Abstract
Background
Although numerous studies have investigated long-term outcomes after surgical treatment of ulnar neuropathy at the elbow with simple decompression, no study has evaluated the trend of postoperative recovery. The authors assessed timing of recovery after simple decompression for ulnar neuropathy at the elbow.
Methods
The five-center Surgery of the Ulnar Nerve Study Group prospectively recruited 58 consecutive subjects with ulnar neuropathy at the elbow and treated them with simple decompression. Patients were evaluated preoperatively and at 6 weeks, 3 months, 6 months, and 1 year postoperatively. Patient-rated outcomes questionnaires included the Michigan Hand Questionnaire; the Disabilities of the Arm, Shoulder and Hand questionnaire; and the Carpal Tunnel Questionnaire. Functional tests used were grip strength, key pinch strength, two-point discrimination, and Semmes-Weinstein monofilament testing. Postoperative improvement was assessed at each time point to establish the trend of recovery in reaching a plateau.
Results
Significant patient-reported symptomatic and functional recovery occurred over the first 6 weeks postoperatively as represented by improvements in questionnaire scores. Symptomatic recovery occurred earlier than functional recovery as measured by sensory and strength testing and the work domain of the Michigan Hand Questionnaire. Improvement in patient-reported outcomes continued and reached a plateau at 3 months, whereas measured strength and sensory recovery continued over 12 months.
Conclusion
The greatest clinical improvement after simple decompression for ulnar neuropathy at the elbow, according to questionnaire scores, occurs in the first 6 weeks postoperatively and reaches a plateau by 3 months.
Ulnar neuropathy at the elbow is the second most prevalent peripheral compressive neuropathy, after carpal tunnel syndrome.1 With an estimated annual incidence of 75,000 cases in the United States, the health care and societal burden of ulnar neuropathy at the elbow continues to grow.2 Similar to carpal tunnel syndrome, various stressors on the ulnar nerve result in pain, numbness, and weakness in ulnar neuropathy at the elbow. Although the biomechanics of the ulnar nerve as it traverses the elbow are different from those on the median nerve in the carpal tunnel, surgical treatments for carpal tunnel syndrome and ulnar neuropathy at the elbow are similar in aiming to relieve nerve compression.3,4
Traditionally, functional outcomes measures used in evaluating treatment for compressive neuropathies have included grip strength and two-point discrimination, among others. Studies using these measures for postoperative evaluation of carpal tunnel syndrome and ulnar neuropathy at the elbow found recovery to take many months, and over 1 year for some.5–8 However, using patient-rated questionnaires, patients report symptomatic improvement far earlier than functional testing indicates. Questionnaires such as the Michigan Hand Questionnaire; the Disabilities of the Arm, Shoulder and Hand questionnaire; and the disease-specific Carpal Tunnel Questionnaire have been validated for carpal tunnel syndrome and ulnar neuropathy at the elbow and are more responsive than the functional tests.9–13 Improvement in these patient-reported outcomes after carpal tunnel surgery occurs in 6 weeks or less.14
Controversy remains as to the optimal surgical treatment for ulnar neuropathy at the elbow; however, numerous prospective trials, systematic reviews, and meta-analyses have shown no difference in outcomes among the various surgical techniques.15–18 These studies advocate simple decompression as the less invasive and less costly procedure, with equivalent outcomes. In all of these trials, long-term outcomes were the main focus. No study has reported the timing of recovery after surgery for ulnar neuropathy at the elbow. Determining how patients recover after surgery is not only critical in understanding the pathophysiology of ulnar neuropathy at the elbow but can guide optimal treatment and management. In addition, it may facilitate establishing appropriate patient and provider expectations, which play a significant role in outcomes.
The Surgery of the Ulnar Nerve Study Group, a collaboration of five centers, was established to further investigate this condition. Patients with ulnar neuropathy at the elbow were treated with simple decompression and evaluated at 6 weeks, 3 months, 6 months, and 1 year postoperatively. All three questionnaires—the Michigan Hand Questionnaire; the Carpal Tunnel Questionnaire; and the Disabilities of the Arm, Shoulder and Hand questionnaire—were administered and have been validated for ulnar neuropathy at the elbow by this group.19 Objective measures were also used. The timing of improvement in all of these metrics was evaluated in this cohort. We hypothesized that recovery after surgery for ulnar neuropathy at the elbow would take longer than reported for carpal tunnel syndrome but would occur earlier than previously believed.
Patients and Methods
Study Sample
Consecutive patients were recruited prospectively to participate in this study. Inclusion criteria included patients 18 years of age or older; electrodiagnostic confirmation of ulnar neuropathy at the elbow; and the ability to read, understand, and complete questionnaires in English. Exclusion criteria included history of trauma to the affected elbow; recurrent ulnar neuropathy at the elbow or prior ulnar nerve surgery on the affected elbow; other diagnosed conditions in the injured extremity; history of substance abuse; and history of dementia, Alzheimer disease, traumatic brain injury, or serious psychiatric disorders. Subjects were recruited from five participating centers: University of Michigan Health System; University of Rochester Medical Center; Indiana Hand Center; Drisko, Fee & Parkins (North Kansas City, Mo.); and OrthoCarolina Hand Center (Charlotte, N.C.). All centers received institutional review board approval before patient recruitment.
Surgical Procedure
Simple decompression was used for all patients in this cohort. All participating surgeons are trained in hand or peripheral nerve surgery and perform these procedures as part of their practice. A 3-cm curvilinear incision is made posterior to the medial epicondyle. The medial antebrachial cutaneous nerve is preserved. The ulnar nerve is identified proximally, and Osborne's ligament is divided to free the nerve. If at the time of simple decompression the nerve subluxed anteriorly with elbow flexion, anterior transposition was performed, and the patient was excluded from the study.
Data Collection
Physical Measurements
Static two-point discrimination was tested with the Mackinnon-Dellon Discriminator (Lafayette Instruments, Lafayette, Ind.) on the radial and ulnar sides of each digit. Light touch sensation was tested using Semmes-Weinstein monofilaments (Smith & Nephew Roylan, Inc., Germantown, Wis.) along the radial and ulnar borders of each digit. Key pinch strength was measured using a pinch gauge (B & L Engineering, Tustin, Calif.). Grip strength in each hand was evaluated using a Jamar dynamometer (Jamar, Bolingbrook, Ill.). Three measurements for each strength test were averaged to obtain the final value used in analysis. For grip strength, a 10 percent strength increase in the right hand was accounted for in right hand–dominant patients, with no compensation used for left hand–dominant patients.20 All physical assessments were performed preoperatively and then at 6 weeks, 3 months, 6 months, and 1 year postoperatively.
Patient-Reported Outcomes
The Michigan Hand Questionnaire21; the Disabilities of the Arm, Shoulder and Hand questionnaire22; and the Carpal Tunnel Questionnaire11 were administered at the five time points. Data were entered separately by two trained research assistants and cross-checked for discrepancies. The Michigan Hand Questionnaire is a 37-item, hand-specific outcomes instrument with six domains: (1) overall hand function, (2) activities of daily living, (3) pain, (4) work performance, (5) aesthetics, and (6) patient satisfaction. All domains assess the left and right hands separately, with the exception of work. Incorporating scores for each domain, a total overall Michigan Hand Questionnaire score is calculated. Michigan Hand Questionnaire scores range from 0 to 100, and a lower score indicates a worse disease state, with the exception of pain. In the pain domain, higher scores indicate worse pain. The Disabilities of the Arm, Shoulder and Hand instrument is a 30-item questionnaire evaluating disability and symptoms in patients with upper extremity abnormality. Scored from 0 to 100, a higher score indicates more symptoms and/or disability. The Carpal Tunnel Questionnaire is a 19-item disease-specific questionnaire divided into a symptom severity score and a functional status score, each ranging from 1 to 5. Higher scores indicate more severe symptoms and more limited function. Although initially designed for evaluating patients with carpal tunnel syndrome, the questions are not disease-specific or muscle-group specific, allowing patients to capture many symptoms of ulnar neuropathy at the elbow and functional changes as well.
Statistical Analysis
Means and SDs were calculated using descriptive statistics. Differences between preoperative and follow-up values were evaluated with repeated measures analysis of variance. To assess outcome trends, we first plotted individual data and cross-sectional means of each outcome variable over time. These plots were designed to (1) show the trend of recovery over time, and (2) indicate how time should be modeled for a linear mixed-effects model. If the plots showed a linear trend for time, time would be modeled as a continuous variable. If the plots showed early change followed by a plateau, time would be modeled as a categorical variable.
We devised a statistical model to determine whether a plateau existed. A plateau point was the time point after which the change between mean scores at successive time points was no longer significantly different from 0. In other words, a plateau is identified at the point the slope (amount of change in mean score) turned back toward 0 after being significantly different from 0. The difference in score between each set of adjacent time points was calculated for each individual patient, resulting in four score changes calculated per patient. For example, score change 1 is the change in score between the preoperative time point and 6 weeks postoperatively. An independent t test was then used to determine whether the collective cohort's score change was significantly different from 0 for each of the time intervals. This analysis was performed for each outcome measure. A Bonferroni correction was applied to adjust for the multiple comparisons.
We then used a linear mixed-effects model to assess the trends in each outcome variable measured over the multiple time points. The model adjusted for age, sex, and Dellon grade. A random subject effect was used to account for correlations between measurements taken from the same subject. The plots of mean scores revealed a nonlinear trend for time. Thus, time was modeled as a categorical variable. Statistical analyses were performed using SPSS Version 19 (IBM Corp., Armonk, N.Y.).
Results
Study Cohort
From the five participating institutions, 75 patients elected to enroll and provided consent for the study. Seventeen patients subsequently did not complete the study and were excluded from final analysis (Fig. 1). Subjects completing the preoperative measurements and at least two of the four postoperative measurements were included in the final analysis, resulting in 58 patients in the final cohort. Table 1 lists complete demographic information for the cohort. The average age of the patients was 49 years. Patients who reported being white and having an income more than $70,000 per year were disproportionately represented in the cohort. Sex, education level, and Dellon staging23 were well distributed in the cohort. Ulnar neuropathy at the elbow severity by Dellon grade was not significantly different between the attrition group and our study cohort (p > 0.1). One patient developed recurrent ulnar neuropathy at the elbow at a second compression site. This patient required anterior subcutaneous transposition and was excluded from the study.
Fig. 1.
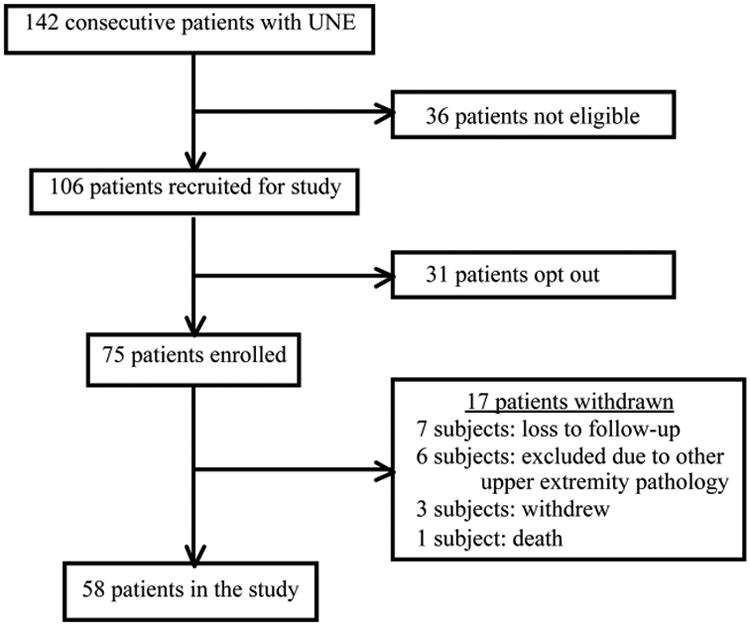
Subject enrollment. UNE, ulnar neuropathy at the elbow.
Table 1. Demographic Information.
| Characteristics | Value (%)* |
|---|---|
| Age, yr | |
| Mean ± SD | 49 ± 13 |
| Range | 23–79 |
| Sex | |
| Men | 27 (46.6) |
| Women | 31 (53.4) |
| Race | |
| White | 46 (79.3) |
| Nonwhite† | 11 (18.9) |
| Education level | |
| Some high school | 2 (3.4) |
| High school graduate | 12 (20.7) |
| Some college | 17 (29.3) |
| College graduate | 15 (25.9) |
| Professional or graduate school | 12 (20.7) |
| Income level | |
| <$29,999 | 13 (22.5) |
| $29,999–$69,999 | 18(30.9) |
| >$70,000 | 26 (44.8) |
| Dellon stage‡ | |
| 1, mild | 22 (37.9) |
| 2, moderate | 22 (37.9) |
| 3, severe | 14 (24.1) |
Values may not sum to 100 percent because some respondents chose not to answer all demographic questions.
Nonwhite includes black or African American, Native Hawaiian or other Pacific Islander, Asian, American Indian, and Alaskan Native.
The mild, moderate, and severe stages for ulnar nerve compression at the elbow are described in Dellon AL. Techniques for successful management of ulnar nerve entrapment at the elbow. Neurosurg Clin North Am. 1991;2:57–73.
Postoperative Change
Mean scores for each questionnaire and objective metrics at the five time points are listed in Table 2. The improvements in questionnaire scores from preoperatively to 6 weeks postoperatively were significant (p = 0.03 for the Disabilities of the Arm, Shoulder and Hand questionnaire and p < 0.01 for all others), with the exception of the work and aesthetic subdomains of the Michigan Hand Questionnaire (p > 0.3). The changes in all of the sensory and strength metrics at 6 weeks were not significant (p > 0.1). At 1 year, all questionnaire scores and all functional scores were significantly improved from preoperative levels (p < 0.05), with the exception of grip strength, which showed clinically substantial but not statistically significant improvement.
Table 2. Mean Scores for All Questionnaires and Functional Tests.
| Preoperatively | 6 Weeks | 3 Months | 6 Months | 1 Year | |
|---|---|---|---|---|---|
| MHQ | |||||
| Overall hand function | 50 ± 21 | 65 ± 24* | 67 ± 25* | 71 ± 22* | 72 ± 23* |
| Activities of daily living | 66 ± 24 | 75 ± 27* | 75 ± 27* | 80 ± 26* | 80 ± 24* |
| Work | 59 ± 25 | 61 ± 30 | 75 ± 25† | 78 ± 24* | 73 ± 27† |
| Pain | 49 ± 26 | 32 ± 29* | 29 ± 30* | 28 ± 32* | 27 ± 27* |
| Aesthetic | 68 ± 31 | 71 ± 27 | 77 ± 26 | 81 ± 24† | 79 ± 22† |
| Satisfaction | 37 ± 22 | 57 ± 29* | 65 ± 29* | 68 ± 28* | 67 ± 29* |
| Overall score | 55 ± 19 | 66 ± 23* | 72 ± 24* | 75 ± 22* | 74 ± 22* |
| CTQ | |||||
| Symptom severity score | 2.8 ± 0.7 | 2.1 ± 0.9* | 2.1 ± 0.9* | 1.9 ± 0.8* | 1.8 ± 0.7* |
| Functional status score | 2.1 ± 0.7 | 1.9 ± 0.9* | 1.8 ± 0.8* | 1.7 ± 0.8* | 1.6 ± 0.7* |
| DASH disability score | 29.6 ± 17.8 | 25.2 ± 20.8† | 20.8 ± 19.3* | 19.5 ± 20.0* | 17.5 ± 17.6* |
| Functional tests | |||||
| 2PD, ulnar border SF, mm | 7.6 ± 4.4 | 7.5 ± 5.3 | 6.3 ± 4.1 | 6.3 ± 4.7 | 5.4 ± 2.8† |
| Key pinch, kg | 6.6 ± 2.8 | 7.0 ± 3.0 | 7.3 ± 3.2 | 8.4 ± 4.9 | 8.6 ± 3.3† |
| Grip strength, kg | 25.0 ± 12.1 | 26.7 ± 12.6 | 28.5 ± 12.5 | 29.8 ± 13.4 | 32.5 ± 14.1 |
| SWM, ulnar border SF | 4.2 ± 1.0 | 4.0 ± 1.2 | 3.9 ± 1.0* | 3.7 ± 0.9* | 3.4 ± 0.6* |
MHQ Michigan Hand Questionnaire; CTQ Carpal Tunnel Questionnaire; DASH, Disabilities of the Arm, Shoulder, and Hand questionnaire; 2PD, two-point discrimination; SWM, Semmes-Weinstein monofilament testing; SF, small finger.
Repeated measures analysis of variance evaluating postoperative time point score and preoperative score, p < 0.01.
Repeated measures analysis of variance evaluating postoperative time point score and preoperative score, p < 0.05.
Recovery Trend
We evaluated trends of recovery over the course of 1 year after simple decompression. The cross-sectional means for Carpal Tunnel Questionnaire functional status score; Carpal Tunnel Questionnaire symptom severity score; Disabilities of the Arm, Shoulder and Hand questionnaire; Michigan Hand Questionnaire overall; and all Michigan Hand Questionnaire subdomains were plotted (Fig. 2). Cross-sectional means for grip strength, pinch strength, and static two-point discrimination of the small finger were also plotted (Fig. 3). From these plots, we see that all questionnaire results showed significant early improvement in the first 6 weeks, followed by a plateau by 3 months postoperatively. All of the sensory and strength metrics showed a slower recovery and never hit a plateau. Patients had improvements in sensory and strength testing over the entire postoperative year.
Fig. 2.

Trend in mean questionnaire scores from preoperatively to 1 year postoperatively. (Above) Michigan Hand Questionnaire overall score. (Center) Michigan Hand Questionnaire sub-domains. (Below) Carpal Tunnel Questionnaires and Disabilities of the Arm, Shoulder and Hand questionnaire. ADL, activities of daily living; MHQ, Michigan Hand Questionnaire; CTQs, Carpal Tunnel Questionnaire symptom severity score; CTQf, Carpal Tunnel Questionnaire functional status score; DASH, Disabilities of the Arm, Shoulder and Hand questionnaire.
Fig. 3.
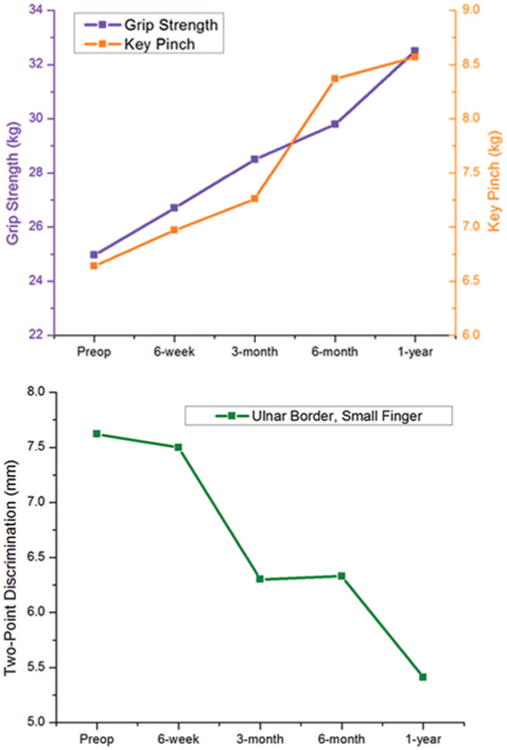
Trend in mean functional metric scores from preoperatively to 1 year postoperatively. (Above) Grip strength and key pinch strength. (Below) Two-point discrimination, ulnar border of the small finger.
For each questionnaire, a plateau was reached by the first 3 months of recovery, with the exception of the aesthetic domain of the Michigan Hand Questionnaire (Table 3). In addition, for Carpal Tunnel Questionnaire functional status and symptom severity scores and Michigan Hand Questionnaire scores, the most significant score change was seen within the first 6 weeks. For Disabilities of the Arm, Shoulder and Hand questionnaire, the score change between 6 weeks and 3 months was the most significant; however, in the first 6 weeks, there was notable but not statistically significant score change with our plateau model. There was no plateau in improvement with any of the sensory or strength metrics.
Table 3. Results of Plateau Analysis for All Questionnaires and Functional Tests.
| Score Change 1†, t (p)‡ | Score Change 2, t (p) | Score Change 3, t (p) | Score Change 4, t (p) | |
|---|---|---|---|---|
| MHQ | ||||
| Overall hand function | 4.71 (<0.001) | 0.85 (0.40)* | 1.27 (0.21)* | −0.39 (0.70)* |
| Activities of daily living | 2.93 (0.005) | −0.20 (0.85)* | 1.58 (0.12)* | −1.38 (0.18)* |
| Work | 0.49 (0.65)* | 4.09 (<0.001) | 0.78 (0.44)* | −1.15 (0.48)* |
| Pain | −4.13 (<0.001) | −0.64 (0.53)* | 0.38 (0.71)* | 0.08 (0.94)* |
| Aesthetic | 1.03 (0.31)* | 1.61 (0.92)* | 0.85 (0.40)* | −1.70 (0.09)* |
| Satisfaction | 4.74 (<0.001) | 3.39 (<0.001) | 0.27 (0.79)* | −0.92 (0.39)* |
| Overallscore | 4.30 (<0.001) | 2.86 (0.005) | 0.82 (0.42)* | −1.19 (0.24)* |
| CTQ | ||||
| Symptom severity score | −5.48 (<0.001) | −2.19 (0.03)* | −0.88 (0.39)* | −0.28 (0.78)* |
| Functional status score | −3.00 (0.005) | −1.87 (0.07)* | −0.62 (0.54)* | −0.37 (0.71)* |
| DASH disability score | −2.24 (0.03)* | −3.02 (0.005) | −0.08 (0.94)* | −0.86 (0.40)* |
| Functional measures | ||||
| 2PD, ulnar border SF | 0.15 (0.88)* | −2.03 (0.05)* | 0.00 (1.00)* | 0.59 (0.56)* |
| SWM, ulnar border SF | −1.55 (0.13)* | −1.81 (0.08)* | −1.78 (0.08)* | −0.86 (0.40)* |
| Key pinch | 1.43 (0.16)* | 0.95 (0.35)* | 1.90 (0.07)* | −0.61 (0.53)* |
| Grip strength | 1.45 (0.16)* | 1.40 (0.17)* | 0.22 (0.83)* | 1.64 (0.11)* |
MHQ Michigan Hand Questionnaire; CTQ Carpal Tunnel Questionnaire; DASH, Disabilities of the Arm, Shoulder, and Hand questionnaire; 2PD, two-point discrimination; SWM, Semmes-Weinstein monofilament testing; SF, small finger.
Score changes are not significantly different from 0. Transition from boxes marked with an asterisk and those not marked with an asterisk indicates a plateau.
Score change indicates the difference in score between each time point, and represents slope on our plots in Figures 2 and 3. Score change 1 is the difference in score between preoperatively and 6 weeks postoperatively. Score change 2 is between 6 weeks postoperatively and 3 months postoperatively. Score change 3 is between 3 months postoperatively and 6 months postoperatively. Score change 4 is between 6 months postoperatively and 1 year postoperatively.
Results are considered statistically significant at p < 0.0125 to allow for a Bonferroni correction accounting for the multiple comparisons.
The linear mixed-effects model showed that the overall trend in questionnaire score changes was not significantly affected by the patient's sex or Dellon grade. However, of the patients with Dellon grade 3 (most severe) disease, early recovery in overall Michigan Hand Questionnaire score; Michigan Hand Questionnaire pain and satisfaction subdomains; Carpal Tunnel Questionnaire; and Disabilities of the Arm, Shoulder and Hand questionnaire score did not reach significance until 3 months (Table 4). The function-related Michigan Hand Questionnaire sub-domains (hand function, activities of daily living, and work) and Carpal Tunnel Questionnaire functional status score did not reach significance until 6 months. Improvement in grip strength and pinch strength did not reach statistical significance. In addition, mean scores were lower at 6 months and 1 year postoperatively compared with the overall sample, although these differences did not reach statistical significance (p > 0.05).
Table 4. Mean Scores for All Questionnaires and Functional Tests in Patients with Dellon Grade 3 Ulnar Neuropathy at the Elbow.
| Preoperatively | 6 Wk | 3 Mo | 6 Mo | 1 Yr | |
|---|---|---|---|---|---|
| MHQ | |||||
| Overall hand function | 40 ± 27 | 51 ± 24 | 60 ± 22 | 65 ± 26* | 66 ± 25* |
| Activities of daily living | 53 ± 24 | 58 ± 32 | 70 ± 31 | 72 ± 32* | 73 ± 29* |
| Work | 57 ±25 | 59 ±28 | 77 ±22 | 78 ± 25* | 74 ±23 |
| Pain | 53 ±28 | 38 ±31 | 19 ± 26† | 18 ± 26† | 27 ± 28* |
| Aesthetic | 54 ± 28 | 68 ± 27 | 75 ± 25 | 81 ± 24* | 73 ± 26 |
| Satisfaction | 25 ± 17 | 48 ± 30 | 64 ± 27* | 65 ± 30* | 64 ± 30† |
| Overall score | 46 ± 17 | 58 ± 26 | 71 ± 23* | 74 ± 25† | 70 ± 23† |
| CTQ | |||||
| Symptom severity score | 3.0 ± 0.7 | 2.5 ± 1.0 | 2.1 ± 0.9* | 2.0 ± 0.8† | 2.1 ± 0.7† |
| Functional status score | 2.4 ± 0.7 | 2.2 ± 1.0 | 1.9 ± 0.8 | 1.7 ± 0.8* | 1.7 ± 0.8* |
| DASH disability score | 35 ± 18 | 29 ± 19 | 21 ± 18* | 22 ± 21* | 19 ± 17* |
| Functional measures | |||||
| 2PD, ulnar border SF mm | 10.8 ± 7.3 | 12.4 ± 8.6 | 8.6 ± 6.3 | 8.5 ± 6.3 | 5.3 ± 3.1* |
| Key pinch, kg | 5.4 ± 2.6 | 5.2 ± 2.1 | 5.8 ± 2.5 | 6.9 ± 2.5 | 7.2 ± 2.2 |
| Grip strength, kg | 21.0 ± 13.4 | 19.9 ± 10.9 | 26.4 ± 13.3 | 33.1 ± 13.8 | 34.3 ± 13.9 |
| SWM, ulnar border SF | 5.1 ± 1.3 | 4.9 ± 1.3 | 4.3 ± 1.1* | 3.8 ± 0.7* | 3.6 ± 0.8* |
MHQ Michigan Hand Questionnaire; CTQ Carpal Tunnel Questionnaire; DASH, Disabilities of the Arm, Shoulder, and Hand questionnaire; 2PD, two-point discrimination; SWM, Semmes-Weinstein monofilament testing; SF, small finger.
Repeated measures analysis of variance evaluating postoperative time point score and preoperative score, p < 0.05.
Repeated measures analysis of variance evaluating postoperative time point score and preoperative score, p < 0.01.
The model did show age to have an effect on the trend of recovery, with increasing age related to lower score changes between time points. The older patients in this study showed slower early recovery with a lesser degree of change between each time point. As a result, mean scores were lower at the early postoperative time points in patients aged 60 years and older (Table 5). Looking at the plots and plateau analysis, the significant early trend in recovery seen in our study sample did not occur for this cohort (Fig 4 and Table 6). However, at 6 months postoperatively, the overall scores were no longer significantly different between this older patient group and the total sample (p > 0.05).
Table 5. Mean Scores for All Questionnaires and Functional Tests in Patients Older Than 59 Years.
| Preoperatively | 6 Wk | 3 Mo | 6 Mo | 1 Yr | |
|---|---|---|---|---|---|
| MHQ | |||||
| Overall hand function | 46 ± 17 | 53 ± 16 | 53 ± 21 | 67 ± 22* | 73 ± 17† |
| Activities of daily living | 65 ± 26 | 65 ± 32 | 62 ± 33 | 75 ± 35 | 81 ± 25 |
| Work | 65 ± 23 | 76 ± 22 | 73 ± 21 | 81 ± 25 | 75 ± 21 |
| Pain | 37 ± 32 | 29 ± 34 | 26 ± 31 | 15 ± 28* | 18 ± 23 |
| Aesthetic | 60 ± 32 | 58 ± 25 | 71 ± 22 | 77 ± 24 | 83 ± 17 |
| Satisfaction | 37 ± 17 | 43 ± 16 | 52 ± 23 | 65 ± 24 | 73 ± 25* |
| Overall score | 56 ± 19 | 61 ± 20 | 64 ± 21 | 75 ± 23* | 78 ± 19† |
| CTQ | |||||
| Symptom severity score | 2.7 ± 0.9 | 2.2 ± 0.8 | 2.3 ± 0.9 | 1.9 ± 0.8 | 1.8 ± 0.6* |
| Functional status score | 2.0 ± 0.8 | 2.1 ± 1.0 | 1.9 ± 0.8 | 1.7 ± 0.7 | 1.5 ± 0.6* |
| DASH disability score | 24.3 ± 17.3 | 28.1 ± 19.5 | 22.7 ± 16.4 | 19.6 ± 18.1 | 14.0 ± 13.9* |
| Functional measures | |||||
| 2PD ulnar border SF, mm | 11.1 ± 7.2 | 9.9 ± 7.7 | 9.4 ± 6.9 | 8.1 ± 7.3 | 6.0 ± 2.0* |
| Key pinch, kg | 4.6 ± 2.7 | 5.6 ± 2.8 | 6.0 ± 2.8 | 6.5 ± 3.3 | 6.9* ± 1.8 |
| Grip strength, kg | 17.9 ± 9.4 | 17.7 ± 8.2 | 21.8 ± 11.6 | 28.3 ± 10.4 | 27.6 ± 11.0 |
| SWM, ulnar border SF | 4.3 ± 1.0 | 3.8 ± 0.4 | 3.6 ± 0.7 | 3.5 ± 0.7* | 3.3 ± 0.5* |
MHQ Michigan Hand Questionnaire; CTQ Carpal Tunnel Questionnaire; DASH, Disabilities of the Arm, Shoulder and Hand questionnaire; 2PD, two-point discrimination; SWM, Semmes-Weinstein monofilament testing; SF, small finger.
Repeated measures analysis of variance evaluating postoperative time point score and preoperative score, p < 0.05.
Repeated measures analysis of variance evaluating postoperative time point score and preoperative score, p < 0.01.
Fig. 4.
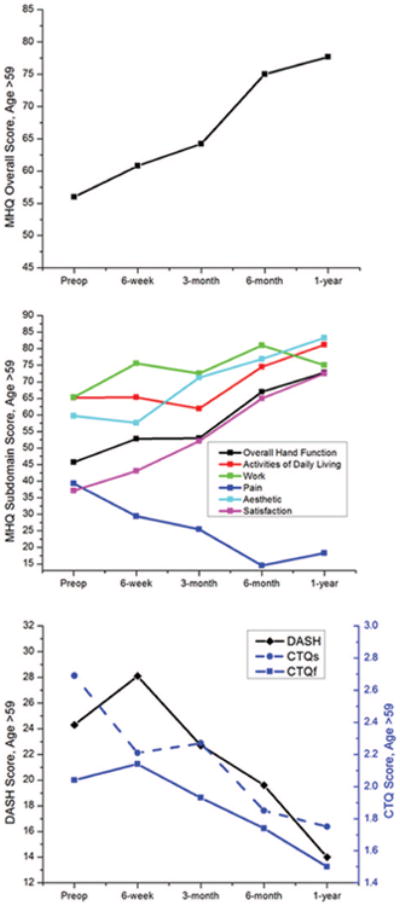
Trend in mean questionnaire scores from preoperatively to 1-year postoperatively in patients older than 59 years. (Above) Michigan Hand Questionnaire overall score; (center) Michigan Hand Questionnaire subdomains; and (below) Carpal Tunnel Questionnaire symptom severity and functional status scores and Disabilities of the Arm, Shoulder and Hand score. ADL, activities of daily living; MHQ, Michigan Hand Questionnaire; CTQs, Carpal Tunnel Questionnaire symptom severity score; CTQf, Carpal Tunnel Questionnaire functional status score; DASH, Disabilities of the Arm, Shoulder, and Hand questionnaire.
Table 6. Results of Plateau Analysis for All Questionnaires and Functional Metrics in Patients Older Than 59 Years*.
| Score Change 1†, t (p)‡ | Score Change 2, t (p) | Score Change 3, t (p) | Score Change 4, t (p) | |
|---|---|---|---|---|
| MHQ | ||||
| Overall and function | 1.70 (0.13) | − 0.64 (0.54) | 1.61 (0.14) | 0.53 (0.61) |
| Activities of daily living | 0.51 (0.62) | −1.02 (0.33) | 1.17 (0.28) | −0.11 (0.92) |
| Work | 0.99(0.34) | −0.68(0.52) | 0.83(0.43) | −1.35(0.21) |
| Pain | −0.72 (0.50) | −0.24 (0.82) | −1.39 (0.20) | 0.56 (0.60) |
| Aesthetic | 0.00 (1.00) | 1.86 (0.40) | 0.12 (0.90) | 0.85 (0.42) |
| Satisfaction | 1.14 (0.29) | 1.57 (0.15) | 1.28 (0.24) | 1.08 (0.31) |
| Overall score | 1.14 (0.29) | 0.20 (0.85) | 1.65 (0.14) | 0.09 (0.93) |
| CTQ | ||||
| Symptom severity score | −2.37(0.05) | 1.64(0.14) | −2.63(0.03) | −1.55(0.16) |
| Functional status score | −1.17 (0.29) | −0.65 (0.53) | −1.03 (0.33) | −1.21 (0.26) |
| DASH disability score | −0.74 (0.49) | −2.53 (0.04) | −0.43 (0.04) | −1.29 (0.68) |
MHQ Michigan Hand Questionnaire; CTQ Carpal Tunnel Questionnaire; DASH, Disabilities of the Arm, Shoulder and Hand questionnaire.
Score change 1 is the difference in score between preoperatively and 6 weeks postoperatively. Score change 2 is between 6 weeks postoperatively and 3 months postoperatively. Score change 3 is between 3 months postoperatively and 6 months postoperatively. Score change 4 is between 6 months postoperatively and 1 year postoperatively. Values represent score changes that are not significantly different from 0. A transition from nonsignificantly different to significantly different corresponds to a plateau. This does not occur, confirming the results shown in Figure 4 that no early plateau occurred in this population.
Score change indicates the difference in score between each time point and represents the slope in the Figure 4 plot.
Results are considered statistically significant at p < 0.0125 to allow for a Bonferroni correction accounting for the multiple comparisons.
Discussion
Studies of recovery after surgery for carpal tunnel syndrome improved our understanding of the unique symptomatic and functional recovery that occurs after nerve decompression procedures.8,24,25 The seminal publication on outcomes after carpal tunnel syndrome surgery by Levine and Katz showed that outcomes are better measured by patient-reported outcomes tools than the traditional measures such as grip strength and sensory testing.11 Patient-reported outcomes have since been found to be more responsive indicators of functional and symptomatic improvement and have provided better understanding of surgical treatment and postoperative recovery.13 Evaluating timing and trend of recovery contributed largely to this evolved understanding. Katz et al. showed that carpal tunnel syndrome patients reported a majority of their symptomatic and functional improvement in the first 6 weeks.14 These findings were confirmed in subsequent studies.26
Methods for evaluating outcomes after surgery for ulnar neuropathy at the elbow have also shifted to using validated, responsive, patient-reported outcomes, and have improved our understanding of the disease. The paradigm in treatment has evolved in using simple decompression as the initial surgical intervention, because it has lower costs, fewer complications, and similar outcomes for patients with all levels of disease severity when compared with the other surgical options.17,18 Although a validated disease-specific instrument is not available for ulnar neuropathy at the elbow, the use of validated system-specific instruments has shown continued reliability.27
It has been reported that recovery for ulnar neuropathy at the elbow is often protracted, but studies on timing and trend of recovery are lacking. The earliest time period any study has evaluated and reported on recovery of ulnar neuropathy at the elbow is at 3 months. Nabhan et al. conducted a prospective trial comparing simple decompression to anterior subcutaneous transposition.16 They evaluated patients at 3 months and again at 9 months postoperatively. For both procedure cohorts, patients showed marked improvements in pain, intrinsic muscle strength, Semmes-Weinstein monofilament testing, and nerve conduction velocity 3 months after surgery; however, no patient-reported outcomes were used. Filippi et al. prospectively evaluated 40 patients after simple decompression using strength testing and a nonvalidated questionnaire.28 At 3 months, 34 of 36 patients reported improvement or complete resolution of pain, and 35 of 40 patients reported improvement or complete resolution of dysesthesia and hypesthesia.
Our study found that recovery after simple decompression has rapid early improvement in all patient-reported outcome metrics over the first 6 weeks, with a plateau by 3 months. Symptomatic recovery, with improvements in patient-reported pain and satisfaction, occurred earlier than functional recovery. Strength and sensory testing, and the work domain for the Michigan Hand Questionnaire, showed early improvement but did not follow the same rapid trend. Improvements in strength and sensory testing continued to 1 year postoperatively.
Using Kaplan-Meier hazard analysis, Dellon et al. showed that over 60 percent of patients with severe ulnar neuropathy at the elbow fail conservative therapy and progress to needing surgery within 20 months.29 Although Bimmler and Meyer reported that the degree of postoperative change in their cohort of 87 ulnar neuropathy at the elbow patients was independent of preoperative severity, timing and progression of postoperative recovery in patients with severe disease are unknown.30 Supporting previous reports that simple decompression is an appropriate treatment in severe ulnar neuropathy at the elbow, recovery trends in this study held true for all Dellon grades in our model.31 Severity of disease did not significantly affect the overall timing of change and improvement. However, the patients with the most severe grade of ulnar neuropathy at the elbow still had worse overall outcomes.
The slower recovery seen in patients aged 60 years and older is also expected. Reports in the carpal tunnel syndrome literature cite concerns regarding neural recovery in an elderly patient group.32 However, long-term results in patients older than 65 years are equivalent.33 Similarly, the long-term results of surgery for ulnar neuropathy at the elbow are equivalent in older patients.30 However, there has not been adequate investigation into early neural recovery in this older cohort. This study shows that these patients take longer to reach the same overall results.
This study is the first to highlight the trend of early recovery after simple decompression for ulnar neuropathy at the elbow. Not only does this serve to improve our understanding of the pathology and pathophysiology of ulnar neuropathy at the elbow, it also illustrates additional similarities between two common peripheral compressive neuropathies—ulnar neuropathy at the elbow and carpal tunnel syndrome. Perhaps more importantly, refining our understanding of postoperative recovery will guide providers in better managing patient expectations. For numerous surgical patient populations, it has been shown that patient expectations, and how these expectations have been met or not, affect decision-making, recovery, and satisfaction.34,35 Having a clear understanding of expectations allows providers to more adequately prepare patients for surgery.36
Our study has limitations. The loss of 23 percent of patients (17 of 75) decreased our sample size and potentially introduced attrition bias. Although we had adequate patient involvement, our subgroups were smaller in size. This may have limited our ability to discern differences in recovery timing attributable to disease grade; however, our model did not find Dellon grade to have a significant effect on trends of improvement in measured outcomes, and this was supported in our subgroup analysis of patients with grade 3 disease. Also, although the patient-reported outcomes reached a plateau, strength and sensory testing showed improvement through 1 year post-operatively. We cannot exclude the possibility that continued improvement would be seen with longer follow-up. In addition, the inclusion and exclusion criteria and the demographic mix of this study must be considered when interpreting and translating our results. This study does have the advantage of patient involvement from academic centers and private specialty practices from various regions, in addition to patients of orthopedic, plastic, and neurosurgery providers.
Conclusions
This multicenter collaborative study is the first to highlight the trend of early recovery after simple decompression for ulnar neuropathy at the elbow. Our cohort reported significant improvement in three validated patient-reported outcomes within 6 weeks, and recovery reached a plateau by 3 months. The findings of this study also serve to confirm numerous previous reports regarding simple decompression for ulnar neuropathy at the elbow, showing great success in alleviating functional debilitation and patient-reported symptoms, and resulting in significant improvements in patient satisfaction. Simple decompression is an appropriate treatment for ulnar neuropathy at the elbow and results in significant early improvement within the first 6 weeks after surgery.
Acknowledgments
This project was supported by the Ruth L. Kirschstein National Research Service Awards for Individual Postdoctoral Fellows (1F32AR058105-01A1) (to J.W.S.), by a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120), by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute of Aging (R01 AR062066), and by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (2R01 AR047328-06) (to K.C.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the following study coordinators for their assistance: Patricia Burns, M.P.H., Connie McGovern, Miriana Popadich, R.N., B.S.N., Ann E. Coppage, P.A.-C., Mollie K. Hanlon, N.P.-C., M.S.N., M.B.A., Benjamin Connell, B.A., Sara Defendorf, B.S., and Allison W. McIntyre, M.P.H. Additional thanks go to Joel M. Vaughan, Ph.D., and Breanna Miller, B.S., at the University of Michigan Center for Statistical Consultation and Research.
Footnotes
CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, IV.
Disclosure: The authors have no financial interest to declare in any of the products or devices mentioned in this article.
References
- 1.Shin R, Ring D. The ulnar nerve in elbow trauma. J Bone Joint Surg Am. 2007;89:1108–1116. doi: 10.2106/JBJS.F.00594. [DOI] [PubMed] [Google Scholar]
- 2.Zlowodzki M, Chan S, Bhandari M, Kalliainen L, Schubert W. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome: A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:2591–2598. doi: 10.2106/JBJS.G.00183. [DOI] [PubMed] [Google Scholar]
- 3.Werner CO, Ohlin P, Elmqvist D. Pressures recorded in ulnar neuropathy. Acta Orthop Scand. 1985;56:404–406. doi: 10.3109/17453678508994358. [DOI] [PubMed] [Google Scholar]
- 4.James J, Sutton LG, Werner FW, Basu N, Allison MA, Palmer AK. Morphology of the cubital tunnel: An anatomical and biomechanical study with implications for treatment of ulnar nerve compression. J Hand Surg Am. 2011;36:1988–1995. doi: 10.1016/j.jhsa.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Asami A, Morisawa K, Tsuruta T. Functional outcome of anterior transposition of the vascularized ulnar nerve for cubital tunnel syndrome. J Hand Surg Br. 1998;23:613–616. doi: 10.1016/s0266-7681(98)80014-4. [DOI] [PubMed] [Google Scholar]
- 6.Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: An analysis of the literature. J Neurosurg. 1998;89:722–727. doi: 10.3171/jns.1998.89.5.0722. [DOI] [PubMed] [Google Scholar]
- 7.Nancollas MP, Peimer CA, Wheeler DR, Sherwin FS. Long-term results of carpal tunnel release. J Hand Surg Br. 1995;20:470–474. doi: 10.1016/s0266-7681(05)80155-x. [DOI] [PubMed] [Google Scholar]
- 8.Le Quesne PM, Casey EB. Recovery of conduction velocity distal to a compressive lesion. J Neurol Neurosurg Psychiatry. 1974;37:1346–1351. doi: 10.1136/jnnp.37.12.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman NB, Kaye MB, Wilgis EF, Zimmerman RM, Dubin NH. Are standardized patient self-reporting instruments applicable to the evaluation of ulnar neuropathy at the elbow? J Shoulder Elbow Surg. 2009;18:463–468. doi: 10.1016/j.jse.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Kotsis SV, Chung KC. Responsiveness of the Michigan Hand Outcomes Questionnaire and the Disabilities of the Arm, Shoulder and Hand questionnaire in carpal tunnel surgery. J Hand Surg Am. 2005;30:81–86. doi: 10.1016/j.jhsa.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75:1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Atroshi I, Breidenbach WC, McCabe SJ. Assessment of the carpal tunnel outcome instrument in patients with nerve-compression symptoms. J Hand Surg Am. 1997;22:222–227. doi: 10.1016/S0363-5023(97)80155-4. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Gelberman RH, Wright EA, Lew RA, Liang MH. Responsiveness of self-reported and objective measures of disease severity in carpal tunnel syndrome. Med Care. 1994;32:1127–1133. doi: 10.1097/00005650-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Katz JN, Fossel KK, Simmons BP, Swartz RA, Fossel AH, Koris MJ. Symptoms, functional status, and neuromuscular impairment following carpal tunnel release. J Hand Surg Am. 1995;20:549–555. doi: 10.1016/S0363-5023(05)80265-5. [DOI] [PubMed] [Google Scholar]
- 15.Biggs M, Curtis JA. Randomized, prospective study comparing ulnar neurolysis in situ with submuscular transposition. Neurosurgery. 2006;58:296–304. doi: 10.1227/01.NEU.0000194847.04143.A1. discussion 296–304. [DOI] [PubMed] [Google Scholar]
- 16.Nabhan A, Ahlhelm F, Kelm J, Reith W, Schwerdtfeger K, Steudel WI. Simple decompression or subcutaneous anterior transposition of the ulnar nerve for cubital tunnel syndrome. J Hand Surg Br. 2005;30:521–524. doi: 10.1016/j.jhsb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Caliandro P, La Torre G, Padua R, Giannini F, Padua L. Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst Rev. 2011;(2):CD006839. doi: 10.1002/14651858.CD006839.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Chung KC. Treatment of ulnar nerve compression at the elbow. J Hand Surg Am. 2008;33:1625–1627. doi: 10.1016/j.jhsa.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JW, et al. An outcome study for ulnar neuropathy at the elbow: A multicenter study by the SUN study group. 2012 doi: 10.1227/NEU.0b013e31828ca327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby CA, Wehbé MA, Mawr B. Hand strength: Normative values. J Hand Surg Am. 1994;19:665–670. doi: 10.1016/0363-5023(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 21.Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23:575–587. doi: 10.1016/S0363-5023(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 22.Beaton DE, Katz JN, Fossel AH, et al. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–146. [PubMed] [Google Scholar]
- 23.Dellon AL. Techniques for successful management of ulnar nerve entrapment at the elbow. Neurosurg Clin North Am. 1991;2:57–73. [PubMed] [Google Scholar]
- 24.Nygaard OP, Trumpy JH, Mellgren SI. Recovery of sensory function after surgical decompression in carpal tunnel syndrome. Acta Neurol Scand. 1996;94:253–257. doi: 10.1111/j.1600-0404.1996.tb07061.x. [DOI] [PubMed] [Google Scholar]
- 25.Schrijver HM, Gerritsen AA, Strijers RL, et al. Correlating nerve conduction studies and clinical outcome measures on carpal tunnel syndrome: Lessons from a randomized controlled trial. J Clin Neurophysiol. 2005;22:216–221. [PubMed] [Google Scholar]
- 26.Gay RE, Amadio PC, Johnson JC. Comparative responsiveness of the disabilities of the arm, shoulder, and hand, the carpal tunnel questionnaire, and the SF-36 to clinical change after carpal tunnel release. J Hand Surg Am. 2003;28:250–254. doi: 10.1053/jhsu.2003.50043. [DOI] [PubMed] [Google Scholar]
- 27.Macadam SA, Bezuhly M, Lefaivre KA. Outcomes measures used to assess results after surgery for cubital tunnel syndrome: A systematic review of the literature. J Hand Surg Am. 2009;34:1482–1491.e5. doi: 10.1016/j.jhsa.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Filippi R, Farag S, Reisch R, Grunert P, Böcher-Schwarz H. Cubital tunnel syndrome: Treatment by decompression without transposition of ulnar nerve. Minim Invasive Neurosurg. 2002;45:164–168. doi: 10.1055/s-2002-34394. [DOI] [PubMed] [Google Scholar]
- 29.Dellon AL, Hament W, Gittelshon A. Nonoperative management of cubital tunnel syndrome: An 8-year prospective study. Neurology. 1993;43:1673–1677. doi: 10.1212/wnl.43.9.1673. [DOI] [PubMed] [Google Scholar]
- 30.Bimmler D, Meyer VE. Surgical treatment of the ulnar nerve entrapment neuropathy: Submuscular anterior transposition or simple decompression of the ulnar nerve? Long-term results in 79 cases. Ann Chir Main Memb Super. 1996;15:148–157. doi: 10.1016/s0753-9053(96)80004-4. [DOI] [PubMed] [Google Scholar]
- 31.Gervasio O, Gambardella G, Zaccone C, Branca D. Simple decompression versus anterior submuscular transposition of the ulnar nerve in severe cubital tunnel syndrome: A prospective randomized study. Neurosurgery. 2005;56:108–117. doi: 10.1227/01.neu.0000145854.38234.81. discussion 117. [DOI] [PubMed] [Google Scholar]
- 32.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 33.Weber RA, Rude MJ. Clinical outcomes of carpal tunnel release in patients 65 and older. J Hand Surg Am. 2005;30:75–80. doi: 10.1016/j.jhsa.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Mandl LA, Burke FD, Shaw Wilgis EF, Lyman S, Katz JN, Chung KC. Could preoperative preferences and expectations influence surgical decision making? Rheumatoid arthritis patients contemplating metacarpophalangeal joint arthroplasty. Plast Reconstr Surg. 2008;121:175–180. doi: 10.1097/01.prs.0000295376.70930.7e. [DOI] [PubMed] [Google Scholar]
- 35.Kadzielski J, Malhotra LR, Zurakowski D, Lee SG, Jupiter JB, Ring D. Evaluation of preoperative expectations and patient satisfaction after carpal tunnel release. J Hand Surg Am. 2008;33:1783–1788. doi: 10.1016/j.jhsa.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Bessette L, Keller RB, Liang MH, Simmons BP, Fossel AH, Katz JN. Patients' preferences and their relationship with satisfaction following carpal tunnel release. J Hand Surg Am. 1997;22:613–620. doi: 10.1016/S0363-5023(97)80117-7. [DOI] [PubMed] [Google Scholar]


