Abstract
Purpose
To assess the tumor response to the smoothened (SMO) inhibitor, sonidegib (LDE225), in patients with an advanced basal cell carcinoma (BCC) resistant to treatment with vismodegib (GDC0449).
Experimental Design
Nine patients with an advanced BCC that was previously resistant to treatment with vismodegib were given sonidegib in this investigational, open-label study. Tumor response was determined using the response evaluation criteria in solid tumors. SMO mutations were identified using biopsy samples from the target BCC location.
Results
The median duration of treatment with sonidegib was 6 weeks (range, 3–58 weeks). Five patients experienced progressive disease with sonidegib. Three patients experienced stable disease and discontinued sonidegib either due to adverse events (n = 1) or due to election for surgery (n = 2). The response of one patient was not evaluable. SMO mutations with in vitro data suggesting resistance to Hh pathway inhibition were identified in 5 patients, and none of these patients experienced responses while on sonidegib.
Conclusion
Patients with advanced BCCs that were previously resistant to treatment with vismodegib similarly demonstrated treatment resistance with sonidegib. Patients who have developed treatment resistance to an SMO inhibitor may continue to experience tumor progression in response to other SMO inhibitors.
Introduction
Smoothened (SMO) inhibitors are a new class of drugs for the treatment of advanced basal cell carcinomas (BCC) that include locally advanced and metastatic tumors (1). Vismodegib (GDC0449) is a first-in-class SMO inhibitor approved by the FDA for the treatment of both locally advanced and metastatic BCCs in 2012 (1, 2). Although patients treated with vismodegib can have partial and complete responses, more than 50% of patients are refractory to treatment, whereas over 20% of initial responders develop resistance and experience disease progression or recurrence (2, 3). Recently, a subset of mutations in the drug target, SMO, has been identified in advanced BCCs resistant to vismodegib (4, 5). Acquired SMO mutations maintain hedgehog signaling by either impairing drug binding or inducing constitutive activity of SMO, resulting in resistance to vismodegib (4, 5). These SMO mutations have also been shown to display functional resistance to vismodegib in vitro (4). The clinical outcome of a chemically distinct SMO inhibitor on an advanced, vismodegib-resistant BCC in vivo is unknown.
Sonidegib (LDE225) is a new SMO inhibitor approved in 2015 by the FDA for locally advanced BCCs. Clinical trials have shown its efficacy against locally advanced BCCs (6, 7). Sonidegib blocks hedgehog signaling by selective inhibition of SMO, even though its chemical structure is different from vismodegib (8). Although antitumor activity has been reported in patients with advanced BCCs who were treated with sonidegib, an in vitro study has shown that cells with SMO mutations display resistance to sonidegib (4, 6, 7). The purpose of this investigator initiated open-label study was to assess the tumor response to sonidegib in patients with an advanced BCC that previously was resistant to treatment with vismodegib.
Materials and Methods
Study patients
Approval was obtained from the Institutional Review Board, and written informed consent was obtained from all participants. An open-label study was conducted at a single academic institution from October 2011 to December 2013. This study was registered as NCT01529450 on clinicaltrials.gov.
Inclusion criteria
Eligible patients were those with advanced (locally advanced or metastatic) BCC that clearly demonstrated either primary or secondary treatment resistance with vismodegib, as documented by their previous treating physician. Composite response endpoint incorporating RECIST 1.0 (1) had been used to quantitatively define resistance to vismodegib (Supplementary Fig. S1). Primary resistance was defined as stable or progressive disease; secondary resistance was defined as progressive disease after initially demonstrating stable, partial, or complete response. Histologic confirmation of the target advanced BCC was required before enrollment into this study. In this study, patients required measurable, evaluable disease as defined by modified RECIST v. 1.1 criteria and standard and annotated color photography (9). Patients with basal cell nevus syndrome could enroll if they met inclusion criteria.
Exclusion criteria
Patients were excluded from enrollment if they previously had been treated with sonidegib; had completed antitumor therapy less than 28 days before sonidegib initiation; or were on concurrent antitumor therapy.
Study design
This was an open-label, investigator-initiated study. Patients received 800 mg oral sonidegib once daily, with treatment cycles defined as every 28 days. This dose of sonidegib has been used in other clinical trials (7). Clinic visits and tumor assessments occurred every 1 to 2 treatment cycles, or sooner, if necessary, as deemed by the investigator. Clinic visits included medical history, vital signs, physical examination, and adverse event recording and grading using the National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.0 (10).
Efficacy analysis
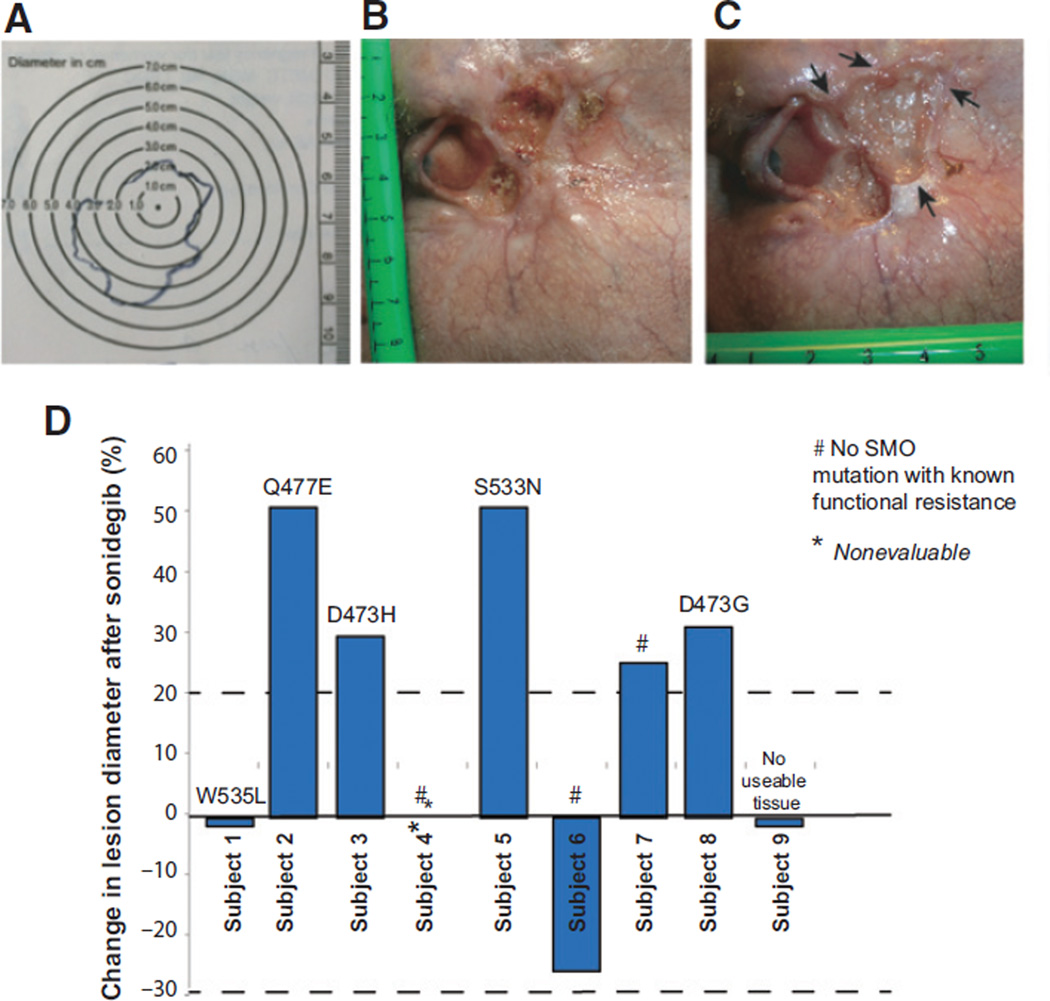
Patients receiving greater than or equal to one dose of sonidegib and having at least one follow-up tumor assessment were included in the efficacy-evaluable population. Tumor responses were investigator-assessed according to modified RECIST v. 1.1, which was a composite multimodal evaluation to integrate imaging by RECIST 1.1 criteria and standard and annotated color photography (WHO criteria; ref. 9). In cases of clinically visible BCC, where the tumor borders were complex, clean plastic grids were used to trace the edges of the tumors to identify the longest diameter (Fig. 1A). Color photographs of target lesions with rulers in the plane of focus were taken at each clinic visit, and longest diameters identified (Fig. 1B and C). In cases where tumor borders were unclear from visual inspection of photographs, the plastic grid tracings were inspected to provide additional information on tumor borders. Sonidegib was administered until either disease progression, as defined by RECIST v 1.1; intolerable toxicity, or patient or physician request to discontinue (9). Dose reduction was not permitted.
Figure 1.
Target BCC size determination of clinically visible lesions, and overall responses of enrolled subjects. Color photographs were taken of all clinically visible lesions with a ruler in the plane of the lesion. A, in cases where the tumor borders are complex, clean plastic grids were placed over the lesions and traced to document the longest diameter. B, photograph of target lesion in a subject before study start. C, photograph of same target lesion after 6 weeks of sonidegib. Arrows indicate areas of tumor growth. D, change in target tumor diameter from baseline (%) after sonidegib. None of the patients met the 30% partial response (top gray dotted line) per RECIST, version 1.1. SMO mutations that are known to lead to functional resistance in vitro found in individual tumors are annotated for each patient.
Genetics
DNA from the target BCCs after vismodegib exposure, but before study entry was isolated and subjected to high-depth sequencing for the entire SMO coding region. Briefly, five to eight 10-mm sections were obtained from the formalin-fixed, paraffin-embedded tumor block and DNA was isolated using the Qiagen DNeasy Blood and Tissue Kit according to the manufacturer's protocol (Qiagen). The exonic regions of SMO were amplified using the Access Array platform (Fluidigm) in a multiplex format with genomic DNA (100 ng) according to the manufacturer's recommendation (Ambry Genetics). The multiplexed library pools were then subjected to deep sequencing using the Illumina MiSeq platform. FASTQ files were generated for the raw data and 150 bp reads aligned to the human reference genome sequence (hg19) using the Burrows-Wheeler Aligner (BWA). Samtools mpileup was used to call variants. Only bases meeting the minimum base quality score of 20 from reads meeting the minimum mapping quality score of 20 were considered, and a minimum allele frequency of 5% at a position with a read depth >100 was required to make calls. SMO mutations defined as functionally resistant mutations were those previously reported in the medical literature to convey resistance to SMO inhibitors in vitro (Supplementary Table S1A; refs. 4, 5, 11). Biopsy samples of the target BCCs were taken before initiation of sonidegib, but results of mutational analysis were not known until after the conclusion of the clinical study.
Statistical analysis
Patient data were summarized by descriptive statistics using Microsoft Excel (version 14.5.1).
Results
Nine patients with an average age of 57.4 years (range, 42–91 years) were enrolled in the study (Table 1). Three patients had basal cell nevus syndrome. Three enrolled subjects had primary resistance to vismodegib and 6 subjects had secondary resistance to vismodegib (Supplementary Fig. S1). Of patients with secondary resistance, 1 demonstrated prior complete response, 3 demonstrated partial response, and 3 had stable disease.
Table 1.
Clinical characteristics and outcomes of 9 patients with vismodegib-resistant advanced BCCs treated with sonidegib
| Patient | Age | Sex | LA vs. MT BCC |
BCNS | Target BCC location |
Sonidegib duration (wk) |
Target tumor size pre- sonidegib (cm) |
Target tumor size post- sonidegib (cm) |
Tumor response |
Reason for discontinuation |
SMO mutation with known functional resistance to sonidegib in vitro (4, 5, 11) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | LA | Paranasal | 3 | 6.8 × 6.2 | 6.8 × 6.2 | SD | Patient election for surgery | W535L | |
| 2 | 45 | F | LA | X | Back | 3 | 4.0 × 3.0 | 6.0 × 4.0 | PD | PD | Q477E |
| 3 | 42 | M | MT | Lymph node | 58 | 1.0 × 1.0 | 1.0 × 1.3 | PD | PD | D473H | |
| 4 | 51 | M | MT | Iliac bone | 5 | 6.4 × 1.2 | N/A | NE | Grade 3 adverse event: rhabdomyolysis | No SMO mutations with known functional resistance | |
| 5 | 91 | M | LA | Temple | 6 | 2.5 × 1.5 | 3.5 × 2.5 | PD | PD | S533N | |
| 6 | 56 | M | LA | X | Tragus | 4 | 2.5 × 1.8 | 2.0 × 1.7 | SD | Patient election for surgery | No SMO mutations with known functional resistance |
| 7 | 50 | M | MT | Femoral bone | 14 | 1.5 × 1.4 | 1.9 × 1.7 | PD | PD | No SMO mutations with known functional resistance | |
| 8 | 59 | M | LA | X | Postauricular | 12 | 5.0 × 5.0 | 6.4 × 6.0 | PD | PD | D473G |
| 9 | 68 | M | MT | Rib | 7 | 4.5 × 3.2 | 4.5 × 3.2 | SD | Grade 3 adverse event: nausea, altered mental status | Tissue unavailable for sequencing |
Abbreviations: BCNS, basal cell nevus syndrome; LA, locally advanced; MT, metastatic; NE, not evaluable; PD, progressive disease; SD, stable disease.
The median duration of treatment with sonidegib was 6 weeks (range, 3–58 weeks). Figure 1A–C shows an example of a study patient and method used to track the lesions. Overall study results are shown in Fig. 1D. Five patients experienced progressive disease within a median of 6 weeks (range, 3–58 weeks) of treatment with sonidegib, and sonidegib was discontinued, per the study protocol (Table 1). Three patients experienced stable disease with a median of 4 weeks of treatment with sonidegib (range, 3–7 weeks). Two subjects elected to discontinue sonidegib for surgery. One patient elected to discontinue sonidegib due to grade 3 adverse events, including nausea and altered mental status. One subject was not evaluable due to study drug discontinuation from grade 3 rhabdomyolysis secondary to sonidegib and subsequent inability to travel to our clinic for a follow-up visit. Rhabdomyolysis was diagnosed on the basis of the overall clinical picture of diffuse myalgias requiring narcotics, markedly dark urine, creatine kinase elevation to 56,564 mcg/L, urine myoglobin elevation to >8,750 mg/dL, and transaminitis. All symptoms and abnormal blood tests resolved after intravenous fluids and discontinuation of sonidegib (10). None of the 8 evaluable patients demonstrated partial or complete response. The study was terminated by the principal investigator due to the lack of response in any of these 9 patients.
Adverse events while on sonidegib included grade 1–2 muscle cramps (N = 5), grade 1 nausea (N = 4), grade 1 and 3 creatine kinase elevation (N = 2), grade 4 altered mental status (N = 1), grade 1 vomiting (N = 1), grade 1 diarrhea (N = 1), grade 2 weight loss (N = 1), and grade 1 loss of appetite (N = 1).
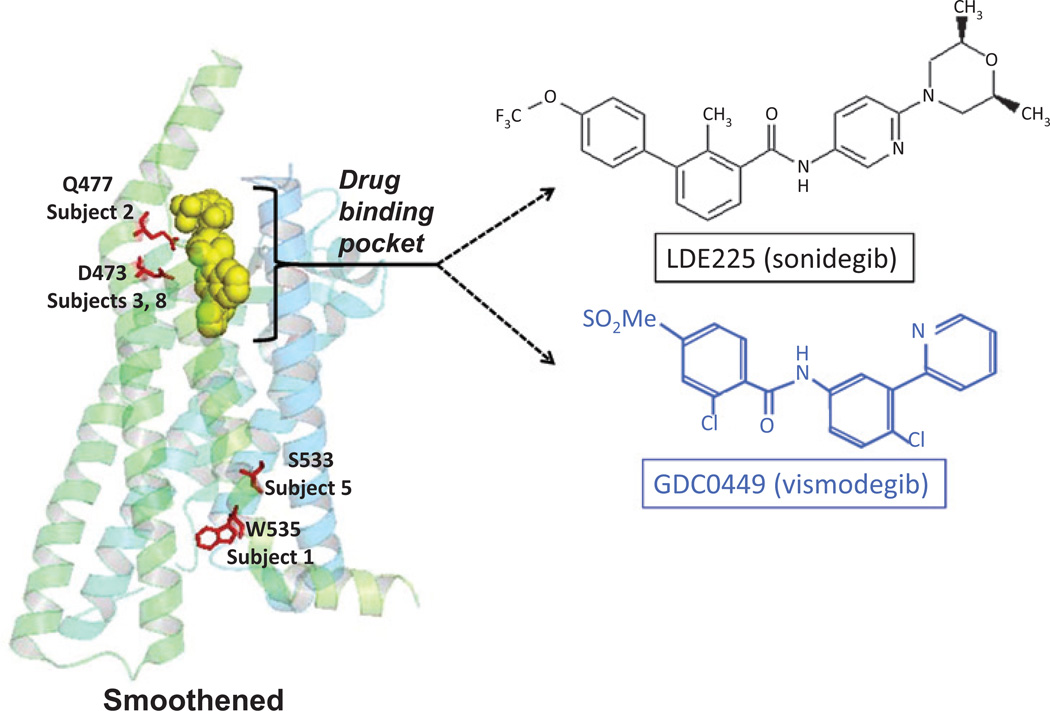
The entire coding regions for SMO were sequenced, and SMO mutations with previously reported functional resistance in vitro to either sonidegib or vismodegib were identified in five of eight available baseline tumor samples (Table 1 and Supplementary Table S1). Four patients with an SMO mutation demonstrated progressive disease on sonidegib, and 1 patient with an SMO mutation demonstrated stable disease for 58 weeks before eventual disease progression. The molecular structures of vismodegib and sonidegib bear some similarity but are chemically distinct (Fig. 2), but both interact with SMO at the drug-binding pocket, where the mutations Q477 and D473 (found in tumors from two different subjects) are located (4, 5, 11). Two subjects' tumors had mutations outside of the drug-binding pocket, S533 and W535, and these are believed to change the conformation of SMO so that the inhibitors cannot access the drug-binding pocket (Table 1, Fig. 1D, Fig. 2 and Supplementary Table S1; refs. 4, 5, 11). In addition, three subjects' BCCs possessed mutations that are not previously known to confer functional resistance in vitro: R302, H304, W549, Q581 R168, and N476 (Supplementary Table S1B).
Figure 2.
Locations of the SMO mutations known to confer resistance through in vitro studies were identified in the BCCs of the study patients. The locations of these mutations within the SMO protein are shown on the left. Mutations Q476 and D473 are within the drug-binding pocket of sonidegib. Mutations S533 and W535 likely cause conformational change in SMO rendering the drug-binding pocket inaccessible to the sonidegib. The chemical structures of sonidegib and vismodegib are shown on the right for comparison.
Discussion
In this study, advanced BCCs that previously were resistant to treatment with vismodegib also were refractory to treatment with sonidegib. All patients demonstrated either progressive or stable disease with sonidegib. These results suggest that chemoresistance can occur between different SMO inhibitors, a clinically important finding.
Clinical trials have reported an objective response in 35% of patients with an advanced BCC who were treated with sonidegib (7). This rate has similarly been reported in trials with vismodegib (2). However, in these studies, patients were not previously exposed to different SMO inhibitors, and the clinical response rate of patients who have been previously exposed to multiple SMO inhibitors has been unclear (2, 7). This is the first study to demonstrate that patients who have been treated with a previous SMO inhibitor such as vismodegib may be resistant to treatment with a different SMO inhibitor such as sonidegib.
SMO mutations previously identified through in vitro functional studies as showing resistance to an SMO inhibitor were identified in 5 patients in this study. Given that patients with an SMO mutation continued to experience progressive disease on sonidegib, these mutations are likely to prevent sonidegib inhibition of SMO. SMO-D473 and SMO-Q477 are mutations that have been reported to be in the SMO binding pocket (Fig. 2; ref. 4). SMOD473 (found in patient 3 and 8) was previously reported to be found in a patient with metastatic medulloblastoma and was shown to abrogate vismodegib binding in vitro (11). SMO-W535 (patient 1) and SMO-S533 (patient 5) are mutations that have been reported as oncogenic drivers located outside of the binding pocket of SMO (Fig. 2; refs. 4, 5, 12). Interestingly, patient 3 continued sonidegib for 58 weeks with stable disease before experiencing disease progression, possibly due to tumor heterogeneity. Although patients with no known SMO mutation similarly demonstrated progressive disease while on sonidegib, other pathways or unknown SMO mutations may be driving tumor growth.
A limitation of the study includes early discontinuation of sonidegib due to patient decision and adverse events. In this study, 4 patients were treated with sonidegib for 5 weeks or less. Of these 4 patients, one had progressive disease after 3 weeks of sonidegib, and 3 patients discontinued the study do to either side effects or election for surgery. The short duration of sonidegib exposure in these 3 patients could have contributed to our inability to detect a response, despite the fact that treatment response in a phase II study has been observed in as early as 4 weeks after starting sonidegib (7).
The population of patients who develop resistance to treatment with an SMO inhibitor is likely to increase as more patients with BCCs are treated with SMO inhibitors. Results of this study suggest that patients with BCCs resistant to vismodegib may continue to experience tumor progression in response to a different SMO inhibitor. Future therapies with new targets in the hedgehog pathway downstream of SMO are needed. Combination therapy with agents such as radiation, other pathway inhibitors, or immunotherapy may be needed to control the advanced BCC. Given the advanced state of disease in many patients, timely initiation of other treatments besides trials of different SMO inhibitors can be critical.
Supplementary Material
Acknowledgments
Grant Support
This study was funded by Novartis.
Footnotes
Disclosure of Potential Conflicts of Interest
A.L.S. Chang is a consultant/advisory board member for and reports receiving commercial research grants from Novartis. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: C. Danial, A.E. Oro, A.L.S. Chang
Development of methodology: C. Danial, A.E. Oro, A.L.S. Chang
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C. Danial, K.Y. Sarin, A.L.S. Chang
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C. Danial, K.Y. Sarin, A.E. Oro, A.L.S. Chang
Writing, review, and/or revision of the manuscript: C. Danial, K.Y. Sarin, A.E. Oro, A.L.S. Chang
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K.Y. Sarin, A.L.S. Chang
Study supervision: A.E. Oro, A.L.S. Chang
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang AL, Solomon JA, Hainsworth JD, Goldberg L, McKenna E, Day BM, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60–69. doi: 10.1016/j.jaad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Chang AL, Oro AE. Initial assessment of tumor regrowth after vismodegib in advanced Basal cell carcinoma. Arch Dermatol. 2012;148:1324–1325. doi: 10.1001/archdermatol.2012.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe HJ, Pau G, Dijkgraaf GJ, Basset-Seguin N, Modrusan Z, Januario T, et al. Genomic analysis of smoothened inhibitor resistance in Basal cell carcinoma. Cancer Cell. 2015;27:327–341. doi: 10.1016/j.ccell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atwood SX, Sarin KY, Whitson RJ, Li JR, Kim G, Rezaee M, et al. Smoothened variants explain the majority of drug resistance in Basal cell carcinoma. Cancer Cell. 2015;27:342–353. doi: 10.1016/j.ccell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20:1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 7.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, et al. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010 Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5_11.pdf.
- 11.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.