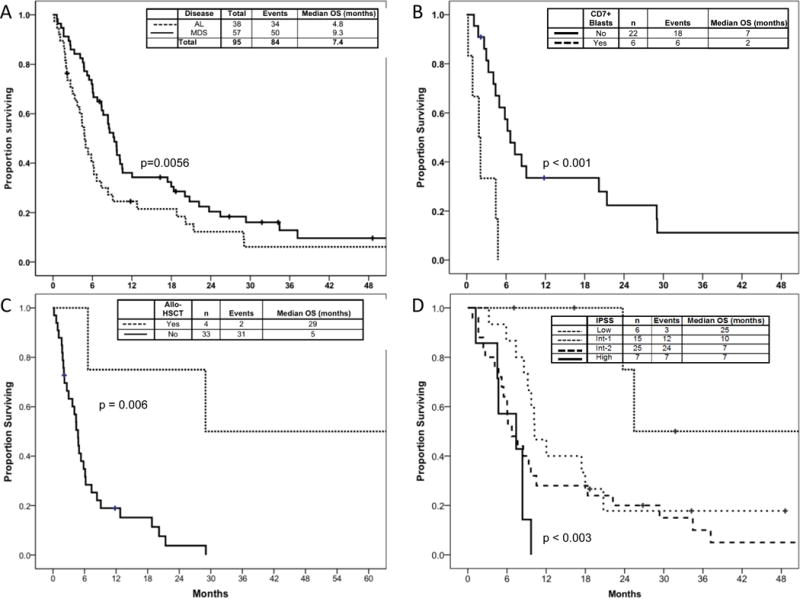
A) overall survival from AL and MDS diagnosis; B) survival in AL by blast surface expression of CD7; C) survival in AL by stem cell transplantation status; D) survival in MDS by IPSS (n=57)
Prior Treatment for CLL
Treatment for CLL prior to AL or MDS diagnosis was given to 89% of patients who developed AL and to 86% of patients who developed MDS (Table 1). The median number of prior CLL treatment regimens before AL and MDS diagnosis was 2 (0–9) and 1 (0–8), respectively; 34% of patients who developed AL and 44% who developed MDS received only 1 prior CLL treatment (Table 1). Patients who did not receive any previous CLL treatment are referred to as de novo cases (Supplemental Tables 1&2).
Nucleoside analogue (fludarabine)-based was the most common first-line or salvage treatment for CLL and was administered to 82% of patients who developed AL and 79% of patients who developed MDS; nearly two-thirds of patients were exposed to combined nucleoside analogue with alkylating agent (Table 1 and Supplemental Table 3). Fludarabine-based CLL therapies are detailed in Supplemental Table 3. None of the AL patients and 7% of MDS patients underwent previous autologous hematopoietic stem cell transplant (ASCT), while 1 patient of each with AL and MDS underwent previous allogeneic hematopoietic stem cell transplantation (allo-HSCT) for CLL (Table 1).
For patients who developed AL after only 1 prior CLL therapy (n=13/38), 85% had received a purine analogue: 15% received fludarabine monotherapy, 8% fludarabine with cyclophosphamide (FC), 54% received fludarabine with cyclophosphamide and rituximab (FCR), 1 patient received CFAR (FCR with alemtuzumab), 1 patient received chlorambucil, and 1 patient CHOP with radiation therapy (data not shown). For patients who developed MDS after only 1 prior CLL treatment (n=25/57), 92% received purine analogue; 8% were treated with fludarabine monotherapy, 8% with fludarabine and cyclophosphamide, 76% with fludarabine, cyclophosphamide, and rituximab, 1 patient received prior chlorambucil, and 1 patient received prior rituximab monotherapy (data not shown).
Characteristics of Patients Diagnosed with Acute Leukemia
Among the 38 patients diagnosed with AL, 33 had AML; 3 had ALL, 1 was Philadelphia chromosome positive (Ph+); 1 had biphenotypic AL; and 1 had extramedullary AML (bladder granulocytic sarcoma); characteristics at AL presentation are shown in Table 2. Among patients with AML, all FAB except M3 and M7 were represented: M0 (n=4); M1 (n=5); M2 (n=5); M4 (n=4); M5 (n=5); and M6 (n=4); FAB was missing for 6 patients. The median proportion of bone marrow and blood blasts was 42% (0–94) and 11% (0–94), respectively (Table 2). CLL was present in BM in 42% patients at AL diagnosis. Blast expression of CD7, identified by flow cytometry in 6 of 17 cases evaluated and was associated with poor outcome in AML. None of the patients had favorable karyotype, 68% and 18% had high- and intermediate-risk, respectively; karyotype was unknown for 13% patients (Table 2). Abnormalities involving chromosomes 5,7,8,11, and 17 were most common among AL karyotype (Table 1). Characteristics of patients with de novo AL are shown in Supplemental Tables 1 and 2.
Table 2.
Characteristics of Patients with Acute Leukemia (n=38)
| Characteristic at AL diagnosis | Number | Range | |
|---|---|---|---|
| White blood count, median | 5.1 K/μL | 0.6–194 | |
| Absolute neutrophil count, median | 0.72 K/μL | 0–14 | |
| Hemoglobin, median | 9.9 g/dL | 7.2–12.9 | |
| Platelet, median | 38 K/μL | 4–403 | |
| BM blasts, median | 42 | 0–94 | |
| Blood blasts, median | 11 | 0–93 | |
| CD7+ blasts, # positive/evaluable | 6/17 | ||
| CLL present in BM @ AL Dx, % positive | 42 | ||
| Multi-lineage dysplasia present, % | 53 | ||
| AL-specific characteristics | Number | % | |
| Subtype | AML | 33 | 86 |
| ALL | 3 | 8 | |
| Biphenotypic | 1 | 3 | |
| Granulocytic sarcoma (bladder) | 1 | 3 | |
| Karyotype | Good | 0 | 0 |
| Intermediate | 26 | 68 | |
| Poor | 7 | 18 | |
| Unknown | 5 | 13 | |
AL, acute leukemia; AML, acute myelogenous leukemia; ALL, acute lymphoid leukemia; BM, bone marrow; Dx, diagnosis
Treatments for Acute Leukemia
The median number of treatments for AL was 1 (0–4). One patient remained untreated and half the patients (n=19) received 1 treatment; 23.7%, 10.5% and 5.3% patients received 2, 3, and 4 treatments for AL, respectively; treatment status was unknown for 8% patients (Table 3). Among all AL patients, 79% received a cytarabine-based regimen (detailed in Supplemental Table 4) and other treatments are detailed in Table 3. Hyper-CVAD was administered to 2 ALL and one AML patients. Five patients underwent Allo-SCT for AL.
Table 3.
Treatments for Patients with Acute Leukemia and Myelodysplastic Syndrome
| Treatment for AL/MDS | Percent of Patients | |
|---|---|---|
| AL (n=38) | MDS (n=57) | |
| HMA (azacitadine or decitabine) | 8 | 28 |
| Cytarabine-based | 79 | 19 |
| Allo-HSCT | 11 | 5 |
| Hyper-CVAD | 8 | 0 |
| IMID | 3 | 11 |
| FTI/TKI | 3 | 9 |
| Other | 3 | 7 |
| None | 3 | 18 |
AL, acute leukemia; MDS, myelodysplastic syndrome; n, number; HSCT, hematopoietic stem cell transplant; hyper-CVAD, hyper-fractionated cyclophosphamide, adriamycin, vincristine, dexamethasone; IMID, immune-modulating agent (thalidomide or lenalidomide); FTI, farnesyl transferase inhibitor; TKI, tyrosine kinase inhibitor, HMA, hypomethylating agent
Survival from Acute Leukemia
The median overall survival from AL was 4.8 months (Figure 1A). Survival from AL was not correlated with age, number of CLL treatments (Supplemental Figure 1), or karyotype category (Supplemental Figure 2). Furthermore, there was no association between presence of CLL at AL diagnosis, white blood count, neutrophil, hemoglobin or platelet counts and survival from AL (data not shown). Survival for patients with CD7 expression by AML blasts was 2 vs. 7 months for patients without CD7 expression (p<.001) (Figure 1B).
Among patients with AL, 4 underwent allo-HSCT and were noted to have improved survival compared to the patients with AL who did not undergo allo-HSCT (29 vs. 5 months; p=0.006) (Figure 1C).
There were 4 long-term (≥ 24 months) survivors with AL (Supplemental Table 5); karyotype was intermediate for 3 (missing for 1) and none had blast expression of CD7. Three of the long-term survivors underwent allo-HSCT (Supplemental Table 5).
Characteristics of Patients Diagnosed with Myelodysplastic Syndrome
The International Prognostic Scoring System (IPSS) category was high-risk for 12%; intermediate-2 for 26%, intermediate-1 for 44%, low-risk for 11%, and could not be determined for 7% (Table 4) of patients with MDS; lab values at MDS diagnosis are shown in Table 4. Karyotype for MDS was favorable in 21%, intermediate in 11% poor in 63% and unknown for 5% of patients (data not shown). More than a fifth of cases had abnormal chromosome 5,7, or 8 among MDS karyotype (Table 1). Among the 57 patients with MDS, 18% subsequently transformed to acute myeloid leukemia; the median time to transformation was 6 months.
Table 4.
Characteristics of Patients with Myelodysplastic Syndrome (n=57)
| Characteristic | Number | % or Range | |
|---|---|---|---|
| IPSS | High Risk | 7 | 12 |
|
| |||
| Intermediate-2 | 15 | 26 | |
|
| |||
| Intermediate-1 | 25 | 44 | |
|
| |||
| Low Risk | 6 | 11 | |
| Missing | 4 | 7 | |
|
| |||
| Laboratory, median | % BM blasts | 3 | 0–19 |
|
| |||
| Blood blasts present | 11 | 19 | |
|
| |||
| HGB (g/dL) | 9.8 | 5–14 | |
|
| |||
| PLT (K/μL) | 53 | 8–476 | |
|
| |||
| CLL present @ MDS diagnosis | 28 | 49 | |
|
| |||
| MDS subsequently transformed to AL, n % | 10 | 18 | |
AL, acute leukemia; MDS, myelodysplastic syndrome; n, number; IPSS, International Prognostic Score System; BM, bone marrow; HGB, hemoglobin; PLT, platelet
Treatments for MDS
The median number of treatments for MDS was 1 (0–4); 15.8% remained untreated, and treatment was unknown for 21% patients. Among those treated, 38.6%, 14%, 7%, 3.5% received 1, 2 3 or 4 treatments, respectively. The most common among all lines of treatment for MDS was a hypomethylating agent, administered to 28% patients (Table 3); 9 received azacitidine and 7 received decitabine ± other agents. Details of treatments for MDS are shown in Table 3 and Supplemental Table 4.
Survival from MDS
The median overall survival from MDS was 9.3 months (Figure 1). Survival from MDS was correlated with IPSS: 25 months for low-, 10.2 months for Int-1, 6.7 months for Int-2, and 7.4 months for high-risk (p=.003) (Figure 1D). In addition, patients who had no prior CLL chemotherapy had median survival of 37 months vs. 9.7 months for patients with 1 prior CLL treatment and 5.9 months for those with ≥2 (p=.007) (Figure 2). Superior survival was observed for patients with favorable karyotype (25 months), compared to patients with intermediate- (7 months) or poor-risk (8 months) karyotype (Figure 3). The estimated median survival from MDS was 18 versus 7.4 months for patients with hemoglobin ≥10 g/dL versus <10 g/dL (p=.002), respectively; and the estimated median survival from MDS was 18 versus 6 months for patients with platelet count ≥ 50 K/L versus < 50 K/L (p=0.002), respectively (data not shown). There was no association between WBC count, neutrophil count, presence of CLL, or age at MDS diagnosis and survival from MDS (data not shown).
Figure 2. Survival in MDS by Number of Prior CLL Treatments (n=57).
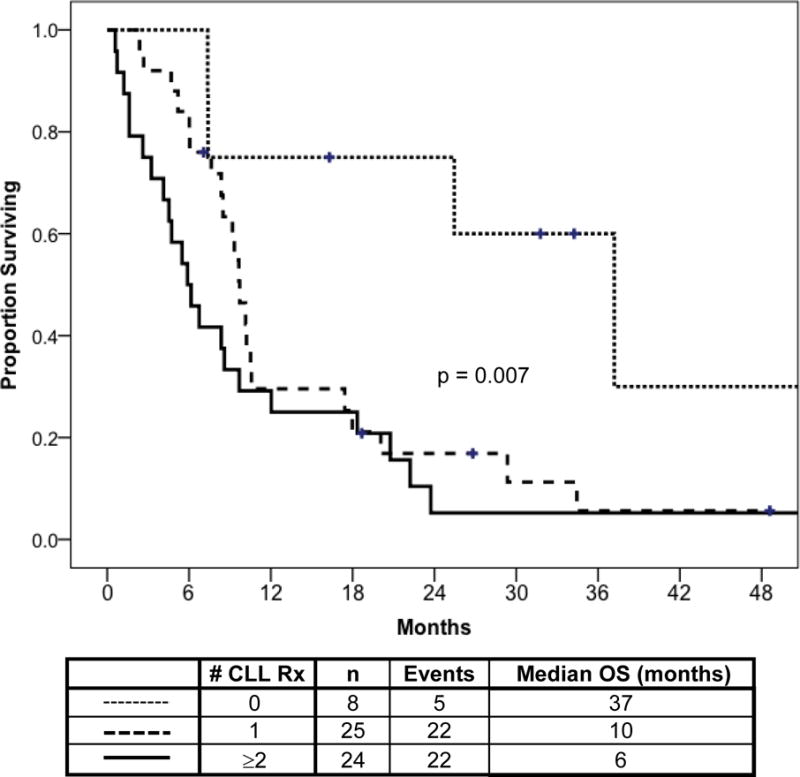
Overall survival time from MDS diagnosis is shown according to the number of prior CLL treatment regimens received. Patients who had no prior CLL treatments had improved survival.
Figure 3. Survival in MDS by Karyotype (n=57).
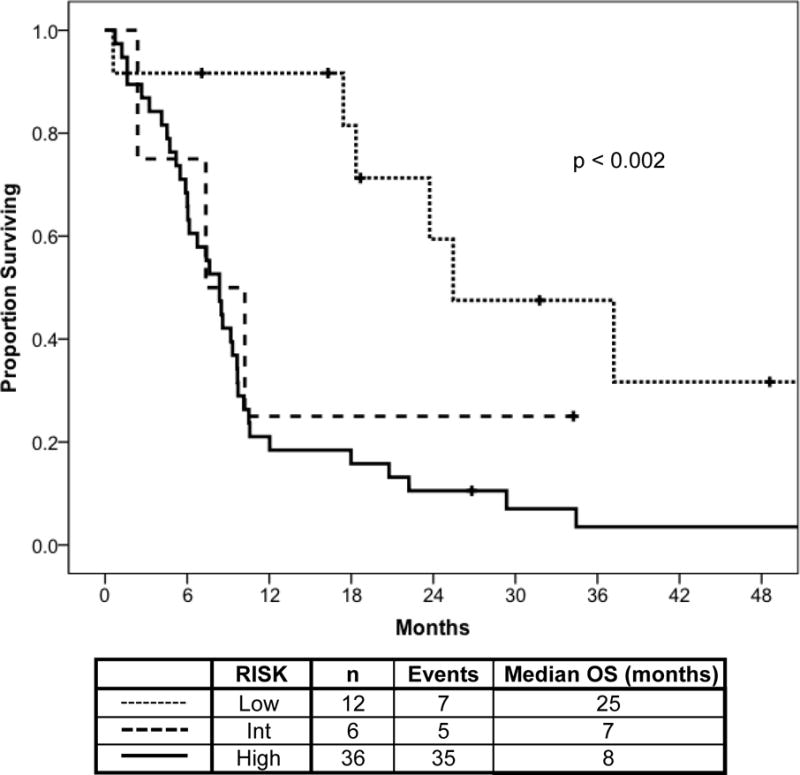
Overall survival time from MDS diagnosis is shown according to MDS-associated karyotype. Longest survival was observed for patients with low-risk karyotype features.
There were 10 long-term (≥ 24 months) MDS survivors, half had favorable karyotype and half were low or intermediate-1 IPSS (Supplemental Table 5). Allo-HSCT did not appear to improve survival for 4 patients with MDS (Supplemental Figure 3).
Characteristics of Patients who Developed De Novo AL or MDS
There were 12 patients who developed AL (n=4) or MDS (n=8) without receiving any prior CLL treatment, reported as de novo cases (Supplemental Tables 1 & 2). These patients were older compared to those who received prior CLL treatment (Supplemental Table 1). Also, the median time from CLL to AL/MDS for these patients was 21 months, shorter than for those who had prior CLL treatment. Superior median survival was observed for de novo MDS at 37 months compared 10 months and 6 months for patients with MDS patients who received 1 and ≥ 2 prior CLL treatments, respectively (p=0.007) (Figure 2); this was not the case for AL in comparing de novo vs. those previously treated for CLL (p=0.69) (Supplemental Figure 1).
DISCUSSION
The strength of this retrospective study is that it delineates the characteristics of second and secondary AL/MDS diagnosed among patients with CLL and describes their poor clinical outcomes. Survival outcomes for MDS were consistent with previously reported prognostic modeling for therapy-related MDS16. The characteristics of MDS or AL in our cohort of patients with CLL are consistent with reports of therapy-related MDS and AL, such as for patients with treated breast cancer and ovarian cancer, as well as non-Hodgkin lymphomas and multiple myeloma16–18. This is indicated by the number and type of associated cytogenetic abnormalities in both AL and MDS. Nearly 90% of patients were exposed to fludarabine-based CLL therapy, making an association of purine analogue exposure with MDS/AL. Nearly 90% of patients with AL had acute myelogenous leukemia and over half these cases had multi-lineage dysplasia present in the marrow among non-leukemia cellular elements, also consistent with therapy-related acute leukemia17. Second hematologic malignancy of lymphoid etiology was rare and is rarely reported in the literature.
Overall survival from AL or MDS in patients diagnosed with CLL was poor. We identified a group of patients with improved survival (≥ 24 months), which tended to have good- or intermediate-risk karyotype, lacked blast expression of CD7, and in the AL group had undergone allo-HSCT. With a limited patient number, it did not appear that allo-SCT was associated with improved outcome for the patients diagnosed with MDS. There did not appear to be any treatment for MDS or AL with better survival outcome, except allo-HSCT for AL.
Among cases with de novo MDS or AL, age was significantly older than patients who had previously been treated for their CLL, and they had a shorter time from CLL diagnosis to diagnosis of AL/MDS. The survival from time of AL diagnosis was similar between those who had prior CLL therapy vs. de novo cases, although small patient numbers limited this comparison. Survival from MDS diagnosis was more favorable for the de novo cases compared to those previously treated for their CLL.
The true incidence of second and secondary hematologic malignancies is difficult to quantify. AL and MDS, as second cancer diagnoses in patients with CLL are uncommon2–15. Several factors potentially contribute to the risk of developing them, including, DNA damage and genotoxicity induced in hematopoietic stem cells by chemotherapeutic agents used to treat CLL; the advanced age of patients with CLL; and immune dysfunction brought on by CLL and by the treatments for CLL. The advanced age at which patients are exposed to these agents also may compound this risk. Better insight into factors that predispose or agents that contribute to development of second hematologic malignancies may come from the study of large cohorts of genetically-characterized patients with CLL followed longitudinally through the course of their disease with monitoring of serial treatments and serial characterization of disease characteristics. Additionally, with newer chemotherapy-free treatment options for CLL, the incidence of hematologic malignancies may diminish.
There are challenges with making a diagnosis of MDS among patients with CLL, especially if there was a significant amount of CLL present in bone marrow. Anemia, neutropenia, and thrombocytopenia are all commonly seen with CLL, and are important features in diagnosis and prognosis of MDS. Making the diagnosis of MDS requires thorough examination of all marrow cellular elements, which may be compromised if there also is CLL present in the marrow sample. The presence of CLL cells may also limit the ability to detect cytogenetic and molecular abnormalities in non-lymphoid lineages. Finally, bone marrow morphologic abnormalities diagnostic for MDS are seen in bone marrow cells of patients treated with chemotherapy, which can resolve over time and do not necessarily herald a diagnosis of MDS. Improved detection of cytogenetic abnormalities and sequencing data may help to clarify cases of MDS in patients with CLL.
Chemotherapy for CLL has been proposed as the primary risk factor for developing a second hematologic malignancy2, 3, 5, 10, 12, 15; however, cases have been reported occurring in the absence of CLL treatment6, 19. Treatment for CLL has evolved over many years starting with alkylating agent monotherapy, such as chlorambucil. Purine analogues such as fludarabine were developed next, then combinations, including the addition of CD20 monoclonal antibody to chemoimmunotherapy regimens. With this evolution have come improved CLL outcomes, including increased overall and complete remission rates, longer progression-free survival and improved overall survival. There is likely heterogeneity in the leukemogenic potency of alkylating agents, purine analogues, and combinations. Concern has been raised for increased risk of AL/MDS with purine analogue combined with alkylating agents5, 10, 12, particularly the FCR regimen2, 15. Up to 13% of patients who underwent autologous hematopoietic stem cell transplantation developed AL/MDS, although the treatment history for these patients was diverse20. Our patient cohort was heterogeneous in prior treatment exposure and included patients with no prior treatment for their CLL.
Our data support CLL treatment as a risk for developing second hematologic malignancy first, because the majority of patients had received prior treatment for their CLL before developing AL/MDS, and second, because of the younger age at diagnosis for the patients who received CLL treatment compared to the advanced age of the de novo cases. It is difficult to gain insight into how the prior treatment for CLL contributed to the risk for developing their second hematologic malignancy, only that fludarabine was the most common chemotherapy exposure. Interestingly, over a third of patients had only 1 prior treatment regimen for their CLL.
Limited numbers of cases of AL/MDS reported have been reported in patients treated on first-line clinical trials10–12, 14, 15. Fortunately the incidence of second hematologic malignancy is low, therefore each of these reports have fewer than 10 cases diagnosed with AL/MDS. The incidence of second MDS in CLL may be under reported, particularly for patients with relapsed and refractory CLL.
The outcome for patients with second AL/MDS is very poor; generally, patients diagnosed with MDS have longer survival. New agents are desperately needed to treat these patients. New treatment strategies are needed for CLL, in order to minimize exposure of patients to potentially genotoxic chemotherapy. With the advent, development, approval and integration of B cell receptor signaling pathway inhibitors for treatment for CLL, we hope to see a reduction in the number of second hematologic cancers among patients with CLL owing to reduced exposure to traditional standard chemotherapy and chemoimmunotherapy. Supplementary information is available at Leukemia’s website.
Supplementary Material
Acknowledgments
None
Footnotes
DISCLOSURES OF CONFLICT OF INTEREST
Nothing to disclose, no authors have any conflict of interest or disclosures for this work.
AUTHORSHIP CONTRIBUTIONS
Designed research: FPT; WGW
Collected data: FPT; LXT; SP
Performed research: FPT; GGM; SMO; SHF; AF; JAB; LXT; SP; XW; K-AD; HMK; MJK; WGW
Analyzed and interpreted data: FPT; XW; K-AD; WGW
Performed statistical analysis: FPT; XW; K-AD
Wrote the manuscript: FPT; WGW
Reviewed the manuscript: FPT; GGM; SMO; SHF; AF; JAB; LXT; SP; XW; K-AD; HMK; MJK; WGW
References
- 1.Wiernik PH. Second neoplasms in patients with chronic lymphocytic leukemia. Current treatment options in oncology. 2004 Jun;5(3):215–223. doi: 10.1007/s11864-004-0013-7. [DOI] [PubMed] [Google Scholar]
- 2.Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011 Mar 17;117(11):3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carney DA, Westerman DA, Tam CS, Milner A, Prince HM, Kenealy M, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010 Dec;24(12):2056–2062. doi: 10.1038/leu.2010.218. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Vena DA, Barrett J, Freidlin B. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol. 1999 Aug;17(8):2454–2460. doi: 10.1200/JCO.1999.17.8.2454. [DOI] [PubMed] [Google Scholar]
- 5.Colovic M, Suvajdzic N, Jankovic G, Tomin D, Colovic N, Fekete MD, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia in patients with chronic lymphocytic leukemia treated with fludarabine and cyclophosphamide. Biomed Pharmacother. 2011 Aug;65(5):319–321. doi: 10.1016/j.biopha.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Dighiero G, Maloum K, Desablens B, Cazin B, Navarro M, Leblay R, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med. 1998 May 21;338(21):1506–1514. doi: 10.1056/NEJM199805213382104. [DOI] [PubMed] [Google Scholar]
- 7.Johnson S, Smith AG, Loffler H, Osby E, Juliusson G, Emmerich B, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet. 1996 May 25;347(9013):1432–1438. doi: 10.1016/s0140-6736(96)91681-5. [DOI] [PubMed] [Google Scholar]
- 8.Keating MJ, O’Brien S, Lerner S, Koller C, Beran M, Robertson LE, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998 Aug 15;92(4):1165–1171. [PubMed] [Google Scholar]
- 9.Leporrier M, Chevret S, Cazin B, Boudjerra N, Feugier P, Desablens B, et al. Randomized comparison of fludarabine, CAP, and ChOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001 Oct 15;98(8):2319–2325. doi: 10.1182/blood.v98.8.2319. [DOI] [PubMed] [Google Scholar]
- 10.Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002 Sep 15;20(18):3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 11.Robak T, Blonski JZ, Gora-Tybor J, Kasznicki M, Konopka L, Ceglarek B, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur J Cancer. 2004 Feb;40(3):383–389. doi: 10.1016/j.ejca.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Neuberg D, Flinn IW, Grever MR, Lazarus HM, Rowe JM, et al. Incidence of therapy-related myeloid neoplasia after initial therapy for chronic lymphocytic leukemia with fludarabine-cyclophosphamide versus fludarabine: long-term follow-up of US Intergroup Study E2997. Blood. 2011 Sep 29;118(13):3525–3527. doi: 10.1182/blood-2011-03-342485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008 Aug 15;112(4):975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woyach JA, Ruppert AS, Heerema NA, Peterson BL, Gribben JG, Morrison VA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol. 2011 Apr 1;29(10):1349–1355. doi: 10.1200/JCO.2010.31.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Tang G, Medeiros LJ, McDonnell TJ, Keating MJ, Wierda WG, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PubMed] [Google Scholar]
- 16.Quintas-Cardama A, Daver N, Kim H, Dinardo C, Jabbour E, Kadia T, et al. A prognostic model of therapy-related myelodysplastic syndrome for predicting survival and transformation to acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2014 Oct;14(5):401–410. doi: 10.1016/j.clml.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008 Aug;35(4):418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003 Jul 1;102(1):43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 19.Charafeddine KM, Ibrahim GY, Mahfouz RA, Zaatari GS, Salem ZM. Chronic lymphocytic leukemia associated with myelodysplastic syndrome with ring sideroblasts. South Med J. 2010 Aug;103(8):823–827. doi: 10.1097/SMJ.0b013e3181e6d2b4. [DOI] [PubMed] [Google Scholar]
- 20.Gribben JG, Zahrieh D, Stephans K, Bartlett-Pandite L, Alyea EP, Fisher DC, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005 Dec 15;106(13):4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


