Anti-thyroglobulin autoantibodies (TgAb) frequently cause false low measurements of serum thyroglobulin (Tg) when immunometric assays (TgIA) are used (1). This problem can be overcome by tryptic digestion of patient serum with subsequent measurement of Tg-proteotypic peptides by LC-MS/MS (TgMS) (2-4). However, it remains uncertain how different TgMS assays compare with each other, a crucial question in today's laboratory testing environment, wherein concern has been raised over patient samples being tested by different methods or in different laboratories over time (5). We therefore performed a pilot study to assess the inter-method concordance between four independently developed TgMS methods. The study was approved by the Mayo Clinic institutional review board.
We tested 40 patient serum samples spanning Tg concentrations from <0.1 to 10ng/mL (assigned by the DxI TgIA, Beckman Coulter). We classified the samples as TgAb positive (N=22) or negative (N=18) based on whether they contained TgAb concentrations that exceeded the functional sensitivity of the Beckman DxI TgAB assays (1.8 IU/mL). Specimens were de-identified and shared between four laboratories for TgMS testing: ARUP Laboratories (ARUP), Laboratory Corporation of America® Holdings (LabCorp), Mayo Clinic, and the University of Washington (UWash). Each assay employs different sample preparation methodologies: ARUP and Mayo utilize different protein precipitation steps to enrich for thyroglobulin prior to proteolysis and LabCorp and UWash digest serum samples directly after different denaturing steps. The peptide targets are also different between the assays: ARUP enriches and monitors the peptide VIFDANAPVAVR, while LabCorp, Mayo, and UWash enrich and monitor the peptide FSPDDSAGASALLR for primary quantification.
Mayo calibrators were also shared between laboratories (Tg-negative/TgAb-negative pooled serum spiked with international reference material, BCR-457®) and run as unknowns in each assay. A different batch of samples (owing to specimen volume limitations) also spanning Tg concentrations of <0.1 to 10ng/mL and with similar TgAb status were assayed across four automated TgIA assays: DxI-Tg, Immulite®-Tg (Siemens Corporation), Elecsys® Tg II (Roche Diagnostics), and Kryptor-Tg (Thermo Fisher).
Comparison of Mayo calibration material between TgMS methods demonstrated excellent correlation, but showed significant differences in calibrator assignment. Utilizing Mayo values as a reference, slopes were: 0.826, 0.878, and 0.814, and r2: 0.999, 0.999, and 0.994 for ARUP, LabCorp, and UWash, respectively. The variation in the observed slopes are likely explained by the different approach to calibration: ARUP utilizes the calibration materials provided with the Beckman DxI assay, Mayo utilizes BCR-457 spiked into Tg-negative serum, and LabCorp and UWash utilize human samples assigned by the Beckman DxI assay.
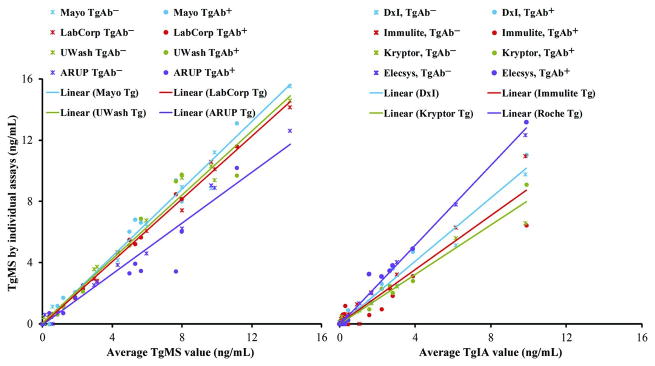
Comparison of TgMS methods in the patient samples also showed good correlation (Figure 1). Defining the mean Tg value (ng/mL) across all four TgMS assays as the reference, slopes across patient specimens were: 0.833, 1.027, 1.043, and 1.102, while r2 were 0.961, 0.996, 0.981 and 0.988 for ARUP, LabCorp, UWash, and Mayo respectively. When the Mayo assay's patient values and calibrators are used as the reference instead, slopes are: 0.740, 0.920, and 0.942, and r2: 0.932, 0.983, and 0.970 for ARUP, LabCorp, and UWash, respectively. The contribution of the calibration bias on the slope can be seen when the values are adjusted for each assay's calibration bias versus the Mayo calibrators. This yields slopes of 0.895, 1.04, and 1.16, with identical r2 values for ARUP, LabCorp, and UWash, respectively.
Figure 1.
Method comparison of all TgMS, and TgIA assays. Mean values of all TgMS and TgIA assays are on the abscissas and differentiated assay values are on the ordinate. In each case a single color represents a single assay, and a star represents a TgAb- specimen, where a solid circle represents a TgAb+ specimen. Linear regression is represented across all specimens (both TgAb- and TgAb+). For TgMS, slopes are: 1.102, 1.027, 1.043, and 0.833, while r2 are: 0.988, 0.996, 0.981, and 0.961 for Mayo, LabCorp, UWash, and ARUP, respectively. For TgIA, slopes are: 1.029, 0.876, 0.803 and 1.297 and r2 are: 0.983, 0.911, 0.971 and 0.993 for the DxI, Immulite, Kryptor and Elecsys TgIA, respectively. As a point of note, the bias induced by TgAb-positivity into the Tg measurements by the TgIAs is of a comparable magnitude in all but the Immulite assay. This explains why there is no apparent large bias between most of the TgIAs in measurements of TgAb positive samples. For further details on this subject we point the reader to the supplemental material published with reference number 2.
These comparative linear fit data for the TgMS assays are similar to those obtained when comparing the different TgIAs, which gave slopes to the all TgIA mean of 1.029, 0.876, 0.803 and 1.297 and r2 of 0.983, 0.911, 0.971 and 0.993 for the DxI, Immulite, Kryptor and Elecsys TgII, respectively.
Judging by the agreement in the slopes (standard deviation of 11.6% for TgMS vs. 21.9% for TgIA) and the more favorable r2 values (mean of 0.98 for TgMS vs. 0.96 for TgIA) the harmonization between the MS-based assays is at least as good as, or probably better, than the harmonization between the TgIAs. This is remarkable given the differences in target peptide, sample preparation, choice of isotopically labeled internal standards, and calibration across the four TgMS assays.
It is important to point out that the monoclonal antibody to the peptide FSPDDSAGASALLR was developed by the Clinical Proteomic Technologies for Cancer Initiative, a multi-center consortium funded by the National Cancer Institute from 2006-2011. Its direct application in the care of patients is a great advance for laboratory medicine. An expanded repertoire of anti-peptide antibodies would help facilitate better patient care in other important disease areas.
The mass spectrometric measurement of Tg is resistant to the effects of autoantibodies. Our small pilot study further demonstrates that even with extensive sample preparation, multiple laboratories can achieve similar results to one another. However, additional steps toward harmonization of Tg assays will surely benefit TgMS assays as much as they would immunoassays in helping to ensure excellent comparability of results across assays in many laboratories.
Acknowledgments
This work was partially funded by the National Institutes of Health (U24 CA160034).
List of non-standard abbreviations used in this manuscript
- Tg
Human thyroglobulin
- TgAb
anti-thyroglobulin autoantibodies
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- TgMS
Thyroglobulin by LC-MS/MS
- TgIA
Tg by immunometric detection
Footnotes
The material described here has not been submitted for consideration of publication to any other journals or books.
References
- 1.Spencer CA. Challenges of serum thyroglobulin (Tg) measurement in the presence of Tg autoan-tibodies. J Clin Endocrinol Metab. 2004;89:3702–4. doi: 10.1210/jc.2004-0986. [DOI] [PubMed] [Google Scholar]
- 2.Netzel BC, Grebe SKG, Carranza Leon G, Castro AR, Clark PM, Hoofnagle AN, Spencer CA, Turcu AF, Algeciras-Schimnich A. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J Clin Endocrinol Metab. 2015;100:E1074–E1083. doi: 10.1210/jc.2015-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoofnagle AN, Becker JO, Wener MH, Heineck JW. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 2008;54(11):1796–1804. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushnir MM, Rockwood AL, Roberts WL, Abraham D, Hoofnagle AN, Meikle AW. Measurement of thyroglobulin by liquid chromatography–tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin Chem. 2013;59(6):982–990. doi: 10.1373/clinchem.2012.195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer C, Petrovic I, Fatemi S, LoPresti J. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications. J Clin Endocrinol Metab. 2014;99(12):4589–4599. doi: 10.1210/jc.2014-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]