Abstract
Lung ventilation fluctuates widely with behavior but arterial PCO2 remains stable. Under normal conditions, the chemoreflexes contribute to PaCO2 stability by producing small corrective cardiorespiratory adjustments mediated by lower brainstem circuits. Carotid body (CB) information reaches the respiratory pattern generator (RPG) via nucleus solitarius (NTS) glutamatergic neurons which also target rostral ventrolateral medulla (RVLM) presympathetic neurons thereby raising sympathetic nerve activity (SNA). Chemoreceptors also regulate presympathetic neurons and cardiovagal preganglionic neurons indirectly via inputs from the RPG. Secondary effects of chemoreceptors on the autonomic outflows result from changes in lung stretch afferent and baroreceptor activity. Central respiratory chemosensitivity is caused by direct effects of acid on neurons and indirect effects of CO2 via astrocytes. Central respiratory chemoreceptors are not definitively identified but the retrotrapezoid nucleus (RTN) is a particularly strong candidate. The absence of RTN likely causes severe central apneas in congenital central hypoventilation syndrome. Like other stressors, intense chemosensory stimuli produce arousal and activate circuits that are wake- or attention-promoting. Such pathways (e.g., locus coeruleus, raphe, and orexin system) modulate the chemoreflexes in a state-dependent manner and their activation by strong chemosensory stimuli intensifies these reflexes. In essential hypertension, obstructive sleep apnea and congestive heart failure, chronically elevated CB afferent activity contributes to raising SNA but breathing is unchanged or becomes periodic (severe CHF). Extreme CNS hypoxia produces a stereotyped cardiorespiratory response (gasping, increased SNA). The effects of these various pathologies on brainstem cardiorespiratory networks are discussed, special consideration being given to the interactions between central and peripheral chemoreflexes.
Introduction
Lung ventilation, cardiac output, and blood pressure are highly labile physiological variables that are continually adjusted by the central nervous system to match the metabolic requirements of specific behaviors (37,117,396). Unlike lung ventilation, PaCO2 usually remains constant around a set-point that is characteristic of a particular individual (163). This classic example of homeostatic regulation suffers few exceptions. For example, under hypoxia and/or during strenuous exercise, PaCO2 falls because maintaining the oxygen supply takes priority over the need to keep CO2 constant. During slow-wave sleep (SWS) PaCO2 rises 3 to 8 mm above the resting level present during restful waking. These temporary deviations from homeostasis are adaptive and in keeping with the concept of allostasis or stability through change (283,396).
The mechanisms that maintain CO2 constant despite large variations in the metabolic production of this gas are complex (123, 166). For example, during exercise, central command, and reflexes originating from muscle mechano-and metabotropic receptors cooperate to activate breathing to a degree roughly commensurate with the rise in whole body metabolism and CO2 production (83, 105, 106). Some combination of central command and reflexes presumably also matches CO2 production and excretion in other physiological contexts such as thermogenesis, changes in diet, and the sleep-wake cycle. The chemoreceptors, which are the subject of this review, are presumed to be responsible for the finer stabilization of PaCO2. By activating central and peripheral chemoreceptors, an increase in arterial PCO2 triggers a reflex, the chemoreflex, that increases breathing and activates the circulation in ways that contribute to restore PCO2 toward normal.
The mechanisms by which the carotid bodies sense CO2 and O2 and other blood borne chemicals (glucose and fixed acids) have been reviewed in great detail recently (224, 324). This topic will therefore not be revisited here. However, the CNS network that is activated by carotid body afferents will be described because central and peripheral chemoreceptors interact and presumably recruit overlapping neural pathways.
To understand how the central respiratory chemoreflex operates, three questions must be answered. The first is the nature of the molecular sensors. Via carbonic anhydrase, CO2 is in equilibrium with three other molecules, namely, bicarbonate, protons, and hydroxide ions, each of which could in theory encode the level of PCO2 by interacting with receptors. Labile but covalent reactions with CO2 (carbamylation) may also be implicated in the actions of this gas on the mammalian brain (284). The second question is the nature of the cells (glia, neurons, and microvasculature) that express such sensors (receptors). The third issue is of an integrative neurobiological nature. Its object is to identify the neuronal network that is recruited by the chemoreceptors to alter breathing and the circulation.
Asphyxia produces gasping and the Cushing response (65, 331, 354). In this article, we will also consider whether, like hypercapnia, CNS hypoxia produces adaptive cardiorespiratory reflexes and whether gasping and the Cushing response are among those adaptive responses.
The Chemoreflexes: Phenomenology
A PubMed search of the term “chemoreflex” yielded 1086 references at the time of this writing. A comprehensive review of this literature is clearly not warranted here but a brief description of the major issues and concepts will be useful as a guide to interpret the CNS neurophysiological literature which is the focus of this review (Fig. 1). The effects elicited by brain hypoxia are not considered in this section.
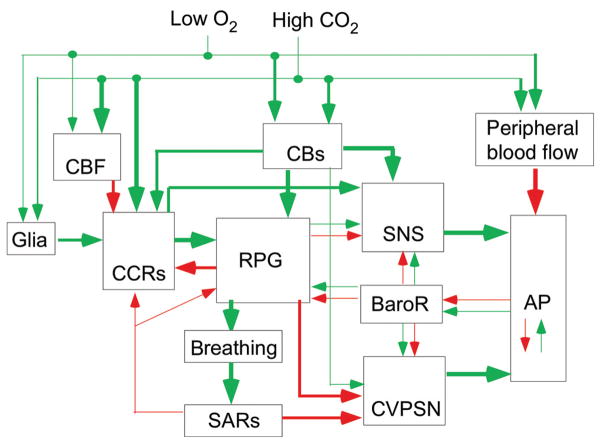
Figure 1.
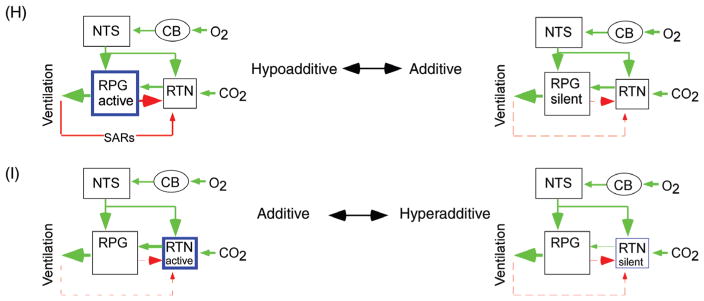
Organigram of the chemoreflexes. Cascade of cardiorespiratory responses elicited in anesthetized mammals by hypoxic stimulation of the carotid bodies or by hypercapnia. These cardiorespiratory responses are elaborated primarily within spinal and pontomedullary circuits. The same circuits are also presumably recruited by small per-turbations of the blood gases in the conscious state to stabilize PCO2. Large acute perturbations of blood gases produce arousal, aversive sensations and stress, responses that involve numerous other brain regions and processes. The direct effects of hypoxia on the CNS are not considered here. Green arrows denote cell activation (e.g., carotid bodies by hypoxia) or a globally excitatory connection (e.g., effect of the carotid bodies, CBs, on the RPG), or an increase in a dependent variable [e.g., effect of CO2 on cerebral blood flow (CBF) resulting in a “washout” of brain CO2]. Red arrows have the opposite meaning. The baroreflex (BaroR) potentiates or attenuates the chemoreflexes depending on the direction of the change in arterial pressure (AP). Slowly adapting lung stretch receptors (SARs) exert a feedback regulation on the RPG and on central chemoreceptors (CCRs) and inhibit the cardiovagal outflow (CVPSN, cardiovagal parasympathetic nerve activity). The chemoreceptors, both central and peripheral, activate the sympathetic nervous system (SNS) both via the RPG and independently of it.
The cardiovascular effects of hypercapnia result from effects of CO2 at multiple levels (264, 438). CO2 dilates the peripheral vasculature which tends to lower AP. Chemoreceptor stimulation activates the sympathetic nervous system (SNS) which counteracts the CO2-induced peripheral vasodilation via neurogenic vasoconstriction and increases the cardiac output. As a result AP may or may not rise or may do both in a time-dependent manner. If AP rises, the effects of chemoreceptor stimulation on the SNS and upstream brainstem neurons will be mitigated by the powerful baroreceptor feedback (BaroR). If AP decreases, the effects of chemoreceptor stimulation will be potentiated by this feedback. Chemoreceptor stimulation activates the respiratory pattern generator (RPG) which increases breathing and has multiple effects on the neural control of the circulation. For example, increased breathing increases the discharge of lung stretch afferents (SARs), which reflexly inhibit the activity of the cardiovagal parasympathetic outflow (CVPSN), increasing heart rate and thereby contributing to raising AP and cardiac output. The heart rate also increases because of direct inhibitory connections between the RPG and cardiovagal preganglionic neurons (CVPGNs). The effects exerted by CO2 on central chemoreceptors (CCRs, Fig. 1) are no less complex. A rise in CNS PCO2 activates the CCRs directly and probably indirectly via astrocytes but these effects are attenuated by an increase in cerebral blood flow which tends to reduce the relative contribution of CO2 produced by the brain to brain tissue PCO2 (washout effect; Fig. 1) (10,110,461). In addition to these three primary mechanisms (direct effects of acid on neurons, paracrine effects of CO2 or acid and CO2-washout effect caused by the CO2-sensitivity of the vasculature), CCRs such as the retrotrapezoid nucleus (RTN) receive polysynaptic excitatory inputs from the carotid bodies and are negatively regulated by lung stretch afferents and by the RPG (Fig. 1) (152, 158, 295, 421). The respiratory and autonomic effects produced by hypoxia via the carotid bodies are no less complex (428) and are also extremely briefly summarized in Figure 1.
Several key points should be emphasized. First, each component of the organigram shown in Figure 1 is likely to be differentially affected by anesthesia or in reduced preparations. For example, the inhibitory Hering-Breuer reflex is facilitated in anesthetized rats to the point where the RPG of a ventilated animal is silent at PaCO2 of less than 8% to 9% if the vagus nerves are left intact. By contrast, in the same preparation, the arterial baroreflex threshold is typically very high (>80 mmHg). The exacerbation of the lung inflation reflex could be related to the potentiation by anesthetics of a crucial GABAergic link between the nucleus solitarius (NTS) and the RPG (424). Thus, the relative importance of each pathway cannot be accurately assessed from experimentation in anesthetized or reduced preparations and, whenever possible, experiments in conscious man or animals should provide the last word.
A second key point is that the responses elicited by chemoreceptor activation depend on the intensity of the stimulus. Low-level stimulation as occurs under physiological conditions produces no sensation, no effect on the level of vigilance, and probably engages mostly the lower brainstem pathways that are the primary focus of this article. When PCO2 is substantially and rapidly elevated such as during diving, airway obstruction, accidental, or deliberate exposure to high levels of CO2, this gas produces arousal from sleep and/or adverse sensations in man (dyspnea, the urge to breathe, even panic) (56, 292). Hypoxia alone has similar wake-promoting effects. Acute stimulation of chemoreceptors with high levels of CO2 or severe hypoxia as is routinely done in animal experiments for the purpose of measuring chemoreflexes also produces arousal and/or emotional responses. The effect of such perturbations on the cardiorespiratory outflows therefore results from both short loop pontomedullary reflexes and long loop reflexes that recruit wake-promoting pathways and pathways involved in the emotional control of cardiorespiratory function (67,159,178,227,362). Brief maximal stimulation of the carotid body with cyanide boluses produces the defense reaction in lightly anesthetized animals (45, 173, 271). The signs include piloerection, vocalizations, increased breathing and blood flow redistribution to the muscles away from the splanchnic region. These signs are elicited by all noxious stimuli; therefore, administration of boluses of cyanide to conscious animals is presumably strongly aversive. The procedure may be of interest to understand the arousal or emotional effect of acute asphyxia but it is of questionable value to understand the normal metabolic control of breathing (148, 345). Similarly, massive acute activation of the carotid bodies with cyanide causes a profound vagally mediated bradycardia in the absence of anesthesia (277). While this response may be relevant in the context of the diving response or accidental asphyxia, its importance for the chemoreceptor control of the circulation under physiological circumstances is debatable.
In summary, the dose-dependent effects of chemoreceptor stimulation should be kept in mind when interpreting the effects of various brain lesions, genetic manipulations, and other experimental procedures on the chemoreflexes. The assumption that the same sensors and the same CNS pathways are recruited by small physiological fluctuations of blood gases as by sudden extreme changes should be questioned a priori. Finally, when comparing the results of human versus animal experiments, consideration should be given to the fact that rodents, unlike man, consciously perceive very low levels of ambient CO2 via the sense of smell and display species-specific behaviors in response to this gas (182).
Brief overview of the Organization of the Central Respiratory Network
This review is not the place for an extensive description of the respiratory network but its components need to be mentioned to convey current hypotheses about how breathing and the autonomic nervous system are regulated by the carotid bodies and such putative chemoreceptors as RTN, raphe, orexin neurons, and others. The chosen nomenclature for the respiratory network is as described in 2013 by Smith et al. (381). The RTN is defined here restrictively as a cluster of CO2-activated Phox2b and neurokinin-receptor-1 expressing glutamatergic neurons located under the facial motor nucleus (401). The term RTN/pfRG (parafacial respiratory group) will not be used because the pfRG is an evolving physiological concept and probably consists of several types of neurons still to be identified (327, 330).
Rhythm and pattern generation for eupneic breathing
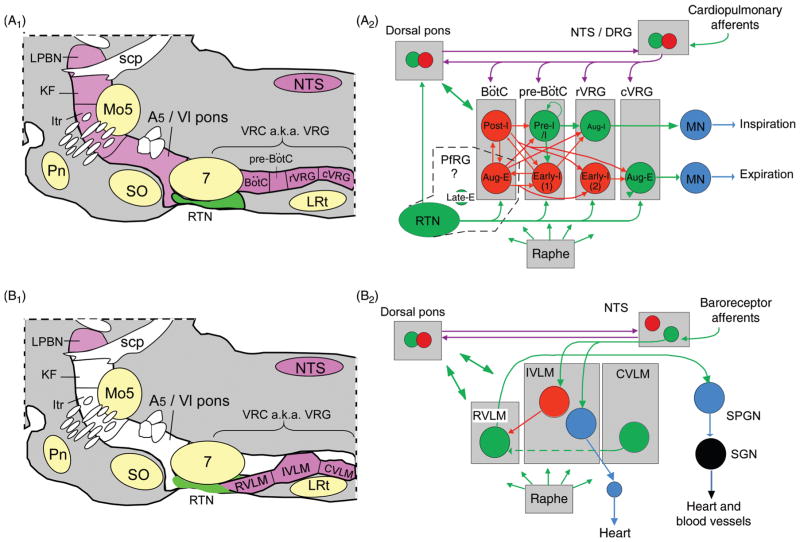
The pontomedullary region contains a set of interconnected structures that are necessary for the production of eupnea, the term referring to regular automatic breathing movements involving the sequential contraction and relaxation of pump and airway muscles according to a pattern closely resembling that of animals that are quietly awake and resting or in non-REM sleep (12, 117, 291, 381) (Fig. 2A1 and A2). A major component of this network, the ventral respiratory column (VRC), resides in a ventrolateral medullary region that contains many other types of neurons including but not limited to neurons that regulate the sympathetic and parasympathetic outflows (Fig. 2B1 and B2). The VRC is divided by respiratory physiologists into at least four functional segments, not counting the RTN nor the still elusive parafacial respiratory oscillator (pfRG, Fig. 2A2) which can be viewed as the rostral-most extension of the VRC (12, 117, 381) (Fig. 2A1 and A2). The most caudal section of the VRC (cVRG), is primarily dedicated to the control of abdominal muscles and expiratory activity (Fig. 2A1 and A2). The next rostral segment called the rostral VRG (rVRG), contains bulbospinal inspiratory premotor neurons (Fig. 2A2) (92). In rats, these neurons also innervate the ipsilateral and contralateral VRC, the hypoglossal nucleus and the NTS (249). At the present time, these inspiratory premotor neurons are assumed to play no role in respiratory rhythm generation (381). Their local collaterals may drive interneurons which, in turn, may influence the cardiovagal and the sympathetic vasomotor outflows. The next rostral section of the VRC is the pre-Bötzinger complex (pre-BötC) which generates the inspiratory rhythm (Fig. 2A2) (116, 192, 381, 384). Rhythm generation relies on recurrent excitatory interactions between two symmetrically located clusters of DBX1 domain-derived glutamatergic neurons (116, 149, 198). The discharge of these neurons (a.k.a. pre-inspiratory/inspiratory, pre-I/I, neurons, Fig. 2A2) usually starts during the late expiratory phase of the respiratory cycle and undergoes an abrupt crescendo during inspiration. The periodicity of the pre-Bötzinger complex cycle is regulated by numerous modulators such as serotonin, substance P, noradrenaline, and adenosine (93, 347). The respiratory rhythm is further regulated by a surround of inhibitory neurons (GABAergic and/or glycinergic) located within the pre-Bötzinger complex and within the next rostral section of the VRC, the Bötzinger region (Fig. 2A2) (381). The former discharge during early inspiration (early-I inhibitory neurons) and the latter (Bötzinger neurons) discharge either in a decrementing fashion during the first phase of expiration (post-I neurons) or fire incrementally during late expiration (late-E neurons) (Fig. 2A2) (381). The network of interconnected neurons described so far sculpts the membrane trajectory of rVRG and other premotor neurons which, in turn, phasically activate pump and airway motoneurons (MNs) located, for example, in the phrenic nucleus (inspiration in Fig. 2A2) and elsewhere (hypoglossal and ambiguus nuclei, not represented). As mentioned, the premotor neurons that drive spinal inspiratory MNs (phrenic and intercostal) reside just caudal to the pre-Bötzinger complex within the rVRG. In cats but possibly not in rodents, a portion of these inspiratory premotor cells also reside in the caudolateral portion of the NTS, a highly heterogeneous portion of this nucleus defined by respiratory physiologists as the dorsal respiratory group (DRG, Fig. 2A2). This region of the NTS receives afferents from the lungs and airways (e.g., slowly adapting lung stretch receptors) (222). It also contains some of the neurons that receive monosynaptic input from these afferents and relay information from cardiopulmonary receptors to the VRC and dorsolateral pons (222). These neurons (second order neurons) are generally glutamatergic. This is probably the case of the neurons that receive carotid body input (see later section). Other second-order neurons such as the “pump cells” which receive direct input from non-adapting lung stretch afferents seem to be GABAergic and or glycinergic [(424) and references therein].
Figure 2.
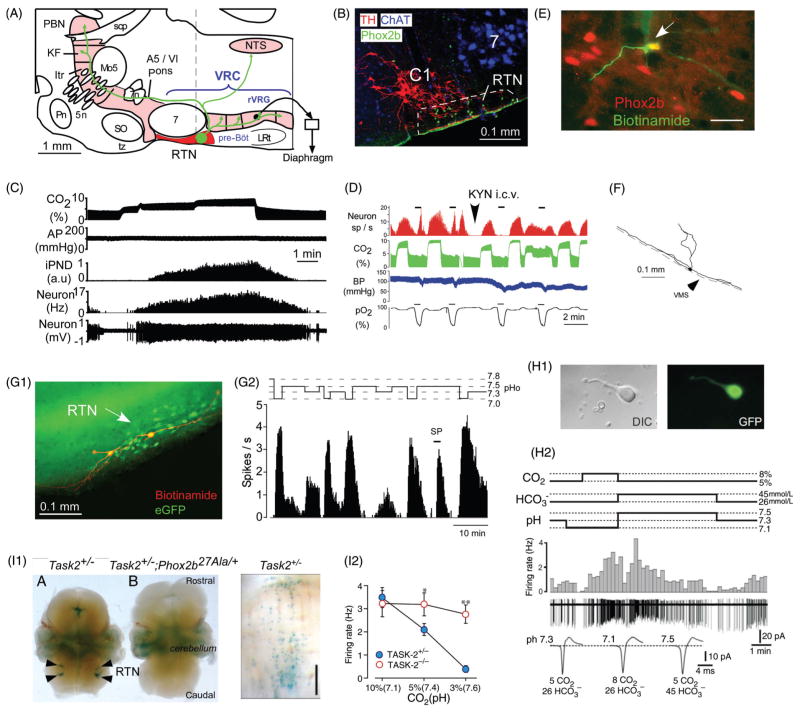
Pontomedullary regions responsible for eupneic breathing and for generating the autonomic outflows to the cardiovascular system: anatomy and simplified circuitry. (A1) Parasagittal section through the pons and medulla oblongata of a rodent. The regions colored in magenta contain the principal building blocks of the respiratory pattern generator. The ventral respiratory column (VRC) contains four functional compartments aligned in rostrocaudal order (Bötzinger Complex (BötC), pre-BötC, rostral ventral respiratory group (rVRG) and caudal VRG (cVRG)). The retrotrapezoid nucleus (RTN) resides at the rostral end of the VRC under the facial motor nucleus. In this article, the term RTN refers specifically to a cluster of about 2000 CO2-activated Phox2b-ir glutamatergic neurons (in rats, 800 in mice). (A2) Minimal circuitry responsible for the generation of eupneic breathing [adapted, with permission, from Lindsey, Ryback & Smith (246)]. The drawing depicts some of the neuronal interconnections within and between the four compartments of the ventral respiratory column and a few of the connections of RTN neurons (for details see text). The parafacial respiratory group (pfRG) is a physiologically defined entity now believed to be specifically involved in the generation of active expiration (330). Its constituent neurons and their location are not yet defined. Bötzinger augmenting expiratory neurons have been included by some authors in the pfRG (327). Inhibitory (GABAergic or glycinergic) neurons are represented in red, glutamatergic neurons in green, motoneurons in blue, connections with both excitatory and inhibitory components in magenta (e.g., neurons transmitting information from arterial baroreceptors, pulmonary stretch afferents, the carotid bodies etc.). (B1) The regions colored in magenta are thought to contain the main components of the network that generates the autonomic outflows to the cardiovascular system. From an autonomic regulation standpoint, the ventrolateral medulla can be subdivided into three regions whose anatomical relationship with the respiratory compartments can be appreciated by comparing panels A1 and B1. (B2) Schematic of cardiovagal parasympathetic neurons, RVLM presympathetic neurons and connections responsible for their regulation by arterial baroreceptors. Abbreviations: aug-E, augmenting expiratory neurons; aug-I, augmenting inspiratory neurons (a.k.a. inspiratory premotor neurons); CVLM, caudal VLM; DRG, dorsal respiratory group (caudolateral portion of the NTS); early-I, early-inspiratory neurons, early-I(1) and early-I(2) are postulated to have distinct input-output functions; IVLM, intermediate VLM; Itr, intertrigeminal region; KF, Kölliker-Fuse nucleus; LPBN, lateral parabrachial nuclei; LRt, lateral reticular nucleus; Mo5, trigeminal motor nucleus; NTS, nucleus of the solitary tract; pfRG, parafacial respiratory group; Pn, pontine nuclei; post-I, postinspiratory; preI/I, preinspiratory/inspiratory (putative rhythmogenic neurons); scp, superior cerebellar peduncle; SO, superior olive; SPGN, sympathetic preganglionic neurons; SPN sympathetic (post)ganglionic neuron; VRC, ventral respiratory column.
Of note, while the inspiratory rhythm relies on excitatory neurons, a large majority of the neurons implicated in generating the eupneic breathing pattern are glycinergic and/or GABAergic (Fig. 2A2) (381). The activity of the entire network depends on multiple sources of tonic excitatory drive that simultaneously depolarize these mutually interacting inhibitory neurons. The chemoreceptors, both central and peripheral, are assumed to be one of the most important excitatory drives to this network. This hypothesis is revisited in further detail later. Two putative CCRs, the RTN and the raphe are represented in Figure 2A2.
The dorsolateral pons contains three regions that are also essential to breathing, the intertrigeminal region, the Kölliker-Fuse nucleus, and the lateral parabrachial complex (Fig. 2A1 and A2). Chemical activation of selected regions of the lateral parabrachial complex increases blood pressure and activates breathing rate and amplitude (53, 54). This region also contributes to the patterning of the inspiratory outflow during hypoxia and CCR stimulation (54, 389). The integrity of the connections between the dorsolateral pons and the medulla oblongata is required for VRC neurons and certain vagal MNs to develop a post-I discharge pattern and/or to be active at all (381). In other words, these connections are necessary for the production of the three phase breathing pattern (inspiration, postinspiration, and late expiration) and for proper control of the airway constrictor muscles that slow expiratory airflow immediately after inspiration. The effects of a surgical interruption of the connections between the dorsolateral pons and the rest of the respiratory network have been interpreted as suggesting that the Kölliker-Fuse nucleus provides an excitatory drive specifically directed to post-I neurons located in the VRC (381). Alternative interpretations are possible because connections between the VRC and the Kölliker-Fuse nucleus are reciprocal and such lesions also interrupt multilateral connections between the Kölliker-Fuse nucleus, the dorsal parabrachial complex, the VRC (RTN included) and the nucleus of the solitary tract (42, 52–54, 390, 391). In addition, connections to and from the intertrigeminal region, a structure that resides in the immediate vicinity of the Kölliker-Fuse nucleus could be responsible for many of the effects attributed to the latter nucleus (52, 54).
Active expiration and its recruitment by chemoreceptor stimulation and exercise
Normally, abdominal muscles are phasically recruited for breathing only when a high level of lung ventilation is required such as during exercise or when chemoreceptors are strongly activated. The phasic expiratory activity of abdominal muscles operates under the control of a specialized network termed the expiratory oscillator that is partially distinct from the inspiratory network described above (191,330). Essential components of this expiratory oscillator seem to reside in the immediate vicinity of the facial motor nucleus in a region that overlaps with the Bötzinger region and the RTN chemoreceptors (191, 330). This region is called the pfRG (Fig. 2A2). RTN chemoreceptors or a subset of these cells could be components of the expiratory oscillator or at least may provide the CO2-dependent excitatory drive that enables this oscillator to be active (7, 8, 269). This issue will be developed in greater detail later. The activity of the expiratory oscillator is always synchronized with the inspiratory oscillator but, in vitro, the expiratory outflow can be intermittently recruited when the chemoreceptor drive is low (7, 191, 269). The expiratory oscillator is presumed to drive expiratory abdominal muscles via premotor neurons located in the cVRG (Fig. 2A2) (209, 367, 381).
Central Pathways that Regulate the Sympathetic and Cardiovagal Outflows
This topic will be also addressed succinctly and solely for the purpose of presenting current views on how chemoreceptors might influence the sympathetic and cardiovagal outflows. Lower brainstem networks are emphasized because these regions contribute to short loop cardiorespiratory reflexes that are presumably recruited in priority by low levels of chemoreceptor stimulation to regulate the cardiorespiratory outflows. High levels of chemoreceptor stimulation, of the type that produce adverse sensations, arousal and emotional responses, must at some point engage a common core of hypothalamic pathways that regulate the sleep-wake cycle and a variety of hypothalamic nuclei that project back to the brainstem and spinal cord. These nuclei (dorsomedial nucleus, paraventricular nucleus, and orexin system) can be viewed as the descending portion of long loop reflexes that are triggered by high levels of chemoreceptor stimulation of the type that produce aversive sensations, stress, or arousal from sleep. Their role is examined in another section.
Spinal presympathetic neurons
The monosynaptic inputs to sympathetic preganglionic neurons a.k.a. presympathetic neurons have been identified using a variety of techniques including the retrograde transsynaptic transport of an attenuated form of the pseudorabies virus (PRV). The latter technique revealed monosynaptic input from surprisingly few regions of the spinal cord, medulla oblongata and hypothalamus (405–407). The PRV method likely understates the number of monosynaptic direct inputs to SPGNs. For example, the orexin neurons which assuredly target sympathetic preganglionic neurons (250) were not identified as presympathetic with this technique (405–407).
In conjunction with electrophysiological evidence, spinal “presympathetic” interneurons are predominantly segmental and reside in laminae 5, 7, and 10 and, probably, also within the intermediolateral cell column [reviewed in (87)]. These interneurons mediate spinal reflexes whose existence was originally identified in spinally transected preparations. These reflexes are inhibited by largely uncharacterized supraspinal pathways with the possible contribution of catecholaminergic neurons (61, 81, 82, 304). Based on electrophysiological recordings in slices, some of these “sympathetic” interneurons are clearly excitatory while others are inhibitory (glycinergic or GABAergic) (88, 101, 220, 221, 289). In intact conscious mammals, these interneurons may regulate blood flow in active skeletal muscles, they may also regulate renal function [reviewed in (87)].
Rostral ventrolateral medulla “presympathetic” neurons
The most studied supraspinal presympathetic neurons are located within the rostral ventrolateral medulla (RVLM; Fig. 2B1 and B2) [for reviews, see (160, 371)]. Most of these cells are inhibited by arterial baroreceptors and are assumed to regulate the activity of the sympathetic efferents that are also under baroreceptor control, that is, muscle and gut vasoconstrictors and cardiac efferents (160,193,371). RVLM presympathetic neurons are glutamatergic (2, 160, 293, 403). About two thirds of them are C1 cells (293, 369). The latter are catecholaminergic as well as glutamatergic and contain the adrenaline-synthesizing enzyme PNMT (160, 371). The C1 cells also express various combinations of peptides such as substance P, PACAP, enkephalin, NPY, and TRH [for review, see (399)]. Non-C1 RVLM presympathetic neurons express at least one peptide, enkephalin (160,371,402). RVLM presympathetic neurons, whether of the C1 or noncatecholaminergic variety receive a heterogeneous complement of synaptic inputs. For example, their central respiratory modulation falls into discrete patterns that are identical to those exhibited by individual sympathetic efferents recorded under the same conditions (72, 168, 193). This particular topic is expanded in a later section. Subsets of RVLM presympathetic neurons are presumed to differentially regulate the activity of sympathetic efferents to various organs. One piece of evidence is the above mentioned heterogeneity of their respiratory modulation, which closely resembles the respiratory pattern of sympathetic ganglionic units (72, 168, 193, 323) (Fig. 3). Also, RVLM presympathetic neurons are differentially affected by i.v. injections of cholecystokin or by stimulation of the carotid bodies and such response heterogeneity is also observed in peripheral sympathetic efferent barosensitive fibers (274, 363). Finally, microstimulation of various subregions of the RVLM with glutamate differentially activates selected types of sympathetic efferents (e.g., muscle vasoconstrictor, visceral, or skin vasoconstrictor) (68, 275, 276). These observations suggest that subsets of RVLM presympathetic neurons could be organized in some kind of viscerotopic manner. Other subsets of RVLM neurons, probably of the C1 variety may exert a more generalized influence on the sympathetic outflow (194).
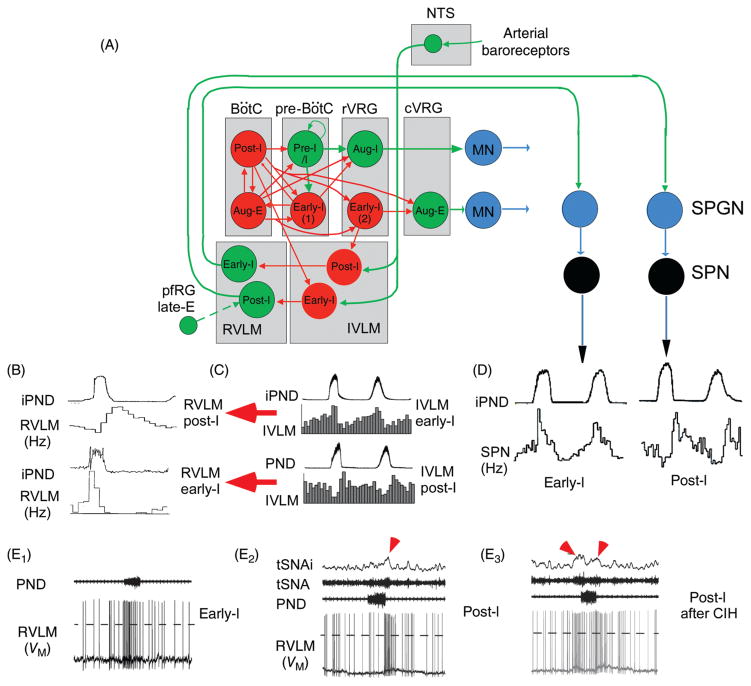
Figure 3.
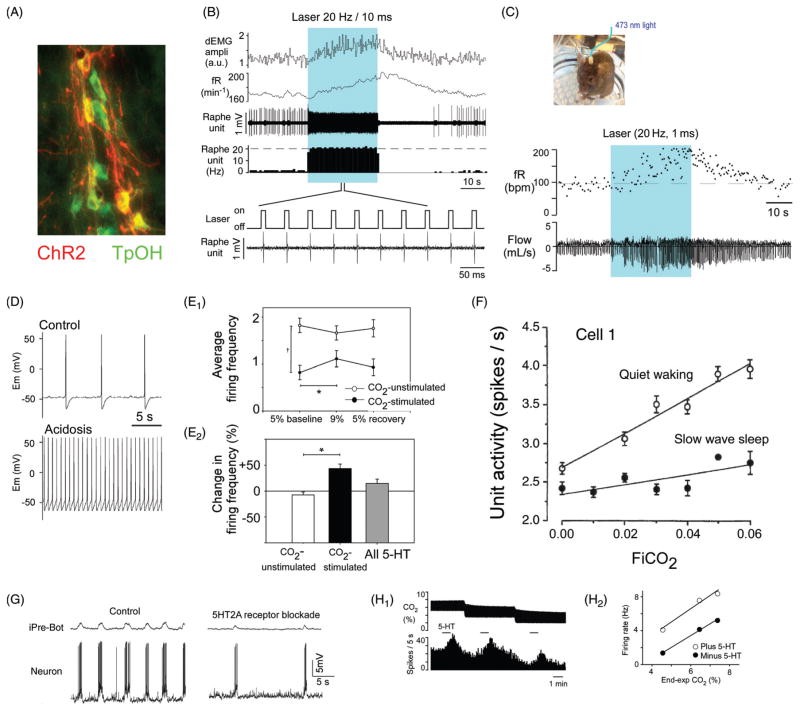
Regulation of the sympathetic vasomotor outflow by the respiratory pattern generator. (A) Plausible circuitry responsible for coupling respiration with the sympathetic nerve activity to the cardiovascular system. Sympathetic ganglionic neurons (SPNs) display stereotyped patterns of respiratory modulation in deafferented preparations (vagotomized, barodenervated). Two of the most commonly found patterns in rats (early-I and post-I) are depicted in D [top traces, average integrated phrenic nerve discharge, iPND; lower trace iPND-triggered rate histogram depicting the probability of firing of a single SPN during the central respiratory cycle; redrawn, with permission, after (72)]. These SPN patterns are virtually identical to those of single RVLM presympathetic neurons [shown in B; redrawn, with permission, from (168)]. The IVLM contains GABAergic neurons that are activated by arterial baroreceptor stimulation and inhibit RVLM presympathetic neurons (panel A). The respiratory modulation of these IVLM GABAergic neurons is approximately the mirror image of that of the RVLM presympathetic neurons [C; reprinted from Mandel and Schreihofer (265) with permission from Wiley & Sons] hence the hypothesis that IVLM neurons with early-inspiratory discharge produce post-I modulation in RVLM presympathetic neurons and vice versa (red arrows in C). The depicted inputs from the RPG to IVLM neurons are plausible but speculative. (E) Intracellular recordings of RVLM presympathetic neurons in an arterially perfused midcollicular transected rat preparation [from Moraes et al. (293) with permission]. The early-I (left trace) and the post-I patterns (middle trace) are instantly recognizable. The right panel shows a presympathetic RVLM neuron that exhibits an extra peak of activity during late expiration. The latter recording was made in a rat subjected to chronic intermittent hypoxia which caused late-expiratory activity in an abdominal nerve at rest (not shown) and an additional late-E peak of activity in the thoracic chain (red arrow pointing down and to the right above the thoracic chain SNA trace, tSNA). An excitatory input from pfRG has been tentatively proposed as the source of this late-expiratory activation (illustrated in A).
In anesthetized animals or in reduced preparations RVLM presympathetic neurons are typically highly active (discharge rate up to 35 Hz), in rodents especially (369). These cells remain active and discharge very regularly after blockade of glutamatergic transmission in vivo (410). These observations are at the root of the theory that the sympathetic vasomotor tone could originate from intrinsic pacemaker properties of the presympathetic neurons (410). Experiments carried out in slices of neonate rat brainstem supported this theory to some extent by showing that the C1 cells remain slowly active under conditions of synaptic blockade, probably under the effect of the persistent sodium current INaP (200, 243). Later dismissed (248) but recently resurrected (293), the theory that RVLM presympathetic neurons have pacemaker properties may well be correct but, because this characteristic relies on INaP, it is prominent only when the interspike membrane potential reaches the voltage range where voltage activated fast sodium channel exhibit a significant window current. Such conditions are probably reached when the powerful GABAergic inhibitory input to RVLM presympathetic neurons is withdrawn such as, for example, during severe hypotension or when INaP is enhanced, for example, during severe brain hypoxia (see Cushing response in later section).
The monosynaptic inputs that originate from RVLM neurons are viewed as determinant for the discharge of preganglionic neurons and ultimately sympathetic vasomotor tone generation (Fig. 2B2). This interpretation is primarily based on the results of experiments performed in anesthetized or reduced preparations in which other sources of input are either surgically eliminated or silent (160, 268, 371). It is also supported to some modest degree by a few experiments carried out in conscious rats. For example, adenovirus-mediated over-expression of endothelial NOS within the RVLM decreases BP for a few days whereas overexpression of inducible NOS has the opposite effect (207,210). However, these effects may not result from the inhibition of the presympathetic neurons since the RVLM innervates many places besides the IML and adenoviruses appear to transfect glial cells rather than RVLM neurons (14). Chronic lesions of the C1 neurons (presympathetic and others) plus collateral damage to lower brainstem noradrenergic neurons produce a modest degree of hypotension (10 mmHg) in conscious rats and very little change in resting plasma catecholamine levels (261,262). However, they do attenuate the increase in circulating catecholamines caused by hypoxia or hemorrhage (261, 262). According to this evidence, the C1 neurons may exert a relatively modest control over mean AP in normal conditions but they appear to mediate a significant fraction of the sympathoexcitation associated with severe hypotension and hypoxia. The effects produced by lesions of the C1 cells could also be mediated by their projections to structures other than the preganglionic neurons that also regulate AP (dorsolateral pons, NTS, medullary reticular formation, periaqueductal gray matter, and various hypothalamic nuclei) (51).
VLM “depressor” area and other inputs to RVLM presympathetic neurons from the medullary reticular formation
Under anesthesia, virtually all sympathetic reflexes are eliminated by blocking glutamatergic or GABAergic transmission in the RVLM, including the peripheral chemoreflex [(217) and, for review, see (371)]. The temptation to interpret such results as definitive evidence that the RVLM is the only or even the main hub for all cardiovascular sympathetic reflexes should be resisted until confirmation is obtained in conscious animals because anesthesia and other procedures to which reduced preparations are subjected exaggerate the contribution of the RVLM to resting sympathetic tone. A comparable mistake would be to conclude that, because the respiratory outflow is entirely dependent on the level of CO2 under anesthesia, respiration is exclusively driven by chemoreceptors in the conscious state.
Unit recordings and/or Fos studies have provided much information regarding the type of stimuli or brain regions that elicit responses in RVLM presympathetic neurons but the intervening pathways including the location of the neurons that are immediately antecedent to RVLM presympathetic neurons is generally not known except in special cases where a particular neuronal marker has helped in tracing the neurons of origin (69, 70). For example, the latter type of evidence suggests that RVLM presympathetic neurons may receive oxytocinergic and orexinergic inputs from the hypothalamus, substance P, and serotonergic inputs from the medullary raphe and cholinergic inputs possibly from the pedunculopontine nucleus. The pontine reticular formation, the lateral parabrachial nuclei, the periaqueductal gray matter and the vestibular nuclei provide input to RVLM presympathetic neurons but the connections have not been shown to be monosynaptic (69, 70). C1 cells receive monosynaptic inputs from the NTS that could be transmitting chemosensory information from the carotid bodies (9). RVLM presympathetic neurons receive monosynaptic inputs from more caudal regions of the ventrolateral medulla. One such region is often called the medullary depressor area in the subfield of AP control (370, 371). The term originally referred to the fact that microstimulation of this region with glutamate inhibits sympathetic tone (457). This region was subsequently called the caudal VLM referring to the fact that it lies immediately caudal to the RVLM. Stimulation of the depressor region causes AP to drop (457) because it contains GABAergic neurons that are activated by arterial baroreceptor stimulation and inhibit RVLM presympathetic neurons (Fig. 2B2) (370,371). These GABAergic interneurons are highly respiratory modulated and probably contribute to the phenomenon known as sympatho-respiratory coupling. Their role in this context is revisited in a later section. These GABAergic neurons are actually distributed in a region of the ventrolateral medulla that closely overlaps the pre-Bötzinger and rVRG subdivisions of the ventrolateral medulla (Fig. 2B2). Sapru’s depressor region (457) is here called IVLM (intermediate ventrolateral medulla; Fig. 2B2) because fully a third of the VLM resides caudal to the region that contains these GABAergic interneurons (160). The latter region, called here caudal VLM (CVLM), lies ventral or ventromedial to the cVRG and contains another “pressor” region, that is, a neuronal network whose mass excitation by glutamate causes BP to rise, at least in anesthetized animals (Fig. 2B2) (50). The rise in BP caused by stimulating the CVLM with glutamate seems partly mediated via excitation of the RVLM presympathetic neurons but opinions vary as to whether the excitatory pathway from CVLM (or its continuation in the upper cervical region) to RVLM is direct or includes a medullary relay (50, 177, 308, 376).
Midline medulla “presympathetic” neurons
Based on PRV data, SPGNs also receive massive projections from the midline medulla oblongata (405–407). These midline neurons (not represented in Fig. 2) are observed when PRV is injected in the adrenal medulla, the stellate ganglia, and other targets; therefore, these midline medullary neurons are presumed to regulate a broad swath of sympathetic efferents including those that control the circulation. These bulbospinal neurons include serotonergic cells, which exert facilitatory effects on all sympathetic outflows tested so far, and inhibitory (GABA and or glycinergic) neurons (400, 407). Midline bulbospinal neurons with putative sympathoinhibitory function have been described in cats (135).
The presympathetic neurons that regulate the sympathetic tone to the brown adipose fat and cutaneous vessels are also located in the midline medulla but they tend to reside more rostrally in the medulla oblongata than those discussed above (297,305). Some of these neurons are serotonergic and reside in raphe pallidus, others express VGLUT3. Brown fat activation is shut off by hypoxia and is therefore under the control of peripheral chemoreceptors (260).
Lower brainstem pathways that regulate the parasympathetic outflow
CVPGNs reside both in the ventrolateral medulla and in the DMV. The connectome of the former is understood to a small degree, the role of the latter is obscure. The anatomical location of CVPGNs was originally inferred from retrograde tracing studies in which a marker had been introduced in the cardiac branch of the vagus nerve and, in cats, by recording brainstem units backfired from the cardiac branch of the vagus. Their activity pattern and inputs are deduced from studies of the cardiac rate (baroreflex and sinus arrhythmia) and a very limited number of electrophysiological recordings performed within the nucleus ambiguus or at the level of cardiac ganglia (277–279). Some of the inputs of presumptive CVPGNs are also known from experiments in which PRV was introduced in the heart muscle or the pericardial fat pads which contain the cardiac parasympathetic ganglia. More recently, investigators have injected a retrogradely transported dye into the pericardiac sac of neonate rats to visualize and record from putative cardiovagal parasympathetic preganglionic neurons in slices (321). Attractive in principle, this method may not be as specific as originally presumed because it seems to also label esophageal MNs located in the compact portion of nucleus ambiguus (150).
Based on this scant body of work, the CVPGNs of the nucleus ambiguus region appear to include subgroups that produce primarily negative chronotropic or dromotropic effects (133). A large fraction of CVPGNs are activated by arterial baroreceptors (Fig. 2B2) but a subgroup of them, perhaps those located in the DMV may not be. The input from arterial baroreceptors reaches CVPGNs via a minimally disynaptic pathway consisting of the baroreceptor primary afferents and glutamatergic second-order neurons located in the dorsolateral subnucleus of the NTS (Fig. 2B2) (55, 157, 279, 371). Cardiovagal MNs are also regulated by both central and peripheral chemoreceptors, a topic that will be developed later in this article.
Chemoreceptors: Definitions and Generalities
Central CO2 sensors, central chemoreceptors, and central respiratory chemoreceptors
A reflex is initiated by the action of a physical variable (stretch, temperature) on a sensor or by the binding of a molecule to a receptor. In cardiorespiratory research, the term central chemoreception usually refers to the process by which CO2 activates the respiratory and the cardiovascular control centers of the brain stem. The problem is that neither the ligand (molecular CO2, bicarbonate, protons, or hydroxide ions) nor its receptors have been definitively identified. Moreover, the types of CNS cells that express the relevant receptors and trigger a cardiorespiratory response, also loosely defined as CCRs, are not fully identified either. For these reasons, the concept of central respiratory chemoreceptors (CRCs) is still work in progress. Furthermore, depending on the author, the term central chemoreception refers indifferently to a hypothetical CO2 or proton molecular sensor, to the cells that express such sensors or to the CNS circuitry that is activated by CO2. A more precise terminology is required. Here, we will call the receptors (probably proteins) with which CO2 or its proxies (bicarbonate, protons, or hydroxide ions) interact “central CO2 sensors.” We will call the cells (neurons, glia) that express such sensors CCRs and we will reserve the name CRCs for cells that express the CO2 sensors and contribute to the respiratory and cardiovascular components of the central chemoreflex. By this definition, both CCRs and CRCs detect changes in brain pCO2 but only CRCs cause a change in ventilation in response to a change in pCO2. Though useful, the distinction between CCRs and CRCs may not be absolute and these definitions may have to be revised as knowledge progresses. For example, high levels of CNS hypercapnia may increase breathing in part by producing an emotional or arousal response with secondary autonomic and respiratory consequences. Identifying CRCs has been and remains a challenge because pH has pervasive effects on protein structure and function and pH sensitivity is only a matter of degree. Researchers have encountered the same type of difficulty in the search for CNS temperature or glucose receptors.
Central oxygen sensors
Severe CNS hypoxia produces a rise in blood pressure, the Cushing response, and a characteristic high amplitude inspiratory activity called gasping (65, 354). Hypoxia eventually depolarizes all neurons, even those that are transiently hyperpolarized by ATP-regulated potassium currents (162, 287). The Cushing response and gasping could be viewed as the last throes of a nervous system in the process of dying from oxygen deprivation and neuronal depolarization. Another view is that gasping and the rise in blood pressure elicited by severe brain asphyxia are, along with arousal, components of a life-saving reflex honed by evolution to overcome airway blockade during sleep. The failure of this reflex could be the primary cause of SIDS (131). An entirely different question is whether levels of brain hypoxia considerably less extreme than those that cause gasping or the Cushing syndrome also trigger adaptive cardiorespiratory responses. The concept that CNS PO2 is a physiological regulator of breathing and of the circulation has been repeatedly evoked but is far from demonstrated (332, 350). This notion assumes the existence of brain oxygen sensors. The existence, location, and putative role of such central oxygen sensors will also be discussed in this article.
Distinguishing the sensors from the reflexes
The literature on central respiratory chemoreflexes is immense and goes back to the late 19th century. Most of the pertinent research has black-boxed the brain and examined the input-output relationship between PaCO2 (or PO2) and lung ventilation (or cardiovascular function). Ignoring issues such as lung and chest compliance, the threshold and the gain of the overall reflex depend on many different factors such as the properties of the central CO2 receptors, the degree of activation of these receptors, the responsiveness of the CRCs, the reactivity of the neuronal network engaged by the CRCs, and the properties of the respiratory muscles. Countless experimental conditions or experimental interventions have been shown to change the CO2 threshold and/or the gain of the hypercapnic ventilatory reflex (HCVR), for example, state of vigilance, brain lesions, administration of CNS-active drugs, genetic modifications, carotid body manipulations (including denervation), changes in renal function that impact on bicarbonate, and acid excretion. Such effects could be caused by a change in any of the above-mentioned components of the reflex.
Evidence for a Distributed Network of Central Respiratory Chemoreceptors
CRCs may be broadly distributed throughout the neuraxis (311, 317). According to this view, the central chemoreflex is an emergent property of the respiratory network at large, including the brainstem rhythm and pattern generating network and its multiple inputs (312). Since many of these inputs are active only during specific states or behaviors, the chemoreflex is also viewed as recruiting partially distinct CNS pathways during different behaviors or states. Yet, at the present time, even the most assertive proponents of this theory seem to think that a hierarchy of CRCs probably exists and they view the RTN, serotonergic, noradrenergic, and orexinergic nuclei as contributing the largest share of the central respiratory chemoreflex. This section presents a critical analysis of the main arguments supporting the existence of a widely distributed network of CRCs. The specific contribution of the RTN and other CRC prima donnas will be examined later on.
Microdialysis and related evidence
The location of CRCs has been probed by acidifying small brain regions in conscious or anesthetized animals using one of two methods: intraparenchymal injection of an inhibitor of carbonic anhydrase (acetazolamide or methazolamide) or microdialysis of CO2 enriched aCSF. Using these methods, increases in breathing or a neurophysiological surrogate of breathing (usually the phrenic nerve discharge, PND) have been produced in many brain regions.
Unilateral microinjection of acetazolamide below the ventral surface of the medulla oblongata of anesthetized cats, within and outside the region of the pre-Bötzinger complex, increases the PND (58, 388). PND is also activated by injecting acetazolamide into the NTS (59). Coates et al. (59) estimated that the local acidification produced in close vicinity of the acetazolamide injection site was equivalent to increasing PaCO2 by 36 Torr above the resting level.
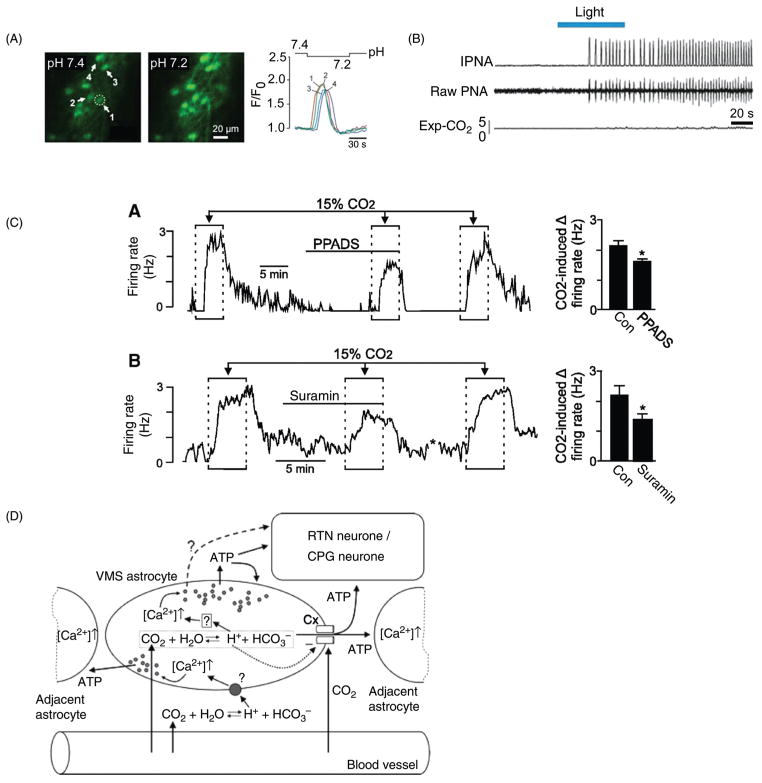
Microdialysis has been most thoroughly and systematically used by Nattie and his colleagues [reviewed in (309,311)]. The technique consists of circulating a physiological buffer equilibrated with CO2 (typically 25%) through a small bore needle fitted at its extremity with a semi-permeable membrane. The device is inserted into the brain of an anesthetized or awake animal. Reversible changes in the animal’s breathing caused by circulating CO2-enriched fluid inside the probe are attributed to the activation of CRCs located in the vicinity of the probe’s tip. The results of many such experiments suggest that CRCs could be present throughout the ventrolateral medulla, including the RTN, within the nucleus of the solitary tract, the medullary raphe and the hypothalamus [e.g., (66, 89, 236, 241) and for review (309)]. These regions correspond to where the core of the breathing network and many of its main excitatory inputs are located (12, 117). The microdialysis technique typically produces very small changes in breathing when compared to the effect of hypercapnia or those produced by direct stimulation of putative chemoreceptors (Fig. 4A–C). A plausible explanation of this discrepancy is that a very small portion of the respiratory network is acidified at one time by a dialysis probe. The method is somewhat destructive because of the large diameter of the probe (0.5 mm) but the ineffectiveness of CSF perfusate at physiological pH indicates that mechanical lesions are not the cause of the respiratory changes. The principal drawback of the dialysis method is the uncertainty regarding the degree of topical brain acidification produced by the hypercapnic fluid. The reported pH change, recorded in vivo with pH electrodes inserted “in the vicinity” of a dialysis probe, seems surprisingly modest (equivalent to that produced by a 6 to 7 mmHg rise in PaCO2) given that the perfusate is equilibrated with 25% CO2 (236). Similar measurements performed in anesthetized animals revealed that 25% CO2 dialysis caused an acidification equivalent to 63 Torr end-expiratory CO2 (240), that is, an increase of around 25 mmHg above resting level, also “in the vicinity” of the probe. This discrepancy could be caused by anesthesia but it also likely denotes the fact that the pH gradient close to a dialysis probe is extremely steep, probably highly blood flow dependent, and virtually impossible to accurately measure in vivo. The cells (neurons and glia, microvessels) closest to the dialysis membrane are likely to be exposed to the same pH as that of artificial CSF equilibrated with 25% CO2, probably as low as 6.5, the effect of which may combine with those of local ischemia caused by an acute lesion. The same uncertainty applies to experiments in which acetazolamide is locally injected (59). In the NTS, severe acidification in vitro (down to pH 7.0) causes astrocyte depolarization and glutamate release via reverse uptake (186). According to these authors, extracellular acidification alters synaptic transmission by compromising glutamate uptake by the astrocytes. Such a mechanism could conceivably explain why an increase in breathing is produced by acidifying any brain region that harbors the respiratory generator or its principal excitatory inputs.
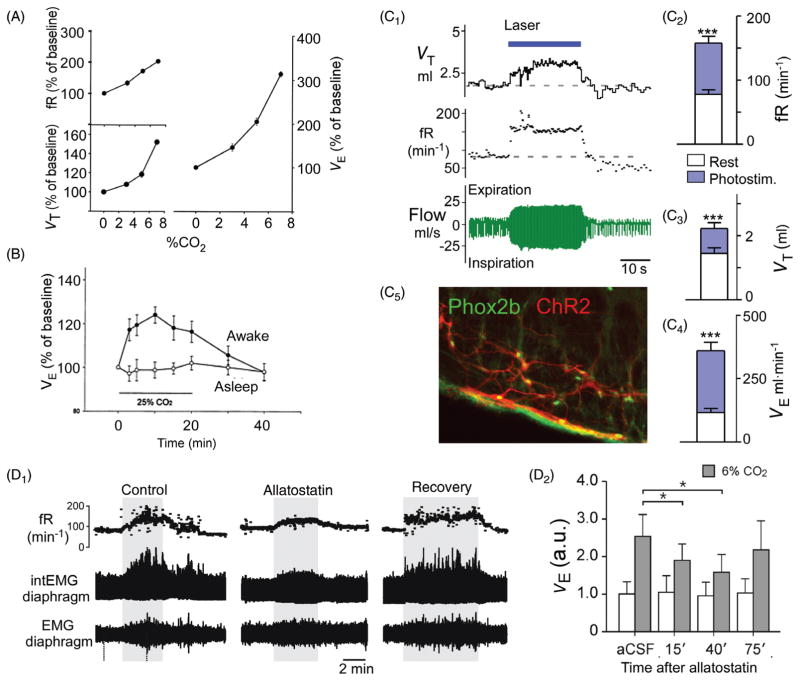
Figure 4.
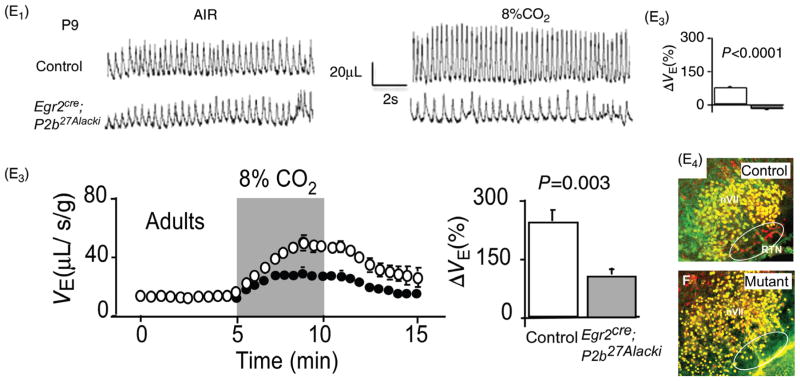
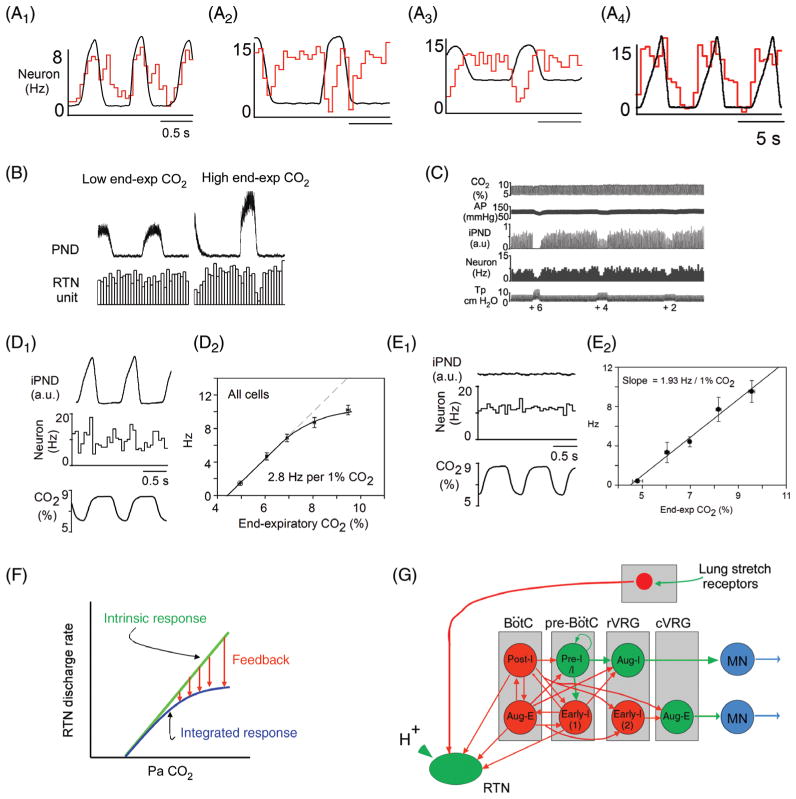
Gain and loss of function of RTN Phox2b neurons in conscious rodents: effect on breathing and the hypercapnic ventilatory reflex (HCVR). (A) Steady-state ventilatory response of a conscious rat exposed to graded levels of hypercapnia. Breathing was measured by whole body plethysmography [reprinted from (236) with permission from Elsevier]. Note that, at 8% FiCO2, fR (respiratory rate) doubles and VE triples. (B) Unilateral acidification of the RTN with a dialysis probe containing a fluid equilibrated with 25% CO2 produces a small (24% average) increase in VE [adapted from Li et al. J. Appl. Physiol. (240) with permission]. (C) Unilateral optogenetic activation (20Hz) of around 35% of RTN Phox2b neurons triples VE in a conscious rat [adapted, with permission, from (4)]. (C1) Typical example (plethysmography); (C2-C4) average responses (from top to bottom: frequency, tidal volume and minute volume) to unilateral optogenetic stimulation of RTN neurons. (C5) Selective expression of ChR2-mCherry fusion protein by RTN (i.e., Phox2b+) neurons in one such animal (Phox2b in green; mCherry in red). (D1) Attenuation of the hypercapnic ventilatory reflex by i.c.v. allatostatin administration to a conscious rat in which an unknown fraction of RTN neurons were transduced with the allatostatin receptor. (D2) Average results from five rats [(from Marina et al. (269) with permission]. (E1) Absence of HCVR in neonatal (day 9) transgenic mice in which a mutated form of transcription factor Phox2b (Phox2b-27ala) is expressed selectively by cells of rhombomere 3 and 5 lineage (Phox2b27Alacki; Egr2cre/0) preventing or aborting RTN neuron development. (E2) Group data. (E3) Partial recovery (one third) of the HCVR in adult Phox2b27Alacki; Egr2cre/0 mice. (E4) Absence of Phox2b-ir neurons in the RTN of a Phox2b27Alacki; Egr2cre/0 mouse (embryonic day 14; RTN identified by white oval, Phox2b in red) [E1–E4 reprinted from Ramanantsoa et al. (346) with permission].
Widespread effects of acidification on neurons in vitro
Fukuda and Honda (126) are usually credited with the first sharp electrode intracellular recordings of CO2-responsive lower brainstem cells in slices (rats). These superficial cells were recorded between the lateral edge of the pyramids and the rootlets of the hypoglossal nerves, that is, in a region that would have roughly corresponded to the caudal chemosensitive region identified by Loeschcke in cats (368). Acidification of this region by microdialysis also produces some respiratory stimulation in rats (66). The cells recorded by Fukuda and Honda were depolarized by about 20 mV by changing bath pH from 7.8 to 7.0. They had a strongly hyperpolarized membrane potential at alkaline pH (−78 mV) and were nonspiking. Given these characteristics, these cells could have been astrocytes since a subset of ventral medullary surface glial cells are pH-sensitive and gap-junction coupled (146, 453). Fukuda and Honda suggested that the effect of CO2 on these cells was entirely caused by the associated pH change because increasing CO2 while maintaining external pH constant was ineffective. Area M, discussed below in the section on the RTN, was also explored by these authors in search of pH-modulated cells, but without success.
Numerous electrophysiological studies subsequently showed that acidification or increases in PCO2 (10%–15%) alters the discharge rate and/or membrane potential of brainstem neurons recorded in such preparations as the whole neonatal brainstem and brain slices, usually from neonatal rodents, or in culture (76,77,167,203,300,328,447,448,458). A substantial proportion of neurons (15%–45%) selected at random within a given region are mildly activated/depolarized and a similar proportion are inhibited/hyperpolarized by acidification in the range of 0.2 to 0.5 pH or more (15% CO2), the balance being unaffected. The effects are typically small and qualitatively similar results are observed in areas that have no obvious link to central respiratory chemosensitivity such as the cerebellum, cortex, and hippocampus although the percentage of activated and inhibited neurons varies somewhat depending on the brain region (448). When the recorded neurons were of a defined type (RTN, nucleus ambiguus, LC) they responded in a qualitatively similar manner to acidification (77, 203, 231, 340, 353). The Kawai et al. studies (203,204) performed using a neonatal preparation in vitro are especially noteworthy. These investigators found that directionally consistent responses to CO2 (range 2%–8%; depolarization vs. hyperpolarization) were elicited in different functional classes of respiratory neurons (inspiratory, postinspiratory, RTN, etc.). Furthermore these responses largely persisted in preparations in which exocytosis was presumed to have been blocked by lowering extracellular calcium and increasing magnesium. The drawback of experimenting with the whole brainstem preparation is that the effects of acidification occur on a background of hypoxia and acidosis present at the core of the preparation. In addition the substantial change in CO2 investigated produced a notable (70%) increase in fictive respiratory frequency but this was associated with a reduction in burst amplitude. In other words large changes in CO2 produced very little if any increase in overall motor out-put. The small effects of acidification on the fictive respiratory rate observed in such preparations could perhaps be explained by the contribution of TASK-1/3 channels to the activity of rhythmogenic neurons (213, 214). However, TASK channels are extremely widespread in this part of the brain where their main role, as elsewhere, is likely to mediate the effect of serotonin, substance P and other G-protein coupled receptors. Finally, the genetic knock-down of TASK-1/3 channels has no influence on the central respiratory chemoreflex in vivo (301, 450). Kawai et al. (204) concluded that the effects of CO2 on the various respiratory neurons might have been cell autonomous. However, their observations are also compatible with the possibility that CO2 exerts its effects in this preparation by causing the release of ATP from astrocytes, either by exocytosis (assuming a minor dependence of glial exocytosis on an influx of extracellular calcium) or through connexin channels (185, 201).
In vitro, the pH response of the neurons considered to be the best CRC candidates (RTN, locus coeruleus, NTS, and serotonergic neurons) is not dramatically larger than that of neurons far less likely to be CRCs given their CNS location (e.g., Figs. 4G and 5E), although the metrics used to define neuronal chemosensitivity vary from author to author and the conditions under which pH sensitivity is measured (slice, neuronal culture, etc.) obviously matter. For example, a neuron whose discharge rate varies from 0.1 to 0.5 Hz over 0.4 pH is said to be highly chemosensitive (to have a high chemosensitivity index) because its firing increases by 400%. Whether an increase of 0.4 Hz over 0.5 pH is meaningful or not cannot be determined without evidence that this type of change alters breathing in vivo. Also, most experiments have been conducted at room temperature, which complicates attempts to compare the response of different neuronal types under more physiological conditions. For example, the pH sensitivity of neonatal RTN neurons increases substantially between 23° and 35° (from 0.59 ± 0.1 Hz/0.1 pH at 23°C to 1.68 ± 0.2 Hz/0.1 pH at 35°C) (158). The same could be true of other putative CRC neurons as suggested by work on the bullfrog’s locus coeruleus (361) but the matter has not been systematically investigated.
Figure 5.
Activation of RTN neurons by CO2. (A) Location and anatomical projections of RTN neurons (definition of RTN in Fig. 2 legend; abbreviations as in Fig. 2). (B) Transverse section through the lower right quadrant of the rat medulla oblongata at the level of the dotted line in A. The facial motor nucleus is in blue (choline acetyl-transferase immunoreactivity), the C1 presympathetic neurons are in red (tyrosine hydroxylase) and phox2b immunoreactivity (green, nuclear localization) identifies RTN neurons [adapted, with permission, from (401)]. (C) Response of an RTN neuron to graded hypercapnia in an anesthetized rat (end-expiratory CO2 shown in top trace) [adapted, with permission, from (401)]. (D) Example of one RTN neuron excited by brief hypoxia (carotid body stimulation, bottom trace) and by hypercapnia (end-expiratory CO2 in green). After i.c.v. administration of the broad spectrum glutamatergic blocker kynurenic acid, the cell no longer responds to hypoxia but its response to hypercapnia is unaffected [adapted, with permission, from (300)]. (E) RTN CO2-activated neuron labeled juxtacellularly with biotinamide in vivo (green fluorescence) has a Phox2b-ir nucleus [adapted, with permission, from (401)]. (F) Structure of an RTN neuron whose cell body was located at the ventral surface of the medulla oblongata (in vivo recording, juxtacellular labeling with biotinamide, and transverse plane projection). Note the extensive dendrites on the ventral surface [adapted, with permission, from (300)]. (G1) Two intracellularly labeled RTN neurons recorded in a Phox2b-eGFP transgenic mouse coronal slice (green eGFP, red biotinamide). (G2) Representative acid sensitivity of one such neuron recorded at room temperature (cell attached recording, integrated rate histogram, 10 s bin) [adapted, with permission, from (231)]. (H1) RTN Phox2b+ neuron acutely isolated from a Phox2b-eGFP mouse. (H2) Acid sensitivity of such an acutely isolated RTN neuron. [adapted, with permission, from (446)]. Note that the cell responds to a change in pH, not to CO2 per se. I1, selective expression of TASK-2 potassium channels by Phox2b+ RTN neurons in mice [adapted from Gestreau et al. (137) with permission]. Left panel shows TASK-2 expression monitored with LacZ reaction product. Middle panel shows that TASK-2 expressing neurons are eliminated in a mouse line in which RTN neurons express the 27-ala Phox2b mutation and fail to develop (Task2+/−; Phox2b27Ala/+). Right panel: Task-2 expressing neurons from a Task2+/− mouse (ventral surface view) showing the superficial location in perfect register with the RTN. I2, reduced acid sensitivity of RTN neurons in Task-2 knock-out mice compared to Task-2+/− control mice [adapted, with permission, from (445)].
An interesting attempt was been made by Su et al. (409) to try and resolve the apparent paradox between the typically modest acid sensitivity of isolated neurons and the exquisite sensitivity of the respiratory network in vivo to CO2. In primary cultures of embryonic rat brainstem (pons and medulla oblongata), these investigators found that 20% of the neurons were CO2 responsive. This subset of neurons responded linearly to changes in PCO2 by doubling their resting firing rate between 38 and 70 Torr and halving their resting rate between 38 and 20 Torr, with some neurons responding detectably (+30% of baseline) to as low as 1 Torr change in CO2. Using channel blockers, albeit of relatively modest selectivity, the authors argued that KIR closure might have mediated the most sensitive effects of pH whereas TASK channel closure may have been responsible for the responses to more substantial levels of acidification. Of particular interest, neuronal sensitivity to CO2 was reduced by blocking glutamate and selected serotonergic receptors. Although a culture of embryonic neurons is unlikely to reassemble spontaneously into an anatomically correct circuit, the study provided proof of principle that a high CO2 sensitivity could be an emergent property of a brainstem network.
In summary, CO2 sensitivity is a fairly common property of neurons in vitro, fueling the speculation that CRCs are widespread. The effects of CO2 are often surprisingly small and frequently counter to expectations (e.g., decrease in amplitude of respiratory motor outflows in slices or en bloc preparations). In most instances, without knowing the connections of the acid-sensitive neurons identified in vitro, nor whether they retain their acid sensitivity in vivo, their contribution to the respiratory chemoreflex remains uncertain.
Hypercapnia produces widespread activation of Fos in the brain of rodents
A rapid rise of the proto-oncogene cFos in neurons denotes an increase in protein synthesis and is typically interpreted as resulting from a sustained increase in neuronal discharge rate (359). In reality, all cells express Fos and even astrocytes can express levels of Fos that are detectable by immunohistochemistry (151, 244, 408, 433).
Fos immunohistochemistry does not permit identification of neurons that are inhibited during a given behavior. In addition, many neurons do not express Fos when activated. This is the case of all respiratory MNs and of most neurons of the ventral respiratory group whose massive activation during hypercapnia is not in any doubt. Finally, neurons that express Fos in animals exposed to hypercapnia are not necessarily CRCs (i.e., directly activated by CO2). They could be synaptically activated by the latter or by any component of the respiratory network. They could also be activated because of the wake-promoting effect of hypercapnia (202, 338). Fos activation by CNS neurons could also result from the activation of the carotid bodies. In short, like pH sensitivity in vitro, Fos activation by hypercapnia is neither necessary nor sufficient to identify CRCs and the results must be cautiously interpreted.
In rats, hypercapnia activates Fos in multiple regions of the medulla oblongata, pons, midbrain and hypothalamus (23, 35, 171, 325, 365, 430). Interestingly, in the lower brainstem of cats, Fos activation by hypercapnia is reportedly confined to the RTN (427). This discrepancy could be technical, for example, a difference in the sensitivity of the Fos detection method, or it could be related to differences in the duration and intensity of the stimulus. Hypercapnia may also produce species-specific behavioral effects in addition to an increase in ventilation. For example, low concentrations of CO2 are detected by the olfactory system of rodents (182). Cats may be unable to detect CO2 concentrations below 30% via the sense of smell, as is the case in humans. In rats, Fos activation by hypercapnia persists in putative RTN neurons when the animals are treated with morphine, hence behaviorally sedated and deprived of a significant hypercapnic ventilatory stimulation (365). These results can be viewed as the first suggestion that RTN neurons might be directly activated by CO2 in vivo.
In the rat (conscious or anesthetized), long-term hypercapnia (3 h) results in Fos expression in both neurons and other cell types (325). Okada et al. (325) found most of the Fos-positive cells in the immediate vicinity of the basilar artery and in a region just lateral to the edge of the pyramids, with additional clusters near penetrating arterioles. Most of these Fos-positive nuclei were associated with superficial blood vessels and, often, with regions of the medulla oblongata that are rich in serotonergic cells (raphe pallidus and the parapyramidal region) although the authors did not test whether the Fos-positive cells were serotonergic. A scattering of Fos-positive superficial cells were also identified over the RTN region. RTN neurons (Phox2b-positive, tyrosine-hydroxylase-negative) do express Fos in rats exposed to hypercapnia but even a strong chemoreceptor stimulus (10% FiCO2 in anesthetized ventilated rats) fails to produce Fos expression in every cell (125). Using an arterially perfused preparation of young rat, Okada et al. (325) found a population of superficial cells that retained the ability to express Fos following hypercapnia under synaptic blockade (TTX, high magnesium). These putative chemoreceptors had smaller than average nuclei and were remarkably numerous. Unfortunately, these cells were not characterized. They could conceivably have been astrocytes, microglia, pericytes, or other types of vascular cells.
The Retrotrapezoid Nucleus
From Mitchell’s “area M” to Feldman’s retrotrapezoid nucleus (1958–2003)
The early research on central chemoreceptors has been reviewed by Severinghaus (374). Very briefly, the first experiments suggesting that chemoreceptors were located in the lower brainstem are usually attributed to Loeschcke and colleagues (251). These experimenters acidified the fourth ventricle CSF of anesthetized cats, which produced an increase in breathing. More detailed experiments conducted in the early 1960s by Mitchell’s group suggested that the CRCs were located at the ventral surface of the medulla oblongata (288). One of the responsive regions was identified immediately caudal to the trapezoid bodies and called area M (288). These experiments consisted of applying acid-soaked pled-gets to various superficial regions of the medullary oblongata of anesthetized cats and, if breathing stimulation was elicited, chemoreceptors were assumed to reside in the immediately underlying tissue. Later experiments in rats confirmed that respiratory stimulation could also be produced by superfusing CO2 enriched solution on the ventral but not dorsal surface of the medulla oblongata (176). These methods had limited anatomical resolution and the interpretation of the results, namely, that chemoreceptors were confined to the ventral medullary surface, was quickly criticized because the pled-gets could have acidified deeper medullary structures which receive their blood supply from the ventral surface via penetrating arterioles and subsequent investigators were unable to find CO2-activated units within the superficial regions out-lined by Mitchell and collaborators (247). Other putatively chemosensitive superficial regions of the medulla oblongata were described by Loeschke and Schlaefke (368); the most frequently mentioned is located between the pyramidal tract and the rootlets of the hypoglossal nerve [(368) and references therein]. Although, the brainstem respiratory network was already the subject of sophisticated electrophysiological explorations in the 1970s, medullary regions located rostral to the area postrema were usually not sampled and the next two decades saw little progress in identifying the chemoreceptors whose existence was postulated by Mitchell and collaborators.
While searching for inputs to the ventral respiratory group with conventional labeling methods, Smith et al. (385) noted the presence of retrogradely labeled neurons between the facial motor nucleus and the ventral medullary surface. They called this cell group the RTN because its rostral end extends up to the trapezoid body. They speculated that these neurons could play a role in respiratory chemosensitivity because their location seemed roughly in register with area M (288, 315). A sparse collection of neurons with axonal projections to the VRC was soon after identified under the facial motor nucleus of rats, also with anatomical techniques (107). The same laboratory also found that the RTN of the cat contained respiratory-modulated or respiratory phasic units with projections to the ventrolateral respiratory group (60). A few of the recorded units were shown to be cell bodies as opposed to axons of passage because they were excited by iontophoretic application of a glutamate analog (60). Microstimulation of the RTN produced premature onset of inspiration when delivered during late expiration (60), suggesting that RTN neurons control the respiratory rhythm. This effect was reproduced more recently by selective optogenetic stimulation of the Phox2b-expressing neurons of the RTN in the rat (4).
Nattie and colleagues (318, 319) injected the powerful glutamate receptor agonist kainic acid into various rostral and superficial regions of the ventral medullary surface of the cat to initially activate and then destroy local neurons (172). This method produced BP and respiratory effects consistent with the existence of two partially segregated neuronal clusters, one located caudal to the facial motor nucleus and involved primarily in BP control [most likely the RVLM highlighted previously by Reis et al. (356, 357)] and a more rostral region, corresponding to the RTN described by Smith et al.(385). These experiments suggested that, under anesthesia, RTN regulates both the resting level of breathing and the stimulation of breathing by CO2 (318, 319). Later experiments in rats supported the same interpretation, namely that a group of neurons located under or close to the facial motor nucleus drives resting breathing and mediates a portion of the HCVR (313, 314). Unilateral injections of glutamate receptor antagonists into this region of the cat’s brain also reduced breathing and its activation by CO2 (315) suggesting that the neuronal network responsible for these respiratory effects is tonically activated by glutamatergic inputs. In anesthetized rats, the presumed RTN chemoreceptors do not appear to be tonically driven by glutamate receptors (300). The respiratory depressant effects caused by injecting glutamate receptor antagonists into the cat RTN could also have been caused by interrupting the on-going glutamatergic drive contributed by the chemoreceptors to other rostral medullary neurons implicated in breathing. Li and Nattie (239) also demonstrated that controlled acidification of the RTN using dialysis probes increases ventilation, providing evidence consistent with the involvement of the RTN region in respiratory chemosensitivity. This interpretation was also consistent with the above-mentioned evidence that rat and cat RTN neurons express c-Fos following exposure of conscious animals to high levels of CO2 [e.g., (365, 427)]. The regions referred to as RTN in rodents and cats are probably homologous but the fact has never been strictly demonstrated using biochemical markers.
In summary, experiments performed up to 2003 suggested that a region located under and or near the facial motor nucleus of rats and cats contains a network of active neurons that innervate the ventral and the DRGs, contribute to breathing automaticity and participate to the stimulation of breathing by CO2.
Cellular studies of the retrotrapezoid nucleus, characterization of the putative chemoreceptors
Detailed cellular studies of the region of the reticular formation located under the facial motor nucleus started in 2004 when many of the superficial neurons described by Ellenberger and Feldman (107) were shown to be nonserotonergic, to contain VGLUT2 mRNA and to be activated by CO2 in vivo and in vitro (300, 456) (Fig. 5C and D). The next major step was the finding that the CO2-sensitive glutamatergic cells of the parafacial region express transcription factor Phox2b (401) (Fig. 5B and E). This observation provided an additional histological marker to identify the putative chemoreceptors in rodents and, later on, in man (358). It also provided the means to identify the putative chemoreceptors neurons in vitro using transgenic mice in which Phox2b drives eGFP (231, 445, 446) (Fig. 5G and H). The finding has also added translational and medical relevance because Phox2b mutations cause the congenital central hypoventilation syndrome (CCHS), a developmental disease whose cardinal neurological signs are sleep apnea and the loss of breathing stimulation by CO2 (17,452). Finally, the presence of Phox2b in RTN neurons has enabled us and others to take advantage of a known Phox2-responsive artificial promoter (PRSx8) (187, 253) to express various transgene products in RTN neurons using lentiviral vectors (5, 269) (Fig. 4C5).
The RTN neurons of the anesthetized rat are highly sensitive to CO2 in vivo (0.5 Hz or 1/20th of their dynamic range per 0.01 arterial pH change) (158, 300). As one would expect of neurons that drive the RPG rather than the reverse, their CO2 threshold is below that of the rest of the respiratory network (Fig. 5C) and they remain active even when the RPG is silenced by morphine (158). The latter result is consistent with the fact that Fos expression is still induced by hypercapnia in RTN neurons in conscious morphinized rats in which this stimulus does not increase breathing above the resting level (365). The brainstem contains countless types of CO2-activated neurons which contribute in various capacities to generate the respiratory and autonomic outflows. By itself, the fact that neurons respond to CO2 in vivo provides only very weak evidence that they might be directly chemosensitive. RTN neurons are unusual in that their response to CO2 in vivo is virtually unaffected by pharmacological blockade of glutamatergic transmission, a condition that silences the RPG and eliminates the polysynaptic input from the carotid bodies to RTN neurons (300) (Fig. 5D). This evidence suggested that, in vivo, RTN neurons could be directly CO2-sensitive or could owe their CO2 response to a local (paracrine) effect of pH rather than from synaptic inputs. In keeping with this interpretation, RTN neurons are activated by CO2 and acidification in coronal slices of neonate rodent brain (Fig. 5G2) even when synaptic activity is reduced by addition of blockers of glutamatergic, glycinergic, and GABAergic ionotropic transmission plus PPADS, a blocker of ATP receptors (231, 300, 355) or by incubation in low calcium/high magnesium solution (328). Finally, these cells also retain their CO2 sensitivity after acute isolation (446) (Fig. 5H). In such experimental models, the effect of CO2 seems mediated primarily by changes in pH because increasing PCO2 is ineffective if pH is maintained constant by the appropriate addition of bicarbonate (300) (Fig. 5H). In vitro, the pH response of RTN neurons is temperature-dependent and at near physiological temperature (30°C), is about 40% of that observed in vivo in adult rats (158). In voltage clamp (−70 mV) and in the presence of tetrodotoxin, acidification causes an inward current consistent with closure of a resting potassium conductance (300). A large portion of this potassium current is mediated by TASK-2 channels (Fig. 5I) as described in another section (445). The acid sensitivity of RTN neurons in slices or after acute dissociation is substantially less than in vivo although the comparison is no trivial matter. Indeed, in vivo sensitivity is expressed as change in firing rate per arterial pH and concerns adult neurons whereas, for in vitro experiments, the norm is neonate neurons incubated at room temperature and chemosensitivity is expressed as changes in firing rate per extracellular pH. A given change in PaCO2 is thought to cause a greater change in brain extracellular pH than in plasma pH because the brain extracellular fluid is protein free and, therefore, has less buffering capacity than the plasma. If correct, this fact would reduce the discrepancy between the pH sensitivity observed in vivo (relative to arterial pH) versus in vitro (relative to extracellular pH). However, even after applying a sensible correction for temperature and the presumed relative buffering capacity of the CSF versus the plasma, the CO2 sensitivity of RTN neurons is still less than in vivo by at least twofold. One potential explanation is that the intrinsic response of mature RTN neurons to acid could be greater. Alternately, the contribution of the surrounding chemosensitive astrocytes to the RTN neuron response to CO2 could be larger in vivo than in vitro (146, 454). Consistent with these speculations, the HCVR is weaker during the postnatal period than in adults (74) although this fact could have many other explanations besides a change in the sensitivity of the pH sensors. The larger pH response of RTN neurons in vivo versus in vitro could also be due to the fact that these cells receive excitatory inputs from additional respiratory chemoreceptors. For example, RTN neurons are activated by stimulation of peripheral chemoreceptors in both cats (43,44) and rats (421) (Fig. 5D). RTN neurons also receive monosynaptic excitatory inputs from other putative CRCs such as serotonergic and orexinergic neurons (232, 299, 449, 458).
The location of the RTN neurons, the structure of their cell bodies and the type of mRNA or enzyme that they express are now known in some detail. The first studies were conducted by electroporating biotinamide into single neurons recorded extracellularly in vivo using Pinault’s method (339). The CO2-activated RTN neurons of the rat were thus shown to have extensive superficial dendrites (up to a mm in length) within the marginal layer (Fig. 5F), a 50-micron-thick sliver of myelin free neuropil located at the ventral surface of the medulla oblongata below the spinocerebellar tract (158,300). These dendrites must be easily accessible to fluids applied to the ventral medullary surface, which may explain why this region of the brain responds to topical acidification. By combining the detection of biotinamide with immunohistochemistry or with situ hybridization (ISH), CO2-activated RTN neurons were shown to express the homeodomain transcription factor Phox2b (Fig. 5E) and VGLUT2 mRNA but neither tryptophan-hydroxylase nor tyrosine-hydroxylase. There are close to 2000 such RTN neurons in the rat (1000 per side) and around 400 per side in mice (231, 423). By ISH, around 50% of rat RTN neurons also express preprogalanin mRNA (404) and, by single cell PCR, a similar proportion of RTN neurons expressed this transcript in a Phox2b-EGFP transgenic mouse (231). Small and very superficial galaninergic neurons are also depicted in the Allen brain atlas (234) within the RTN region (e.g., preprogalanin ISH, P56 mouse, coronal section, and image 92). Ultrastructural studies of RTN neurons carried out using a Phox2b-EGFP mouse (231) revealed that the dendrites of these neurons are covered with presumptive inhibitory (symmetric) and glutamatergic synapses (asymmetric) (231). This finding indicates that RTN neurons are not mere pH detectors but are subject to highly complex synaptic regulations. An unusual aspect of RTN neurons is that their plasma membrane is frequently in direct contact with what appears to be pericytes, which could suggest the existence of direct interactions with the microvasculature (231). Like all brain neurons, RTN neurons are enwrapped by astrocytic processes (231). In this case, the relationship may be of special functional significance because a subset of astrocytes located at the ventral medullary surface are depolarized by acidification and may contribute to the CO2-sensitivity of RTN neurons by releasing ATP or other paracrine signals (146, 302, 303, 453). In the neonate, the somata of the CO2-sensitive neurons reside in close proximity of blood vessels (329). Close apposition to blood vessels could be viewed as favorable for these cells to detect arterial PCO2 and, potentially, other circulating factors. The soma of RTN neurons is occasionally pierced by a capillary although a glial sheath always separates the neuronal membrane from the basement membrane of the blood vessel (231). The proximity of serotonergic neurons to blood vessels has also contributed to the theory that these cells detect arterial PCO2 (375). In all cases, RTN included, the argument is less than persuasive. First, there is no control, that is, no evidence that such cells are statistically closer to capillaries than any other brain neuron. Secondly, the C1 catecholaminergic neurons of the ventrolateral medulla also have extensive superficial dendrites in the RTN region and they are also occasionally pierced by capillaries (286). Yet, these cells do not respond to CO2 in slices (231).
RTN gain of function: Effects on breathing
By the late 1990s, it was clear that the vicinity of the facial motor nucleus contained a network of neurons that activate breathing. Recent optogenetic experiments have shown that the Phox2b-positive population of RTN neurons is an essential component of this network. RTN neurons express high levels of Phox2b (401); Phox2b also binds the artificial promoter PRSX8 (187). Abbott et al. (5) injected PRSx8-ChR2-mCherry lentiviral vector in the RTN region and were able to achieve a substantial degree of selectivity of ChR2 expression by Phox2b-expressing neurons (60%–70%), the balance consisting largely of C1 catecholaminergic neurons plus a few cholinergic cells. Neither GABAergic nor glycinergic neurons nor nearby facial motor neurons expressed detectable levels of ChR2 despite their immediate proximity to RTN neurons (5). Photostimulation of this population of ChR2-expressing neurons in anesthetized rats produced strong respiratory activation that was monitored by recording the mass discharge of the phrenic nerve PND (5). In a subset of rats, the lentiviral vector was injected into the RTN after treating the animals with a saporin-based toxin that selectively reduced the number of C1 neurons that reside within the RTN region (5). In these animals, >90% of the ChR2-mCherry-expressing cells were RTN neurons. Photostimulation of these ChR2-positive neurons increased PND to a degree comparable to that observed in the rats in which 30% to 40% of the ChR2-expressing neurons were C1 cells, showing that selective activation of RTN neurons is capable of vigorously increasing breathing under anesthesia. Similar experiments were later conducted in conscious rats (4) (Fig. 4C). Unilateral photostimulation of ChR2-expressing RTN neurons at 20 Hz (around 35% of the total population) increased VE to the same degree as normoxic hypercapnia with 8% CO2 and precisely reproduced the changes in respiratory rate and tidal volume elicited by this degree of hypercapnia (compare Fig. 4A and C). Breathing could also be paced by applying the light in short bursts, each burst producing a stereotyped sequence of forced expiration followed by enhanced inspiration (4). These experiments suggested that the CO2 responsive glutamatergic Phox2b-positive neurons (RTN neurons) activate all aspects of breathing including rate, inspiratory activity, and active (abdominal) expiration in both anesthetized and conscious rats. This interpretation is consistent with the loss of function experiments described below.
RTN loss of function: Effects on breathing and the hypercapnic ventilatory response
Acute nonselective inhibition of RTN neurons
The effects produced in an anesthetized animal by an acute nonselective lesion of RTN neurons with kainic acid have been described in a prior section (318, 319). More recently, Takakura et al. (422) injected the GABA-mimetic agent muscimol bilaterally into the RTN region of conscious rats, presumably causing a generalized inhibition of all the neurons located near the sites of injection. Results similar to those of Nattie et al. were obtained, namely, a severe reduction of baseline ventilation accompanied by an equally massive reduction of the HCVR (422). These pharmacological results established that the parafacial reticular formation contains neurons that contribute both to resting breathing and to the HCVR in conscious, anesthetized, or decerebrate mammals. The neurons responsible for the effects of the injected drugs could not be identified by such experiments. The fact that kainic acid or muscimol injection into the parafacial reticular formation reduced baseline breathing to about the same extent as they reduced the HCVR makes it difficult to determine whether this region of the brain contains CRCs, neurons that regulate the excitability of the central respiratory pattern generator or whether the drugs directly depressed the respiratory rhythm and pattern generator.
Chronic lesions of RTN neurons
Saporin conjugated to a substance P analog (SSP-saporin) has been used to destroy RTN and other parafacial neurons (316,423). Receptor internalization following agonist binding (266) translocates the ribosomal inactivating protein saporin into the cytoplasm of the cells that express NK1 receptors eventually killing them by inhibiting protein synthesis. Nattie and Li’s experiments were done before RTN neurons could be identified histologically. They examined the effects of the toxin on the HCVR of conscious rats and gauged the destructive effect of the toxin by the loss of highly NK1R-ir processes, presumably dendritic, located in the parafacial region. Later evidence revealed that most of these intensely immunoreactive processes do not belong to RTN neurons which only express a low and hard to detect NK1R immunoreactivity (299). The unilateral lesions produced by Nattie and Li had virtually no effect on breathing whereas bilateral lesions reduced baseline ventilation and the HCVR, albeit modestly (316). Takakura et al. (423) examined the rats with SSP-SAP lesions under anesthesia and were able to relate the observed respiratory deficits to the proportion of RTN neurons destroyed by the toxin by using Phox2b as a marker of these cells. Chronic bilateral lesions of RTN neurons exceeding 70% of the population produced rats that had extremely elevated apneic thresholds under anesthesia (concentration of inhaled CO2 required to elicit a PND, 8% compared to 4.5% in controls) (423). Smaller lesions produced virtually no effect. Except for the high apneic threshold, the rhythm and pattern of the phrenic outflow were normal suggesting that the network responsible for rhythm and pattern generator was largely unaffected. These two sets of experiments concur with the notion that the RTN contains neurons that are equally important for baseline breathing as for its stimulation by CO2. Both studies show that SSP-SAP is not sufficiently selective to exclusively lesion the Phox2b-positive putative RTN chemoreceptors. The Takakura study (423) suggested that the breathing deficit could have been directly related to the degree of loss of the RTN neurons but the contribution of additional neurons could not be ruled out. Finally, these studies showed that even massive (70%) RTN lesions are survivable and cause limited deficits in the conscious state. Such results can be interpreted in two ways. One is that RTN makes a relatively modest contribution to breathing and the HCVR, the other that plasticity of surviving RTN neurons and countervailing regulations compensate for the loss of a majority of these neurons.
Genetic deletion of RTN neurons
Mouse genetics studies have contributed critical evidence that the Phox2b-expressing RTN neurons are required for quiet automatic breathing and respiratory chemoreception (144). Using intersectional genetics, RTN development was prevented or aborted with varying degree of selectivity culminating with a mouse in which RTN appears to have been selectively deleted (346). This animal survived, had a complete absence of HCVR for three weeks after birth and recovered about a third of this response as adult (Fig. 3E1-E4). These models are examined in greater detail in a later section because of their relevance to CCHS.
Acute pharmacogenetic inhibition of RTN neurons
Reversible and relatively selective pharmacogenetic inhibition of RTN neurons has been accomplished using the allato-statin receptor method designed by Callaway et al. (425). The receptor was introduced into RTN neurons using the PRSx8-lentivirus approach first used by Abbott et al. (5,269). Allato-statin produced a 60% average reduction in HCVR without change in baseline ventilation in these experiments (269) (Fig. 4D). The lack of effect of allatostatin on baseline ventilation could suggest that the RTN is specifically involved in the HCVR and has little to do with resting breathing. However, this interpretation is at odds with the fact that injection or dialysis of muscimol into the RTN of conscious rats reduces baseline ventilation and the HCVR to a similar extent (310, 422). The discrepancy between these pharmacogenetic experiments and more conventional approaches used previously is unresolved because neither method targets exclusively (muscimol) or completely (lentivirus) the RTN neurons. In addition, the cell selectivity of the lentiviral vector used by Marina et al. (269) is the same as that used by Abbott et al.(5) and, as shown by the latter, this vector targets the nearby C1 cells, which also control breathing (1, 2, 5). Finally, such vectors typically transfect at most half of the RTN neurons. Inhibiting a fraction of the RTN neurons may cause a small degree of hypercapnia at rest that quickly restores diaphragmatic activity to control levels by activating the carotid bodies and the untransfected RTN neurons.
In summary opto- and pharmacogenetic experiments based on the use of lentiviral vectors that express their transgene under the control of the PRSx8 promoter demonstrated that RTN neurons stimulate breathing and strongly suggested that these neurons mediate a large portion of the chemoreflex. The contribution of the C1 neurons to the respiratory effects observed in these experiments generally remains to be determined.
RTN regulates every aspect of breathing
The Phox2b-expressing RTN neurons regulate the breathing rate even prenatally in mice (431). They undoubtedly do so immediately after birth (326, 327) and their ability to regulate the respiratory rate remains prominent during both quiet waking and non-REM sleep (1). During REM sleep and while the animal is awake and behaviorally engaged, RTN stimulation produces a negligible effect on the breathing rate (1). These observations suggest that RTN could be a key component of the circuit that generates automatic (unconscious) breathing movements and that this nucleus or the respiratory rhythm generator may be disengaged when the respiratory centers are recruited for purposes other than quiet breathing such as whisking and sniffing, or during REM sleep. The ability of RTN to increase inspiratory amplitude is also strongly suggested by optogenetic studies in which the diaphragm EMG or the activity of the phrenic nerve have been recorded (5). Finally, there is also excellent evidence that RTN neurons are capable of facilitating active expiration (4, 269).
How RTN regulates these various aspects of breathing is uncertain. The axonal projections of RTN neurons have been traced most comprehensively using the PRSx8- lentivirus-vector approach (5) following selective lesions of the C1 cells or by tracking the projections of the subset of RTN neurons that express galanin (42). These two approaches produced indistinguishable results. Information on the axonal projections of RTN neurons also derives from antidromic mapping experiments in cats and rats (43,300). Collectively, RTN neurons innervate the entire length of the VRC and dorsolateral pontine nuclei implicated in respiratory control (Kölliker-Fuse, intertrigeminal region, and lateral parabrachial nucleus). The only additional targets of RTN neurons are commissural and caudolateral regions of the nucleus of the solitary tract, regions which receive afferents from the carotid bodies and other cardiopulmonary organs (5, 43, 222). From this evidence, it appears that RTN neurons could directly excite the rhythmogenic neurons of the pre-Bötzinger complex and that they could exert synergistic effects with carotid body input in several places (NTS, parabrachial region, and ventrolateral medulla). Under anesthesia, RTN neurons are variously modulated by the breathing network (44, 158) but tonic optogenetic activation of RTN neurons at 10 to 20 Hz in conscious or anesthetized rats decreases the duration of the respiratory cycle without disrupting it (4, 5). The discharge of RTN neurons, therefore, need not be phasic to increase the breathing rate. However, when RTN neurons are phasically activated, also optogenetically, the stimulus “settles” during the late expiratory phase which is also the period of the cycle during which the rhythmogenic cells of the pre-Bötzinger complex begin their depolarization (4). RTN neurons may therefore increase fR by accelerating the late-expiratory depolarization phase of the pre-Bötzinger rhythmogenic core (116). RTN neurons acquire a stronger respiratory modulation under conditions of high CO2 and under certain experimental conditions some of these neurons develop a preinspiratory discharge (158, 326, 327). This preinspiratory discharge could conceivably enhance the depolarization of the rhythm generating neurons of the pre-Bötzinger complex during the period of the cycle that is most critical for the timing of the inspiratory burst. In adult anesthetized rats exposed to very high levels of CO2, RTN neurons exhibit several types of multi-phasic phase-spanning respiratory patterns (158). This multiplicity of patterns suggests that separate classes of RTN chemoreceptors may exist. Such cells could conceivably be specialized in regulating breathing rate versus the amplitude of various inspiratory or expiratory outflows. The parafacial region contains cells that discharge selectively during late expiration in unanesthetized preparations when hypercapnia triggers a late expiratory discharge in abdominal motor neurons (7). These neurons have not yet been identified as Phox2b RTN neurons. They could conceivably be facial MNs or belong to the anatomically undefined parafacial expiratory oscillator (330).
Contrary to other putative CRCs (orexin, locus coeruleus, and serotonergic neurons) RTN neurons do not innervate the spinal cord nor the raphe (5, 42). The effects of RTN neurons on inspiratory and expiratory amplitude are therefore presumably exerted at the level of premotor neurons or on neurons antecedent to the latter (e.g., the rhythmogenic pre-Bötzinger core). The effects of RTN on inspiratory amplitude could be mediated by their projections to the rVRG (5, 42, 107, 385). RTN neurons also undoubtedly drive or facilitate abdominal (active) expiration (4, 269). The hypothesis that RTN neurons selectively drive expiratory muscles has been proposed (191, 269, 460). This view partly comes from experiments performed in an arterially perfused midcollicular transected preparation in which the cardiorespiratory efferent pattern (respiratory and autonomic) under normal levels of PCO2 resembles that of a maximally exercising animal or an animal exposed to extremely high levels of hypercapnia (7, 290). In such a preparation the inspiratory drive seems to be saturated under normocapnia and, as expected, is the last to yield to both hypocapnia and RTN inhibition. The respiratory activity of abdominal muscles is much less sensitive to CO2 and, accordingly, is more sensitive to both hypocapnia and RTN neuron inhibition than the inspiratory outflow. The fact that in neonates, a subset of RTN neurons burst during late expiration (326) has also been interpreted by some investigators as evidence that such cells drive expiration selectively (460). As discussed above, this late expiratory discharge could actually be viewed as a particularly efficient way to decrease the cycle period of the inspiratory rhythmogenic neurons of the pre-Bötzinger complex. RTN neurons may drive expiratory motor neurons by activating the network currently referred to as the parafacial oscillator, a group of neurons located within the reticular formation, lateral, and caudal to RTN (191,330). Pagliardini et al. (330) used a lentivirus to introduce ChR2 nonselectively into these neurons with a synapsin promoter. By activating this mixed population of neurons, these authors were able to elicit increases in abdominal EMG indicative of active expiration. They tentatively attributed these effects to the activation of neurons other than RTN because the majority of the ChR2-expressing neurons did not express Phox2b. A third possibility, supported only by light microscopic anatomical evidence, is that RTN neurons drive the expiratory pre-motor neurons located in the cVRG (5, 19, 21, 28, 42).
Conclusions and remaining issues
RTN neurons respond vigorously to hypercapnia in vivo and in vitro and have the correct connections to influence the respiratory pattern generator. In fact, RTN neurons are the only known group of putative central chemoreceptors that exclusively target the pontomedullary regions that harbor the breathing rhythm and pattern generating network. Selective activation of the RTN produces large increases in breathing under all experimental conditions tested including in conscious animals and inhibition of these neurons always attenuates the HCVR. RTN neurons are intrinsically activated by acidification via closure of a resting potassium conductance largely mediated by TASK-2 channels, a type of two-pore potassium channel expressed by very few CNS neurons other than RTN (137, 445). The CO2 sensitivity of RTN neurons is also partially dependent on the release of ATP, presumably from surrounding astrocytes as detailed later. In sum, RTN neurons provide a CO2-regulated excitatory drive to the respiratory system and undoubtedly function as CRCs.
The fraction of the central chemoreflex that is relayed through RTN neurons is undetermined. All that can be said at this time is that RTN neurons mediate at least 60% of the HCVR (269). The real percentage is certainly larger. Chronic lesions in adult animals or genetic manipulations always trigger countervailing changes in brain function, therefore, such experimental procedures have not definitively tested the quantitative importance of RTN neurons to respiratory regulation in the intact brain. Existing vector-based pharmaco- or opto-genetics experiments have not provided a definitive answer because it has not been possible to express any transgene product (ChR2, allatostatin) in all or even a majority of RTN neurons and currently published experiments have not targeted these putative chemoreceptors with complete specificity (2). Acute injections of nonspecific drugs like muscimol into the RTN region lack the required selectivity because they could reach the dendrites of neurons located at least a millimeter away from the injection site. It seems increasingly probable that several other types of neurons reside in close vicinity to the RTN, particularly at the lateral, caudal, and medial edge of the cluster of Phox2b-ir RTN neurons. These neurons are currently referred to as the parafacial respiratory group or oscillator (330). They represent a collection of neurons of still undetermined phenotype that form a network implicated in active expiration (191). Subsets of RTN neurons could be components of this oscillator or simply provide a CO2-dependent drive to the oscillator (330).
Role of Serotonergic Neurons in Central Respiratory Chemoreflexes
Loss and gain of function experiments support a contribution of serotonergic neurons to the regulation of breathing and the hypercapnic ventilatory response
Subsets of serotonergic neurons clearly activate breathing. One of the most direct evidence is that selective optogenetic stimulation of raphe obscurus serotonergic neurons increases breathing frequency and amplitude in anesthetized or conscious mice (86, 155) (Fig. 6A–C). Although serotonin exerts both inhibitory and excitatory effects at the cellular level via countless receptors, this optogenetic evidence shows that the overall effect produced by increasing serotonin release from raphe obscurus neurons is a robust activation of breathing, even in the conscious state. This effect is presumably mediated partly by direct projections of raphe obscurus serotonergic neurons to the ventral respiratory column because in transverse slices, application of appropriate serotonin (5HT-2) agonists to the region of the pre-Bötzinger complex, or direct stimulation of the raphe obscurus increases the respiratory-like outflow of the rodent “breathing” slice (94, 337, 344) and serotonin receptor-2A blockade has the opposite effect in such a system (337) (Fig. 6G). However, medullary serotonergic nuclei also innervate every brainstem and spinal region of importance to respiratory control such as the dorsolateral pons, phrenic MNs, the dorsal vagal complex, and the RTN. Each of these regions presumably mediates a portion of the respiratory response elicited in conscious animals by activation of serotonergic neurons.
Figure 6.
Serotonergic neurons and breathing. (A) Selective expression of ChR2-mCherry fusion protein by raphe obscurus serotonergic neurons in an e-PET Cre mouse [adapted, with permission, after (86)]. (B) Photostimulation of ChR2-expressing raphe obscurus serotonergic neurons in such a mouse (ketamine/dexmedetomidine initial anesthesia with 0.3% to 0.6% isoflurane supplementation) increases diaphragm EMG amplitude (top trace) and respiratory rate (fR, second from top). Bottom traces shows a single raphe serotonergic unit being photoactivated every time the laser light was pulsed [adapted, with permission, after (86)]. (C) Selective optogenetic photostimulation of ChR2-expressing raphe obscurus serotonergic neurons in a conscious mouse (whole body plethysmography). The response has the same slow ON-slow OFF kinetics as in the anesthetized mouse [adapted, with permission, after (155)]. (D) Robust acid sensitivity of a serotonergic neuron in culture [reprinted from Richerson (353)] adapted with permission from Macmillan Publishers Ltd., Nature Neuroscience (5), 2004). (E1) In an arterially perfused rat preparation, serotonergic neurons are either modestly inhibited or modestly excited by CO2. (E2) In this preparation, hypercapnia had no effect on the mean activity of the serotonergic population at large (all 5-HT) [from Iceman et al. (188) with permission]. (F) Putative serotonergic neuron recorded in conscious cats showing a modest but dose-dependent increase in discharge rate (2.7 to 3.9 Hz; +44% at 6% FiCO2). The effect of CO2 essentially disappeared during non-REM sleep [reproduced from Veasey et al. (440) with permission]. (G) Serotonin2A receptor antagonism dramatically slows the pre-Bötzinger complex rhythm in a slice (top traces, mass activity of pre-Bötzinger complex neurons, bottom traces whole cell recording of a presumed rhythmogenic neuron) [reproduced from Peña and Ramirez (337) with permission]. (H1–2) Iontophoretic application of serotonin activates RTN neurons by a constant amount regardless of the level of end-expiratory CO2 [H1, representative example, H2 average results; adapted, with permission, from (299)]. (I) Selective global inhibition of CNS serotonergic neurons (DREADD methodology) attenuates the hypercapnic ventilatory reflex of a conscious mouse [after Ray et al. (349) reprinted with permission from AAAS]. (J) Interpretations: serotonergic neurons activate breathing via innumerable mechanisms (e.g., direct projection to RTN, the RPG, dorsolateral pons, motoneurons, and indirect effects via changes in vigilance). The integrity of serotonergic neurons is required for full expression of the hypercapnic ventilatory reflex, possibly because a fraction of serotonergic neuron is directly responsive to acidification in vivo.
The ability of the serotonergic system to activate breathing is also clearly supported by loss of function experiments. The HCVR is reduced by about 50% in mice in which the development of all serotonergic neurons has been aborted (174). These deficits could also conceivably be caused by cascading developmental abnormalities of neurons other than the serotonergic cells. However, acute inhibition of CNS serotonergic neurons using designer-receptor-exclusively activated-by-designer drugs (DREADDs; RC::PDi; Slc6a4-cre mice) also reduces the HCVR of conscious adults by about 50% (22,349) indicating that serotonergic neurons are required for the full expression of the chemoreflex (Fig. 6I). In the latter studies, breathing was assessed by whole body plethysmography. The VE reduction could also have been partially caused by muscle weakness since serotonin neurons innervate the spinal cord and facilitate the activity of skeletal motor neurons, including phrenic MNs (245,281) (Fig. 5J). Also, generalized inhibition of serotonergic neurons would have impaired the level of vigilance of the animals and could have reduced the arousal effect of CO2 which likely contributes to the HCVR (49, 228).
CO2-sensitivity of serotonergic neurons in vitro and in vivo
The midbrain dorsal raphe of the rat contains acid-activated cells in slices (16 out of a sample of 100; increase in discharge rate of around one Hz for a 0.2 pH change) many of which could have been serotonergic based on the regularity of their discharge (375). Supporting this view, 9 of 12 serotonergic neurons cultured from the midbrain raphe were activated to about the same degree (375). A majority of lower brainstem raphe serotonergic neurons recorded in slices or in culture responded in similar fashion to acidification in rats and mice (46, 349, 353, 449) (Fig. 6D).
The results obtained in vivo have not been nearly as consistent. In conscious cats, only 8 out of 36 dorsal raphe cells, presumed but not proven to be serotonergic, responded to a hypercarbic ventilatory challenge with increased firing rates that were roughly proportional to the fraction of inspired carbon dioxide (441). A similar fraction of nucleus raphe obscurus and pallidus putatively serotonergic neurons (6 of 27) responded to hypercarbia in conscious cats (440). Hypercarbia-responsive neurons typically had greatly reduced responses to CO2 during SWS (440) which could mean that the effect might have been a network effect rather than a direct effect of CO2/pH (Fig. 6F). In an arterially perfused preparation of juvenile rat, reputed to be a highly reactive model for cardiorespiratory studies, of 16 neurons identified histologically as serotonergic, 7 were judged to be activated by raising PaCO2 from 5% to 9% (188) (Fig. 6E1). However, the mean increase in rate for the activated neurons was only 0.3Hz (from 0.82 to 1.1 Hz). Furthermore, the remainder of the identified serotonergic cells were unaffected or slightly inhibited by CO2 such that CO2 had no significant effect overall on the discharge of the 16 identified serotonergic neurons recorded in that study (188) (Fig. 6E2). In anesthetized mice, the addition of 5% FiCO2 produced no effect overall on the spontaneous discharge of optogenetically identified raphe obscurus serotonergic neurons (86). As in the Iceman et al. study (188), the latter authors did find a few serotonergic neurons that were modestly stimulated by hypercapnia (by 10%–30% of baseline firing) but the distribution of responses to CO2 appeared Gaussian, therefore, CO2-activated and inhibited neurons were not separated for statistical purposes. Serotonergic neurons located in the parapyra-midal region tended to be slightly inhibited by a hypercapnic condition of 10% end-expiratory CO2 under conditions where the respiratory outflow was vigorously activated and RTN neurons firing rate changed from 0 to approximately 10 Hz (300). This observation was later reproduced by Takakura and Moreira (420) who also showed, using a different anesthetic (urethane and chloralose vs. 1% halothane) that these parapyramidal serotonergic neurons are inhibited by carotid body activation. These particular serotonergic neurons likely facilitate shivering and BAT thermogenesis, responses that are inhibited by hypoxia, which may explain the inhibitory polysynaptic input that these serotonergic cells receive from the carotid bodies (263).
The ionic mechanisms responsible for the effects of pH on serotonergic neurons have not been clarified. In slices of neonatal mouse brain, TASK channels appear to underlie the bulk of the pH sensitivity of dorsal raphe serotonergic neurons since pH-dependent current is all but eliminated in TASK KO mice (301). Because TASK channel knock-out does not change detectably the central respiratory chemoreflex, Mulkey et al. (301) argued that a direct effect of pH on serotonergic neurons was not required for the HCVR (301). This interpretation is still tentative because the in vitro evidence was obtained in neonatal mice and the HCVR was measured in adults.
Contribution of serotonin to the peripheral chemoreflex
Genetic deletion of serotonergic neurons produced no effect on the hypoxic ventilatory response of mice (174). Interestingly, acute inhibition of serotonergic neuronal activity with the DREADD approach prevented the arousal effect of hypercapnia but not the arousal response to hypoxia (8% O2) (49). As mentioned later, locus coeruleus lesions also selectively reduce the HCVR without impairing the hypoxic ventilatory reflex (38, 39). These intriguing results could mean that the effects of hypoxia on respiration and vigilance, unlike those of CO2, depend neither on the serotonergic system nor on the locus coeruleus. Another interpretation is that severe acute hypoxia is a much stronger arousal stimulus than normoxic hypercapnia. Accordingly, lesion of a single neuromodulator system (serotonergic neurons or locus coeruleus) might be sufficient to attenuate CO2-induced arousal but may be incapable of preventing arousal and the attending cardiorespiratory changes caused by hypoxia.
Serotonergic neurons as CRCs: Summary and remaining issues
Subsets of serotonergic neurons clearly activate breathing and enhance the HCVR. This conclusion is supported by abundant experimental evidence, much of which parallels the type of data that supports the role of RTN neurons in respiratory control. Evidence that a majority of serotonergic neurons are activated by hypercapnia in vitro is also apparent and supports the notion that these cells could be CRCs. Against this notion, in vivo evidence that serotonergic cells are activated by hypercapnia is surprisingly modest. A possible explanation is that only a small fraction of serotonergic neurons are CRCs. The rodent brain contains tens of thousands of serotonergic neurons with multiple developmental lineages, inputs, and probably diverse physiological roles (190, 195). A subset of yet to be located serotonergic neurons may be particularly responsive to the direct effect of CO2. Also, the extremely weak CO2 sensitivity of serotonergic cells observed in rodents in vivo, regardless of the presence or nature of the anesthesia (fraction of one Hz for a 4%–5% change in PaCO2) (86, 188, 300) could be because the pH sensing mechanism expressed by serotonergic cells, or by the surrounding glia, is saturated at rest in these preparations. Also, a diminutive increase in the discharge rate of the serotonergic neurons could conceivably produce a large activation of the HCVR. Finally, discharge synchrony among the raphe serotonergic neurons could hypothetically be more important functionally than their absolute firing rate. Unfortunately, none of the above speculations has the benefit of evidence. For example, optogenetic stimulation of raphe obscurus serotonergic neurons does elicit a robust increase in breathing in anesthetized and conscious mice in a frequency-dependent manner (Fig. 6B and C). Very weak if any effect was produced by stimulating below 2 to 5 Hz, although the stimulus was applied using a pulsed laser which synchronizes the action potentials of the activated neurons (86, 155).
The discrepancies highlighted above require considering other types of interpretations, besides or in addition to a central chemoreceptor function, to account for the powerful effect of serotonin on breathing, vigilance, thermoregulation, and the many other roles that have been attributed to these cells. The most tempting is that some level of serotonin is simply permissive for the HCVR. Three arguments support this interpretation. The HCVR deficit observed in mice with genetic deletion of serotonergic neurons can be fully reversed by intracerebral infusion of serotonin (174). This outcome seems contrary to the view that CO2 increases breathing by releasing serotonin because, if this were the case, serotonin administration should raise resting ventilation and then occlude the effect of a subsequent hypercapnia. Also, CO2 chemosensitivity is restored to normal in a mouse model of Rhett syndrome by the simple addition of citalopram, a serotonin-selective reuptake blocker that also causes a brain-wide increase in serotonin concentration (436). Finally, serotonin depolarizes RTN neurons, an action that is likely to enhance the HCVR (170, 299) (Fig. 6H). All these considerations hardly clarify our understanding of the actual function of the medullary raphe in breathing and the last word should probably be left to Jacobs (190) who thoroughly examined the discharge pattern of serotonergic neurons in conscious cats. According to this author, the most likely role of the medullary serotonergic system is a generalized facilitating effect on motor behaviors and their autonomic (i.e. cardiorespiratory) correlates. To quote: “The strongest relationship was between [serotonergic] neuronal activity and motor output, especially tonic and repetitive motor activity. We hypothesize that the primary functions of this motor-related activity are to facilitate motor output, suppress processing of some forms of afferent activity, and to coordinate autonomic functioning with the current motor demand” (190).
Locus Coeruleus Neurons as Central Respiratory Chemoreceptors
CO2 activates LC neurons in vivo and in vitro
Hypercapnia increases the discharge rate of LC neurons in anesthetized rats. Specifically, increasing PaCO2 from 35 (resting) to over 100 Torr increased the resting discharge rate of these neurons by about 90% (104). This effect persisted after severing baroreceptor and carotid body afferents and was therefore primarily of central origin. The effect of hypercapnia could have partly resulted from a direct of CO2 because hypercapnia also modestly activates LC neurons in slices (132, 340).
Lesions of the LC attenuate the hypercapnic ventilatory reflex
Partial lesions of lower brainstem catecholaminergic neurons were produced in rats by administering the noradrenergic neuron-selective toxin antidopamine-β-hydroxylase-saporin (237). These lesions reduced waking time, baseline ventilation and the HCVR during waking and non-REM sleep by about 28%. The lesions reduced the effect of CO2 on breathing frequency but had no effect on tidal volume. The toxin destroyed an estimated 65% to 85% of the LC, plus other brainstem noradrenergic and adrenergic neurons, therefore, the effects could not be attributed to any specific brainstem catecholaminergic cell group. More complete lesions of the LC were made by injecting 6-OHDA directly into this structure in rats (38). These lesions caused a larger reduction of the HCVR (~64%) that was entirely accounted for by a change in tidal volume [the opposite of (237)]. The neurotoxin 6-OHDA is only partially selective for catecholaminergic neuronal somata and destroys catecholaminergic axons of passage. Accordingly, such lesions could have destroyed pontine neurons other the LC and the axons of noradrenergic or dopaminergic cell groups other than the LC. Interestingly, 6-OHDA lesions of the LC, just like lesions of the serotonergic neurons, attenuated the hypercapnic ventilatory response without changing the ventilatory response to hypoxia (38, 39, 91). In both cases this selectivity was tentatively attributed to the fact that the neurons are bona fide CRCs that respond to CO2 but not to hypoxia. However, in anesthetized rats at least, LC neurons are activated to about the same degree by hypoxia as by hypercapnia (104), therefore, a selective effect of CO2 on LC neurons does not account particularly well for the selective effect of LC lesions on the HCVR relative to the hypoxic response. Other interpretations include a generalized decrease in vigilance caused by a reduction of noradrenaline release throughout the brain.
Summary and interpretations
The effects of LC lesions on cardiorespiration function cannot be properly interpreted without considering the role of these neurons from a broader perspective, namely, the effect of arousal on their activity and, in turn, their contribution to the waking state. LC neuron activity is highly state-dependent (23, 189). During conscious waking their activity increases with nociception, abnormalities of the internal milieu including but not limited to hypercapnia (e.g., hypoglycemia, hypotension, hypovolemia, and inflammation), attention and psychological stress (23, 189). As noted by Jacobs (189) “When any of these physiological challenges induce (or are accompanied by) an increase in the level of behavioral arousal, the resulting unit response is more dramatic, suggesting an additive effect.” In other words, some portion of the activating effect of CO2 on LC neurons may well be direct (the CRC hypothesis), but another portion is probably mediated by synaptic inputs, certainly from the C1 cells and, possibly, from the NTS (3, 25, 108, 160). The rest of the effect of hypercapnia is very likely the consequence of hypercapnia-induced arousal and the result of inputs to the LC that originate from the hypothalamus including the orexinergic system and other suprapontine structures (24, 181). LC neurons are not required for the basic alternation between the major states of vigilance (waking non-REM and REM sleep), processes that are largely defined by hypothalamic circuits (362). However, LC neurons “contribute to the initiation and maintenance of behavioral and forebrain neuronal activity states appropriate for the collection of sensory information (e.g., waking). Second, within the waking state, (the LC) modulates the collection and processing of salient sensory information through a diversity of concentration-dependent actions within cortical and subcortical sensory, attention, and memory circuits” (36). In other words, LC lesions likely reduce the salience of a hypercapnic stimulus hence the portion of the HCVR that is due to behavioral arousal which may well be substantial. Via their brainstem projections, LC neurons also probably produce some of the more elementary reflex components of the effect of hypercapnia. For example, LC neurons innervate the pre-Bötzinger complex and all MNs including those that innervate respiratory muscles. The effect of noradrenaline on MNs and on the pre-Bötzinger complex is α1-adrenoreceptor mediated and facilitatory (94,180,322). The loss of this modulatory input likely contributes to the reduced HCVR stimulation that has been observed following noradrenergic neuron lesions.
The Orexinergic System and Central Respiratory Chemoreception
Like the LC, the orexinergic neurons stimulate breathing and the circulation, their brain projections are extensive, they are selectively active during waking, orexinergic cell lesions attenuate the HCVR and some evidence suggests that they might be CRCs.
Three pieces of evidence argue that orexinergic neurons might be CRCs. First, these neurons are activated by CO2/acidification in slices via closure of a resting potassium conductance (458). This unidentified conductance is unaffected by eliminating TASK1 or TASK3 channels (143). Second, 28% of orexin neurons located in the perifornical area and dorsal hypothalamus express Fos after a 3-hour-long exposure to hypercapnia compared to 16% in control rats (416). A direct effect of pH on these cells in vivo might cause this relatively small change. It could also be an indirect consequence of CO2-induced arousal. Indeed orexin neurons express Fos during waking and sleep deprivation which are anticipated consequences of the several hour-long exposure to high CO2 levels required for Fos studies (114). Finally, microdialysis of CO2 enriched fluid (25% CO2) into the orexin neuron-rich perifornical region of the hypothalamus increases resting ventilation somewhat (“up to 15% of baseline”) but only when the rats were awake (241). The orexin cells are active during waking and silent during sleep (233); therefore, one might presume that they would be less excited by acidification during sleep regardless of how acid activates them. However these experiments do not demonstrate that the orexin cells were specifically responsible for the effects of acidification because this region of the hypothalamus contains several other types of neurons with state-dependent activity (169). In addition, acidification of the perifornical area increased resting ventilation by increasing breathing frequency whereas pharmacological blockade of orexin receptors decreases the hypercapnic ventilatory response by reducing both tidal volume and frequency (238, 241).
The theory that orexinergic neurons regulate cardiorespiratory outflows specifically during waking is firmly grounded in experimental evidence. This evidence is of three types. First, mice with genetic deletion of orexin or orexin neurons have normal baseline ventilation but reduced chemoreflexes and blood pressure reductions during their waking period only, a deficit that was remedied by orexin supplementation (226). Secondly, administration of orexin receptor antagonists to normal mice mimicked the genetic abnormality (226). Orexin receptor antagonists produced similar effects in rats (238, 312). Based on the locations of orex-inergic terminals and the widespread excitatory effects of orexin, orexinergic neurons presumably activate cardiorespiratory function via cumulative effects at many sites, including the ventral respiratory group, the spinal cord, the RTN, the locus coeruleus, the C1 cells, and raphe serotonergic neurons (48, 223, 228, 232, 238, 250, 360, 386, 387, 465). Orexin has powerful excitatory effects on RTN neurons and, based on injections of orexin antagonists in rats, 30% of the HCVR of awake rats may be mediated by the stimulatory effect of orexin on RTN neurons and another 15% may be mediated by activation of the medullary raphe (90, 232). Again two interpretations are possible. The first is that hypercapnia causes arousal by a variety of means (sensory feedback from chest and lungs, olfactory inputs, direct small effects on vast numbers of CNS neurons, and activation of the carotid bodies) and orexinergic neurons mediate around 30% of the “waking drive” on cardiorespiratory centers. The other is that orexin neurons contribute to the HCVR simply because they directly respond to CO2.
In summary, the orexinergic neurons facilitate the breathing and cardiovascular responses to CO2 during waking (228). However, orexin neurons also mediate the cardiorespiratory responses to other types of stresses (227). Hypercapnia may activate the orexinergic neurons via a direct effect of pH on these cells. The activation of these neurons by hypercapnia could also be the result of CO2-induced stress and arousal.
Other Hypothalamic Regions
The brainstem projections of the paraventricular nucleus of the hypothalamus are extensive (136, 255). These projections originate from the parvocellular subdivision of the nucleus where many neurons are oxytocinergic and putatively glutamatergic (397). Based on these projections, PVH may modulate a range of homeostatic functions, including cerebral and ocular blood flow, corneal and nasal hydration, ingestive behavior, sodium intake, and glucose metabolism, as well as cardiovascular, gastrointestinal, and respiratory activities [for references, see (136)]. Physiological studies have implicated these neurons in the regulation of blood volume and pressure and, more recently, in stress and appetite control (13, 26, 62, 398, 462). However, with few exceptions, the connectome of the PVH is largely deduced from light microscopic studies, that is, the nature of the cells directly contacted by these neurons is for the most part unknown. Lower brainstem catecholaminergic neurons and sympathetic pre-ganglionic neurons are a notable exception to this statement (136).
The evidence that implicates PVH in breathing control and, in particular, in the control of breathing by hypercapnia is weak. Although the PVH does project to the ventrolateral medullary reticular formation, this region is extremely heterogeneous and evidence of direct synaptic contact between PVN neurons and the neurons that make up the respiratory network is yet to be obtained (136). Activation of the PVH region with bicuculline or glutamate produced activation of phrenic and hypoglossal outflows in some studies (258,463). Such experimental procedures do not necessarily activate PVH neurons selectively and the cardiorespiratory activation described in such studies could have been caused or relayed by many types of hypothalamic neurons including the dorsomedial nucleus and the orexin system. A possible connection between PVH and respiration could involve the catecholaminergic neurons of the VLM which are excited by oxytocin or vasopressin and are in turn capable of stimulating breathing (2, 397). Based on anterograde tracing data the direct projections from the PVH to the hypoglossal nucleus, deduced from PRV studies (259) do not appear to exist (136). The projections from PVH to the ventral horn of the spinal cord, postulated on the basis of retrograde transport evidence (463) are also not observed by anterograde tracing. The remaining evidence implicating PVH in respiratory control is based on Fos studies, the limitations of which have been indicated previously (35, 205).
In summary, the PVH could potentially play some role in the chemoreflexes but the evidence remains weak. There is no evidence that these neurons are directly pH-responsive and more selective methods to activate or inhibit specific subsets of PVH neurons in conscious animals need to be developed to accurately assess their role in respiratory control as well as in other functions. Secondly, the connectome of PVH neurons will have to be elaborated much more precisely. PVH is activated by all manner of stresses, be they physical, for example, hemorrhage, hypotension, hypoxia, or psychological (366). Sustained hypercapnia which belongs to the category of stressful stimuli, could activate the PVH in any number of ways including via direct projections from lower medullary noradrenergic and adrenergic neurons [for review, see (160)] and via many other autonomic relays such as the NTS and the parabrachial nuclei.
Regulation of the Autonomic Outflows by Central Chemoreceptors
Central chemoreceptors regulate the sympathetic and cardiovagal outflows in two ways. The first mechanism operates via activation of the respiratory network and is considered later on in the section on cardiorespiratory coupling. Central chemoreceptors also increase sympathetic tone by mechanisms that are independent of the respiratory pattern generator. In anesthetized rats, the increased sympathetic tone is probably largely mediated by an increase in the discharge rate of RVLM presympathetic neurons. This interpretation is based on the following evidence. Hypercapnia activates RVLM presympathetic neurons in rats, in which the carotid sinus nerves, the vagus nerves, and the aortic depressor nerves have been cut (294). This activation persists after injection of muscimol into the IVLM, a procedure that silences the RPG.
The mechanism responsible for the activation of RVLM neurons by central chemoreceptors is unknown. Whereas administration of the glutamate receptor antagonist kynurenic acid into the RVLM blocks the peripheral chemoreflex (Fig. 5D), this treatment does not change the increase in sympathetic nerve activity (SNA) caused by hypercapnia (294). The response of RVLM neurons is unlikely to denote a direct effect of CO2 on the presympathetic neurons because the discharge of the C1 cells in unaffected by CO2 in slices (231). The effect of hypercapnia does not originate from CRCs located within the NTS because muscimol injection in this structure has no effect on the sympathetic response to hypercapnia (294). Other unexplored hypotheses include an excitatory input from RTN neurons mediated by metabotropic (kynurenic acid-resistant) glutamatergic receptors, a serotonergic connection or an effect of CO2 mediated via glial cells.
As indicated in a prior section, like the respiratory system, the sympathetic vasomotor outflow is regulated by noradrenergic, orexinergic, and serotonergic neurons at multiple levels including the RVLM and the intermediolateral cell column. In conscious animals, these neuronal systems presumably contribute to some degree to the activation of the SNS by CNS hypercapnia.
Whether central chemoreceptors regulate the cardiovagal outflow independently of the respiratory system is an open question.
Astrocytes as Central Chemoreceptors
Role of astrocytes in central chemosensitivity
Astrocytes have recently been credited with an increasing number of physiological roles, including pH detection and central respiratory chemosensitivity (109,110,183,201,393). As mentioned above, the first electrophysiologically recorded brainstem cells that showed some acid sensitivity are likely to have been glia (126, 127). Acute astrocytic dysfunction produced by local injection of the toxin fluorocitrate into a small portion of the RTN produced tissue acidification and breathing stimulation (111). One interpretation of this experiment is that astrocytes normally buffer pH and thus regulate the chemosensitivity of RTN chemoreceptors. Another would be that the disruption of astrocytic glutamate reuptake and potassium buffering produced nonspecific activation of nearby neurons.
A subset of ventral medullary surface astrocytes are depolarized by acid and display increases in intracellular free calcium, albeit transient, when exposed to acidic pH (146) (Fig. 7A). A causal link between these effects and the activation of RTN neurons was suggested by the following five pieces of evidence. ATP is released by astrocytes in general. ATP is released from the ventral medullary surface in vivo when animals are subjected to hypercapnia (146). ATP receptor antagonists of generally accepted selectivity attenuate the HCVR when applied to the ventral medullary surface in vivo (146). The acid-induced depolarization of RTN neurons maintained in culture was also attenuated by the same blockers (146). Finally, ChR2-mediated depolarization of the marginal glia activated breathing (Fig. 7B) and this activation was attenuated by ATP receptor blockers (146).
Figure 7.
Glial cells and central respiratory chemosensitivity. (A) Acidification increases intracellular calcium fluorescence in medullary astrocytes [from Gourine et al. (146) reprinted with permission from AAAS]. (B) Optogenetic depolarization of ChR2-expressing ventrolateral medullary astrocytes activates the phrenic outflow in an anesthetized rat. Note the very slow onset of the response to light and its persistence [adapted, with permission, from (146) reprinted with permission from AAAS]. (C) Activation of RTN neurons by CO2 in a slice of neonate rat brain is attenuated by application of the P2 receptor antagonists PPADS or suramin. Summary data (right panels): about a quarter of the overall response of RTN neurons to 15% CO2 could be mediated by ATP release in this preparation [reproduced from Wenker et al. (453) with permission]. (D) Schema of the contribution of ventral medullary surface (VMS) astrocytes to central respiratory chemosensitivity according to (145) [after Gourine and Kasparov, Exp. Phsiol. (96), 2011 reprinted with permission from John Wiley & Sons]. VMS astrocytes are thought to mediate the hypercapnic ventilatory reflex by simultaneously activating RTN neurons and undefined components of the central pattern generating (CPG). ATP release by astrocytes may be a calcium-dependent exocytotic process triggered by intracellular acidification and/or a leak through connexin channels (Cx26 primarily) opened by molecular CO2 via carbamylation.
However, the following caveats regarding astrocytes as the main transduction agents for the HCVR should be considered. The effects produced by depolarizing the glia have extremely slow on and off kinetics compared to the breathing effects produced by optogenetic activation of RTN neurons (compare Fig. 4C1 and 7B). The consequences of a large astrocytic depolarization on the local microvasculature are unknown. Reduced local blood flow is a distinct possibility, which could increase breathing by reducing CO2 wash-out (Fig. 1). Such a mechanism would account for the above-mentioned slow kinetics of the breathing response to astrocyte stimulation in vivo (146).
Molecular basis of glial chemosensitivity
The mechanism by which astrocytes detect CO2 is not altogether clear. Dale and colleagues (183) as well as Mulkey (302,303) reached different conclusions than Gourine (146) in terms of the molecule responsible for the effects of hypercapnia (CO2 vs. pH). The mechanism of ATP release by astrocytes is also uncertain. Gourine (146) considers that the release is exocytotic whereas others argue for a release through connexin channels (185, 453). A subset of ventral medullary astrocytes are clearly depolarized by CO2, by a mechanism that includes inhibition of heteromeric Kir4.1-Kir5.1 channels and activation of a depolarizing inward current generated by the sodium bicarbonate cotransporter (453). Connexins are hexameric pore forming transmembrane proteins that are widely expressed by glial cells throughout the CNS (138). The notion that connexins, notably connexin 26 (Cx26) contribute to central respiratory chemosensitivity is based on the following evidence (185). Connexins are hererogeneously expressed in the brainstem. Cx26 is especially abundant at the ventral surface of the medulla oblongata (leptomeninges, glia limitans, and blood vessel entry zones) as judged by qPCR, knock-in reporter expression and immunohistochemistry (185). Also, as mentioned already, ATP is released from the ventral medullary surface by hypercapnia in vivo (146). Third, ATP is also released from the ventral surface when horizontal slices of the medulla oblongata are exposed to hypercapnia (185). This release is not mimicked by extracellular acidification, it is observed by increasing CO2 at constant extracellular pH (i.e., by increasing bicarbonate), is calcium independent, and is inhibited by connexin hemichannel blockers (185). Application to the ventral medullary surface of connexin blockers reduced the HCVR in vivo by 20% (185). Finally, heterologous expression of Cx26 in HeLa cells is sufficient to endow them with the capacity to release ATP in a CO2-sensitive manner (184).
CO2 may open connexin hemichannels via a carbamylation reaction, not unlike the effect of CO2 on hemoglobin (284). The connexins that are opened by increases in PCO2 (Cx26, Cx30, and Cx32) have a carbamylation motif that is absent from a CO2-insensitive connexin (Cx31). Introducing the carbamylation motif into Cx31 created a mutant hemichannel (mCx31) that was opened by increases in PCO2 and, conversely, mutation of the carbamylation motif in Cx26 and mCx31 eliminated CO2 sensitivity measured by dye loading in Hela cells (284).
Thus at the ventral medullary surface CO2 could have direct effects on the respiratory network by opening astrocytic Cx26 hemichannels. The presence of connexin 26 at the ventral surface is certainly intriguing since this region contains a large portion of the dendrites of RTN neurons as well as the terminal arborizations of the distal dendrites of many respiratory neurons (158, 203).
Conclusions and remaining uncertainties
As summarized above, evidence that astrocytes participate somehow to the central respiratory chemoreflex by releasing ATP is substantial. Yet several inconsistencies of the experimental record need to be resolved. Contrary evidence includes the fact that neurons like RTN and serotonergic neurons respond quite well to acidification after complete isolation and the observation that purinergic blockers have no (serotonergic neurons, NTS) or minimal (~25%) effects on neuronal acid sensitivity in neonatal brain slices (Fig. 7C) (453). Additionally, deleting TASK-2, a channel expressed by RTN neurons but not by glial cells, attenuates the chemoreflex of adult mice and decreases considerably the pH-sensitivity of neonatal RTN neurons (137, 445). Finally, genetic manipulations that eliminate RTN neurons eliminate the chemoreflex of mice. There is no obvious reason to expect that these genetic manipulations should have broadly affected astrocytic development in the medulla oblongata. Therefore, these observations seem to leave room for a single alternative, either RTN neurons are the only CRCs, a hypothesis which could be correct but which much evidence reviewed above seems to contradict, or astrocytes outside the RTN could not be responsible for the chemoreflex. The absent to weak effects of purinergic blockers in slices of neonate brain could perhaps be explained by the immaturity of the astrocytes. Alternately, the CO2 response of astrocytes could require a blood filled vasculature which could explain the seemingly greater efficacy of purinergic blockers in vivo than in slices.
The theory that connexin channels are a CO2 receptor and the vehicle for ATP release by glial cells also has a few loose ends (183–185, 284). ATP release was indirectly measured using a biosensor whose selectivity for this molecule cannot be fully demonstrated in vivo. Even if this selectivity is granted, CO2-induced ATP release was observed primarily over the parapyramidal sulcus, particularly medial to the hypoglossal rootlets (Schlaefke area) but no release of ATP was detected over the RTN region (185) which contains neurons thought by proponents of the glial theory of chemosensitivity to derive their CO2 response both in vivo and in vitro, wholly or partly, from astrocytes (146,454). The parapyramidal sulcus is overlaid by meninges and major blood vessels. ATP released over this region could originate, partly or totally, from cells other than the marginal glia. Connexins including Cx26, Cx30.2, Cx36, Cx45, and Cx57 are also expressed by neurons (138). The application of connexin blockers directly to the VMS produced relatively small (20%) reductions of the HCVR in vivo (185). This result could be explained by a limited access of the drugs to the relevant chemoreceptors. On the other hand, the limited efficacy of the connexin blockers could simply result from their notorious lack of selectivity or from an increase in blood flow. Connexin hemichannel opening is typically voltage-dependent and the interaction between CO2 and voltage is unknown (142). Glial cells, unlike Hela cells are extremely hyperpolarized and would need extremes of pH to reach threshold for connexin channel opening. Also, human Cx26 hemichannels are reportedly closed by CO2 (141) and, in man Cx26 defects cause hearing loss but respiratory problems have not been reported (426).
In conclusion, ventral surface astrocytes probably contribute to the CO2 response of RTN neurons by releasing ATP and other gliotransmitters (summarized in Fig. 7D). The remaining questions are under which conditions and to what extent this phenomenon contributes to the central respiratory chemoreflex.
Molecular Basis of Neuronal Chemosensitivity
The putative contribution of glial cells to central chemosensitivity has not eliminated the possibility that certain neurons are also intrinsically responsive to pH.
TASK potassium channels
TASK-1/3 potassium channels (homo- and heterodimers) are closed by acidification and their pH50 is near physiological (31, 103). These channels are widely expressed in brainstem cardiorespiratory centers including the VRC and serotonergic cells suggesting that they might contribute to central respiratory chemosensitivity (450). However, this hypothesis has not been substantiated experimentally. TASK-1/3 double KO mice have a normal central respiratory chemoreflex (HCVR under hyperoxia) and the pH-sensitivity of TASK-expressing neurons such as the orexinergic neurons is unaffected in these mice (143,301). Furthermore, the pH sensitivity of RTN neurons is unaffected by the absence of TASK1/3 (301).
Cloned in 1998 from human kidneys, TASK-2 (TWIK-related acid-sensitive potassium channel-2, a.k.a. K2P5.1, a.k.a. KCNK5) belongs to a large family of tandem-pore potassium channels (2-P K-channels) (31, 57, 235, 352). TASK-2 produces noninactivating, outwardly rectifying K+ currents with activation potential thresholds that closely follow the K+ equilibrium potential (352). TASK-2 mRNA was originally thought to be absent from adult human and rodent brains (15, 352). In fact, the gene is selectively expressed by RTN neurons and very few other brainstem neurons (137, 445). This anatomical information derives from the detailed inspection of a TASK-2 KO mouse in which the cells that should normally express TASK-2 express lacZ (137) (Fig. 5I1). TASK-2 expression by RTN neurons in normal mice and its absence in TASK-2 KO mice was verified by single cell PCR (445). Based on this mouse model, astrocytes, including those that reside at the ventral medullary surface, do not appear to express TASK-2. Immunohistochemical studies have suggested a more widespread expression of TASK-2 in brain, notably in the forebrain [(206) and references therein]. This evidence is at odds with the reported lack of TASK-2 mRNA in the forebrain (137) and suggests a possible issue with the selectivity of the antibodies that were used for immunostaining. Conscious TASK-2 KO mice exhibit several respiratory abnormalities, namely, a slightly exaggerated ventilatory response to low levels of CO2 (1.5%), a severely depressed response to high CO2 (FiCO2, 6%) and an absence of posthypoxic respiratory frequency decline (137). Interpretation of these global effects is complicated by the fact that TASK-2 deletion in the kidneys causes metabolic acidosis and the absence of TASK-2 in the pulmonary arteries could interfere with the effects of hypoxia (140). However, the in vitro pH sensitivity of RTN neurons is severely decreased in TASK-2 KO mice (445), suggesting that the reduced ability of RTN neurons to be activated by acidification likely contributes to the reduced HCVR in these mice (Fig. 5I2).
In vivo, and in vitro in the presence of bicarbonate-based artificial CSF, RTN neurons respond to a range of pH expected from CRCs (7.0–7.5) (158, 300). TASK-2 channels are technically speaking “alkaline-activated” meaning that, at least in heterologous expression systems, these channels are closed at physiological pH and are opened by alkalization with a pH50 of around 8.0 (235), that is, largely outside the expected range for central chemoreceptors. This discrepancy raises the question of whether the intrinsic pH-sensitivity of TASK-2 channels actually underlies the pH response of RTN neurons. TASK-2 channels are inhibited by G-proteins (Gβγ subunit) (18) and therefore must be regulated by as yet unknown GPCRs. One of these receptors could be a pH sensor or could mediate the effects of a gliotransmitter released in a CO2-dependent manner by neighboring astrocytes.
Other molecular sensors
The potential role of connexins and Kir as CO2/pH sensors has been reviewed above in the section on astrocytes. The general topic of pH/CO2 sensors has been aptly covered in a recent review by Huckstepp and Dale (183) to which the reader is referred. To summarize, a plethora of ion channels have been proposed to play some role in the pH response of brainstem neurons such as the locus coeruleus and the NTS. The level of evidence is uniformly low because of the quasi ubiquitous expression of many of these channels in brain and the failure to establish a causal relationship between the acid-sensitivity of a given channel, the in vivo CO2-sensitivity of the neurons that express such a channel and the HCVR. Various G-protein coupled receptors have also been proposed to serve as pH or CO2 sensors in invertebrate and/or mammalian systems. The contribution of such receptors to respiratory chemoreception has not been tested or demonstrated. A form of adenylate cyclase has also been proposed to contribute to pH sensitivity [reviewed in (183)].
The Carotid Bodies and the Peripheral Chemoreflexes
Carotid body physiology and reflexes evoked by carotid body stimulation
Carotid body (CB) stimulation produces different effects according to the intensity and duration of the stimulus. Low level continuous stimulation such as caused by moderate hypoxia and or hypercapnia in conscious animals stimulates breathing, increases cardiac output and AP and causes tachycardia. Brief high levels of carotid body stimulation such as produced by cyanide boluses or brief anoxia, produce a primary vagally mediated cardioinhibition that overrides the sympathetic stimulation and, if the preparation is intact, arousal and the defense reaction (indices of a nociceptive stimulus). Carotid body stimulation also reduces brown adipose fat thermogenesis especially during cold exposure, resulting in a drop in core temperature and metabolism which appears to be an oxygen-saving strategy (134, 260).
Brainstem projections of carotid body primary afferents
Carotid body afferents project mostly to the commissural portion of the NTS although one report suggests that some of them continue on to innervate the ventrolateral medulla (118). As of 1990, there was very little information regarding the central projections of carotid body afferents and the synaptic connections that these afferents make in the NTS (392). Chemoresponsive neurons with cell bodies in the petrosal ganglion were shown by antidromic mapping techniques to access the NTS via the tractus solitarius and to consistently innervate the medial and commissural nuclei in cats (96). In this species, NTS neurons were almost uniformly excited by stimulating a carotid sinus nerve (95). The carotid nerve contains a mixture of baro- and chemoreceptor afferents (364) but the fact that excitatory responses were almost always elicited by activating this nerve provided the first suggestion that carotid body inputs might be excitatory. In anesthetized rats, the peripheral chemoreflex is blocked by injecting powerful antagonists of ionotropic glutamate receptors (DNQX plus AP7) into the NTS commissural nucleus (439). This evidence confirmed that the commissural nucleus of the NTS is essential for the respiratory stimulation elicited by carotid body stimulation. It also suggested that carotid body afferents could be glutamatergic, although intraparenchymal microinjection of drugs influences entire circuits and the resulting effect cannot be attributed to specific synapses such as, in this instance, those established by carotid body afferents. In conscious or arterially perfused unanesthetized rats, injecting a mixture of ATP receptor blocker and ionotropic glutamatergic antagonist (PPADS plus kynurenic acid) attenuated the pressor and bradycardic responses but had no effect on the tachypneic response to carotid body stimulation (47). The lack of effect of kynurenic acid on the respiratory component of the reflex could be explained by the fact that an unanesthetized preparation is far more reactive and, unlike the much more powerful mixture of CNQX and AP7 used by Vardhan et al. (439), Braga et al. (47) used kynurenic acid, a weak blocker of glutamatergic transmission which may have been insufficient to interrupt glutamate transmission triggered by massive stimulation of the carotid bodies with cyanide. Another interesting but highly speculative interpretation would be that distinct carotid body sensory afferents mediate the respiratory versus the cardiovascular responses.
Processing of carotid body afferent input within the nucleus tractus solitarius
Carotid body afferents, like many other types of visceral sensory afferents, include myelinated and unmyelinated fibers. Like virtually all visceral afferents, both types of neurons are probably glutamatergic because stimulation of the tractus solitarius, which contains the axons of ninth and tenth nerve primary afferents, produces monosynaptic EPSCs in NTS neurons that are always blocked by ionotropic glutamate receptor antagonists (196). Also as a rule, the release of glutamate by unmyelinated tractus solitarius afferents is capsaicin-sensitive due to the presence of presynaptic TRPV1 channels, whereas glutamate release from myelinated afferents is facilitated by ATP via activation of P2X receptors, maybe of the P2X3 homomeric variety (196). These general rules presumably also apply to carotid body afferents.
Carotid body stimulation with cyanide affects about a third of the caudal NTS neurons, causing a variety of responses such as pure ePSPs, ePSP/iPSP sequence, pure iPSPs, or respiration synchronous PSPs (334). The intrinsic membrane properties of the responsive neurons vary considerably, suggesting that carotid body afferents activate or inhibit a complex network of NTS neurons (334). As a caveat, strong activation of carotid afferent fibers such as resulting from administration of cyanide may recruit brain circuits that elicit arousal and underlie the nociceptive/aversive effects of CB stimulation (272). The pathways engaged by low level activity of the chemoreceptors, of the type that regulates the cardiorespiratory system selectively under normal circumstances, could be much more restricted.
Usually, different NTS neuronal populations respond to activation of baroreceptors versus carotid bodies (333) and, also in general, there seems to be very limited convergence between various visceral sensory modalities onto second-order neurons (282). According to Paton et al. (334) many carotid body responsive NTS neurons have an unmyelinated axon that courses in the direction of the ventrolateral medulla. These cells are very likely to be second-order neurons because, according to Andresen and colleagues, virtually all NTS neurons with projections to the ventrolateral medulla receive monosynaptic glutamatergic input from the solitary tract (27). Based on this evidence, second-order neurons involved in the respiratory and cardiovascular components of the peripheral chemoreflexes probably directly innervate the cardiorespiratory regions of the ventrolateral medulla but many of these cells may also have axon collaterals within the NTS (334). Ninety percent of NTS neurons with axonal projections to the ventrolateral medulla that express Fos after a hypoxic stimulus contain VGLUT2 mRNA (421). Based on the work of Bailey et al. (27), most, if not all these, VLM projecting glutamatergic neurons are carotid body second-order neurons. In anesthetized rats, about a third of commissural NTS neurons with axonal projections to the ventrolateral medulla were activated tonically by hypoxia (215), a proportion in good agreement with the percentage of commissural NTS neurons that receive input from the carotid bodies (334). The commissural NTS also innervates the Kölliker-Fuse nucleus and lateral parabrachial subnuclei, regions that contain essential components of the lower brainstem circuit that generates eupneic breathing (52, 54, 383). This innervation originates in part from commissural neurons that express Fos following hypoxia (391). These hypoxia-responsive NTS neurons could therefore be the very same neurons that innervate the ventrolateral medulla (215, 421).
The commissural nucleus of the NTS innervates many additional brain structures including the locus coeruleus, the periaqueductal gray matter, hypothalamic regions such as the dorsomedial and paraventricular nuclei, the median preoptic nucleus and the lateral hypothalamic area, and the amygdala (252). Based on electrophysiological considerations the hypothalamic projections from NTS may originate from third or higher order neurons rather than from neurons that receive direct input from carotid body afferents (27). However this may not be a general rule because the A2 noradrenergic neurons, which project widely to suprapontine structures, seem to be second-order neurons (20). The type of visceral afferents that the A2 cells receive has not been determined.
Peripheral chemoreflex pathways beyond the NTS
To summarize the previous paragraph, the breathing stimulation elicited by carotid body stimulation is probably mediated by glutamatergic second-order neurons located in the commissural NTS, which innervate multiple segments of the ventral respiratory group, the RTN and pontine structures such as the Kölliker-Fuse nucleus and the dorsolateral parabrachial nuclei (11, 215, 252, 334, 391, 421) (Fig. 8). The same pontomedullary regions are targeted by the RTN neurons and by serotonergic neurons. Integration between the peripheral and the central components of the respiratory chemoreflexes therefore probably occurs to some degree within all of these regions. The projections from NTS to the pre-Bötzinger complex seem lighter than to other regions of the VLM (11). However, this observation does not exclude the possibility that the tachypnea produced by carotid body stimulation could result at least partly from the direct activation of the respiratory rhythm generating neurons (preI-I neurons of the pre-Bötzinger complex; Fig. 2).
Figure 8.
Sympathetic nerve activation by carotid body stimulation: lower brainstem pathways. The four experiments depicted around the interpretative schematic (A–D, clockwise from top right) suggest that, in anesthetized rodents, carotid body stimulation increases sympathetic vasomotor tone via two pathways that converge on RVLM presympathetic neurons, only one of which depends on the respiratory pattern generator. (A) Microinjection of the GABA mimetic agonist muscimol into the commissural portion of the NTS abolishes the response to cyanide [adapted, with permission, from (294)]. (B) Injection of muscimol into the rVRG eliminates the phrenic nerve discharge but does not change the sympathetic response to carotid body activation (N2, 10 s nitrogen inhalation) [adapted, with permission, from (217)]. (C) Injection of muscimol into the pre-Bötzinger complex, eliminates the phrenic nerve discharge and eliminates the respiratory oscillations of the sympathetic nerve discharge elicited by carotid body stimulation. However, the sympathoexcitation produced by carotid body stimulation persists. The response of a simultaneously recorded single RVLM presympathetic neuron is also shown. Note that muscimol produces the same effect on the neuron as on SNA, that is, muscimol changes the response from respiratory synchronous oscillations to a tonic activation [adapted, with permission, from (216)]. (D) Administration of kynurenic acid into the RVLM dramatically reduces SNA activation caused by carotid body stimulation [after (294) reprinted with permission from the Society for Neuroscience]. The respiratory dependent pathway of the chemoreflex is considered in greater detail in Fig. 3).
The existence of direct projections from carotid body-activated NTS neurons to the presympathetic neurons of the RVLM, including the C1 cells, is very probable for the following reasons (Fig. 8A–D). These RVLM cells are robustly activated by hypoxia in vivo (Fig. 8C) (216, 412, 413). This excitation persists when respiration is blocked by introducing muscimol into the IVLM (Fig. 8C), a procedure that silences the RPG because the IVLM coincides with the pre-Bötzinger and rVRG (Fig. 2, Fig. 8). Finally, the C1 cells receive predominantly asymmetric synapses from commissural NTS neurons (9).
In sum, the effects of carotid body stimulation on the sympathetic outflows are mediated in part by direct excitatory inputs from the NTS to RVLM presympathetic neurons. Indirect routes undoubtedly exist, the best documented is via the respiratory network as described in the next section.
Carotid body stimulation by hypoxia also produces tachypnea and sighs. These responses could be partly caused by activation of subsets of C1 cells because sighs are evoked by injections of beta-receptor agonists into the pre-Bötzinger complex (442) and they are also evoked by optogenetic stimulation of the C1 neurons in conscious rats or mice (1, 2).
Central Cardiorespiratory Coupling
In anesthetized animals, the sympathetic and cardiovagal tones fluctuate during the central respiratory cycle in a stereotyped, species and anesthetic-dependent manner [for recent reviews see (153, 161, 193)]. These fluctuations persist after bilateral vagotomy and in paralyzed animals subjected to a pneumothorax, therefore, they are independent of feedback from cardiopulmonary receptors and chest muscle and joint mechanoreceptors. These fluctuations are therefore generated centrally by inputs from lower brainstem neurons whose discharge is driven by the central respiratory pattern/rhythm generator. Since the discovery of this phenomenon, the key questions have been to explain the oscillations of the cardiovagal and sympathetic outflow in terms of brainstem circuitry, to determine whether the phenomenon exists in conscious mammals and whether it has any sort of physiological importance in human physiology.
Influence of the respiratory centers on the sympathetic outflow
Central and peripheral chemoreceptors regulate the sympathetic vasomotor outflow in part via the respiratory network. Under anesthesia or in reduced preparations, this regulation operates largely via the presympathetic neurons of the RVLM. Whether this is also the case in conscious animals is undocumented. The principal evidence is that, in a given preparation, the central respiratory modulation of RVLM presympathetic neurons exhibits several stereotypic patterns that closely mimic that of individual sympathetic efferents (72, 168) (Fig. 3B and D). Activation of central or peripheral chemoreceptors produces identical patterns of respiratory modulation in a given RVLM presympathetic neuron. Under anesthesia, the discharge probability of rat RVLM presympathetic neurons and of sympathetic fibers usually displays two main patterns characterized by a maximum discharge probability during the postinspiratory phase or by a maximum during the early inspiratory phase (218, 323) (Fig. 3B and D). Neurons with peak discharge probability coinciding broadly with inspiration have also been found. In the unanesthetized perfused rodent preparation, the discharge of RVLM neurons with post-I maxima exhibits a second activity peak during late expiration when the respiratory drive is sufficient to trigger late-expiratory abdominal activity (293) (Fig. 3E3).
Various theories have been proposed regarding the identity of the respiratory neurons that modulate the presympathetic neurons. According to one theory this modulation would be driven by direct inputs from phase-spanning (inspiratory-expiratory) excitatory pontine neurons and from inhibitory inputs from Bötzinger post-I and rVRG early-I(2) neurons (290). This model (not illustrated) downplays the contribution of the GABAergic neurons of the IVLM that relay the sympathetic baroreflex to RVLM presympathetic neurons. It also assumes that these IVLM neurons receive an input from an excitatory population of Bötzinger post-I neurons. The model postulated by Molkov et al. (290) works in silico but the postulated inputs to RVLM neurons are not documented anatomically. In this article, we propose an alternative scheme which is that the respiratory modulation of RVLM presympathetic neurons operates via the IVLM neurons that mediate the baroreflex. The reason for this choice is that the baroactivated IVLM neurons have respiratory patterns that are the mirror image of those displayed by RVLM presympathetic neurons (265) (Fig. 3C). These IVLM neurons are located in a region that closely overlaps the pre-Bötzinger complex/rVRG region (Figs. 2 and 3A). If these IVLM neurons are indeed the source of the respiratory fluctuations of the RVLM presympathetic neurons, the respiratory maxima exhibited by RVLM presympathetic neurons must be caused by disinhibition, that is, by periodic inhibitory inputs from components of the RPG to subsets of IVLM GABAergic neurons. Input from early-I, possibly early-I(2) (290) inhibitory neurons to IVLM neurons could therefore account for the existence of RVLM presympathetic neurons with a peak discharge during the early-I phase. Input from Bötzinger post-I inhibitory neurons to other populations of IVLM neurons could account for the second major class of RVLM presympathetic neurons which exhibit a peak firing probability during the postinspiratory phase (Fig. 3B). RVLM presympathetic neurons with an inspiratory peak could be receiving input from IVLM cells that in turn receive input from inhibitory inspiratory neurons. All the postulated inhibitory inputs to the IVLM neurons (early inspiratory, inspiratory, or postinspiratory) are commonly found in the rVRG and pre-Bötzinger region, that is, in the immediate vicinity of the IVLM neurons (246, 296, 381). The late-expiratory peak observed in some presympathetic neurons when the respiratory drive is very elevated could plausibly originate from excitatory pfRG neurons with late-expiratory activity (246, 296, 381) (Fig. 3).
In summary, RVLM presympathetic neurons may derive their respiratory modulation from respiratory modulated IVLM GABAergic interneurons but additional sources are possible. Some uncertainty remains as to whether the respiratory modulation of the presympathetic neurons causes an increase or decrease in their average discharge rate. Because the IVLM neurons are also regulated by baroreceptors, the overall effect of a chemosensory stimulus on their activity will depend on the concomitant change in AP and may vary depending on the type of presympathetic cells, that is, whether they regulate the splanchnic circulation, muscle blood vessels, or the heart (274).
Another facet of central cardiorespiratory coupling is the influence of the network that regulates the cardiovascular outflows on the breathing network. This phenomenon has been more difficult to detect than the reverse. The most prominent example yet is the excitatory input from the C1 cells to the RPG which can be demonstrated by selective optogenetic stimulation of these cells in conscious animals (2). This input modulates respiratory frequency selectively and causes sighs, thus mimicking the effects of hypoxia on the respiratory system.
Influence of the respiratory centers on the cardiovagal outflow
The cardiovagal outflow is also prominently modulated by the respiratory system. These oscillations are largely responsible for the respiratory fluctuations of the heart rate called sinus arrhythmia (33,130). The CVPGNs receive an inhibitory input from pulmonary stretch receptors that contributes to increasing the heart rate when ventilation increases (Fig. 9B). The pathway could be as simple as a direct inhibitory input from GABAergic pump cells located within the NTS. It could also be indirect via elements of the RPG. The central respiratory modulation of CVPGNs has been accurately documented in the arterially perfused rat preparation. In this preparation in which the feedback from cardiac and lung stretch receptors is inoperative, cardiac ganglionic neurons clearly receive respiratory phasic input with maxima coinciding with the postinspiratory phase (277) (Fig. 9A1 and A3). The interpretation of this data in terms of synaptic input to the CVPGNs is tentative. Figure 9B represents a few possibilities. The first one is that the respiratory modulation of the CVPGNs is caused by early-I and aug-E inhibitory inputs on a background of tonic (respiration-independent) excitation. The existence of inhibitory inputs during inspiration is supported by evidence of IPSCs synchronized with the hypoglossal discharge in putative CVPGNs recorded in slices (Fig. 9C) (320). An input from respiratory excitatory neurons with post-I activity could most simply account for the respiratory modulation of the discharge of CVPGNs. Post-I excitatory neurons are also hypothesized to drive the post-I discharge of laryngeal vasoconstrictor MNs (246, 381) but there is no direct evidence yet that such post-I neurons exist. Arterial baroreceptors and serotonergic neurons presumably are the main source of tonic excitatory drive to CVPGNs.
Figure 9.
Regulation of the cardiovagal parasympathetic outflow by the respiratory pattern generator. (A1–3) [Reproduced from McAllen et al. (277), reprinted with permission.] Intracellular recordings of cardiovagal ganglionic neurons from an arterially perfused rat preparation in which the lungs are not inflated therefore reflexes from lung stretch receptors are absent (A1). The firing properties of cardiovagal preganglionic neurons (CVPGN) were inferred from the discharge pattern of the ganglionic neurons (A2) or the EPSPs recorded from these neurons (A3). Activation of the carotid bodies with cyanide or of arterial baroreceptors by raising perfusion pressure produced the expected robust excitation of cardiovagal neurons, and so did the activation of the diving reflex (A2). A3, PND-triggered histograms of EPSPs recorded in cardiovagal ganglionic neurons (CVGNs) indicate that, in this preparation, CVPGNs discharge preferentially during the postinspiratory phase. (B) Schematic interpretation based on A2–3 and C. Rat cardiovagal preganglionic neurons could be receiving inhibitory inputs from early-I and Aug-E neurons. The existence of inhibitory input during inspiration has been documented in slices as shown in C. Pulmonary stretch receptors (inactive in an arterially perfused preparation) inhibit cardiovagal preganglionic neurons, possibly via a direct input from GABAergic “pump cells” located within the NTS. Carotid body stimulation produces opposite effects on CVPGN activity depending on the intensity of the stimulus. Mild stimulation inhibits these neurons via the RPG, presumably via inhibitory inputs from early-I and post-I neurons as shown in B. Acute and intense stimulation of the carotid bodies (e.g., with cyanide) activates cardiovagal preganglionic neurons as shown in A2. This effect may be mediated by polymodal NTS neurons that also respond to noxious stimulation of the airways (pathway not represented). Post-I glutamatergic neurons are an alternative plausible source of excitation of cardiovagal preganglionic neurons and other vagal efferents with a post-I discharge pattern but the existence of such neurons has yet to be demonstrated. (Panel C was originally published in (153), and has been reproduced by permission of Oxford University Press [http://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780195306637.001.0001/acprof-9780195306637]).
Interplay between Peripheral and Central Chemoreceptors
Two facts about chemoreceptors are well accepted. Acute bilateral carotid body excision all but eliminates the hypoxic ventilatory reflex, and peripheral chemoreceptors have 5–10 times faster response kinetics to a change in PaCO2 than central chemoreceptors [e.g., (34)]. However, the relative contribution of the carotid bodies versus the CRCs to ventilation, both at rest and in the presence of hypercapnia, is still debated and so is the type of interaction between the central and the peripheral chemoreflexes (100, 429, 459).
One approach to determining the relative importance of peripheral versus central chemoreceptors has been to examine the effects of carotid body resection or denervation (carotid sinus nerve section) on the ventilatory response to hypoxia or hypercapnia. Bilateral carotid body resection preserves the carotid baroreceptors whereas carotid body denervation eliminates these afferents. In man, bilateral resection of the carotid bodies virtually eliminates the hypoxic ventilatory response and this effect is essentially permanent (175,254,298,380). In animals, the hypoxic ventilatory response recovers partially or totally (rats) over time presumably because of an increase in the activity of the aortic bodies. Postsurgically, carotid body denervation also produces resting hypoventilation and hypercapnia, an effect that decreases days to week after surgery but may never recover completely depending on the species (45, 285, 298). Similar hypoventilation has been observed in man [(443) and references therein]. For example, resting PaCO2 also increased after denervation of the carotid bifurcation in patients who had undergone bilateral endarterectomy of the carotid bifurcation (38.9–44.7 Torr). This procedure was implemented to increase cerebral perfusion but it effectively denervated both carotid bodies and carotid sinuses (443). Long-term follow-up studies made at varying intervals from 16 days to 10 months after surgery revealed a sustained loss of ventilatory response to hypoxia and a persistent elevation in resting PaCO2, but no notable effects on blood pressure under normoxia. Bilateral carotid body resection (as opposed to denervation) was implemented in man almost 50 years ago under the now disproved assumption that the procedure would be of therapeutic value in bronchial asthma (254). Patients with carotid body resection had virtually no hypoxic ventilatory response, a 30% reduced hypercapnic ventilatory response but otherwise had essentially normal PaCO2 at rest and a normal exercise hyperpnea (254). On balance, these human and animal experiments indicate that combined section of carotid baro- and chemoreceptor afferents causes a sustained increase in resting PaCO2 but this effect may be smaller or absent when the carotid bodies are selectively removed without damaging the baroreceptors. Lugliani et al. (254) speculated that carotid baroreceptor denervation might increase cerebral blood flow thereby reducing PCO2 in the vicinity of the CRCs and resetting the central respiratory chemoreflex toward higher levels of PCO2. These lesion experiments suggested that, at steady-state in man around 30% of the HCVR may be mediated by the carotid bodies. Similar estimates have been obtained in animal experiments (34).
A less invasive way to gauge the relative contribution of central and peripheral chemoreceptors to resting ventilation and the respiratory chemoreflex consists of activating these receptors separately or in combination. In conscious dogs, silencing the carotid bodies using extracorporeal perfusion with a hyperoxic hypocapnic mixture produced an initial 49% to 80% drop in eupneic ventilation that settled to 31% reduction at steady state despite a 9 Torr increase in PaCO2 (40). In this experimental model, carotid body inhibition reduced the ventilatory response to CNS hypercapnia by 80% whereas carotid body stimulation increased this response by roughly twofold (41). In anesthetized mammals central and peripheral chemoreceptors typically have roughly additive effects on ventilation (99, 124). A hypoadditive hypercapnic-hypoxic interaction on breathing has also been reported, first in a hypothermic arterially perfused midcollicular transected rat preparation (75) and, more recently, in urethane-anesthetized atropinized rats (434).
In summary, the relative contribution of the carotid bodies and the CRCs to the HCVR depends on the species and the preparation and, very probably on the concurrent behavior (rest vs.exercise) and state (sleep vs. awake) although this has not been specifically tested. Depending on the experimental model, the interaction between peripheral and central chemoreflexes can be either hypoadditive, additive, or hyper-additive. Hypoadditivity has only been observed in reduced preparations or in anesthetized rats.
These various interactions could be predicted by considering some key features of the wiring of the RTN. The response of RTN neurons to hypercapnia is saturable (Fig. 10D2), probably because these neurons receive inhibitory inputs from the RPG and from pulmonary stretch receptors (Fig. 10A–G). In addition, RTN neurons receive polysynaptic excitatory inputs from the carotid bodies (Fig. 5D). A change in the resting membrane potential of RTN neurons could shift the interaction between central and peripheral chemoreflexes from simply additive (when RTN neurons are sufficiently depolarized at rest to respond linearly to incremental changes in brain PCO2, Fig. 10I, left panel) to potentiative (hyperadditive) when these cells are hyperpolarized at rest (Fig. 10I, right panel). In the latter case, the excitatory input from the carotid bodies would be required to prime RTN neurons to respond to CO2. In the presence of carotid body input to RTN, the central chemoreflex would be much more powerful because RTN neurons would have reached action potential threshold. On the other hand, if and when the RPG was unusually active or excitable (Fig. 10H, left panel), the strong feedback from the RPG (and potentially from pulmonary stretch receptors if present) to RTN neurons would minimize the contribution of central chemoreceptors, resulting in hypoadditivity between the effects of carotid body stimulation and central chemoreceptor stimulation. Hypoadditivity has been observed only in anesthetized or reduced preparations, probably because in such situations the network is somewhat reconfigured by the surgical or pharmacological interruption of critical inputs. The condition of reduced RTN excitability (Fig. 10I, right side) could explain the potentiative interaction observed in conscious resting dogs as well as the long-term depression of resting ventilation following carotid body excision. However, one should not lose sight of the fact that, in resting conscious man, carotid bodies and central chemoreceptors seem to exert roughly additive effects on breathing (63). This would be consistent with a moderately active RPG and an RTN that is responsive to CO2 and receives little feedback from the RPG (Fig. 10H, right panel or 10I, left panel).
Figure 10.
Feedback regulation of RTN neurons and interaction between central and peripheral respiratory chemoreflexes. (A1–4) Four examples of RTN neurons showing different types of respiratory modulation which can be interpreted as post-I and E-aug inhibition in cell 1, early-I and post-I inhibition in cell 2, early-I only in cell 3, and early-I, post-I and E-AUG in cell 4 [adapted, with permission, from (158)]. (B) Typical RTN neuron devoid of respiratory modulation at low end-expiratory CO2. This cell exhibits the early-I/post-I pattern when FiCO2 is increased (unpublished example from P. Guyenet). C, single RTN neuron inhibited by lung-inflation [Tp, tracheal pressure, iPND integrated rectified phrenic nerve discharge; adapted, with permission, from (295)]. (D1) Single RTN neuron recorded in a vagally intact anesthetized rat showing the complex respiratory modulation of the cell (integrated rate histogram and rectified PND triggered on expiratory CO2 shown as bottom trace). (D2) Average steady-state response of RTN neurons to end-expiratory CO2 in the same preparation as D1 [D1–D2 adapted, with permission, from (158)]. (E1 and E2) Similar recordings after i.c.v. administration of the glutamatergic antagonist kynurenic acid. (E1) PND and the respiratory modulation of the RTN neuron was abolished by kynurenic acid. (E2) The relationship between the discharge rate of RTN neurons and end-expiratory CO2 became linear after kynurenic acid administration [adapted, with permission, from (158)]. (F) Interpretation of the results shown in A–E: the response of RTN neurons to CO2 is saturable because of the existence of inhibitory feedback from lung stretch receptors and from the RPG. G, schematic wiring diagram based on A–E. (H) Hypothetical scenario in which the RPG is unusually active or excitable (left). In such a case, respiratory feedback to the RTN would minimize the contribution of this nucleus to ventilation causing hypoadditivity between the peripheral and central chemoreflexes. On the other hand, additivity would result from a situation in which the RPG is moderately excitable (right panel). (I) Possible hyperadditivity scenario. RTN hyperpolarization at rest (right panel) could result in hyperadditivity between the central and peripheral chemoreflexes. In this configuration, moderate hypercapnia alone would produce a minimal stimulation of breathing because RTN depolarization would be largely subthreshold. Carotid body stimulation would depolarize RTN neurons above their discharge threshold causing them to respond vigorously to the previously ineffective hypercapnic stimulus, hence the apparent hyperadditivity of the reflexes.
How important are the chemoreflexes?
Haldane and Priestley (163) first proposed the view that because hypercapnia is such a powerful breathing stimulant—an increase of PaCO2 of 1 Torr increases lung ventilation by 30% in man—the chemoreceptors must be essential to stabilize PaCO2. The traditional argument against this view is that PaCO2 hardly fluctuates at all during exercise despite the massive increase in CO2 production (78,123,166,273). Thus, the argument goes, a feedback from chemoreceptors could not be important in regulating PaCO2 because there is essentially no error signal. This classic riddle relies on the premise that the chemoreflexes should operate in a simple feedback manner which seems doubtful in view of what we currently know of chemoreceptors. Peripheral chemoreceptors (carotid bodies) are regulated by at least two neural efferent systems and by multiple blood borne factors besides arterial PCO2 and PO2 (224). As reviewed here, central chemoreceptors such as RTN neurons, serotonergic neurons and others are merely CO2-regulated neurons whose activity is also modified by synaptic inputs. Central command and feedback from muscles likely stimulate breathing to a degree commensurate with the anticipated metabolic expenditure, presumably with increasing accuracy through a still unknown learning process, such that only very minor CO2 error signals need be corrected by chemoreceptors in adulthood (123). Judging from CCHS patients, RTN neurons are nonessential for the hyperpnea of exercise (377) although experiments in rats suggest that a component of central command might be mediated by these cells (30). However, RTN neurons likely “factor in” the effects of central command and peripheral feedback via their negative inputs from the respiratory network and lung stretch receptors and their excitatory inputs from the carotid bodies and the hypothalamus.
The carotid bodies are not required for PCO2 stability although their excision tends to reduce ventilation. How essential central chemoreception is to maintain CO2 stability is still an open question. Genetic elimination of RTN neurons abolishes the HCVR during the neonatal period but the effect of such lesions on PCO2 stability and long-term survival has not been examined (346). Reduced chemoreceptor function, such as observed in CCHS, produces frequent and severe sleep related hypoventilation which has adverse consequences throughout the brain including within regions whose development is not directly influenced by Phox2b mutations (225, 257). However, the neural control of the circulation is also dysfunctional in CCHS patients (256,346,451). Furthermore, CCHS is a degenerative disease in which RTN neurons are presumably absent (144). Such neurons are not simply CO2 sensors. Their absence could be compromising an excitatory drive to the breathing network that is important for reasons other than CO2 sensitivity. A stricter test of how important chemosensitivity is for CO2 stabilization during various behaviors will require selective deletion of the mechanism responsible for the CO2 sensitivity of the postulated chemoreceptors rather than the physical elimination of these neurons. This goal has been partially achieved by deleting TASK-2 from RTN neurons (137, 445). This deletion attenuated the HCVR but its effect of PCO2 stability remains unknown.
Chemoreflex-mediated CO2 homeostasis versus effects of asphyxia: The role of wake-promoting pathways
Low-level stimulation of chemoreceptors such as occurs under physiological conditions produces no sensation, no change in the state of vigilance, and presumably engages a very small core of neurons that promote PCO2 stability via very small adjustments of the respiratory network responsible for breathing automaticity. Under these conditions, the key elements of this regulatory system could conceivably be limited to NTS glutamatergic neurons that receive carotid body afferents (second-order chemoreceptor neurons) and RTN neurons that respond directly or indirectly to changes in brain PCO2. Excitatory inputs from second-order chemoreceptor NTS neurons to the RTN represent one mechanism by which peripheral and central chemoreflexes are presumably integrated under such circumstances. However, second-order chemoreceptor NTS- and RTN neurons innervate overlapping pontomedullary regions implicated in cardiorespiratory function. Integration between peripheral and central chemoreflexes likely occurs in these regions as well, although convergence of inputs from RTN and NTS second-order chemoreceptor neurons onto the same brainstem neurons has not yet been demonstrated.
Abrupt large changes in chemoreceptor activation are consciously perceived, usually as a noxious stimulus and produce hypervigilance and arousal from sleep, presumably via numerous mechanisms. The transition from quiet waking to full arousal or from sleep to waking is orchestrated by hypothalamic circuits and accompanied by the activation of serotonergic, orexinergic and noradrenergic neurons. During severe hypercapnia, arousal is probably caused by the cumulative effects of numerous mechanisms. These include sensory feedback from chest or airway receptors activated by a sudden increase in ventilatory movements, a mechanism that may be especially important in obstructive sleep apnea (OSA) (139). Given the extremely aversive quality of even modest hypercapnia or asphyxia in paralyzed ventilated man, wake-promoting pathways are likely to also be activated by stimulation of the carotid bodies and central chemoreceptors independently of mechanosensory feedback (29).These arousal pathways are imperfectly understood but could include projections of carotid body second-order neurons, the C1 cells, RTN, and other CRCs to the lateral parabrachial nucleus (1, 154, 202). Direct projections from NTS and the C1 cells to various wake promoting structures (hypothalamus, locus coeruleus) could also participate (1, 154, 202) as well as projections from the respiratory rhythm generator (efferent copy theory). A portion of the chemoreflex (both respiratory and cardiovascular) is currently attributed to the central chemoreceptor properties of serotonergic, orexinergic and catecholaminergic neurons. The pronounced reduction in chemoreflex observed with lesions of such neurons are state-dependent and are likely caused by a mixture of direct effects of pH and activation by arousal pathways from the hypothalamus. The relative importance of the two mechanisms is unknown at present.
Chemoreflexes and Pathophysiology
Obstructive sleep apnea, chronic intermittent hypoxia, and cardiorespiratory coupling
Airway obstruction during sleep is caused by the combined effect of a reduction of airway muscle tone and narrower than normal airways, most often, but not always, because of obesity (85, 147). Each obstruction produces a severe blood oxygen desaturation combined with hypercapnia, arousal, or lightening of sleep and a surge in blood pressure, heart rate and sympathetic nerve activity (307). Breathing eventually resumes under the combined effect of increased inspiratory efforts and some recovery of upper airway tone due to arousal and increased chemoreceptor stimulation. In OSA, these episodes may occur dozens of time per hour. Their frequent repetition causes sleep deprivation and has detrimental effects during waking hours. These include fatigue and mild hypertension which is associated with and perhaps partly caused by a rise in sympathetic tone (85).
Since its introduction by Fletcher and colleagues (122), chronic intermittent hypoxia (CIH) in rats has commonly been used to study the cardiorespiratory consequences of OSA (122, 378). In rats, CIH produces a persistent mild hypertensive state (10–14 mmHg increase in resting mean AP) that lasts for several weeks after cessation of the procedure. This AP increase is attenuated by carotid body denervation, sympathetic nerve ablation, renal sympathectomy, adrenal medullectomy, and the angiotensin-l receptor blocker losartan (119). Accordingly, sympathetic nerve hyperactivity must play a role in the hypertension. However, other factors also contribute to increasing peripheral resistance such as decreased production of nitric oxide (419). The addition of CO2 during the chronic bouts of hypoxia increases the rise in AP and the bradycardia elicited by each hypoxic episode but makes no obvious difference as far as the chronic hypertension is concerned (120, 121). Accordingly, and for simplicity, CIH rather than chronic asphyxia is used to model the effects of OSA on cardiorespiratory function. However, more recent studies suggest that CIH in rats causes other untoward effects besides hypertension, namely a severe hyperventilation that is manifested by the presence of abdominal expiratory contractions (active expiration) even at rest (290, 293, 467). The phenomenon persists in arterially perfused preparations of such CIH-exposed rats. In these preparations, a very low PCO2 in the perfusate is sufficient to produce late expiratory activity in lumbar motor nerves and withdrawing CO2 in the perfusate fails to silence the phrenic nerve (290, 467). This increased respiratory drive also causes the appearance of additional respiratory phasic activation of the sympathetic outflow during expiration (Fig. 3E). These effects suggest that the cardiorespiratory network could have become hyper-sensitive to CO2 in CIH-exposed rats and the experimental results can indeed be modeled by assuming that the relationship between RTN neuron activity (and/or other CCRs) and CO2 is shifted upward by a constant amount (290). The late-expiratory peak of activity observed in the presympathetic neurons of the RVLM is assumed to originate from excitatory late-E neurons located in the parafacial region (290). These plausible assumptions need both experimental confirmation that RTN neurons are overexcited and a mechanistic explanation. One issue with the arterially perfused preparation is that the apneic threshold is exceptionally low even in normal animals (~2% CO2 in the perfusate). The given and indeed plausible explanation is that brain PCO2 around the central chemoreceptors is substantially higher because of the brain’s metabolic activity (290), which basically means that tissue perfusion and/or the CO2-carrying ability of the perfusate are much below normal in such a preparation. If PCO2 within the brain is not in equilibrium with CO2 in the perfusate, it also means that the relationship between the two could be further altered by changes in vascular resistance. In other words, the differences between control and CIH-exposed rats, in vivo as in vitro, are difficult to definitively interpret without further information regarding brain PCO2 and pH in the region surrounding the chemoreceptors. In fact, reduced brain perfusion of such a region would be sufficient to explain the observed increase in respiratory outflow and the very low apneic threshold. Another way to explain the absence of apneic threshold in the arterially perfused preparation of CIH-exposed rats could be an increased excitatory drive from the carotid bodies such that the central chemoreceptor drive is no longer required for the respiratory network to be active. Indeed, these rat preparations have intact carotid bodies and carotid body afferents discharge spontaneously under normoxia in CIH-exposed rats (224, 335, 343). CIH also sensitizes the carotid bodies to chemoreceptor stimuli (224,335,343). Finally, CIH also modifies synaptic transmission through the NTS in a way that could further potentiate the effect of overactive carotid bodies or exert independent stimulatory effects on the respiratory network (211, 212). For example sustained and CIH reduces a KATP-mediated outward current in NTS neurons that receive input from the carotid bodies (464). Such an effect could boost the peripheral chemoreflex under chronic hypoxic conditions.
In summary, CIH in rats produces cardiorespiratory effects that only partially reproduce those of OSA in man. In rats, these cardiovascular effects are associated with massive increases in respiratory outflow under normocapnic conditions which have not been reported in OSA human patients despite the fact that OSA-dependent hypertension is approximately of the same magnitude as in CIH rodents. The increase in respiratory activity observed in rodents is particularly dramatic in arterially perfused preparations and has been attributed to an increase in central chemoreceptor activity but it could also be caused by increased tonic activity of carotid body afferents or by any form of hyperexcitability of the central pattern generator. The apparent sensitization of central chemoreceptors could also result from a reduction in brain perfusion causing PCO2 to accumulate abnormally next to the central chemoreceptors. As discussed below, the same mechanism (increased vascular resistance) could explain why breathing and/or cardiorespiratory coupling are also increased in arterially perfused preparations of spontaneously hypertensive rats (SHRs) (379, 466). The fact that there is no reported increase in resting breathing in OSA puts into question, if not the validity of severe CIH as a model of OSA, at least the theory that the hypertension caused by OSA could be primarily caused by an increase in “cardiorespiratory coupling.”
Hypertension, chemoreceptors, and cardiorespiratory coupling
In 1985, Trzebski and collaborators (418) reported that the peripheral chemoreflex is enhanced in hypertensive patients and proposed that increased carotid body function could be a contributing factor. Their evidence was based on the Dejours maneuver, the use of a very brief period of hyperoxia to transiently silence arterial chemoreceptors. This maneuver caused a greater breathing reduction and a greater decrease in BP and forearm resistance in hypertensive than in normotensive individuals. This phenomenon could in theory have had many causes including increased activity of carotid body afferents at rest, increased loop gain of the reflex or, more simply, increased vascular reactivity. Experiments conducted in adult SHRs by these investigators showed that the responsiveness of rat carotid chemoreceptors to hypoxia was greater in the SHR although the response to hypercapnia was unchanged (128). Confirming Trzebski’s hypothesis, bilateral resection of the carotid sinus nerves slows the development of hypertension in SHRs and substantially reduces its magnitude (6). Furthermore, in the arterially perfused rat preparation, thoracic SNA has a more pronounced respiratory-related bursting pattern at all ages in spontaneously hypertensive rats than in WKY rats (genetic controls) (280,379). The mean level of SNA may not be higher in SHRs except in neonates although this variable is extremely difficult to quantify across animals (379). According to Simms et al. (379), the increased respiratory modulation of SNA in SHRs is not caused by a sensitization of the central chemoreceptors, unlike in rats exposed to CIH, because the phrenic outflow was silenced at approximately the same level of perfusate CO2 concentration in SHRs as in WKY rats. The authors also saw no change in the SNA response to carotid body stimulation with i.v. injection of cyanide and therefore concluded that, in SHRs, the respiratory chemoreflexes are normal but the central coupling between the respiratory network and the sympathetic outflow is exacerbated. McBryde et al. (280) reached a different conclusion. They showed that, even in the arterially perfused preparation, carotid body denervation acutely reduces vascular resistance and the respiratory modulation of SNA. Oddly, the full effect of carotid denervation took an hour to reach its maximum. CNS pH and PCO2 were not measured in these experiments. A virtuous cycle of reduced sympathetic outflow causing reduced cerebral vascular resistance producing improved brain perfusion thereby reducing central chemoreceptor and begetting further reductions in sympathetic drive could perhaps explain the gradual effect of carotid body denervation observed by McBryde et al. (280).
The observations of Simms et al. (379) and McBryde et al. (280) seem to imply that increased cardiorespiratory coupling or increased central respiratory drive contributes to the increased sympathetic tone and consequent hypertension of the SH rat. These observations raise two questions. The first is the extent to which a change in SNA that is limited to a very small portion of the respiratory cycle (late expiratory phase) matters in terms of neurogenic vascular contraction. The second and more important one is whether a similar change in SNA or resting breathing pattern is present in hypertension in man. The answer to the second question seems negative for the moment (115). More troubling in fact, a central respiratory-sympathetic coupling analogous to what has been observed in anesthetized animals or in reduced preparations has never been clearly demonstrated in resting conscious man in whom the respiratory modulation of SNA seems caused primarily by lung afferent activity and by the respiratory fluctuations of baroreceptor afferent discharges (84,394). The existence of a strong central coupling between the RPG and the sympathetic outflow is an unassailable dogma in the field of cardiorespiratory control. While the phenomenon as observed in anesthetized or reduced preparations is not a matter of debate, its existence in conscious man or conscious animals has very little experimental support and, therefore, its importance should be questioned. Such coupling could well be a peculiarity of reduced or anesthetized preparations in which central respiratory drive is typically maintained at a very high level (half to two-third of its maximum level) and the brainstem neurons that generate the sympathetic tone receive few inputs other than those from the RPG and therefore could be artificially entrained to this oscillator.
In summary, the well-documented increase SNA present at rest in human essential hypertension (112) may partly result from increased carotid body function but there is yet no clear evidence of increased central respiratory drive or of increased central coupling between RPG and SNA in hypertensive man (115). This possibility is perhaps not definitively excluded but it is worth noting that, based on current understanding of how the peripheral sympathetic chemoreflex operates, neither increased coupling nor increased central respiratory drive are required for peripheral chemoreceptors to raise SNA. Indeed, a direct respiratory-independent pathway very probably links the carotid bodies to RVLM presympathetic neurons via the NTS (Fig. 8C). If this view is correct, the central respiratory gating of SNA may actually oppose the effect of this direct pathway on RVLM neurons. Alternately, this gating may simply adjust the response of these neurons to the chemoreceptor stimulus in a way that depends on AP and the baroreceptor feedback (Fig. 8C). Regardless of whether the increase in SNA is respiratory phasic or not, if it is driven by the carotid bodies, excision of these organelles could perhaps alleviate drug refractory hypertension in man as proposed by McBryde et al. (280). However, such a procedure could also have adverse consequences during or after anesthesia, under hypoxic conditions or during sleep apnea (254, 280).
Chemoreflexes in congestive heart failure
CHF is characterized by an elevated sympathetic tone to the heart and blood vessels, the degree of SNA activation rising steeply as the disease progresses (113). In advanced stages of the disease, breathing is also commonly affected (apneas and Cheyne-Stokes breathing). The efflux of epinephrine, norepinephrine, and their metabolites from the brain is greatly increased in CHF patients suggesting that CNS adrenergic and noradrenergic neurons are activated by the disease (229). Chronically elevated central catecholamine release may contribute to CNS inflammation as does increased SNA in peripheral organs including the kidney (280). The increase in CNS catecholaminergic neuron activity, probably including the C1 cells, the presumed source of the adrenaline overflow from the brain, likely contributes to the hypersympathetic state. Central chemoreceptor activation by hypercapnia produces a greater ventilatory and sympathetic stimulation in CHF patients than in normal individuals (306). This exacerbation of the central chemoreflex may contribute to the periodic breathing and central sleep apnea experienced by patients with severe CHF (435). However, enchanced carotid body function may be the most important factor contributing to the hypersympathetic state and respiratory disturbances typical of this disease (80, 242, 267, 342, 373). In CHF, carotid body afferents are firing even under normoxia and their activation by hypoxia is greatly enhanced (242, 342). Consistent with such hyperactivity, carotid body denervation dramatically reduces renal SNA while improving breathing stability and cardiac function (267). Increased coherence between SNA and plethysmography air flow signals has also been observed in this model, suggesting that sympatho-respiratory couping is enhanced (267). The nature of the coupling is yet to be determined in CHF. It could be central as has been sugeested in the case of CIH or hypertension (see preceding section). Many factors contribute to enhanced carotid body function in CHF including elevated levels of circulating angiotensin II, blood flow reduction, oxidative stress, abnormal production of gaseous transmitters (NO, CO2, and H2S) and changes in the expression level of Kv3.4 potassium channels [for recent reviews, see (372,373)]. In brief, both peripheral and central chemoreflexes are overactive in CHF. These abnormalities contribute to excessive sympathetic tone, increased loop gain of the respiratory chemoreflex and periodic breathing. The increased SNA observed in CHF also depends on other factors besides increased chemoreflexes such as hyperactivity of cardiac afferents and muscle metabotropic receptors, reduced baroreflex function and a hyperactive renin-angiotensin-aldosterone system (129, 444).
The congenital central hypoventilation syndrome
CCHS is a developmental disease caused by mutations of the transcription factor Phox2b, usually a polyalanine extension (from the normal 20 repeats up to 30) and more rarely a frameshift (16, 17, 451). The cardinal signs of the disease are hypoventilation, especially pronounced during sleep, and a dramatically reduced if not absent HCVR. Various degrees of autonomic insufficiency including cardiovascular and gastrointestinal dysfunction are also present. The severity of the disease in general and respiratory dysfunction in particular increases with the degree of expansion of the polyalanine moiety, 27 repeats typically causing the inability to breathe during sleep. Phox2b is implicated in the development of cardiopulmonary afferents, the carotid bodies, sympathetic and enteric ganglia, NTS and area postrema neurons, selected lower brainstem neurons including catecholaminergic cells, the RTN (specifically the Phox2b-expressing neurons), and vast numbers of pontine neurons (17, 73). This transcription factor remains expressed in many lower brainstem neurons of the adult (199).
Transgenic mice carrying the disease-defining mutation for CCHS (Phox2b27Ala/+) failed to respond to CO2 and died shortly after birth of respiratory failure (97). These mice lacked the RTN Phox2b-expressing glutamatergic neurons at birth. Because no obvious defect in the other Phox2b-expressing neuronal groups was detected, the loss of the RTN was thought to have been the critical factor underlying the respiratory failure. These authors then proceeded to ablate RTN neurons more selectively by intersectional genetics. Phox2blox/lox mice were crossed with mice expressing Cre recombinase under the control of the Lbx1 or Egr2 promoters (98). The resulting mice (Phox2blox/lox; Lbx1cre/0 and Phox2blox/lox; and Egr2cre/0) had characteristics similar to the Phox2b27ala/+ mice. They lacked RTN neurons, exhibited severely compromised breathing and lack of respiratory chemosensitivity at birth, and died shortly thereafter. In their most recent experiments, the same authors created a mouse that expresses Phox2b27ala conditionally upon Cre recombination (Phox2b27Alacki mice) (346). By crossing these animals with the above mentioned Egr2cre/0 mice, the toxic Phox2b27ala mutation could be selectively expressed in neurons of pontine (rhombomere 3 and 5) lineage. This protocol added yet another level of specificity since RTN neurons seem more vulnerable to the cytotoxic effect of the Phox2b mutation than many other Phox2b-expressing neurons (97). The offspring (Phox2b27Alacki; Egr2cre/0) lacked almost all RTN Phox2b neurons but unexpectedly survived to adulthood despite a complete loss of respiratory stimulation by CO2 for approximately 3 weeks after birth. The survival of these mice is in and of itself remarkable since it suggests that central chemosensitivity may not be necessary to breathe, at least postnatally. The survival of the mice was tentatively attributed to a respiratory compensation via peripheral chemoreceptors (346). The preceding transgenic models (Phox2blox/lox; Lbx1cre/0 and Phox2blox/lox; Egr2cre/0) which died shortly after birth probably had unrecognized neuronal abnormalities besides the loss of the RTN. The survival of a small number of functional RTN neurons in the Phox2b27Alacki; Egr2cre/0 mice may also explain why a portion of the HCVR (around a third) recovered in adulthood.
Genetic approaches such as these have their own interpretative limitations. For example, an abnormality in RTN development produced by the Phox2b27ala mutation could produce cascading defects on the function of other non-Phox2b-dependent pathways. Even the Phox2b27Alacki; Egr2cre/0 mice could have had additional defects besides the loss of RTN. Finally, the fact that RTN is indispensable to the chemoreflex does not prove that these neurons are the only CRCs, it could also mean that these neurons are the main nodal point through which CRCs activate the respiratory centers.
In conclusion, transgenic mice carrying the disease-defining mutation for CCHS (Phox2b27Ala/+) fail to respond to CO2 and die shortly after birth of respiratory failure. This model reproduces the human condition since humans with 27ala mutations have little or no HCVR and would not survive without respiratory assistance during sleep. Mutations that prevent RTN neuron development more selectively always produce major reduction of the HCVR at birth but at least one of them (Phox2b27Alacki; Egr2cre/0 mice) is survivable (346). The survival of these mice suggests that the respiratory deficits seen in humans with 27ala mutations could be caused by a combination of RTN loss and some other brainstem dysfunction. An additional deficit involving the peripheral chemoreflex is a plausible explanation. This reflex, which is also greatly reduced in CCHS, includes an uninterrupted chain of Phox2b-dependent cells (carotid body type 2 cells, carotid body afferents, and NTS glutamatergic neurons) (199) which could be dysfunctional in Phox2b27Ala/+ mice despite a seemingly normal gross morphological appearance (97). Although these speculations are appealing, RTN neurons have only been tentatively identified in man (358) and their loss in CCHS remains to be proven. Finally, CCHS presents with many other signs, notably cardiovascular and gastrointestinal, which indicates that Phox2b-dependent neurons other than RTN must be dysfunctional or absent in CCHS. The neural control of the circulation (sympathetic and parasympathetic alike) also relies on numerous neurons whose development is Phox2b-dependent and could potentially be affected by mutations of this gene. These neurons include cardiopulmonary afferents such as the baroreceptors, the NTS, C1, and other brainstem catecholaminergic neurons, cholinergic parasympathetic preganglionic neurons and sympathetic postganglionic noradrenergic neurons (17, 73).
SIDS, Central Oxygen Sensing, Gasping, and Autoresuscitation
SIDS: General theories
SIDS is tentatively attributed to the rare and fateful combination of a genetic predisposition, an immature and inherently unstable respiratory network and environmental factors (triple risk model) (32, 208). A defect in asphyxia-induced arousal probably contributes to a fraction of SIDS cases [for recent reviews, see (71)] although other causes of death are also being considered (see above-mentioned reviews). The failure to arouse may prevent a life-saving shift in body position during sleep that would free obstructed airways. A dysfunction of peripheral and/or central chemoreceptors could partly contribute to this defect. For example, hypoplasia of the carotid bodies has been observed in SIDS, although this problem may have resulted from exposure to intermittent hyperoxia as premature newborns (341). CNS defects may also contribute to SIDS although the evidence is largely of a postmortem neurohistological nature and typically relies on few specimens. The serotonergic system has received the greatest attention in terms of SIDS research. Abnormally high numbers of neurons and a reduction of selected serotonergic receptor subtypes have been reported suggesting delayed or abnormal development of these neurons (102). Serotonergic defects may reduce the gasping response which underlies the ability to resuscitate following repeated bouts of severe hypoxia (102). Since CNS serotonergic neurons are required for CO2-induced arousal in mice (49, 64), the collective evidence suggests that a defect in the development of the serotonergic system in man could perhaps contribute to the failure to arouse or to resuscitate via gasping and therefore to SIDS. Other brain regions or transmitters (e.g., cholinergic system, RTN) have also been found to be abnormal in SIDS (230, 336).
Mechanisms of autoresuscitation: Role and mechanism of brainstem hypoxia
The term autoresuscitation describes a powerful cardiorespiratory response elicited by asphyxia and which is probably triggered primarily by severe CNS hypoxia. It is unclear at present whether this response is a lifesaving reflex honed by evolution or the sometimes fortuitous last throes of a dying brain. Its main respiratory manifestation, gasping, is a brief and intense series of inspiratory efforts which, if unsuccessful in restoring oxygenation and eupnea, precedes death. Cardiovascular stimulation via increased sympathetic tone occurs concomitantly with gasping. During gasping, the inspiratory outflow resembles that produced in a preparation in which the brain is transected immediately rostral to the pre-Bötzinger complex (382). Specifically, the mass activity of the phrenic nerve is brief and lacks the ramp-like configuration typical of eupnea. One interpretation is that during severe brain hypoxia, many of the respiratory neurons that normally sculpt the membrane trajectory of inspiratory premotor neurons during the respiratory cycle are silenced by cerebral hypoxia or ischemia and the excitatory drive to these premotor neurons derives predominantly from the rhythmogenic neurons of the pre-Bötzinger complex (219, 331, 395). Hypoxia appears to reinstate intrinsic bursting properties in these neurons, a characteristic normally observed only in the neonate medulla oblongata (331,348). The mechanism underlying the bursting includes an increase in persistent sodium current, INaP, the TTX-sensitive window current derived from voltage-dependent sodium channels (79, 331). During hypoxia, this additional inward current presumably enables the excitatory rhythmogenic neurons of the pre-Bötzinger complex to burst in spite of the fact that the excitatory inputs that they receive from the respiratory network and elsewhere are severely depressed by hypoxia. The ability of hypoxia to increase the open probability of TTX-sensitive, inactivation-resistant sodium channels is not unique to the pre-Bötzinger complex. The phenomenon has been described in the hypothalamus and the hippocampus, and in cardiac myocytes (164, 165, 179, 197). In many neurons INaP is sufficient to drive spontaneous nonbursting (i.e., tonic) activity (417). INaP has this effect in the presympathetic C1 neurons (200, 243, 293). In vivo, C1 and other RVLM presympathetic neurons are powerfully activated by cerebral ischemia and by local application of cyanide (156,411,412); this increase in C1 activity most likely contributes to the Cushing response (65). Hypoxic depolarization of the C1 cells may also occur via ATP release from astrocytes. Indeed, ATP depolarizes the C1 cells in vivo (415) and in vitro [via P2Y1 receptors (455)] and channelrhodopsin-mediated depolarization of RVLM astrocytes increases sympathetic tone and excites the C1 cells in an ATP-dependent manner (270). Fast sodium channels are not known to be regulated by purinergic receptors, therefore, an increase in INaP and ATP release are presumably independent contributors to the hypoxic depolarization of brainstem neurons.
During exposure to hypoxia, an INaP-dependent gasp-like inspiratory pattern (fictive gasps) is also observed in reduced preparations such as thick slices of neonate rodent medulla oblongata (336, 437). These fictive gasps are no longer present when 5-HT2A receptors are blocked indicating that serotonin exerts an important facilitatory effect on this response (437). Consistent with this notion, survival to repeated bouts of hypoxia is severely compromised in rats or mice with genetic or chemical lesions of serotonergic neurons (64).
Summary and conclusions
Gasping is caused, at least in part, by an increase of INaP in the inspiratory rhythmogenic neurons of the pre-Bötzinger complex, a mechanism that is probably cell-autonomous (astrocyte-independent) but seems to be modulated by ATP release from glia (468). This effect of hypoxia on INaP has been observed in many brain regions and most certainly occurs elsewhere than in the pre-Bötzinger complex. The same mechanism (increased INaP and ATP release) likely contributes to the activation of the C1 neurons by severe hypoxia, an effect which is probably responsible for some portion of the CNS mediated and carotid body-independent activation of sympathetic nerves observed under this condition (156, 351, 412–414). Hypoxic activation of the C1 cells could also contribute to breathing stimulation and promote arousal (2). ATP release by hypoxia probably affects most respiratory neurons, however, since these neurons are typically activated by iontophoretically applied ATP in vivo (432). However, the by-product of ATP, adenosine, has profound depressant effects on brainstem neurons and many of these cells may be further hyperpolarized by the opening of KATP channels. As a result, hypoxia-induced ATP release may have inhibitory effects on multiple components of the network (468). Such an effect, combined with the direct activation of the pre-Bötzinger complex could contribute to the network reconfiguration triggered by severe hypoxia that causes gasping. In sum, severe hypoxia, like CO2 or acid seems to have both cell-autonomous (e.g., INaP and KATP) and paracrine (astrocyte-derived) effects on neurons. The relative contribution of each mechanism is uncertain and probably depends on the level of hypoxia and the type of neuron.
General Conclusions
Central respiratory chemoreception is not fully deciphered. Divergent views exist regarding the number, location, and types of cells that detect changes in CNS PCO2 and produce the cardiorespiratory adjustments in response to such changes. Levels of gases that produce arousal and stress recruit additional brain circuits besides those that are at work when blood gases hover around normal levels, making the identification of the CO2-responsive cells even more complex. Central chemosensitivity could be a widespread and emergent property of the brainstem respiratory network at large as proposed by many authors but the supporting evidence relies very heavily on a single technique, microdialysis and on the effect of brain lesions, genetic, or otherwise. Such lesions can interfere with motor performance in general. They can also interfere with arousal, vigilance, and stress responses, all of which are consequences of hypercapnia and hypoxia. In short, no definitive judgment can be made at present regarding how many CRCs there are because CRC candidates are still in the process of being vetted. The most critical experiments, which consist of deleting the molecules responsible for pH detection from these candidate CRCs, have rarely been done. At present, the RTN has satisfied the greatest number of criteria required of a CRC, including the fact that these neurons express a rare channel, TASK-2, whose deletion reduces both their pH sensitivity and the HCVR (445). However, TASK-2 deletion does not eliminate the pH sensitivity of every RTN neuron, which implies the existence of some molecular redundancy in the way these particular cells detect or process acidification. Although RTN neurons detect pH in a cell-autonomous manner, they also derive a portion of their CO2 sensitivity from surrounding astrocytes. The relative importance of these two modes of CO2 detection is unclear. The astrocytic component offers another potential example of molecular diversity since molecular CO2 may directly react with connexin channels while CO2-derived protons may depolarize the same cells via Kir channel closure. The pH sensitivity of other putative CRCs such as serotonergic neurons may again be different and the contribution of astrocytes to the chemosensitivity of the latter neurons is untested.
Regardless of whether CO2 activates CRCs directly or indirectly, these neurons undoubtedly receive countless synaptic inputs. These connections are poorly deciphered but enough is known to assert that central chemoreception does not operate as a simple feedback mechanism. RTN neurons, for example, are activated by CO2 but factor in information from the respiratory pattern generator, cardiopulmonary receptors such as lung stretch receptors and the carotid bodies and from the hypothalamus. The serotonergic neurons that regulate breathing presumably have a very different set of inputs which still need to be characterized.
RTN and the second-order neurons of the peripheral chemoreflex pathway innervate a common set of pontomedullary structures where breathing and the autonomic outflows are elaborated (NTS, VLM, and dorsolateral pons). These structures are presumably primarily responsible for the homeostatic regulation of CO2 that takes place under normal circumstances, that is, when the chemoreceptors exert small corrective effects in response to small changes in arterial PCO2 or pH.
Breathing is under triple control, metabolic, emotional, and voluntary. Severe hypercapnia or hypoxia produces arousal from sleep and, in awake mammals and man, adverse sensations and emotions which in turn produce cardiorespiratory responses. By definition, such effects engage brain circuits far beyond the core pontomedullary regions involved in generating the cardiorespiratory outflows. Some of the pathways involved in CO2 and hypoxia-induced stress and arousal include the NTS, C1 neurons, the parabrachial region, plus the noradrenergic, orexinergic, and serotonergic systems. All of these systems are activated by intense chemoreceptor stimulation and contribute to the cardiorespiratory changes elicited by such stimuli. Several of these systems (orexinergic, noradrenergic, and serotonergic) may be directly activated by increases in CNS PCO2 and therefore may also function as CRCs in these particular circumstances. What percentage of their response to hypercapnia in vivo is caused by their direct sensitivity to CO2 versus synaptic drives related to arousal or stress is a wide open question.
Central chemoreceptors such as the RTN appear to regulate every aspect of breathing including rate, inspiration, and active expiration. These various effects presumably rely on RTN projections to all regions of the RPG but, again, the details of these connections remain to be determined. RVLM presympathetic neurons have heterogeneous roles (e.g., control of blood flow in muscles versus gastrointestinal system, control of the heart, breathing, and arousal). By analogy, the 2000-odd neurons that make up the RTN in rats almost certainly consist of several subgroups of neurons that have specialized functions. Similar issues arise regarding the serotonergic neurons, only subsets of which are likely to function as CRCs.
Central and peripheral chemoreceptors regulate the sympathetic outflow both via the respiratory pattern generator and independently of it. The most direct route for sympathetic regulation by the carotid bodies is probably a direct excitatory input to RVLM presympathetic neurons from the commissural NTS. The respiratory-dependent route probably operates through the GABAergic neurons of the IVLM that also relay the baroreflex to the presympathetic neurons. These neurons in turn derive their respiratory modulation from GABAergic or glycinergic early inspiratory, inspiratory and postinspiratory neurons that are commonly found in the pre-Bötzinger complex and neighboring regions of the VRC. The respiratory-related neurons that modulate CVPGNs could be the same as those that regulate the IVLM. Although these connections “make sense” on anatomical grounds and their plausibility is often supported by modeling studies, none are proven. Also, these interpretations derive from experimentation in anesthetized animals where the role of the RVLM may predominate simply because a multitude of other circuits (spinal cord, midline medulla, and suprapontine inputs) are silent. The validity of these concepts should be verified in conscious animals. For example, the existence of a central coupling between the respiratory network and the sympathetic outflow has been very difficult to prove in conscious man or animals. Its functional importance is therefore still in question.
Dysfunctional chemoreceptors cause numerous pathologies. Mutations of the transcription factor Phox2b result in CCHS, a developmental disease in which the chemoreflexes are extremely weak, plausibly because RTN neurons fail to develop. Excessive peripheral chemoreceptor activity contributes to the abnormally high level of sympathetic tone present in essential hypertension, OSA and heart failure. In the first two diseases, increased carotid body activity produces hypertension but apparently no change in breathing. A possible explanation is that central chemoreceptors such as the RTN mitigate the respiratory stimulation caused by an increase in carotid body input. No such compensatory mechanism may exist to attenuate the effect of a chronically increased carotid body input on the sympathetic out-flow, especially under conditions where the baroreflex is also attenuated.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health (HL28785 and HL74011).
References
- 1.Abbott SB, Coates MB, Stornetta RL, Guyenet PG. Optogenetic stimulation of C1 and retrotrapezoid nucleus neurons causes sleep state-dependent cardiorespiratory stimulation and arousal in rats. Hypertension. 2013;61:835–841. doi: 10.1161/HYPERTENSIONAHA.111.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott SB, Depuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci. 2013;33:3164–3177. doi: 10.1523/JNEUROSCI.1046-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott SB, Kanbar R, Bochorishvili G, Coates MB, Stornetta RL, Guyenet PG. C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats. J Physiol. 2012;590:2897–2915. doi: 10.1113/jphysiol.2012.232157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci. 2011;31:16410–16422. doi: 10.1523/JNEUROSCI.3280-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: Patterns, origins, and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:19–25. doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: Comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 11.Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neurosci. 2011;190:207–227. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respir Physiol Neurobiol. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 14.Allen AM, Dosanjh JK, Erac M, Dassanayake S, Hannan RD, Thomas WG. Expression of constitutively active angiotensin receptors in the rostral ventrolateral medulla increases blood pressure. Hypertension. 2006;47:1054–1061. doi: 10.1161/01.HYP.0000218576.36574.54. [DOI] [PubMed] [Google Scholar]
- 15.Aller MI, Wisden W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neurosci. 2008;151:1154–1172. doi: 10.1016/j.neuroscience.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: Lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol. 2009;168:125–132. doi: 10.1016/j.resp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Amiel J, Laudier B, Attie-Bitach T, Trang H, de PL, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- 18.Anazco C, Pena-Munzenmayer G, Araya C, Cid LP, Sepulveda FV, Niemeyer MI. G protein modulation of K potassium channel TASK-2: A role of basic residues in the C terminus domain. Pflugers Arch. 2013;465:1715–1726. doi: 10.1007/s00424-013-1314-0. [DOI] [PubMed] [Google Scholar]
- 19.Anders K, Ballantyne D, Bischoff AM, Lalley PM, Richter DW. Inhibition of caudal medullary expiratory neurones by retrofacial inspiratory neurones in the cat. J Physiol. 1991;437:1–25. doi: 10.1113/jphysiol.1991.sp018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987;401(2):258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- 22.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 25.Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: Restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- 26.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: Target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bainton CR, Kirkwood PA. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banzett RB, Lansing RW, Brown R, Topulos GP, Yager D, Steele SM, Londono B, Loring SH, Reid MB, Adams L. ‘Air hunger’ from increased PCO2 persists after complete neuromuscular block in humans. Respir Physiol. 1990;81:1–17. doi: 10.1016/0034-5687(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 30.Barna BF, Takakura AC, Moreira TS. Pontomedullary and hypothalamic distribution of Fos-like immunoreactive neurons after acute exercise in rats. Neurosci. 2012;212:120–130. doi: 10.1016/j.neuroscience.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 31.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker LE. Neural maturational delay as a link in the chain of events leading to SIDS 176. Can J Neurol Sci. 1990;17:361–371. doi: 10.1017/s0317167100030894. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Tal A, Shamailov SS, Paton JF. Evaluating the physiological significance of respiratory sinus arrhythmia: Looking beyond ventilation-perfusion efficiency. J Physiol. 2012;590:1989–2008. doi: 10.1113/jphysiol.2011.222422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger AJ, Krasney JA, Dutton RE. Respiratory recovery from CO2 breathing in intact and chemodenervated awake dogs. J Appl Physiol. 1973;35:35–41. doi: 10.1152/jappl.1973.35.1.35. [DOI] [PubMed] [Google Scholar]
- 35.Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: A Fos study in adult rats. Brain Res. 2000;857:30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- 36.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 37.Bevan AT, Honour AJ, Stott FH. Direct arterial pressure recording in unrestricted man. Clin Sci. 1969;36:329–344. [PubMed] [Google Scholar]
- 38.Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 39.Biancardi V, da Silva LT, Bicego KC, Gargaglioni LH. Role of Locus coeruleus noradrenergic neurons in cardiorespiratory and thermal control during hypoxia. Respir Physiol Neurobiol. 2010;170:150–156. doi: 10.1016/j.resp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol. 2009;106:1564–1573. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO(2) J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520:1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodineau L, Frugière A, Marlot D, Wallois F. Connections between retrotrapezoid nucleus and nucleus tractus solitarii in cat. Neurosci Lett. 2000a;280:111–114. doi: 10.1016/s0304-3940(00)00770-9. [DOI] [PubMed] [Google Scholar]
- 44.Bodineau L, Frugiere A, Marlot D, Wallois F. Effect of hypoxia on the activity of respiratory and non-respiratory modulated retrotrapezoid neurons of the cat. Auton Neurosci. 2000b;86:70–77. doi: 10.1016/S1566-0702(00)00237-X. [DOI] [PubMed] [Google Scholar]
- 45.Bowes G, Townsend ER, Kozar LF, Bromley SM, Phillipson EA. Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs. J Appl Physiol. 1981;51:40–45. doi: 10.1152/jappl.1981.51.1.40. [DOI] [PubMed] [Google Scholar]
- 46.Bradley SR, Pieribone VA, Wang WG, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 47.Braga VA, Soriano RN, Braccialli AL, De Paula PM, Bonagamba LG, Paton JF, Machado BH. Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol. 2007;581:1129–1145. doi: 10.1113/jphysiol.2007.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 49.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos RR, McAllen RM. Tonic drive to sympathetic premotor neurons of rostral ventrolateral medulla from caudal pressor area neurons. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1209–R1213. doi: 10.1152/ajpregu.1999.276.4.R1209. [DOI] [PubMed] [Google Scholar]
- 51.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- 52.Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143:115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol. 1992;326:245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14:6500–6510. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: Implications for understanding baroreceptor reflex circuitry. J Neurosci. 1998;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chonan T, Mulholland MB, Leitner J, Altose MD, Cherniack NS. Sensation of dyspnea during hypercapnia, exercise, and voluntary hyperventilation. J Appl Physiol. 1990;68:2100–2106. doi: 10.1152/jappl.1990.68.5.2100. [DOI] [PubMed] [Google Scholar]
- 57.Cid LP, Roa-Rojas HA, Niemeyer MI, Gonzalez W, Araki M, Araki K, Sepulveda FV. TASK-2: a K2P K(+) channel with complex regulation and diverse physiological functions. Front Physiol. 2013;4:198. doi: 10.3389/fphys.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- 59.Coates EL, Li AH, Nattie EE. Acetazolamide on the ventral medulla of the cat increases phrenic output and delays the ventilatory response to CO2. J Physiol. 1991;441:433–451. doi: 10.1113/jphysiol.1991.sp018760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol. 1990;258:L33–L44. doi: 10.1152/ajplung.1990.258.2.L33. [DOI] [PubMed] [Google Scholar]
- 61.Coote JH. Spinal and supraspinal reflex pathways of cardio-cardiac sympathetic reflexes. Neurosci Lett. 1984;46:243–247. doi: 10.1016/0304-3940(84)90106-x. [DOI] [PubMed] [Google Scholar]
- 62.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 63.Cui Z, Fisher JA, Duffin J. Central-peripheral respiratory chemoreflex interaction in humans. Respir Physiol Neurobiol. 2012;180:126–131. doi: 10.1016/j.resp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurons compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol. 2011;589:5247–5256. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cushing H. Concerning a definitive regulatory mechanism of the vasomotor centre which controls blood pressure during cerebral compression. B Johns Hopkins Hosp. 1901;12:290–292. [Google Scholar]
- 66.da Silva GS, Li A, Nattie E. High CO2/H +dialysis in the caudal ventro- lateral medulla (Loeschcke’s area) increases ventilation in wakefulness. Respir Physiol Neurobiol. 2010;171:46–53. doi: 10.1016/j.resp.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dampney RA, Horiuchi J, McDowall LM. Hypothalamic mechanisms coordinating cardiorespiratory function during exercise and defensive behaviour. Auton Neurosci. 2008;142:3–10. doi: 10.1016/j.autneu.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Dampney RA, McAllen RM. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol. 1988;395:41–56. doi: 10.1113/jphysiol.1988.sp016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 70.Dampney RAL. The subretrofacial vasomotor nucleus: Anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog in Neurobiol. 1994;42:197–228. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 71.Darnall RA. The carotid body and arousal in the fetus and neonate. Respir Physiol Neurobiol. 2013;185:132–143. doi: 10.1016/j.resp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Darnall RA, Guyenet P. Respiratory modulation of pre- and postganglionic lumbar vasomotor sympathetic neurons in the rat. Neurosci Lett. 1990;119:148–152. doi: 10.1016/0304-3940(90)90820-y. [DOI] [PubMed] [Google Scholar]
- 73.Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 74.Davis SE, Solhied G, Castillo M, Dwinell MR, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- 75.Day TA, Wilson RJ. A negative interaction between brainstem and peripheral respiratory chemoreceptors modulates peripheral chemoreflex magnitude. J Physiol. 2009;587:883–896. doi: 10.1113/jphysiol.2008.160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neurosci. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- 77.Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- 78.Dejours P, Raynoud J, CUENOD CL, LABROUSSE Y. Instantaneous modifications of ventilation at the beginning and at the cessation of muscular exercise; interpretation. J Physiol (Paris) 1955;47:155–159. [PubMed] [Google Scholar]
- 79.Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- 80.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: Rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62:2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dembowsky K, Czachurski J, Amendt K, Seller H. Tonic descending inhibition of the spinal somato-sympathetic reflex from the lower brain stem. J Auton Nerv Syst. 1980;2:157–182. doi: 10.1016/0165-1838(80)90043-0. [DOI] [PubMed] [Google Scholar]
- 82.Dembowsky K, Lackner K, Czachurski J, Seller H. Tonic catecholaminergic inhibition of the spinal somato-sympathetic reflexes originating in the ventrolateral medulla oblongata. J Auton Nerv Syst. 1981;3:277–290. doi: 10.1016/0165-1838(81)90069-2. [DOI] [PubMed] [Google Scholar]
- 83.Dempsey JA. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol. 2012;590:4129–4144. doi: 10.1113/jphysiol.2012.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 85.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deuchars SA. Multi-tasking in the spinal cord–do ‘sympathetic’ interneurones work harder than we give them credit for? J Physiol. 2007;580:723–729. doi: 10.1113/jphysiol.2007.129429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deuchars SA, Milligan CJ, Stornetta RL, Deuchars J. GABAergic neurons in the central region of the spinal cord: A novel substrate for sympathetic inhibition. J Neurosci. 2005;25:1063–1070. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dias MB, Li A, Nattie EE. Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus (RTN) J Appl Physiol. 2008;105:83–90. doi: 10.1152/japplphysiol.00120.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dias MB, Li A, Nattie EE. Antagonism of orexin receptor 1 (OX1R) in the retrotrapezoid nucleus (RTN) inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dias MB, Nucci TB, Margatho LO, ntunes-Rodrigues J, Gargaglioni LH, Branco LGS. Raphe Magnus Nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol. 2007;103:1780–1788. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- 92.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 93.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol. 1985;360:261–273. doi: 10.1113/jphysiol.1985.sp015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: A neurophysiological study. J Physiol. 1984;347:397–409. doi: 10.1113/jphysiol.1984.sp015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc Natl Acad Sci U S A. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duffin J. The role of the central chemoreceptors: A modeling perspective. Respir Physiol Neurobiol. 2010;173:230–243. doi: 10.1016/j.resp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 100.Duffin J, Mateika JH. Cross-Talk opposing view: Peripheral and central chemoreflexes have additive effects on ventilation in humans. J Physiol. 2013;591:4351–4353. doi: 10.1113/jphysiol.2013.256800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dun NJ, Mo N. Inhibitory postsynaptic potentials in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1989;410:267–281. doi: 10.1113/jphysiol.1989.sp017532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duprat F, Lauritzen I, Patel A, Honore E. The TASK background K2P channels: Chemo- and nutrient sensors. Trends Neurosci. 2007;30:573–580. doi: 10.1016/j.tins.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 104.Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and Hypoxia: Chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathetic nerve. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- 105.Eldridge FL. Central integration of mechanisms in exercise hyperpnea. Med Sci Sports Exerc. 1994;26:319–327. [PubMed] [Google Scholar]
- 106.Eldridge FL, Millhorn DE, Kiley JP, Waldrop TG. Stimulation by central command of locomotion, respiration and circulation during exercise. Respir Physiol. 1985;59:313–337. doi: 10.1016/0034-5687(85)90136-7. [DOI] [PubMed] [Google Scholar]
- 107.Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- 108.Ennis M, Aston-Jones G. Potent inhibitory input to locus coeruleus from the nucleus prepositus hypoglossi. Brain Res Bull. 1989;22:793–803. doi: 10.1016/0361-9230(89)90022-1. [DOI] [PubMed] [Google Scholar]
- 109.Erlichman JS, Leiter JC. Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol (1985) 2010;108:1803–1811. doi: 10.1152/japplphysiol.01321.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir Physiol Neurobiol. 2010;173:305–311. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- 112.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 113.Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol. 2000;35:S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- 114.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fatouleh R, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity is not increased in essential hypertension or chronic obstructive pulmonary disease. J Physiol. 2011;589:4997–5006. doi: 10.1113/jphysiol.2011.210534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: So near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Finley JCW, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;571(2):108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- 119.Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Resp Physiol. 2000;119:189–197. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 120.Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: Systemic blood pressure. J Appl Physiol (1985) 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 121.Fletcher EC, Bao G, Miller CC., III Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol (1985) 1995;78:1516–1521. doi: 10.1152/jappl.1995.78.4.1516. [DOI] [PubMed] [Google Scholar]
- 122.Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 123.Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol. 2012;2:743–777. doi: 10.1002/cphy.c100045. [DOI] [PubMed] [Google Scholar]
- 124.Forster HV, Smith CA. Contributions of central and peripheral chemoreceptors to the ventilatory response to CO2/H+ J Appl Physiol. 2010;108:989–994. doi: 10.1152/japplphysiol.01059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fukuda Y, Honda Y. pH-sensitive cells at ventro–lateral surface of rat medulla oblongata. Nature. 1975;256:317–318. doi: 10.1038/256317a0. [DOI] [PubMed] [Google Scholar]
- 127.Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch. 1978;376:229–235. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- 128.Fukuda Y, Sato A, Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst. 1987;19:1–11. doi: 10.1016/0165-1838(87)90139-1. [DOI] [PubMed] [Google Scholar]
- 129.Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension. 2005;45:1173–1181. doi: 10.1161/01.HYP.0000168056.66981.c2. [DOI] [PubMed] [Google Scholar]
- 130.Garcia AJ, III, Koschnitzky JE, Dashevskiy T, Ramirez JM. Cardiorespiratory coupling in health and disease. Auton Neurosci. 2013;175:26–37. doi: 10.1016/j.autneu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garcia AJ, III, Koschnitzky JE, Ramirez JM. The physiological determinants of Sudden Infant Death Syndrome. Respir Physiol Neurobiol. 2013;189:288–300. doi: 10.1016/j.resp.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir Physiol Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gatti PJ, Johnson TA, Massari VJ. Can neurons in the nucleus ambiguus selectively regulate cardiac rate and atrio-ventricular conduction? J Auton Nerv Syst. 1996;57:123–127. doi: 10.1016/0165-1838(95)00104-2. [DOI] [PubMed] [Google Scholar]
- 134.Gautier H. Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol (1985) 1996;81:521–527. doi: 10.1152/jappl.1996.81.2.521. [DOI] [PubMed] [Google Scholar]
- 135.Gebber GL, Barman SM. Studies on the origin and generation of sympathetic nerve activity. Clin Exp Hypertens A. 1988;10(Suppl 1):33–44. doi: 10.3109/10641968809075962. [DOI] [PubMed] [Google Scholar]
- 136.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes A, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giaume C, Leybaert L, Naus CC, Saez JC. Connexin and pannexin hemichannels in brain glial cells: Properties, pharmacology, and roles. Front Pharmacol. 2013;4:88. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 140.Gonczi M, Szentandrassy N, Johnson IT, Heagerty AM, Weston AH. Investigation of the role of TASK-2 channels in rat pulmonary arteries; pharmacological and functional studies following RNA interference procedures. Br J Pharmacol. 2006;147:496–505. doi: 10.1038/sj.bjp.0706649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gonzalez D, Gomez-Hernandez JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. Faseb J. 2006;20:2329–2338. doi: 10.1096/fj.06-5828com. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez D, Gomez-Hernandez JM, Barrio LC. Molecular basis of voltage dependence of connexin channels: An integrative appraisal. Prog Biophys Mol Biol. 2007;94:66–106. doi: 10.1016/j.pbiomolbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 143.Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goridis C, Brunet JF. Central chemoreception: Lessons from mouse and human genetics. Respir Physiol Neurobiol. 2010;173:312–321. doi: 10.1016/j.resp.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 145.Gourine AV, Kasparov S. Astrocytes as brain interoceptors. Exp Physiol. 2011;96:411–416. doi: 10.1113/expphysiol.2010.053165. [DOI] [PubMed] [Google Scholar]
- 146.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 148.Granjeiro EM, Scopinho AA, Correa FM, Resstel LB. Prelimbic but not infralimbic cortex is involved in the pressor response to chemoreflex activation in awake rats. Exp Physiol. 2011;96:518–527. doi: 10.1113/expphysiol.2011.057596. [DOI] [PubMed] [Google Scholar]
- 149.Gray PA. Transcription factors and the genetic organization of brainstem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- 150.Grkovic I, Fernandez K, McAllen RM, Anderson CR. Misidentification of cardiac vagal pre-ganglionic neurons after injections of retrograde tracer into the pericardial space in the rat. Cell Tissue Res. 2005;321:335–340. doi: 10.1007/s00441-005-1145-1. [DOI] [PubMed] [Google Scholar]
- 151.Guo L, Bi X, Li Z, Zhang C. Effect of methylmercuric chloride on apoptosis and c-fos expression of brain glial cells. Wei Sheng Yan Jiu. 2002;31:7–9. [PubMed] [Google Scholar]
- 152.Guyenet PG. The 2008 Carl Ludwig Lecture: Retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guyenet PG. Cardiorespiratory Integration. In: Llewellyn-Smith IJ, Verberne AJ, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 2011. pp. 180–201. [Google Scholar]
- 154.Guyenet PG, Abbott SB. Chemoreception and asphyxia-induced arousal. Respir Physiol Neurobiol. 2013;188:333–343. doi: 10.1016/j.resp.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guyenet PG, Abbott SB, Stornetta RL. The respiratory chemoreception conundrum: Light at the end of the tunnel? Brain Res. 2012;1511:126–137. doi: 10.1016/j.brainres.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guyenet PG, Brown DL. Unit activity in nucleus paragigantocellularis lateralis during cerebral ischemia in the rat. Brain Res. 1986;364:301–314. doi: 10.1016/0006-8993(86)90843-7. [DOI] [PubMed] [Google Scholar]
- 157.Guyenet PG, Filtz TM, Donaldson SR. Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Res. 1987;407:272–284. doi: 10.1016/0006-8993(87)91105-x. [DOI] [PubMed] [Google Scholar]
- 158.Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2-chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010;108:995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. Invited Review EB 2012: C1 neurons: The body’s EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–R204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Habler HJ, Janig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol. 1994;43:567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 162.Haddad GG, Jiang C. Mechanisms of anoxia-induced depolarization in brainstem neurons - invitro current and voltage clamp studies in the adult rat. Brain Res. 1993;625:261–268. doi: 10.1016/0006-8993(93)91067-3. [DOI] [PubMed] [Google Scholar]
- 163.Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol. 1905;32:225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hammarstrom AK, Gage PW. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J Physiol. 1998;510:735–741. doi: 10.1111/j.1469-7793.1998.735bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hammarstrom AK, Gage PW. Hypoxia and persistent sodium current. Eur Biophys J. 2002;31:323–330. doi: 10.1007/s00249-002-0218-2. [DOI] [PubMed] [Google Scholar]
- 166.Haouzi P. Theories on the nature of the coupling between ventilation and gas exchange during exercise. Respir Physiol Neurobiol. 2006;151:267–279. doi: 10.1016/j.resp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 167.Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Med Biol. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- 168.Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol. 1989;256:R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- 169.Hassani OK, Henny P, Lee MG, Jones BE. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32:448–457. doi: 10.1111/j.1460-9568.2010.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci. 2012;32:16943–16952. doi: 10.1523/JNEUROSCI.3043-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Resp Physiol. 1996;105:35–45. doi: 10.1016/0034-5687(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 172.Herndon RM, Coyle JT. Selective destruction of neurons by a transmitter agonist. Science. 1977;198:71–72. doi: 10.1126/science.197604. [DOI] [PubMed] [Google Scholar]
- 173.Hilton SM, Marshall JM. The pattern of cardiovascular response to carotid chemoreceptor stimulation in the cat. J Physiol. 1982;326:495–513. doi: 10.1113/jphysiol.1982.sp014208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Honda Y. Respiratory and circulatory activities in carotid body-resected humans. J Appl Physiol. 1992;73:1–8. doi: 10.1152/jappl.1992.73.1.1. [DOI] [PubMed] [Google Scholar]
- 176.Hori T, Roth GI, Yamamoto WS. Respiratory sensitivity of rat brainstem surface to chemical stimuli. J Appl Physiol. 1970;28:721–724. doi: 10.1152/jappl.1970.28.6.721. [DOI] [PubMed] [Google Scholar]
- 177.Horiuchi J, Dampney RA. Evidence for tonic disinhibition of RVLM sympathoexcitatory neurons from the caudal pressor area. Auton Neurosci. 2002;99:102–110. doi: 10.1016/s1566-0702(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 178.Horiuchi J, McDowall LM, Dampney RA. Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol. 2009;587:5149–5162. doi: 10.1113/jphysiol.2009.179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Horn EM, Waldrop TG. Hypoxic augmentation of fast-inactivating and persistent sodium currents in rat caudal hypothalamic neurons. J Neurophysiol. 2000;84:2572–2581. doi: 10.1152/jn.2000.84.5.2572. [DOI] [PubMed] [Google Scholar]
- 180.Horner RL. Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2553–2564. doi: 10.1098/rstb.2009.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, Van den Pol AN. Hypocretin (Orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 182.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 183.Huckstepp RT, Dale N. Redefining the components of central CO2 chemosensitivity–towards a better understanding of mechanism. J Physiol. 2011;589:5561–5579. doi: 10.1113/jphysiol.2011.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huckstepp RT, Eason R, Sachdev A, Dale N. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huckstepp RT, Id BR, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2- dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huda R, McCrimmon DR, Martina M. pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol. 2013;110:368–377. doi: 10.1152/jn.01074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in nora-drenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 188.Iceman KE, Richerson GB, Harris MB. Medullary serotonin neurons are CO2-sensitive in situ. J Neurophysiol. 2013;110:2536–2544. doi: 10.1152/jn.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog in Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 190.Jacobs BL, Martín-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 191.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Janig W, Habler HJ. Neurophysiological analysis of target-related sympathetic pathways–from animal to human: Similarities and differences. Acta Physiol Scand. 2003;177:255–274. doi: 10.1046/j.1365-201X.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 194.Jansen ASP, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system:basis of the fight-or flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 195.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497:337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBotzinger complex. J Neurosci. 2013;33:9235–9245. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- 200.Kangrga IM, Loewy AD. Whole-cell recordings from visualized C1 adrenergic bulbospinal neurons: Ionic mechanisms underlying vasomotor tone. Brain Res. 1995;670:215–232. doi: 10.1016/0006-8993(94)01282-m. [DOI] [PubMed] [Google Scholar]
- 201.Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci. 2013;33:435–441. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kawai A, Onimaru H, Homma I. Mechanisms of CO2/H+ chemoreception by respiratory rhythm generator neurons in the medulla from newborn rats in vitro. J Physiol. 2006;572:525–537. doi: 10.1113/jphysiol.2005.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kc P, Haxhiu MA, Trouth CO, Balan KV, Anderson WA, Mack SO. CO2-induced c-Fos expression in hypothalamic vasopressin containing neurons. Resp Physiol. 2002;129:289–296. doi: 10.1016/s0034-5687(01)00321-8. [DOI] [PubMed] [Google Scholar]
- 206.Kim JE, Kwak SE, Kang TC. Upregulated TWIK-related acid-sensitive K+ channel-2 in neurons and perivascular astrocytes in the hippocampus of experimental temporal lobe epilepsy. Epilepsia. 2009;50:654–663. doi: 10.1111/j.1528-1167.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 207.Kimura Y, Hirooka Y, Sagara Y, Ito K, Kishi T, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of inducible nitric oxide synthase in rostral ventrolateral medulla causes hypertension and sympathoexcitation via an increase in oxidative stress. Circ Res. 2005;96:252–260. doi: 10.1161/01.RES.0000152965.75127.9d. [DOI] [PubMed] [Google Scholar]
- 208.Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kirkwood PA. Synaptic excitation in the thoracic spinal cord from expiratory bulbospinal neurones in the cat. J Physiol. 1995;484:201–225. doi: 10.1113/jphysiol.1995.sp020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kishi T, Hirooka Y, Sakai K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension. 2001;38:896–901. [PubMed] [Google Scholar]
- 211.Kline DD. Chronic intermittent hypoxia affects integration of sensory input by neurons in the nucleus tractus solitarii. Respir Physiol Neurobiol. 2010;174:29–36. doi: 10.1016/j.resp.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: Evidence for homeostatic plasticity. J Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci. 2010;30:4273–4284. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Botzinger complex in vitro. J Neurosci. 2008;28:1773–1785. doi: 10.1523/JNEUROSCI.3916-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1996a;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- 216.Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol. 1996b;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res. 1993;609:174–184. doi: 10.1016/0006-8993(93)90871-j. [DOI] [PubMed] [Google Scholar]
- 218.Koshiya N, Numao Y. The central respiratory drive-related discharge patterns in the sympathetic nerves of the rat in unanesthetized and anesthetized states. Neurosci Res. 1988;7:S33. [Google Scholar]
- 219.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 220.Krupp J, Feltz P. Excitatory postsynaptic currents and glutamate receptors in neonatal rat sympathetic preganglionic neurons in vitro. J Neurophysiol. 1995;73:1503–1512. doi: 10.1152/jn.1995.73.4.1503. [DOI] [PubMed] [Google Scholar]
- 221.Krupp J, Larmet Y, Feltz P. Postnatal change of glycinergic IPSC decay in sympathetic preganglionic neurons. NeuroReport. 1994;5:2437–2440. doi: 10.1097/00001756-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 222.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kukkonen JP, Holmqvist T, Ammoun S, Åkerman KEO. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- 224.Kumar P, Prabhakar NR. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kumar R, Ahdout R, Macey PM, Woo MA, Avedissian C, Thompson PM, Harper RM. Reduced caudate nuclei volumes in patients with congenital central hypoventilation syndrome. Neurosci. 2009;163:1373–1379. doi: 10.1016/j.neuroscience.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kuwaki T. Hypothalamic modulation of breathing. Adv Exp Med Biol. 2010a;669:243–247. doi: 10.1007/978-1-4419-5692-7_49. [DOI] [PubMed] [Google Scholar]
- 227.Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2010b;161:20–27. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 228.Kuwaki T, Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respir Physiol Neurobiol. 2010;174:43–54. doi: 10.1016/j.resp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 229.Lambert GW, Kaye DM, Lefkovits J, Jennings GL, Turner AG, Cox HS, Esler MD. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation. 1995;92:1813–1818. doi: 10.1161/01.cir.92.7.1813. [DOI] [PubMed] [Google Scholar]
- 230.Lavezzi AM, Weese-Mayer DE, Yu MY, Jennings LJ, Corna MF, Casale V, Oneda R, Matturri L. Developmental alterations of the respiratory human retrotrapezoid nucleus in sudden unexplained fetal and infant death. Auton Neurosci. 2012;170:12–19. doi: 10.1016/j.autneu.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 231.Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lazarenko RM, Stornetta RL, Bayliss DA, Guyenet PG. Orexin A activates retrotrapezoid neurons in mice. Respir Physiol Neurobiol. 2011;175:283–287. doi: 10.1016/j.resp.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 235.Lesage F, Barhanin J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology (Bethesda) 2011;26:424–437. doi: 10.1152/physiol.00029.2011. [DOI] [PubMed] [Google Scholar]
- 236.Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: Stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 237.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 diffusion pipette in vivo. J Appl Physiol. 1997;83:420–428. doi: 10.1152/jappl.1997.83.2.420. [DOI] [PubMed] [Google Scholar]
- 240.Li A, Randall M, Nattie EE. CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- 241.Li N, Li A, Nattie E. Focal microdialysis of CO(2) in the perifornical-hypothalamic area increases ventilation during wakefulness but not NREM sleep. Respir Physiol Neurobiol. 2013;185:349–355. doi: 10.1016/j.resp.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: Role of angiotensin II. J Physiol. 2006;575:215–227. doi: 10.1113/jphysiol.2006.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li YW, Bayliss DA, Guyenet PG. C1 neurons of neonatal rats: Intrinsic beating properties and α2-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1356–R1369. doi: 10.1152/ajpregu.1995.269.6.R1356. [DOI] [PubMed] [Google Scholar]
- 244.Lindqvist N, Liu Q, Zajadacz J, Franze K, Reichenbach A. Retinal glial (Muller) cells: Sensing and responding to tissue stretch. Invest Ophthalmol Vis Sci. 2010;51:1683–1690. doi: 10.1167/iovs.09-4159. [DOI] [PubMed] [Google Scholar]
- 245.Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lindsey BG, Rybak IA, Smith JC. Computational models and emergent properties of respiratory neural networks. Compr Physiol. 2012;2:1619–1670. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lipscomb WT, Boyarsky LL. Neurophysiological investigations of medullary chemosensitive areas of respiration. Respir Physiol. 1972;16:362–376. doi: 10.1016/0034-5687(72)90065-5. [DOI] [PubMed] [Google Scholar]
- 248.Lipski J, Kanjhan R, Kruszewska B, Rong WF. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: An intracellular study ‘in vivo’. J Physiol. 1996;490:729–744. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- 250.Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE. Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett. 2003;351:115–119. doi: 10.1016/s0304-3940(03)00770-5. [DOI] [PubMed] [Google Scholar]
- 251.Loeschcke HH, Koepchen HP, GERTZ KH. Effect of hydrogen ion concentration and carbon dioxide pressure in the cerebrospinal fluid on respiration. Pflugers Arch. 1958;266:569–585. doi: 10.1007/BF00363036. [DOI] [PubMed] [Google Scholar]
- 252.Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. Central Autonomic Pathways; pp. 88–103. [Google Scholar]
- 253.Lonergan T, Teschemacher AG, Hwang DY, Kim KS, Pickering AE, Kasparov S. Targeting brain stem centers of cardiovascular control using adenoviral vectors: Impact of promoters on transgene expression. Physiol Genomics. 2005;20:165–172. doi: 10.1152/physiolgenomics.00120.2004. [DOI] [PubMed] [Google Scholar]
- 254.Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med. 1971;285:1105–1111. doi: 10.1056/NEJM197111112852002. [DOI] [PubMed] [Google Scholar]
- 255.Luiten PGM, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 256.Macey PM, Macey KE, Woo MA, Keens TG, Harper RM. Aberrant neural responses to cold pressor challenges in congenital central hypoventilation syndrome. Pediatr Res. 2005;57:500–509. doi: 10.1203/01.PDR.0000155757.98389.53. [DOI] [PubMed] [Google Scholar]
- 257.Macey PM, Richard CA, Kumar R, Woo MA, Ogren JA, Avedissian C, Thompson PM, Harper RM. Hippocampal volume reduction in congenital central hypoventilation syndrome. PLoS One. 2009;4:e6436. doi: 10.1371/journal.pone.0006436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mack SO, Kc P, Wu M, Coleman BR, Tolentino-Silva FP, Haxhiu MA. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J Appl Physiol. 2002;92:826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- 259.Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J Appl Physiol. 2007;102:189–199. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Madden CJ, Stocker SD, Sved AF. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla (RVLM) neurons. Am J Physiol Regul Integr Comp Physiol. 2006;291:R751–R759. doi: 10.1152/ajpregu.00800.2005. [DOI] [PubMed] [Google Scholar]
- 262.Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol. 2003;285:H2734–H2748. doi: 10.1152/ajpheart.00155.2003. [DOI] [PubMed] [Google Scholar]
- 263.Madden CJ, Tupone D, Cano G, Morrison SF. alpha2 adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci. 2013;33:2017–2028. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Magosso E, Ursino M. A mathematical model of CO2 effect on cardiovascular regulation. Am J Physiol Heart Circ Physiol. 2001;281:H2036–H2052. doi: 10.1152/ajpheart.2001.281.5.H2036. [DOI] [PubMed] [Google Scholar]
- 265.Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, Simone DA. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 267.Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Marina N, Abdala AP, Korsak A, Simms AE, Allen AM, Paton JF, Gourine AV. Control of sympathetic vasomotor tone by catecholaminergic C1 neurones of the rostral ventrolateral medulla oblongata. Cardiovasc Res. 2011;91:703–710. doi: 10.1093/cvr/cvr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Marina N, Tang F, Figueiredo M, Mastitskaya S, Kasimov V, Mohamed-Ali V, Roloff E, Teschemacher AG, Gourine AV, Kasparov S. Purinergic signalling in the rostral ventrolateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res Cardiol. 2013;108:317. doi: 10.1007/s00395-012-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- 272.Marshall JM. The Joan Mott Prize Lecture. The integrated response to hypoxia: From circulation to cells. Exp Physiol. 1999;84:449–470. [PubMed] [Google Scholar]
- 273.Mateika JH, Duffin J. A review of the control of breathing during exercise. Eur J Appl Physiol Occup Physiol. 1995;71:1–27. doi: 10.1007/BF00511228. [DOI] [PubMed] [Google Scholar]
- 274.McAllen RM. Actions of carotid chemoreceptors on subretrofacial bulbospinal neurons in the cat. J Auton Nerv Syst. 1992;40:181–188. doi: 10.1016/0165-1838(92)90199-q. [DOI] [PubMed] [Google Scholar]
- 275.McAllen RM, Dampney RA. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci Lett. 1990;110:91–96. doi: 10.1016/0304-3940(90)90793-9. [DOI] [PubMed] [Google Scholar]
- 276.McAllen RM, May CN, Shafton AD. Functional anatomy of sympathetic premotor cell groups in the medulla. Clin Exp Hypertens. 1995;17:209–221. doi: 10.3109/10641969509087066. [DOI] [PubMed] [Google Scholar]
- 277.McAllen RM, Salo LM, Paton JF, Pickering AE. Processing of central and reflex vagal drives by rat cardiac ganglion neurones: An intracellular analysis. J Physiol. 2011;589:5801–5818. doi: 10.1113/jphysiol.2011.214320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.McAllen RM, Spyer KM. The location of cardiac vagal preganglionic motoneurones in the medulla of the cat. J Physiol. 1976;258:187–204. doi: 10.1113/jphysiol.1976.sp011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.McAllen RM, Spyer KM. The baroreceptor input to cardiac vagal motoneurones. J Physiol. 1978;282:365–374. doi: 10.1113/jphysiol.1978.sp012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- 281.McCall RB, Aghajanian GK. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979;169:11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- 282.McDougall SJ, Peters JH, Andresen MC. Convergence of cranial visceral afferents within the solitary tract nucleus. J Neurosci. 2009;29:12886–12895. doi: 10.1523/JNEUROSCI.3491-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 284.Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife. 2013;22:e01213. doi: 10.7554/eLife.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Hodges MR, Forster HV. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J Appl Physiol (1985) 2013;115:1088–1098. doi: 10.1152/japplphysiol.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Milner TA, Pickel VM, Morrison SF, Reis DJ. Adrenergic neurons in the rostral ventrolateral medulla: Ultrastructure and synaptic relations with other transmitter-identified neurons. Prog Brain Res. 1989;81:29–47. doi: 10.1016/s0079-6123(08)61998-6. [DOI] [PubMed] [Google Scholar]
- 287.Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. J Physiol. 1998;509:755–766. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol. 1963;18:523–533. doi: 10.1152/jappl.1963.18.3.523. [DOI] [PubMed] [Google Scholar]
- 289.Mo N, Dun NJ. Excitatory postsynaptic potentials in neonatal rat sympathetic preganglionic neurons: Possible mediation by NMDA receptors. Neurosci Lett. 1987;77:327–332. doi: 10.1016/0304-3940(87)90522-2. [DOI] [PubMed] [Google Scholar]
- 290.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Moosavi SH, Golestanian E, Binks AP, Lansing RW, Brown R, Banzett RB. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol. 2003;94:141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- 293.Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Autunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci. 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol. 2006;577:369–386. doi: 10.1113/jphysiol.2006.115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol. 2007;580:285–300. doi: 10.1113/jphysiol.2006.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Morrison SF. 2010 Carl Ludwig distinguished lectureship of the APS neural control and autonomic regulation section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J Physiol. 2012;590:3335–3347. doi: 10.1113/jphysiol.2012.234658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 301.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mulkey DK, Wenker IC. Astrocyte chemoreceptors: Mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp Physiol. 2011;96:400–406. doi: 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mulkey DK, Wenker IC, Kreneisz O. Current ideas on central chemoreception by neurons and glial cells in the retrotrapezoid nucleus. J Appl Physiol. 2010;108:1433–1439. doi: 10.1152/japplphysiol.01240.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Muller UW, Dembowsky K, Czachurski J, Seller H. Tonic descending inhibition of the spinal cardio-sympathetic reflex in the cat. J Auton Nerv Syst. 1988;23:111–123. doi: 10.1016/0165-1838(88)90075-6. [DOI] [PubMed] [Google Scholar]
- 305.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Narkiewicz K, Pesek CA, van de Borne PJ, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100:262–267. doi: 10.1161/01.cir.100.3.262. [DOI] [PubMed] [Google Scholar]
- 307.Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: Implications for hypertension. J Hypertens. 1997;15:1613–1619. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- 308.Natarajan M, Morrison SF. Sympathoexcitatory CVLM neurons mediate responses to caudal pressor area stimulation. Am J Physiol Regul Integr Comp Physiol. 2000;279:R364–R374. doi: 10.1152/ajpregu.2000.279.2.R364. [DOI] [PubMed] [Google Scholar]
- 309.Nattie E, Comroe Julius H., Jr distinguished lecture: Central chemoreception: Then . . . and now. J Appl Physiol. 2011;110:1–8. doi: 10.1152/japplphysiol.01061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- 311.Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Nattie E, Li A. Central chemoreception in wakefulness and sleep: Evidence for a distributed network and a role for orexin. J Appl Physiol. 2010;108:1417–1424. doi: 10.1152/japplphysiol.01261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nattie E, Shi J, Li A. Bicuculline dialysis in the retrotrapezoid nucleus (RTN) region stimulates breathing in the awake rat. Resp Physiol. 2001;124:179–193. doi: 10.1016/s0034-5687(00)00212-7. [DOI] [PubMed] [Google Scholar]
- 314.Nattie EE. Chemoreception and tonic drive in the retrotrapezoid nucleus (RTN) region of the awake rat: Bicuculline and muscimol dialysis in the RTN. Adv Exp Med Biol. 2001;499:27–32. doi: 10.1007/978-1-4615-1375-9_4. [DOI] [PubMed] [Google Scholar]
- 315.Nattie EE, Gdovin M, Li A. Retrotrapezoid nucleus glutamate receptors: Control of CO2-sensitive phrenic and sympathetic output. J Appl Physiol. 1993;74:2958–2968. doi: 10.1152/jappl.1993.74.6.2958. [DOI] [PubMed] [Google Scholar]
- 316.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol. 2002;544:603–616. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nattie EE, Li A. Multiple central chemoreceptor sites: Cell types and function in vivo. Adv Exp Med Biol. 2008;605:343–347. doi: 10.1007/978-0-387-73693-8_60. [DOI] [PubMed] [Google Scholar]
- 318.Nattie EE, Li AH. Fluorescence location of RVLM kainate microinjections that alter the control of breathing. J Appl Physiol. 1990;68:1157–1166. doi: 10.1152/jappl.1990.68.3.1157. [DOI] [PubMed] [Google Scholar]
- 319.Nattie EE, Li AH, St John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol. 1991;71:1364–1375. doi: 10.1152/jappl.1991.71.4.1364. [DOI] [PubMed] [Google Scholar]
- 320.Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: Implications for sudden infant death syndrome. J Neurosci. 2004;24:9261–9268. doi: 10.1523/JNEUROSCI.1918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: Endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circulation Research. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- 322.Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27:4435–4442. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: The regional differences. Neurosci Lett. 1987;81:279–284. doi: 10.1016/0304-3940(87)90396-x. [DOI] [PubMed] [Google Scholar]
- 324.Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol. 2010;95:657–667. doi: 10.1113/expphysiol.2009.049312. [DOI] [PubMed] [Google Scholar]
- 325.Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- 326.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Onimaru H, Ikeda K, Kawakami K. CO2-Sensitive Preinspiratory Neurons of the Parafacial Respiratory Group Express Phox2b in the Neonatal Rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Onimaru H, Ikeda K, Kawakami K. Postsynaptic mechanisms of CO2 responses in parafacial respiratory neurons of newborn rats. J Physiol. 2012;590:1615–1624. doi: 10.1113/jphysiol.2011.222687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Onimaru H, Ikeda K, Kawakami K. Relationship between the distribution of Phox2b expressing cells and blood vessels in the parafacial region of the ventral medulla of neonatal rats. Neurosci. 2012;212:131–139. doi: 10.1016/j.neuroscience.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 330.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- 332.Paton JF, Dickinson CJ, Mitchell G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: Introducing ‘Cushing’s mechanism’. Exp Physiol. 2009;94:11–17. doi: 10.1113/expphysiol.2008.043455. [DOI] [PubMed] [Google Scholar]
- 333.Paton JFR. Convergence properties of solitary tract neurones driven synaptically by cardiac vagal afferents in the mouse. J Physiol. 1998;508:237–252. doi: 10.1111/j.1469-7793.1998.237br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Paton JFR, Deuchars J, Li YW, Kasparov S. Properties of solitary tract neurones responding to peripheral arterial chemoreceptors. Neurosci. 2001;105:231–248. doi: 10.1016/s0306-4522(01)00106-3. [DOI] [PubMed] [Google Scholar]
- 335.Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 337.Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Phillipson EA, Kozar LF, Rebuck AS, Murphy E. Ventilatory and waking responses to CO2 in sleeping dogs. Am Rev Respir Dis. 1977;115:251–259. doi: 10.1164/arrd.1977.115.2.251. [DOI] [PubMed] [Google Scholar]
- 339.Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of jux-tacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 340.Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neurosci. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- 341.Porzionato A, Macchi V, Stecco C, De CR. The carotid body in Sudden Infant Death Syndrome. Respir Physiol Neurobiol. 2013;185:194–201. doi: 10.1016/j.resp.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 342.Prabhakar NR. Sensing hypoxia: Physiology, genetics and epigenetics. J Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Prabhakar NR, Peng YJ, Kumar GK, Pawar A. Altered carotid body function by intermittent hypoxia in neonates and adults: Relevance to recurrent apneas. Respir Physiol Neurobiol. 2007;157:148–153. doi: 10.1016/j.resp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 344.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Queiroz EA, Okada MN, Fumega U, Fontes MA, Moraes MF, Haibara AS. Excitatory amino acid receptors in the dorsomedial hypothalamus are involved in the cardiovascular and behavioural chemoreflex responses. Exp Physiol. 2011;96:73–84. doi: 10.1113/expphysiol.2010.054080. [DOI] [PubMed] [Google Scholar]
- 346.Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ramirez JM, Doi A, Garcia AJ, III, Elsen FP, Koch H, Wei AD. The cellular building blocks of breathing. Compr Physiol. 2012;2:2683–2731. doi: 10.1002/cphy.c110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ramirez JM, Quellmalz UJ, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. J Physiol. 1998;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Reis DJ, Golanov EV, Galea E, Feinstein DL. Central neurogenic neuroprotection: Central neural systems that protect the brain from hypoxia and ischemia. Ann N Y Acad Sci. 1997;835:168–186. doi: 10.1111/j.1749-6632.1997.tb48628.x. [DOI] [PubMed] [Google Scholar]
- 351.Reis DJ, Golanov EV, Ruggiero DA, Sun M-K. Sympathoexcitatory neurons of the rostral ventrolateral medulla are oxygen sensors and essential elements in the tonic and reflex control of the systemic and cerebral circulations. J Hypertens. 1994;12(Suppl 10):S159–S180. [PubMed] [Google Scholar]
- 352.Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 353.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 354.Richter DW. Commentary on eupneic breathing patterns and gasping. Resp Physiol. 2003;139:121–130. doi: 10.1016/s1569-9048(03)00196-4. [DOI] [PubMed] [Google Scholar]
- 355.Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: Selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- 357.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: Effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci. 1984;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Rudzinski E, Kapur RP. PHOX2B immunolocalization of the candidate human retrotrapezoid nucleus. Pediatr Dev Pathol. 2010;13:291–299. doi: 10.2350/09-07-0682-OA.1. [DOI] [PubMed] [Google Scholar]
- 359.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science. 1988;240:1328–1330. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 360.Sakurai T, Mieda M, Tsujino N. The orexin system: Roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- 361.Santin JM, Watters KC, Putnam RW, Hartzler LK. Temperature influences neuronal activity and CO2/pH sensitivity of locus coeruleus neurons in the bullfrog, Lithobates catesbeianus. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1451–R1464. doi: 10.1152/ajpregu.00348.2013. [DOI] [PubMed] [Google Scholar]
- 362.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sartor DM, Verberne AJ. The sympathoinhibitory effects of systemic cholecystokinin are dependent on neurons in the caudal ventrolateral medulla in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1390–R1398. doi: 10.1152/ajpregu.00314.2006. [DOI] [PubMed] [Google Scholar]
- 364.Sato A, Fidone S, Eyzaguirre C. Presence of chemoreceptor and baroreceptor C-fibers in the carotid nerve of the cat. Brain Res. 1968;11:459–463. doi: 10.1016/0006-8993(68)90041-3. [DOI] [PubMed] [Google Scholar]
- 365.Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol. 1992;73:96–100. doi: 10.1152/jappl.1992.73.1.96. [DOI] [PubMed] [Google Scholar]
- 366.Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: A tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 367.Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurones. J Physiol. 2007;579:765–782. doi: 10.1113/jphysiol.2006.122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Schlaefke ME, See WR, Loeschcke HH. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respir Physiol. 1970;10:198–212. doi: 10.1016/0034-5687(70)90083-6. [DOI] [PubMed] [Google Scholar]
- 369.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 370.Schreihofer AM, Guyenet PG. Baroactivated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol. 2003;89:1265–1277. doi: 10.1152/jn.00737.2002. [DOI] [PubMed] [Google Scholar]
- 371.Schreihofer AM, Sved AF. The Ventrolateral Medulla and Sympathetic Regulation of Arterial Pressure. In: Llewellyn-Smith IJ, Verberne AJ, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 2011. pp. 78–97. [Google Scholar]
- 372.Schultz HD, Del Rio R, Ding Y, Marcus NJ. Role of neurotransmitter gases in the control of the carotid body in heart failure. Respir Physiol Neurobiol. 2012;184:197–203. doi: 10.1016/j.resp.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep. 2013;15:356–362. doi: 10.1007/s11906-013-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Severinghaus JW. Hans Loeschcke, Robert Mitchell and the medullary CO2 chemoreceptors: A brief historical review. Respir Physiol. 1998;114:17–24. doi: 10.1016/s0034-5687(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 375.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are central pH chemoreceptors. Nat Neurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 376.Seyedabadi M, Li Q, Padley JR, Pilowsky PM, Goodchild AK. A novel pressor area at the medullo-cervical junction that is not dependent on the RVLM: Efferent pathways and chemical mediators. J Neurosci. 2006;26:5420–5427. doi: 10.1523/JNEUROSCI.1190-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Shea SA, Andres LP, Shannon DC, Banzett RB. Ventilatory responses to exercise in humans lacking ventilatory chemosensitivity. J Physiol. 1993;468:623–640. doi: 10.1113/jphysiol.1993.sp019792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: A model of sympathetic activation in the rat. Respir Physiol. 2000;121:173–184. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
- 379.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: Does it contribute to hypertension? J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: Evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: Building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: A hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex–a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- 386.Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- 387.Soffin EM, Evans ML, Gill CH, Harries MH, Benham CD, davies CH. SB-334867-A antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology. 2002;42:127–133. doi: 10.1016/s0028-3908(01)00156-3. [DOI] [PubMed] [Google Scholar]
- 388.Solomon IC, Edelman NH, O’Neill MH. CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol. 2000;88:1996–2007. doi: 10.1152/jappl.2000.88.6.1996. [DOI] [PubMed] [Google Scholar]
- 389.Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol. 2009;165:1–8. doi: 10.1016/j.resp.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Song G, Wang H, Xu H, Poon CS. Kolliker-Fuse neurons send collateral projections to multiple hypoxia-activated and nonactivated structures in rat brainstem and spinal cord. Brain Struct Funct. 2012;217:835–858. doi: 10.1007/s00429-012-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Song G, Xu H, Wang H, Macdonald SM, Poon CS. Hypoxia-excited neurons in NTS send axonal projections to Kolliker-Fuse/parabrachial complex in dorsolateral pons. Neurosci. 2011;175:145–153. doi: 10.1016/j.neuroscience.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Loewy AD, Spyer KM. The Central Nervous Organization of Reflex Circulatory Control. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 168–188. [Google Scholar]
- 393.Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2603–2610. doi: 10.1098/rstb.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.res.85.5.457. [DOI] [PubMed] [Google Scholar]
- 395.St-John WM, Stornetta RL, Guyenet PG, Paton JF. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J Physiol. 2009;587:3175–3188. doi: 10.1113/jphysiol.2009.170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sterling P. Principles of Allostasis: Optimal Design, Predictive Regulation, Pathophysiology and Rational Therapeutics. In: Jay Schulkin., editor. Allostasis, Homeostasis, and the Costs of Physiological Adaptation. New York, NY: Cambridge University Press; 2004. pp. 1–36. [Google Scholar]
- 397.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Stojicic S, Milutinovic-Smiljanic S, Sarenac O, Milosavljevic S, Paton JF, Murphy D, Japundzic-Zigon N. Blockade of central vasopressin receptors reduces the cardiovascular response to acute stress in freely moving rats. Neuropharmacology. 2008;54:824–836. doi: 10.1016/j.neuropharm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 399.Stornetta RL. Neurochemistry of bulbospinal presympathetic neurons of the medulla oblongata. J Chem Neuroanat. 2009;38:222–230. doi: 10.1016/j.jchemneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J Comp Neurol. 2004;479:257–270. doi: 10.1002/cne.20332. [DOI] [PubMed] [Google Scholar]
- 401.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Stornetta RL, Schreihofer AM, Pelaez NM, Sevigny CP, Guyenet PG. Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. J Comp Neurol. 2001;435:111–126. doi: 10.1002/cne.1196. [DOI] [PubMed] [Google Scholar]
- 403.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- 404.Stornetta RL, Spirovski D, Moreira TS, Takakura AC, West GH, Gwilt JM, Pilowsky PM, Guyenet PG. Galanin is a selective marker of the retrotrapezoid nucleus in rats. J Comp Neurol. 2009;512:373–383. doi: 10.1002/cne.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Strack AM, Loewy AD. Pseudorabies virus: A highly specific transneuronal cell body marker in the sympathetic nervous system. J Neurosci. 1990;10:2139–2147. doi: 10.1523/JNEUROSCI.10-07-02139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 407.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 408.Su CK, Ho CM, Kuo HH, Wen YC, Chai CY. Sympathetic-correlated c-Fos expression in the neonatal rat spinal cord in vitro. J Biomed Sci. 2009;16:44. doi: 10.1186/1423-0127-16-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: A paradoxical problem and its solutions in cultured brainstem neurons. J Physiol. 2007;578:831–841. doi: 10.1113/jphysiol.2006.115758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Sun MK, Hackett JT, Guyenet PG. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in the presence of a glutamate-receptor antagonist. Brain Res. 1988;438:23–40. doi: 10.1016/0006-8993(88)91320-0. [DOI] [PubMed] [Google Scholar]
- 411.Sun MK, Jeske IT, Reis DJ. Cyanide excites medullary sympathoexcitatory neurons in rats. Am J Physiol. 1992;262:R182–R189. doi: 10.1152/ajpregu.1992.262.2.R182. [DOI] [PubMed] [Google Scholar]
- 412.Sun MK, Reis DJ. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol. 1994a;44:197–219. doi: 10.1016/0301-0082(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 413.Sun MK, Reis DJ. Hypoxia selectively excites vasomotor neurons of rostral ventrolateral medulla in rats. Am J Physiol Regul Integr Comp Physiol. 1994b;266:R245–R256. doi: 10.1152/ajpregu.1994.266.1.R245. [DOI] [PubMed] [Google Scholar]
- 414.Sun MK, Reis DJ. Medullary vasomotor activity and hypoxic sympathoexcitation in pentobarbital-anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 1996;270:R348–R355. doi: 10.1152/ajpregu.1996.270.2.R348. [DOI] [PubMed] [Google Scholar]
- 415.Sun MK, Wahlestedt C, Reis DJ. Action of externally applied ATP on rat reticulospinal vasomotor neurons. Eur J Pharmacol. 1992;224:93–96. doi: 10.1016/0014-2999(92)94824-f. [DOI] [PubMed] [Google Scholar]
- 416.Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO(2) activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 417.Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 418.Tafil-Klawe M, Trzebski A, Klawe J, Palko T. Augmented chemoreceptor reflex tonic drive in early human hypertension and in normotensive subjects with family background of hypertension. Acta Physiol Pol. 1985;36:51–58. [PubMed] [Google Scholar]
- 419.Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol. 2001;90:2007–2013. doi: 10.1152/jappl.2001.90.5.2007. [DOI] [PubMed] [Google Scholar]
- 420.Takakura AC, Moreira TS. Arterial chemoreceptor activation reduces the activity of parapyramidal serotonergic neurons in rats. Neurosci. 2013;237:199–207. doi: 10.1016/j.neuroscience.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 421.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Takakura AC, Moreira TS, De Paula PM, Menani JV, Colombari E. Control of breathing and blood pressure by parafacial neurons in conscious rats. Exp Physiol. 2012;98:304–315. doi: 10.1113/expphysiol.2012.065128. [DOI] [PubMed] [Google Scholar]
- 423.Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Takakura AC, Moreira TS, West GH, Gwilt JM, Colombari E, Stornetta RL, Guyenet PG. GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J Neurophysiol. 2007;98:374–381. doi: 10.1152/jn.00322.2007. [DOI] [PubMed] [Google Scholar]
- 425.Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 426.Tarkan O, Sari P, Demirhan O, Kiroglu M, Tuncer U, Surmelioglu O, Ozdemir S, Yilmaz MB, Kara K. Connexin 26 and 30 mutations in paediatric patients with congenital, non-syndromic hearing loss treated with cochlear implantation in Mediterranean Turkey. J Laryngol Otol. 2013;127:33–37. doi: 10.1017/S0022215112002587. [DOI] [PubMed] [Google Scholar]
- 427.Teppema LJ, Berkenbosch A, Veening JG, Olievier CN. Hypercapnia induces c-fos expression in neurons of retrotrapezoid nucleus in cats. Brain Res. 1994;635:353–356. doi: 10.1016/0006-8993(94)91462-1. [DOI] [PubMed] [Google Scholar]
- 428.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: Mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 429.Teppema LJ, Smith CA. CrossTalk opposing view: Peripheral and central chemoreceptors have hyperadditive effects on respiratory motor control. J Physiol. 2013;591:4359–4361. doi: 10.1113/jphysiol.2013.256818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 431.Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- 432.Thomas T, Ralevic V, Gadd CA, Spyer KM. Central CO2 chemoreception: A mechanism involving P2 purinoceptors localized in the ventrolateral medulla of the anaesthetized rat. J Physiol. 1999;517:899–905. doi: 10.1111/j.1469-7793.1999.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Tian JB, Bishop GA. Stimulus-dependent activation of c-Fos in neurons and glia in the rat cerebellum. J Chem Neuroanat. 2002;23:157–170. doi: 10.1016/s0891-0618(01)00153-3. [DOI] [PubMed] [Google Scholar]
- 434.Tin C, Song G, Poon CS. Hypercapnia attenuates inspiratory amplitude and expiratory time responsiveness to hypoxia in vagotomized and vagal-intact rats. Respir Physiol Neurobiol. 2012;181:79–87. doi: 10.1016/j.resp.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Topor ZL, Johannson L, Kasprzyk J, Remmers JE. Dynamic ventilatory response to CO(2) in congestive heart failure patients with and without central sleep apnea. J Appl Physiol. 2001;91:408–416. doi: 10.1152/jappl.2001.91.1.408. [DOI] [PubMed] [Google Scholar]
- 436.Toward MA, Abdala AP, Knopp SJ, Paton JF, Bissonnette JM. Increasing brain serotonin corrects CO2 chemosensitivity in methyl-CpG-binding protein 2 (Mecp2)-deficient mice. Exp Physiol. 2013;98:842–849. doi: 10.1113/expphysiol.2012.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: A rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ursino M, Magosso E, Avanzolini G. An integrated model of the human ventilatory control system: The response to hypercapnia. Clin Physiol. 2001;21:447–464. doi: 10.1046/j.1365-2281.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 439.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- 440.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neurosci. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 442.Viemari JC, Garcia AJ, III, Doi A, Elsen G, Ramirez JM. beta-Noradrenergic receptor activation specifically modulates the generation of sighs in vivo and in vitro. Front Neural Circuits. 2013;7:1–14. doi: 10.3389/fncir.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Wade JG, Larson CP, Jr, Hickey RF, Ehrenfeld WK, Severinghaus JW. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:823–829. doi: 10.1056/NEJM197004092821501. [DOI] [PubMed] [Google Scholar]
- 444.Wang HJ, Li YL, Zucker IH, Wang W. Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1260–R1270. doi: 10.1152/ajpregu.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to acidification and CO2. J Neurosci. 2013;33:7756–7761. doi: 10.1523/JNEUROSCI.5550-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wang WA, Richerson GB. Chemosensitivity of non-respiratory rat CNS neurons in tissue culture. Brain Res. 2000;860:119–129. doi: 10.1016/s0006-8993(00)02033-3. [DOI] [PubMed] [Google Scholar]
- 449.Wang WG, Tiwari JK, Bradley SR, Zaykin AV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- 450.Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 451.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Keens TG, Loghmanee DA, Trang H. An official ATS clinical policy statement: Congenital central hypoventilation syndrome: Genetic basis, diagnosis, and management. Am J Respir Crit Care Med. 2010;181:626–644. doi: 10.1164/rccm.200807-1069ST. [DOI] [PubMed] [Google Scholar]
- 452.Weese-Mayer DE, Rand CM, Berry-Kravis EM, Jennings LJ, Loghmanee DA, Patwari PP, Ceccherini I. Congenital central hypoventilation syndrome from past to future: Model for translational and transitional autonomic medicine. Pediatr Pulmonol. 2009;44:521–535. doi: 10.1002/ppul.21045. [DOI] [PubMed] [Google Scholar]
- 453.Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol. 2012;590:2137–2150. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wenker IC, Sobrinho CR, Takakura AC, Mulkey DK, Moreira TS. P2Y1 receptors expressed by C1 neurons determine peripheral chemoreceptor modulation of breathing, sympathetic activity, and blood pressure. Hypertension. 2013;62:263–273. doi: 10.1161/HYPERTENSIONAHA.113.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Weston MC, Stornetta RL, Guyenet PG. Glutamatergic neuronal projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J Comp Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]
- 457.Willette RN, Barcas PP, Krieger AJ, Sapru HN. Vasopressor and depressor areas in the rat medulla. Identification by microinjection of L-glutamate. Neuropharmacology. 1983;22:1071–1079. doi: 10.1016/0028-3908(83)90027-8. [DOI] [PubMed] [Google Scholar]
- 458.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Wilson RJ, Day TA. CrossTalk opposing view: Peripheral and central chemoreceptors have hypoadditive effects on respiratory motor output. J Physiol. 2013;591:4355–4357. doi: 10.1113/jphysiol.2013.256578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Xie A, Skatrud JB, Morgan BJ, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Yang Z, Coote JH. The role of supra-spinal vasopressin and glutamate neurones in an increase in renal sympathetic activity in response to mild haemorrhage in the rat. Exp Physiol. 2006;91:791–797. doi: 10.1113/expphysiol.2006.034082. [DOI] [PubMed] [Google Scholar]
- 463.Yeh ER, Erokwu B, LaManna JC, Haxhiu MA. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci Lett. 1997;232:63–66. doi: 10.1016/s0304-3940(97)00579-x. [DOI] [PubMed] [Google Scholar]
- 464.Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained and intermittent hypoxia reduce function of ATP-sensitive potassium channels in nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1555–R1562. doi: 10.1152/ajpregu.90390.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 466.Zoccal DB, Machado BH. Coupling between respiratory and sympathetic activities as a novel mechanism underpinning neurogenic hypertension. Curr Hypertens Rep. 2011;13:229–236. doi: 10.1007/s11906-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 467.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Zwicker JD, Rajani V, Hahn LB, Funk GD. Purinergic modulation of preBotzinger complex inspiratory rhythm in rodents: The interaction between ATP and adenosine. J Physiol. 2011;589:4583–4600. doi: 10.1113/jphysiol.2011.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]