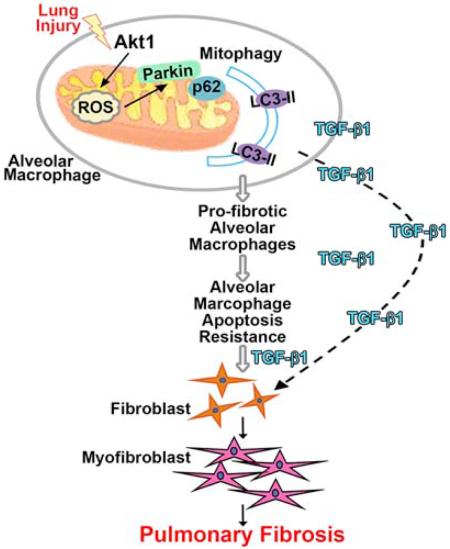
Summary
Idiopathic pulmonary fibrosis (IPF) is a devastating lung disorder with increasing incidence. Mitochondrial oxidative stress in alveolar macrophages is directly linked to pulmonary fibrosis. Mitophagy, the selective engulfment of dysfunctional mitochondria by autophagasomes, is important for cellular homeostasis and can be induced by mitochondrial oxidative stress. Here, we show Akt1 induced macrophage mitochondrial reactive oxygen species (ROS) and mitophagy. Mice harboring a conditional deletion of Akt1 in macrophages (Akt1−/−Lyz2-cre) and Park2−/− mice had impaired mitophagy and reduced active transforming growth factor-β1 (TGF-β1). Although Akt1 increased TGF-β1 expression, mitophagy inhibition in Akt1-overexpressing macrophages abrogated TGF-β1 expression and fibroblast differentiation. Importantly, conditional Akt1−/− Lyz2-cre mice and Park2−/− mice had increased macrophage apoptosis and were protected from pulmonary fibrosis. Moreover, IPF alveolar macrophages had evidence of increased mitophagy and display apoptosis resistance. These observations suggest that Akt1-mediated mitophagy contributes to alveolar macrophage apoptosis resistance and is required for pulmonary fibrosis development.
Introduction
Pulmonary fibrosis is a devastating disorder that has an increasing prevalence of over 60 per 100,000 persons, and the cost of care has had a dramatic upward trend in recent years (Collard et al., 2015; Collard et al., 2012). This devastating disease has a median life expectancy of 3-5 years after diagnosis for certain forms of pulmonary fibrosis, such as idiopathic pulmonary fibrosis (IPF) (Collard et al., 2012; Raghu et al., 2004; Schwartz et al., 1994). Unfortunately, there are no current therapies to halt the development and/or progression of pulmonary fibrosis; thus, understanding the basic molecular mechanisms may uncover additional therapeutic modalities.
Alveolar macrophages play an integral role in the pathogenesis of pulmonary fibrosis by initiating an immune response and generating reactive oxygen species (ROS). Lung remodeling during pulmonary fibrosis is poorly understood, but the generation of ROS, particularly mitochondrial H2O2, from alveolar macrophages plays an integral role in fibrosis development by increasing the expression of transforming growth factor-β (TGF-β1) (He et al., 2011; Jain et al., 2013). The abrogation of mitochondrial oxidative stress reduces Tgfb1 and attenuates the development of pulmonary fibrosis in mice (He et al., 2011; Osborn-Heaford et al., 2012).
Protein kinase B, or Akt1, a pro-survival kinase, is known to mediate mitochondrial H2O2 generation (Larson-Casey et al., 2014), and mitochondrial ROS plays an important role in macrophage innate immunity (West et al., 2011). Although mitochondrial ROS is usually considered toxic, it also has beneficial effects, such as modulating mitochondrial dynamics. For example, mitophagy, a form of macroautophagy, is the selective engulfment of dysfunctional mitochondria by autophagasomes and can be induced by mitochondrial ROS (Chen et al., 2009; Lee et al., 2011; Patel et al., 2015; Wang et al., 2012). Mitochondrial dysfunction results in PTEN-induced putative kinase 1 (PINK1) binding to the outer mitochondrial membrane where it recruits the E3 ubiquitin ligase Parkin (Narendra et al., 2008). These mitochondria are targeted for mitophagy that is dependent on Parkin-mediated ubiquitination of mitochondrial proteins. Thus, mitophagy is important for the quality control of the mitochondrial population and cell homeostasis.
Mitophagy is present in several lung diseases, such as lung cancer, sepsis-induced acute lung injury, and chronic obstructive pulmonary disease (Chang et al., 2015; Chen et al., 2014; Mizumura et al., 2014). In type II alveolar epithelial cells from the IPF lung, PINK1 expression is decreased, while no difference is seen in IPF lung fibroblasts compared to normal subjects (Bueno et al., 2015). However, PINK1 protein is increased in IPF whole lung homogenates (Patel et al., 2015). The role mitophagy plays in alveolar macrophages in pulmonary fibrosis has not been determined. Our data show mitophagy contributes to alveolar macrophage apoptosis resistance and is required for macrophage-derived Tgfβ1 expression and is modulated, in part, by Akt1 activation.
Results
Alveolar macrophage Akt1 is required for the development of pulmonary fibrosis
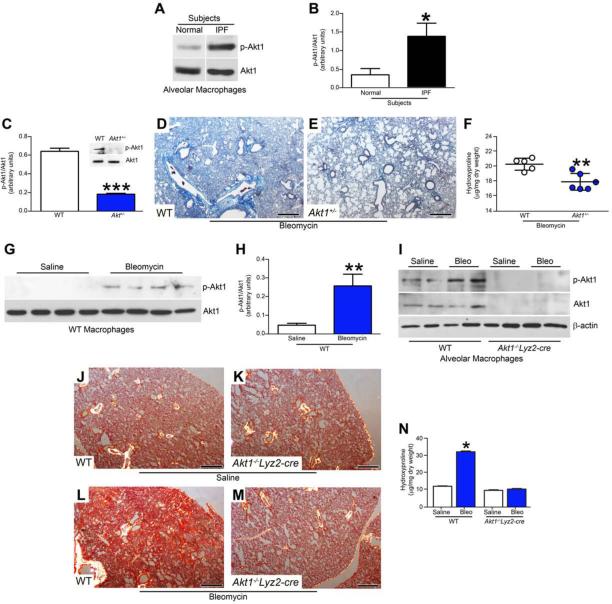
Because Akt is often altered in human disease and can be activated by many cellular stimuli or toxic insults (Govindarajan et al., 2007; Larson-Casey et al., 2014), we measured the expression of Akt1 in alveolar macrophages from normal subjects and IPF patients. Alveolar macrophages showed a 3-fold increase of p-Akt1 in IPF patients compared to normal subjects (Fig. 1A and B).
Figure 1. Akt1 activation in alveolar macrophages is associated with pulmonary fibrosis.
(A) Immunoblot and (B) densitometric analysis of alveolar macrophages isolated by BAL from normal subjects (n = 4) and IPF patients (n = 5), Student’s t-test. (C) Quantitative analysis and representative immunoblot (Inset) of alveolar macrophages isolated by BAL from WT (n =5) and Akt1+/− mice (n =5) exposed to bleomycin (2 U/kg) intratracheally, Student’s t-test. After bleomycin exposure, mice were euthanized 21 days later and lungs from (D) WT and (E) Akt1+/− mice were removed and processed for Masson’s trichrome staining. Representative micrographs from 1 of 6 mice are shown. Bar, 600 μm. (F) Hydroxyproline assay of lungs removed from WT (n = 5) and Akt1+/− mice (n = 6) after bleomycin exposure, Student’s t-test. WT mice were exposed to saline (n = 4) or bleomycin (n = 5) intratracheally, BAL was performed 21 days later. (G) Immunoblot and (H) densitometry analysis. Student’s t-test. (I) Immunoblot analysis of macrophages isolated by BAL from WT (n = 4 saline n = 5 bleo) and Akt1−/−Lyz2-cre mice (n = 4 saline; n = 6 bleo). Excised lungs from WT and Akt1−/−Lyz2-cre mice exposed to (J) and (K) saline or (L) and (M) bleomycin were stained with Sirius red. Representative micrographs from WT (n = 4 saline; n = 5 bleo) and Akt1−/−Lyz2-cre mice (n = 4 saline; n = 7 bleo) are shown. Bar, 500 μm. (N) Hydroxyproline of lungs from WT (n = 4 saline; n = 5 bleo) and Akt1−/−Lyz2-cre mice (n = 4 saline; n = 6 bleo). One-way ANOVA with Tukey’s comparison. *, p < 0.05; **, p < 0.002; ***, p < 0.0001. A minumun of three independent experiments were conducted. Please see Figure S1.
To investigate the relationship of macrophage Akt1 activation to pulmonary fibrosis in vivo, WT and Akt1+/− mice were subjected to bleomycin-induced lung injury. Alveolar macrophages from Akt1+/− mice had a decrease in p-Akt1 expression compared to WT mice (Fig. 1C). Lungs from WT mice showed widespread destruction of lung architecture and aberrant collagen deposition (Fig. 1D), whereas Akt1+/− mice had a marked reduction in collagen accumulation and preserved lung architecture (Fig. 1E). The histological findings were verified biochemically by hydroxyproline assay (Fig. 1F).
We assessed if Akt1 was activated in alveolar macrophages from fibrotic mice. Macrophages isolated from the lungs of bleomycin-injured mice had increased p-Akt1 expression (Fig. 1G), which was 5-fold greater compared to control mice (Fig. 1H). The PI3K inhibitor, LY294002, inhibited phosphorylation of Akt1 and the PI3K regulatory subunit (p85) at Tyr458, which is necessary for Akt1 activation (Fig. S1A). Alveolar macrophages from bleomycin-injured WT mice had increased phosphorylation of p85 (Fig. S1B).
To investigate the pathological significance of macrophage Akt1 in fibrosis, we generated mice harboring a conditional deletion of Akt1 in macrophages (Akt1−/−Lyz2-cre). Alveolar macrophages isolated from Akt1−/−Lyz2-cre mice had a complete absence of p-Akt1 and Akt1 expression, while bleomycin-injured WT mice showed an increase in p-Akt1 expression (Fig. 1I). Type II alveolar epithelial cells isolated from WT and Akt1−/−Lyz2-cre mice had similar expression of p-Akt1 and Akt1, suggesting efficient recombination only in macrophages (Fig S1C). WT and Akt1−/−Lyz2-cre mice had similar numbers of BAL cells after bleomycin (Fig. S1D); however, WT mice had greater loss of barrier function, indicating more lung injury (Fig. S1E).
The conditional deletion of Akt1 from alveolar macrophages had no effect on the lung parenchyma in saline exposed mice (Fig. 1J and K). There was dense collagen deposition in bleomycin-injured WT mice (Fig. 1L), whereas the Akt1−/−Lyz2-cre mice had essentially normal lungs (Fig. 1M). The histological findings were confirmed biochemically (Fig. 1N). These data suggest Akt1 activation in alveolar macrophages is required in the pathogenesis of bleomycin-induced pulmonary fibrosis.
IPF patients have increased pro-fibrotic alveolar macrophages
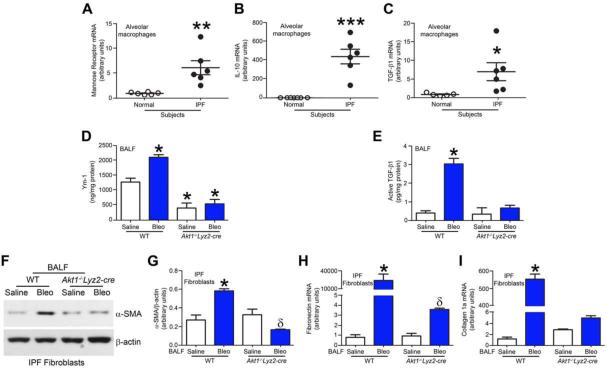
Pro-fibrotic macrophages are responsible for the generation of anti-inflammatory cytokines and are often associated with fibrotic conditions, including pulmonary fibrosis (He et al., 2013; Redente et al., 2014). We questioned if alveolar macrophages from IPF patients have a predominant pro-fibrotic phenotype. Mannose receptor (Fig. 2A) and IL-10 (Fig. 2B) mRNA expression were more than 6-fold and 300-fold greater in IPF alveolar macrophages. Moreover, TGF-β1 mRNA expression in alveolar macrophages was greater than 7-fold more in IPF patients compared to normal volunteers (Fig. 2C).
Figure 2. Alveolar macrophages from IPF patients have a pro-fibrotic phenotype.
Total RNA was isolated from alveolar macrophages obtained from normal subjects and IPF patients by BAL. (A) Mannose receptor (n = 6), (B) IL-10 (n = 7 normal; n = 6 IPF), and (C) TGF-β1 mRNA (n = 5 normal; n = 6 IPF) were measured by quantitative PCR, Student’s t-test. WT and Akt1−/−Lyz2-cre mice were exposed to saline or bleomycin (bleo) intratracheally and BAL was performed 21 days later. (D) Ym-1 and (E) Active TGF-β1 were measured in BAL fluid by ELISA. n = 4 saline; n = 5 bleo. (F) Immunoblot and (G) densitometry analysis of IPF fibroblasts cultured in BAL fluid from exposed WT and Akt1−/−Lyz2-cre mice. n = 3 saline; n = 4 bleo. Total RNA was isolated from IPF fibroblasts conditioned with BAL fluid from WT and Akt1−/−Lyz2-cre mice. (H) Fibronectin and (I) collagen 1A mRNA were measured. n = 4 saline; n = 5 bleo. *, p < 0.05 vs WT+saline; **, p < 0.001; ***, p < 0.0001; δ, p < 0.05 vs Akt1−/−Lyz2-cre +saline. One-way ANOVA with Tukey’s comparison. . A minumun of three independent experiments were conducted. Please see Figure S2.
Because the deletion of Akt1 in macrophages in vivo is protective, we hypothesized that Akt1 expression led to the polarization of macrophages to a pro-fibrotic phenotype. The pro-fibrotic marker, Ym1, was significantly increased in the BAL fluid from bleomycin-injured WT mice (Fig. 2D). In contrast, Akt1−/−Lyz2-cre mice had Ym1 concentrations below controls. Furthermore, active TGF-β1 in the BAL fluid from Akt1−/−Lyz2-cre mice was similar to saline controls from WT mice (Fig. 2E). The anti-fibrotic markers (TNF-α, IL-1β, and IL-6) had an opposite expression trend in BAL fluid (Fig. S2A-C).
Because TGF-β1 is known to induce differentiation of fibroblasts to myofibroblasts, we determined if BAL fluid from mice promoted IPF fibroblast differentiation. IPF fibroblasts incubated with BAL fluid from WT mice induced an increase in α-smooth muscle actin (α-SMA) (Fig. 2F and G). The α-SMA expression was less in fibroblasts incubated with BAL fluid from Akt1−/−Lyz2-cre mice than WT controls suggesting that macrophage-derived TGF-β1 production is critical for fibroblast differentiation.
The difference in fibroblast differentiation correlated with altered myofibroblast function. IPF fibroblasts incubated in BAL fluid from bleomycin-injured WT mice showed a 29,000-fold increase in fibronectin (Fig. 2H) and 400-fold increase in collagen 1α mRNA compared to IPF fibroblasts incubated in BAL fluid from Akt1−/−Lyz2-cre mice (Fig. 2I). Normal human lung fibroblasts showed a similar trend. (Fig. S2D-E). Taken together, these data suggest that alveolar macrophages from Akt1−/−Lyz2-cre mice are anti-fibrotic and have reduced production of the pro-fibrotic factor(s) required for myofibroblast function.
Macrophage-derived TGF-β1 is required for pulmonary fibrosis
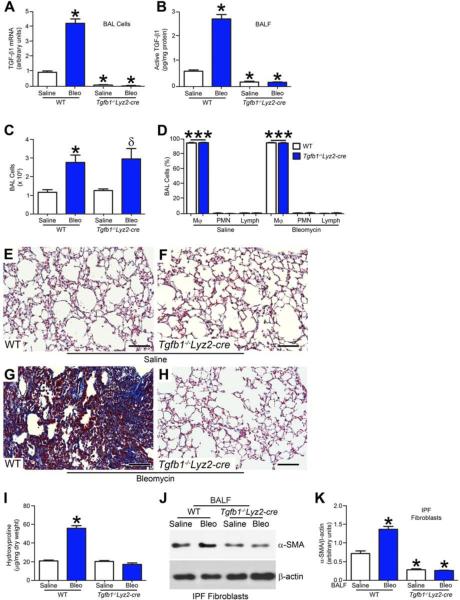
Based on these observations in fibroblast differentiation and function, we hypothesized these findings were due to decreased macrophage-derived TGF-β1 in Akt1−/−Lyz2-cre mice. To determine if macrophage TGF-β1 mediated these changes, we generated mice harboring a conditional deletion of Tgfb1 in macrophages (Tgfb1−/− Lyz2-cre). Bleomycin-injured WT mice had a 4-fold increase in TGF-β1 mRNA expression compared to saline controls, while TGF-β1 mRNA expression in Tgfb1−/−Lyz2-cre mice was at the limit of detection (Fig. 3A). Confirming these results, active TGF-β1 measured in BAL fluid in Tgfb1−/−Lyz2-cre mice was below WT saline controls (Fig. 3B). The total number of BAL cells was increased with bleomycin exposure (Fig. 3C), and alveolar macrophages were the predominant (>90%) cell type (Fig. 3D).
Figure 3. Macrophage-derived TGF-β1 is required for pulmonary fibrosis.
WT and Tgfb1−/− Lyz2-cre mice were exposed to saline or bleomycin intratracheally and BAL was performed 21 days later. (A) Total RNA was isolated from alveolar macrophages obtained by BAL. TGF-β1 mRNA was measured. (B) Active TGF-β1 was measured in BAL fluid by ELISA from WT and Tgfb1−/− Lyz2-cre mice; n = 6. (C) Total number of BAL cells and (D) cell differential determined using Wright-Giemsa stain from BAL; n = 6. Lungs were excised from WT and Tgfb1−/− Lyz2-cre mice exposed to (E) and (F) saline or (G) and (H) bleomycin and stained using Masson’s trichrome. Representative micrographs from WT and Tgfb1−/− Lyz2-cre mice; n = 6. Bar, 200 μm. (I) Hydroxyproline of lungs removed from WT and Tgfb1−/− Lyz2-cre mice; n = 6. (J) Immunoblot and (K) densitometry analysis of IPF fibroblasts cultured in BAL fluid from exposed WT and Tgfb1−/− Lyz2-cre mice; n = 6. *, p < 0.05 vs WT+saline; ***, p < 0.0001 vs PMN and Lymph. One-way ANOVA with Tukey’s comparison. . A minumun of three independent experiments were conducted. Please see Figure S3.
To verify that macrophage-derived TGF-β1 mediates lung fibrosis, trichrome staining revealed deletion of TGF-β1 from alveolar macrophages had no effect on lung parenchyma (Fig. 3E and F). Bleomycin-injured WT mice had widespread accumulation of collagen in lungs (Fig. 3G), whereas Tgfb1−/−Lyz2-cre mice had normal lung architecture similar to saline controls (Fig. 3H). These results were confirmed by hydroxyproline assay (Fig. 3I). Bleomycin increased p-Akt1 in alveolar macrophages from WT and Tgfb1−/−Lyz2-cre mice, although the conditional deletion of Tgf-β1 resulted in greater expression of p-Akt1 (Fig. S3A). Moreover, alveolar macrophages isolated from Tgfb1−/−Lyz2-cre mice polarize to an anti-fibrotic phenotype, with increased IL-1β, IL-6, and TNF-α and decreased Ym-1 mRNA expression compared to WT mice (Fig. S3B-E).
Further evidence to support the role of macrophage-derived TGF-β1 in development of a fibrotic phenotype is that α-SMA was attenuated in IPF fibroblasts incubated in Tgfb1−/−Lyz2-cre BAL fluid compared to WT saline controls (Fig. 3J and K). These observations support the notion that macrophage-derived active TGF-β1 expression mediates development of a fibrotic phenotype in vivo.
Mitophagy is increased in IPF alveolar macrophages
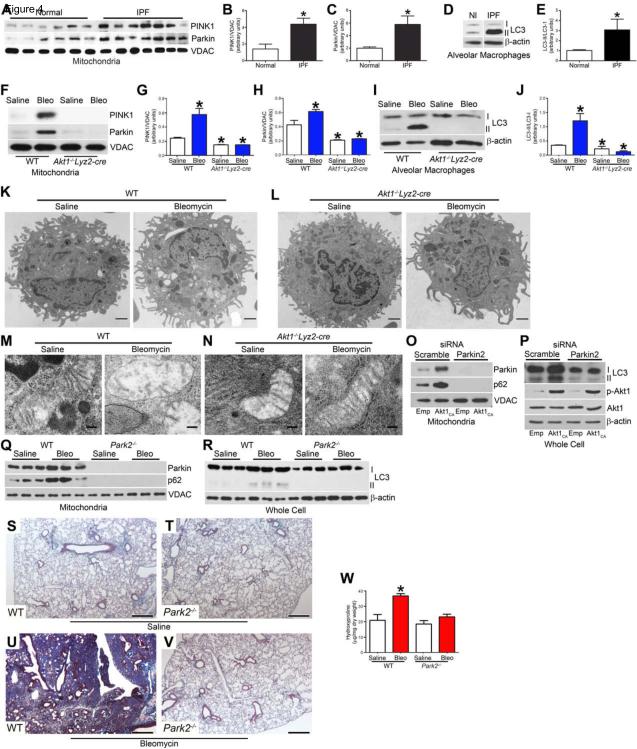
Studies have conflicting data on the role of mitophagy in type II alveolar epithelial cells and fibroblasts from IPF patients (Araya et al., 2013; Bueno et al., 2015; Kim et al., 2012; Patel et al., 2015; Ricci et al., 2013); however, the contribution of mitophagy in alveolar macrophages to fibrosis development is not known. We found that mitochondria isolated from IPF alveolar macrophages had greater immunoreactive PINK1 (Fig. 4A and B) and Parkin expression (Fig. 4A and C) compared to normal subjects. The increase corresponded to an increase of LC3-II expression in alveolar macrophages (Fig. 4D and E), suggesting mitophagy is occurring as indicated by autophagosome formation.
Figure 4. Mitophagy is enhanced in pro-fibrotic alveolar macrophages.
(A) Mitochondria isolated from alveolar macrophages from normal subjects (n = 6) and IPF patients (n = 7) were subjected to immunoblot analysis. Densitometry analysis of (B) PINK1 and (C) Parkin immunoblots normalized to VDAC, Student’s t-test. Alveolar macrophages from normal subjects (n = 7) and IPF patients (n = 6) were subjected to (D) immunoblot and (E) densitometry analysis, Student’s t-test. (F) Macrophages isolated from exposed WT and Akt1−/−Lyz2-cre mice were subjected to immunoblot analysis. Quantitative analysis of (G) PINK1 and (H) Parkin immunoblots normalized to VDAC. (I) Immunoblot and (J) densitometry analysis in alveolar macrophages from exposed WT and Akt1−/−Lyz2-cre mice. WT (n = 4 saline; n = 5 bleo) and Akt1−/−Lyz2-cre mice (n = 4 saline; n = 6 bleo). Macrophages isolated from exposed WT and Akt1−/−Lyz2-cre mice were analyzed by transmission electron microscopy. Images representative of n = 5. (K and L) Bar = 1 μm. (M and N) Bar = 100 nm. (O) Mitochondrial Parkin and p62, and (P) p-Akt1, Akt1, LC3-I and -II expression were measured in THP-1 cells transfected with scrambled or Parkin siRNA in combination with empty or Akt1CA vectors. Exposed WT and Park2−/− mice were subjected to BAL 21. Immunoblot analysis of (Q) mitochondrial Parkin and p62, (R) LC3-I and -II expression were measured. Lungs were excised from WT and Park2−/− mice exposed to (S) and (T) saline or (U) and (V) bleomycin and stained with Masson’s trichrome. Representative micrographs of 1 of 5 mice. Bar, 500 μm. (W) Hydroxyproline assay; n = 5. *, p < 0.05 vs WT+saline. One-way ANOVA with Tukey’s comparison. A minumun of three independent experiments were conducted. Please see Figure S4.
We investigated if macrophage mitophagy was recapitulated in vivo. WT mice with bleomycin-induced injury had increased PINK1 and Parkin expression in isolated mitochondria from alveolar macrophages (Fig. 4F-H), and LC3-II expression was also increased (Fig. 4I and J). In contrast, Akt1−/− Lyz2-cre mice showed no evidence of PINK1, Parkin, or LC3-II expression.
To determine the effect of Akt1 expression in alveolar macrophages visually, we used transmission electron microscopy to examine alveolar macrophage mitophagy. Bleomycin increased the presence of vacuoles in alveolar macrophages isolated from bleomycin-injured WT mice compared to macrophages isolated from saline controls (Fig. 4K). Conversely, alveolar macrophages isolated from Akt1−/−Lyz2-cre mice had no visualized vacuoles (Fig. 4L). Bleomycin-injured WT alveolar macrophages had mitochondria with an irregular shape and disorganized cristae (Fig. 4M), whereas alveolar macrophages isolated from Akt1−/−Lyz2-cre mice had normal mitochondria. To confirm this difference was secondary to Akt1, overexpression of constitutive active Akt1 (Akt1CA) in macrophages in vitro increased the number of vacuoles compared to the saline controls (Fig. S4A and B). The number increased dramatically in cells expressing Akt1CA after bleomycin treatment. These findings provide direct evidence that Akt1 activation in alveolar macrophages induces mitophagy.
Although increased LC3-II expression suggests induction of autophagy, we analyzed flux through the autophagy–lysosome pathway in macrophages by several methods. Macrophages treated with bafilomycin A (Baf A), an inhibitor of autophagosome fusion with lysosomes, had increased mitochondrial accumulation of PINK1 and Parkin (Fig. S4C). The autophagy adaptor protein, p62, and LC3-II expression were increased as well. Overexpression of Akt1CA with Baf A treatment showed a further induction (Fig. S4C and D). The autophagy inhibitor, 3-methyladenine (3-MA), inhibited autophagy flux in macrophages even in the presence of Akt1CA (Fig. S4E and F).
Because mitophagy results in degradation of several mitochondrial proteins, including TOM20 (Wauer et al., 2015), cells expressing Akt1CA treated with vehicle had a decrease in TOM20, whereas Baf A treatment increased TOM20 expression in the presence or absence of Akt1CA (Fig. S4G). Similar results were obtained with leupeptin, a lysosomal enzyme inhibitor, (Fig. S4H), providing further evidence that Akt1 regulates macrophage mitophagy.
To evaluate the role of Akt1 using a genetic approach, mitochondria isolated from Parkin-deficient macrophages showed an absence of mitophagy with no Parkin and p62 with Akt1CA overexpression (Fig. 4O). LC3-II expression was also absent with Parkin2 silencing (Fig. 4P). To determine the biological relevance of mitophagy inhibition, alveolar macrophages isolated from bleomycin-treated Park2−/− mice showed no Parkin or p62 in isolated mitochondria and no corresponding LC3-II expression (Fig. 4Q and R).
The deletion of Parkin in vivo did not alter lung architecture (Fig. 4S and T). Bleomycin-injured WT mice had widespread collagen deposition with destruction of lung architecture (Fig. 4U), whereas the bleomycin-treated Park2−/− mice had essentially normal lungs (Fig. 4V). These results were confirmed by hydroxyproline analysis (Fig. 4W). These data suggest macrophage mitophagy is critical for the pathogenesis of fibrosis.
To determine if inducing mitophagy had the opposite effect, alveolar macrophages isolated from rapamycin-treated mice had increased expression of PINK1, Parkin, and p62 as well as LC3-II (Fig. S4I and J). Moreover, fibrosis development was augmented by rapamycin (Fig. S4K).
Akt1-mediated mitochondrial ROS in alveolar macrophages induces mitophagy
One measure of mitochondrial dysfunction and an indicator of mitophagy is the loss of mitochondrial membrane potential (Δψm) (Narendra et al., 2008; Vives-Bauza et al., 2010). Macrophages exposed to bleomycin showed a loss in Δψm (Fig. S5A), and increasing concentrations of bleomycin induced a dose-dependent decrease in Δψm (data not shown). Overexpression of Akt1CA did not reverse or lead to the loss of Δψm.
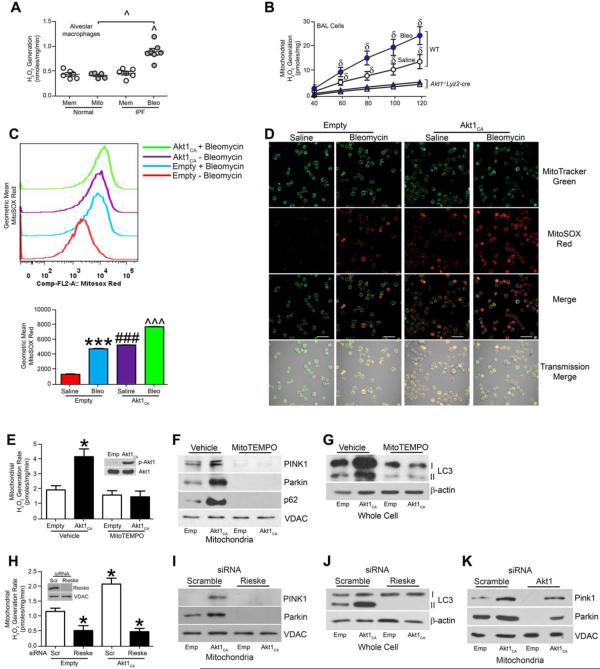
Because mitophagy is induced by ROS and Akt1 increases mitochondrial ROS (Chen et al., 2009; Larson-Casey et al., 2014; Lee et al., 2011; Patel et al., 2015; Wang et al., 2012), we determined that IPF alveolar macrophages had significantly greater mitochondrial H2O2 produced while normal subjects showed no difference in H2O2 produced in membrane or mitochondrial fractions (Fig. 5A). This was also seen in vivo. Mitochondria isolated from bleomycin-injured WT mice produced significantly more H2O2 than saline controls (Fig. 5B). In contrast, mitochondria isolated from Akt1-/-Lyz2-cre mice had decreased H2O2 production compared to WT mice, and there was no increase in H2O2 generation with bleomycin. Similar observations were seen in the lungs of mice; bleomycin-injured WT mice had significantly greater percentage of glutathione in its oxidized form, GSSG, than Akt1−/−Lyz2-cre mice (Fig. S5B).
Figure 5. Akt1-meditated ROS generation induces mitophagy in macrophages.
(A) H2O2 production was measured in membrane (mem) and mitochondrial fractions (mito) from isolated alveolar macrophages from normal subjects (n = 6 mem; n = 5 mito) and IPF patients (n = 6 mem; n = 7 mito). (B) Mitochondria were isolated from alveolar macrophages and H2O2 production was measured. WT (n = 4 saline; n = 5 bleo) and Akt1−/−Lyz2-cre mice (n = 4 saline; n = 6 bleo). (C) Flow cytometry with MitoSOX geometric mean and (D) Representative confocal images of MH-S cells transfected with empty or Akt1CA vector treated with saline or bleomycin (12.5 mU/ml). Macrophages were co-stained with MitoTracker and MitoSOX. Bar = 40 μm. n = 5. (E) Mitochondrial H2O2, (F) PINK, Parkin, p62, and (G) LC3-I and -II expression were determined in THP-1 cells treated with vehicle or MitoTEMPO transfected with empty or Akt1CA vectors. Inset, Akt1 immunoblot analysis. n = 5. (H) Mitochondrial H2O2, (I) mitochondrial PINK1 and Parkin, and (J) LC3-I and -II expression were measured in THP-1 cells transfected with scrambled or Rieske siRNA in combination with empty or Akt1CA vectors. Inset, Rieske immunoblot analysis. n = 5. (K) Mitochondrial PINK1 and Parkin expression were measured in THP-1 cells transfected with scrambled (Scr) or Akt1 siRNA in combination with empty or Akt1CA vectors; n = 5. *, p < 0.05 vs Vehicle+empty or Empty+scr; ***, p < 0.0001 vs Empty+saline; δ, p < 0.05 Akt1−/−Lyz2-cre +saline and Akt1−/−Lyz2-cre+bleo; ^, p < 0.05 vs IPF+mem; ^^^, p < 0.001 vs Empty+ Bleo; ###, I < 0.0001 vs all other conditions. One-way ANOVA with Tukey’s comparison. A minumun of three independent experiments were conducted. Please see Figure S5.
To confirm Akt1 mediates mitochondrial ROS, we co-stained macrophages with MitoSOX red and MitoTracker green to quantitate mitochondrial ROS by flow cytometry. Bleomycin increased fluorescence in cells expressing an empty vector (Fig. 5C). Overexpression of Akt1CA increased MitoSOX fluorescence to a greater extent than bleomycin alone, and bleomycin treatment further enhanced MitoSOX intensity in cells expressing Akt1CA (Fig. 5C). These results were confirmed visually by confocal microscopy (Fig. 5D).
Verifying that ROS production induced mitophagy via Akt1, H2O2 production in isolated mitochondria from macrophages expressing Akt1CA was significantly inhibited by the mitochondrial targeted antioxidant, MitoTEMPO (Fig. 5E). Immunoblot analysis showed MitoTEMPO treatment inhibited mitophagy (Fig. 5F) and reduced LC3-II expression (Fig. 5G) in the presence of Akt1CA. Silencing of Rieske mRNA, encoding a mitochondrial iron-sulfur protein, significantly reduced mitochondrial H2O2 in the presence or absence of Akt1CA (Fig. 5H). Immunoblot analysis revealed that silencing of Rieske mRNA abolished PINK1 and Parkin expression (Fig. 5I) and decreased LC3-II expression (Fig. 5J). The role of mitochondrial H2O2 inducing mitophagy was further confirmed using Antimycin A (Fig. S5C-E).
Because Akt1 induces mitophagy, we assessed if overexpression of Akt1CA in Akt1-deficient cells rescued mitophagy. Silencing of Akt1 mRNA resulted in absence of these markers of mitophagy, whereas Akt1CA overexpression partially increased PINK1 and Parkin (Fig. 5K). These observations suggest Akt1-mediated mitochondrial H2O2 modulates macrophage mitophagy.
Mitophagy is required for active TGF-β1 expression
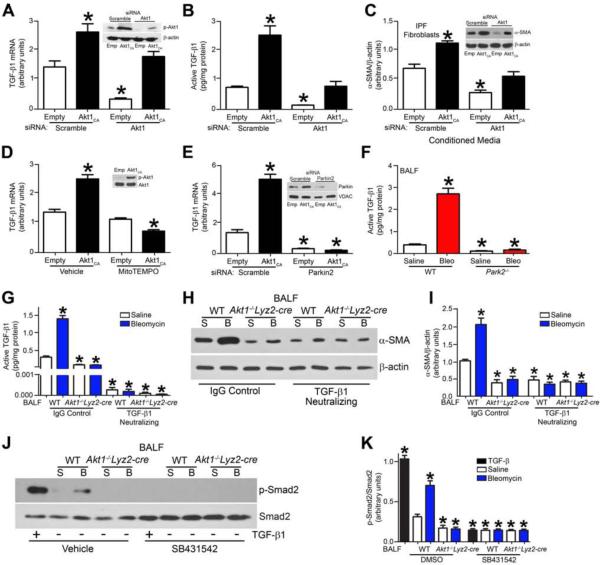
Because Akt1−/−Lyz2-cre mice have reduced active TGF-β1, we determined if this reduction was only due to Akt1 deficiency. Immunoblot analysis showed overexpression of Akt1CA in cells with Akt1 mRNA silencing partially rescued Akt1 expression (Fig. 6A, Inset). Akt1CA overexpression increased TGF-β1 mRNA expression (Fig. 6A) and active TGF-β1 in the conditioned media (Fig. 6B). Silencing of Akt1 mRNA reduced TGF-β1 below control, whereas Akt1CA overexpression partially rescued gene expression and activation of TGF-β1.
Figure 6. Macrophage mitophagy regulates TGF-β1 gene expression and function.
(A) TGF-β1 mRNA was measured in THP-1 cells transfected with scrambled (Scr) or Akt1 siRNA in combination with empty or Akt1CA vectors; n = 5. Inset, Akt1 immunoblot analysis. (B) Active TGF-β1 was measured by ELISA in conditioned media from THP-1 cells transfected with scrambled or Akt1 siRNA in combination with empty or Akt1CA vectors; n = 5. (C) α-SMA and β-actin were measured by immunoblot (Inset) and densitometry analysis in IPF fibroblasts incubated with conditioned media from THP-1 cells transfected with scramble or Akt1 siRNA in combination with empty or Akt1CA vectors; n = 5. (D) TGF-β1 mRNA expression were measured in MH-S cells expressing empty or Akt1CA and treated with mitoTEMPO. Inset, Akt1 overexpression verified by immunoblot analysis; n = 5. (E) TGF-β1 mRNA expression was measured in THP-1 cells transfected with scramble or Parkin siRNA in combination with empty or Akt1CA vectors. Inset, Parkin2 mRNA silencing verified by immunoblot analysis; n = 5. (F) Active TGF-β1 was measured in BAL fluid from exposed WT and Park2−/− mice; n = 5. (G) Active TGF-β1 was measured in BAL fluid from WT and Akt1−/−Lyz2-cre mice incubated with IgG1control or TGF-β1 neutralizing antibody; BAL from n = 6. (H) Representative immunoblot and (I) densitometry analysis of IPF fibroblasts cultured in BAL fluid from saline (S) or bleomycin (B) from exposed WT and Akt1−/−Lyz2-cre mice. BAL fluid was pre-incubated with IgG1 control or neutralized with TGF-β1 antibody (10 μg/ml); BAL from n = 6 mice for each group on IPF fibroblasts. (J) Representative immunoblot and (K) densitometry analysis in IPF fibroblasts pre-treated with vehicle (DMSO) or SB431542 (10 μM) and then incubated with either rhTGF-β1 (10 ng/ml) or BAL fluid from saline (S) or bleomycin (B) exposed WT and Akt1−/−Lyz2-cre mice; BAL from n = 6 mice for each group on IPF fibroblasts. *, p < 0.05 vs Scr+empty, Vehicle+empty, WT+Saline, or WT+Vehicle. One-way ANOVA with Tukey’s comparison. A minumun of three independent experiments were conducted. Please see Figure S6.
We determined if the rescue of Akt1 had a physiologic effect on fibroblast differentiation. IPF fibroblasts incubated in conditioned media from macrophages overexpressing Akt1CA had significantly increased α-SMA, while IPF fibroblasts cultured in conditioned media from Akt1-deficient macrophages decreased α-SMA expression (Fig. 6C). Conditioned media from Akt1-deficient macrophages transfected with Akt1CA increased α-SMA expression in IPF fibroblasts, suggesting Akt1-mediated mitophagy is linked to TGF-β1.
Because Akt1 regulates TGF-β1 mRNA expression, we investigated if Akt1 modulated AP-1-driven transcription, as it regulates the promoter of many growth factors, including TGF-β1 (Birchenall-Roberts et al., 1990; Kim et al., 1990). We determined that Akt1 increased phosphorylation of c-Jun and nuclear localization of c-Fos (Fig. S6A). This was associated with a significant increase in AP-1-driven luciferase and Tgfb1 promoter activity (Fig. S6B and C).
Because MitoTEMPO treatment and Parkin2 mRNA silencing inhibited mitophagy in macrophages, we assessed if macrophage mitophagy was required for TGF-β1 mRNA expression. Akt1CA overexpression increased TGF-β1 mRNA expression in vehicle-treated cells, whereas inhibition of mitophagy with MitoTEMPO decreased TGF-β1 mRNA expression significantly (Fig. 6D). Similarly, Parkin2 mRNA silencing significantly reduced TGF-β1 mRNA below control in the presence or absence of Akt1CA overexpression (Fig. 6E). This was also present in vivo. Active TGF-β1 in BAL fluid from Park2−/− mice was below saline controls (Fig. 6F).
Because mitophagy regulated macrophage-derived TGF-β1 mRNA expression and several growth factors can induce fibroblast differentiation and myofibroblast function, we investigated if macrophage-derived TGF-β1 was the responsible for fibroblast differentiation. Utilizing a TGF-β1 neutralizing antibody, we reduced active TGF-β1 in BAL fluid (Fig. 6G). IPF fibroblasts incubated with neutralized BAL fluid failed to differentiate, shown by markedly less α-SMA compared to the saline control (Fig. 6H). The quantification of these observations showed a greater than 75 percent reduction in α-SMA expression in bleomycin-injured WT mice (Fig. 6I).
This was further confirmed by assessing TGF-β1 signaling. IPF fibroblasts incubated in BAL fluid from Akt1−/−Lyz2-cre mice lacked p-Smad2 expression compared to cells exposed to BAL fluid from bleomycin-injured WT mice (Fig. 6J). In fact, p-Smad2 expression in IPF fibroblasts incubated in BAL fluid from Akt1−/−Lyz2-cre mice was similar to cells treated with SB431542, the TGF-β type 1 receptor (ALK5) inhibitor (Fig. 6K). Rapamycin-treated mice had increased active TGF-β1 in BAL fluid and bleomycin injury leading to further induction (Fig. S6D). In aggregate, these data indicate that Akt1-induced mitophagy in alveolar macrophages is required for macrophage-derived TGF-β1 expression, which promotes fibrosis development.
IPF alveolar macrophages have apoptosis resistance
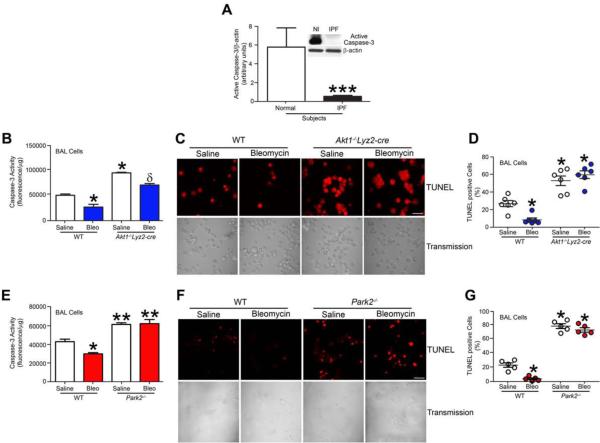
Macrophages in chronic disease typically exhibit apoptosis resistance, which is generally associated with disease progression (Duffield et al., 2005; Redente et al., 2014). We determined that IPF alveolar macrophages were resistant to apoptosis with greater than 85 percent less active caspase-3 compared to normal subjects (Fig. 7A). Although alveolar macrophages were the primary cell type in murine BAL fluid, Akt1−/−Lyz2-cre mice had a significant reduction in the number of alveolar macrophages compared to WT mice (Fig. S7A). Moreover, macrophages isolated from bleomycin-injured WT mice had reduced caspase-3 activity compared to saline controls, whereas caspase-3 activity in macrophages from Akt1−/−Lyz2-cre mice was significantly increased (Fig. 7B). These observations were confirmed visually by TUNEL staining alveolar macrophages from WT and Akt1−/−Lyz2-cre mice (Fig. 7C). Akt1−/−Lyz2-cre alveolar macrophages had a 6-fold increase in TUNEL positive cells (Fig. 7D). To confirm that the apoptosis was secondary to loss of Akt1, Akt1CA overexpression resulted in apoptosis resistance, which was further increased by bleomycin treatment (Fig. S7B). These results suggest that apoptosis resistance in alveolar macrophage promotes the development of a fibrotic phenotype in mice.
Figure 7. IPF alveolar macrophages have apoptosis resistance.
(A) Densitometry analysis in normal subjects and IPF patients (n = 5), Student’s t-test. Inset, representative immunoblot for cleaved caspase-3 and β-actin. (B) Caspase-3 activity measured in alveolar macrophages isolated from exposed WT and Akt1−/−Lyz2-cre mice; n = 4 saline, n = 6 bleo. (C) Representative images of TUNEL staining and (D) quantification from WT and Akt1−/−Lyz2-cre mice exposed to bleomycin or saline; n = 6, each dot represents 5 analyzed images. Bar = 40 μm. (F) Caspase-3 activity measured in alveolar macrophages isolated from exposed WT and Park2−/− mice. n = 5. (G) Representative images of TUNEL staining in BAL cells and (H) quantification from exposed WT and Park2−/− mice; n = 5, each dot represents 5 analyzed images. Bar = 40 μm. *, p < 0.05 vs WT+saline; **, p < 0.001 vs WT+saline and WT+bleo; ***, p < 0.0001. δ, p < 0.05 vs Akt1−/−Lyz2-cre+saline. One-way ANOVA with Tukey’s comparison. A minumun of three independent experiments were conducted. Please see Figure S7.
To directly determine if mitophagy is necessary for macrophage apoptosis resistance, we found that alveolar macrophages in Park2−/− mice were significantly reduced compared to WT mice (Fig. S7C). Alveolar macrophages isolated from Park2−/− mice exhibited increased apoptosis, while bleomycin-injured WT mice had reduced caspase-3 activity (Fig. 7F). These observations were visually confirmed by TUNEL staining (Fig. 7G). Park2−/− alveolar macrophages had 17-fold greater TUNEL positive cells than WT macrophages (Fig. 7H). Similar finding were found in vitro with Parkin-deficient macrophages (Fig. S7D), which had 21-fold increase in TUNEL positive cells (Fig. S7E).
Using an inhibitor of mitophagy, caspase-3 activity was significantly increased in macrophages treated with 3-MA compared to vehicle controls, and Akt1CA overexpression did not alter caspase-3 activity in the presence of 3-MA (Fig. S7F). In contrast, rapamycin treatment in vivo significantly reduced caspase-3 activity in alveolar macrophages compared to vehicle-treated mice (Fig. S7G). This reduction in caspase-3 activity was further enhanced by bleomycin injury. Taken together, these results suggest that mitophagy is required for apoptosis resistance in macrophages. Moreover, these observations suggest that Akt1-mediated macrophage mitophagy promotes apoptosis resistance, which regulates myofibroblast differentiation by modulating macrophage-derived TGF-β1, and thus, has a critical role in the pathogenesis of pulmonary fibrosis.
Discussion
Macrophages in chronic disease typically exhibit apoptosis resistance and their prolonged survival is generally associated with disease progression. In fact, conditional macrophage depletion has been shown to attenuate models of liver and lung injury in vivo (Duffield et al., 2005; Redente et al., 2014). Phagocytosis of apoptotic cells, which are frequently present in tissue injury, also augments cell survival (Weigert et al., 2006). Our observations suggest that dysfunctional mitochondria are removed in alveolar macrophages, promoting apoptosis resistance during the fibrotic process and Akt1-mediated mitophagy modulates TGF-β1 and the effector cells, myofibroblasts.
Mitophagy is a cell survival mechanism that is increased in conditions of stress to attempt to retain cell homeostasis. Genetic deletion of PINK1 and PARK2 leads to progressive mitochondrial damage that is associated with Parkinson’s disease (Lin and Beal, 2006). Mitophagy is known to play an important role in many physiological processes. It has been implicated in aging, tumorigenesis, and it contributes to many diseases, including the neurological diseases Parkinson’s, Alzheimer’s, and Huntington’s, and immunological disorders (Cui et al., 2006; Inami et al., 2011; Lemasters, 2005; Lin and Beal, 2006; Maurer et al., 2000; Zhou et al., 2011). Mitophagy is impaired in the IPF lungs, and the role mitophagy plays in alveolar macrophages in pulmonary fibrosis is not known (Araya et al., 2013; Bueno et al., 2015; Patel et al., 2015; Ricci et al., 2013). The fact that IPF patients taking the rapamycin analogue, everolimus, had progression of disease (Malouf et al., 2011) uncovers the fact that mitophagy contributes to macrophage apoptosis resistance, which appears to be an important feature in the pathogenesis of the disease.
Akt activation is strongly associated with regulating survival and differentiation of myofibroblasts in the setting of pulmonary fibrosis, as it is known to regulate many fibrotic remodeling processes (Xia et al., 2008). Studies indicate TGF-β1 regulates Akt activation in myofibroblasts, and inhibition of Akt diminishes TGF-β1-induced fibrosis (Horowitz et al., 2007; Kang et al., 2007). Integrin αvβ6, expressed on epithelial cells, has been implicated in regulating the local activity of latent TGF-β1 in response to lung injury (Munger et al., 1999). β6−/− mice are protected from bleomycin-induced pulmonary fibrosis due to the inability to activate TGF-β1. Our results do not refute the necessity of αvβ6 in activating latent TGF-β1. Rather, our data demonstrates that alveolar macrophages are the primary source of TGF-β1. However, no study to our knowledge, has implicated Akt1 in regulating TGF-β1 production in macrophages, especially in the pathogenesis of pulmonary fibrosis.
TGF-β1 is known to decrease autophagy in human lung fibroblasts by activation of Akt in the IPF lung (Araya et al., 2013; Mi et al., 2011). Studies suggest that mitophagy is induced in type II alveolar epithelial cells treated with TGF-β1, and TGF-β1 increases autophagy in other cell types as well (Ghavami et al., 2015; Patel et al., 2015). Furthermore, Akt is known to dampen TGF-β1 signaling by direct interaction with SMAD3 (Conery et al., 2004; Remy et al., 2004). This diversity in Akt, TGF-β1, and autophagy may be cell type or stimulus specific; however, our observations show that Akt1 regulates the expression of macrophage-derived TGF-β1 by increasing mitophagy, which results in the development of a fibrotic phenotype in mice.
Pulmonary fibrosis is characterized by aberrant wound healing leading to collagen deposition and distortion of normal lung architecture. Macrophages are known to regulate multiple cell types by secreting growth factors and cytokines (Bitterman et al., 1982; Lemaire et al., 1986). Macrophages not only initiate an inflammatory response after injury, but they are also involved in resolution and repair of the injury. These divergent functions of macrophages are determined by their plasticity and ability to differentiate into distinct macrophage sub-populations. Anti-fibrotic macrophages generate pro-inflammatory cytokines and kill microorganisms and tumor cells, whereas pro-fibrotic macrophages are anti-inflammatory, promote angiogenesis and tissue remodeling, and can be associated with fibrotic conditions, including pulmonary fibrosis (He et al., 2013; Nair et al., 2009). In fact, pro-fibrotic macrophages are often found in the fibrotic tissues (He et al., 2013; Redente et al., 2014). Studies show that alternatively activated macrophages have a prolonged survival because they are involved in repair of injured tissue (Duffield et al., 2005; Redente et al., 2014). In aggregate, these observations in this study suggest that Akt1-mediated macrophage mitophagy is linked to apoptosis resistance, pro-fibrotic polarization, and is required for fibrosis development.
Experimental Procedures
Human Subjects
We obtained human alveolar macrophages, as previously described (He et al., 2011), from normal subjects and IPF patients under an approved protocol by the Human Subjects Institutional Review Board of the University of Iowa Carver College of Medicine and the University of Alabama at Birmingham. Primary alveolar macrophages and lung fibroblasts isolated as previously described (Hecker et al., 2009; Osborn-Heaford et al., 2015), from lung of human subjects with IPF.
Mice
Wild-type C57BL/6, Akt1+/− (B6.129P2-Akt1tm1Mbb/J), and Park2−/− (B6.129S4-Park2tm1Shn/J ) mice were purchased from JAX Laboratories. Akt1−/−Lyz2-cre and Tgfb1−/−Lyz2-cre mice were generated by selective disruption of Akt1 or Tgfb1 gene in the cells of the granulocyte and/or monocyte lineage. All protocols were approved by the University of Iowa and the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Mice were intratracheally administered 1.75-2 U/kg of bleomycin or saline, as a negative control, as previously described.
Cell Culture
Human monocyte (THP-1) and mouse alveolar macrophage (MH-S) cell lines were obtained from American Type Culture Collection. Cell culture maintenance is listed in Supplemental Experimental Procedures.
Quantitative Real Time PCR
Total RNA was isolated, reverse transcribed, and quantitative real-time PCR was performed as described previously (Larson-Casey et al., 2014). Data was calculated by the cycle threshold (ΔΔCT) method, normalized to β-actin or HPRT, and expressed in arbitrary units. Primer information is listed in Supplemental Experimental Procedures.
Plasmids, Transfections, and Luciferase Assays
The constitutively active Akt1 (NM_005163.2) plasmid (Akt1CA) was a generous gift from Rama K. Mallampalli (Department of Medicine, University of Pittsburgh, Pittsburgh, PA). Human AP-1 and TGF-β1 gene expression were evaluated using a luciferase reporter plasmid as previously described (Carter et al., 2001; Jaffer et al., 2015). Cells were transfected using X-treme GENE 9 Transfection Reagent (Roche Applied Scientific) according to the manufacturer’s protocol. Renilla and firefly luciferase activity was determined in cell lysates using the Dual Luciferase reporter assay kit (Promega) and normalized to control (firefly).
Small Interfering RNA (siRNA)
Cells were transfected with 100 nM scramble, human Akt1, human Parkin2, or human Rieske siRNA duplex (IDT), utilizing Dharmafect 2 or Duo (Thermo Scientific) according to manufacturer’s protocol. 8 h after transfection, media was replaced and cells were allowed to recover for 24-72 h.
Determination of H2O2 Generation
H2O2 production was determined fluorometrically, as previously described (Larson-Casey et al., 2014). ROS was also measured in live cells using dihydroethidium derivative (MitoSOX) (Molecular Probes) at 5 μM. Mitochondrial localization of the staining was confirmed by colocalization with MitoTracker green (Molecular Probes) at 50 nM. Cells were imaged using Zeiss LSM 710 confocal microscope; all images were taken at the same time and same imaging settings. Mitochondrial H2O2 was determined using MitoTEMPO (Enzo) at 10 μM.
Flow Cytometry
Macrophage apoptosis was assessed using annexin V staining (BD Biosciences, San Jose, CA), according to the manufacturer's recommendations. For determination of mitochondrial superoxide, cells were incubated with MitoSOX red (5 μM) and MitoTracker green (50 nM) for mitochondrial localization. Data (30,000 events) were acquired on Becton Dickinson LSR II flow cytometer using FACS Diva software (BD Biosciences, San Jose, CA) and were further analyzed using FlowJo 8.5 (Tree Star, Ashland, OR).
Assay for α-SMA Expression
Normal or IPF fibroblasts were incubated in equal concentrations of BAL fluid from WT and Akt1−/−Lyz2-cre or Tgfb1−/−Lyz2-cre mice exposed to saline or bleomycin or conditioned media from transfected macrophages. Fibroblasts were harvested after 24 h of incubation with BAL fluid or conditioned media and lysates were analyzed for α-SMA expression.
TGF-β1 Neutralization
BAL fluid from WT and Akt1−/−Lyz2-cre mice was incubated with TGF-β1 neutralizing antibody (clone #9016) or IgG1 control antibody (clone #11711) (R & D Systems) and then placed on IPF fibroblasts. IPF fibroblasts were incubated with DMSO or SB 431542 (TGF-β type 1 receptor inhibitor) (R & D Systems) and then cultured with BAL fluid from WT and Akt1−/−Lyz2-cre mice.
Transmission Electron Microscopy
Macrophages were fixed in 2.5% paraformaldehyde and 2.5% glutaraldehyde in Sorenson's phosphate buffer at pH 7.4. Cells were processed and sectioned with a diamond knife (Diatome, Electron Microscopy Sciences, Fort Washington, PA) at 70-80 nm and sections were placed on copper mesh grids. Sections were stained with uranyl acetate and lead citrate for contrast and viewed on a Tecnai Twin 120kv TEM (FEI, Hillsboro, OR).
Statistical Analysis
Statistical comparisons were performed using either an unpaired two-tailed t test or one-way ANOVA with a Tukey’s post hoc test. All statistical analysis was expressed as ±S.D. and p < 0.05 was considered to be significant.
Supplementary Material
Acknowledgements
We thank Dr. Stephen Barnes and Ray Moore II with the UAB Targeted Metabolomics & Proteomics Laboratory for assistance with GSH analysis by HPLC, Melissa Foley Chimento and the UAB High Resolution Imaging Facility for assistance with TEM, and M. Taaj Khan for assistance in generating the Tgfb1−/−Lyz2-cre mice.
Funding Research reported in this publication was supported in whole or in part, by National Institute of Health Grants 2R01ES015981-08 to ABC, 2T32HL105346-06 to the division of Pulmonary, Allergy, and Critical Care, and the Targeted Metabolomics and Proteonomics Laboratory supported by P30DK079337 (UAB O’Brien Acute Kidney Center), the UAB Lung Health Center, and the UAB Center for Free Radical Biology. This work was also supported by a Merit Review from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biological Laboratory Research and Development BX001135-03 to ABC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions JLC and ABC developed the concept and design of the study. JLC, JSD, AJR, VJT, and ABC assisted with anlysis and iterpretation of experiments and results. JLC and ABC wrote the manuscript.
Competing Interests The authors declare that they have no competing financial interest.
References and Notes
- Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56–69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- Birchenall-Roberts MC, Ruscetti FW, Kasper J, Lee HD, Friedman R, Geiser A, Sporn MB, Roberts AB, Kim SJ. Transcriptional regulation of the transforming growth factor beta 1 promoter by v-src gene products is mediated through the AP-1 complex. Mol Cell Biol. 1990;10:4978–4983. doi: 10.1128/mcb.10.9.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman PB, Rennard SI, Hunninghake GW, Crystal RG. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982;70:806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AB, Tephly LA, Hunninghake GW. The absence of activator protein 1-dependent gene expression in THP-1 macrophages stimulated with phorbol esters is due to lack of p38 mitogen-activated protein kinase activation. J Biol Chem. 2001;276:33826–33832. doi: 10.1074/jbc.M100209200. [DOI] [PubMed] [Google Scholar]
- Chang AL, Ulrich A, Suliman HB, Piantadosi CA. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic Biol Med. 2015;78:179–189. doi: 10.1016/j.freeradbiomed.2014.10.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BB, Coon TA, Glasser JR, Zou C, Ellis B, Das T, McKelvey AC, Rajbhandari S, Lear T, Kamga C, et al. E3 ligase subunit Fbxo15 and PINK1 kinase regulate cardiolipin synthase 1 stability and mitochondrial function in pneumonia. Cell Rep. 2014;7:476–487. doi: 10.1016/j.celrep.2014.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- Collard HR, Chen SY, Yeh WS, Li Q, Lee YC, Wang A, Raghu G. Health Care Utilization and Costs of Idiopathic Pulmonary Fibrosis in U.S. Medicare Beneficiaries Aged 65 Years and Older. Annals of the American Thoracic Society. 2015;12:981–987. doi: 10.1513/AnnalsATS.201412-553OC. [DOI] [PubMed] [Google Scholar]
- Collard HR, Ward AJ, Lanes S, Cortney Hayflinger D, Rosenberg DM, Hunsche E. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ. 2012;15:829–835. doi: 10.3111/13696998.2012.680553. [DOI] [PubMed] [Google Scholar]
- Conery AR, Cao Y, Thompson EA, Townsend CM, Jr., Ko TC, Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol. 2004;6:366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Cunnington RH, Gupta S, Yeganeh B, Filomeno KL, Freed DH, Chen S, Klonisch T, Halayko AJ, Ambrose E, et al. Autophagy is a regulator of TGF-beta1-induced fibrogenesis in primary human atrial myofibroblasts. Cell death & disease. 2015;6:e1696. doi: 10.1038/cddis.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn-Superoxide Dismutase Mediates Pulmonary Fibrosis by Augmenting H2O2 Generation. J Biol Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Ryan AJ, Murthy S, Carter AB. Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J Biol Chem. 2013;288:20745–20757. doi: 10.1074/jbc.M112.410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer OA, Carter AB, Sanders PN, Dibbern ME, Winters CJ, Murthy S, Ryan AJ, Rokita AG, Prasad AM, Zabner J, et al. Mitochondrial-targeted antioxidant therapy decreases transforming growth factor-beta-mediated collagen production in a murine asthma model. Am J Respir Cell Mol Biol. 2015;52:106–115. doi: 10.1165/rcmb.2013-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med. 2007;204:1083–1093. doi: 10.1084/jem.20061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. J Biol Chem. 2012;287:11677–11688. doi: 10.1074/jbc.M111.308460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, Karin M, Roberts AB. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Casey JL, Murthy S, Ryan AJ, Carter AB. Modulation of the mevalonate pathway by akt regulates macrophage survival and development of pulmonary fibrosis. J Biol Chem. 2014;289:36204–36219. doi: 10.1074/jbc.M114.593285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire I, Beaudoin H, Masse S, Grondin C. Alveolar macrophage stimulation of lung fibroblast growth in asbestos-induced pulmonary fibrosis. Am J Pathol. 1986;122:205–211. [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Malouf MA, Hopkins P, Snell G, Glanville AR. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology (Carlton, Vic.) 2011;16:776–783. doi: 10.1111/j.1440-1843.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol. 2011;187:3003–3014. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn-Heaford HL, Murthy S, Gu L, Larson-Casey JL, Ryan AJ, Shi L, Glogauer M, Neighbors JD, Hohl R, Carter AB. Targeting the isoprenoid pathway to abrogate progression Of pulmonary fibrosis. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn-Heaford HL, Ryan AJ, Murthy S, Racila AM, He C, Sieren JC, Spitz DR, Carter AB. Mitochondrial Rac1 GTPase Import and Electron Transfer from Cytochrome c Are Required for Pulmonary Fibrosis. J Biol Chem. 2012;287:3301–3312. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, et al. Epithelial Cell Mitochondrial Dysfunction and PINK1 Are Induced by Transforming Growth Factor-Beta1 in Pulmonary Fibrosis. PLoS One. 2015;10:e0121246. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91(Suppl 2):S3–10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redente EF, Keith RC, Janssen W, Henson PM, Ortiz LA, Downey GP, Bratton DL, Riches DW. Tumor necrosis factor-alpha accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respir Cell Mol Biol. 2014;50:825–837. doi: 10.1165/rcmb.2013-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- Ricci A, Cherubini E, Scozzi D, Pietrangeli V, Tabbi L, Raffa S, Leone L, Visco V, Torrisi MR, Bruno P, et al. Decreased expression of autophagic beclin 1 protein in idiopathic pulmonary fibrosis fibroblasts. J Cell Physiol. 2013;228:1516–1524. doi: 10.1002/jcp.24307. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Davis CS, Merchant JA, Bunn WB, Galvin JR, Van Fossen DS, Dayton CS, Hunninghake GW. Longitudinal changes in lung function among asbestos-exposed workers. Am J Respir Crit Care Med. 1994;150:1243–1249. doi: 10.1164/ajrccm.150.5.7952547. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8:1462–1476. doi: 10.4161/auto.21211. [DOI] [PubMed] [Google Scholar]
- Wauer T, Simicek M, Schubert A, Komander D. Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature. 2015;524:370–374. doi: 10.1038/nature14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A, Johann AM, von Knethen A, Schmidt H, Geisslinger G, Brune B. Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.