Abstract
Activating KRAS mutations are nearly ubiquitous in pancreatic cancer occurring in more than 95% of clinical cases. MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression by binding sequences within the 3′UTRs of target mRNAs. An integral role for miRNAs in cancer pathogenesis is well established; however, the role of miRNAs in KRAS-mediated tumorigenesis is poorly characterized. Here it is demonstrated that expression of miR-31 is coupled to expression of oncogenic KRAS and activity of the MAPK pathway. MiR-31 is highly expressed in patient derived xenografts and a panel of pancreatic and colorectal cancer cells harboring activating KRAS mutations. The miR-31 host gene is a large non-coding RNA that correlates with miR-31 expression and enabled identification of the putative miR-31 promoter. Using luciferase reporters, a minimal RAS responsive miR-31 promoter was found to drive robust luciferase activity dependent on expression of mutant KRAS and the transcription factor ELK1. Furthermore, ELK1 interacts directly with the endogenous miR-31 promoter in a MAPK-dependent manner. Expression of enforced miR-31 significantly enhanced invasion and migration of multiple pancreatic cancer cells resulting from activation of RhoA through regulation of the miR-31 target gene RASA1. Importantly, acute knockdown of RASA1 phenocopied enforced miR-31 expression on the migratory behavior of pancreatic cancer cells through increased RhoA activation.
Keywords: KRAS, miR-31, pancreatic, RASA1, invasion-migration
Introduction
MicroRNAs (miRNAs) are small non-coding RNAs typically 18–24 nucleotides in length that cause accelerated turnover and reduced translation of target messenger RNAs. MiRNAs have been implicated in the regulation of a wide range of cellular processes including differentiation, proliferation, and apoptosis (1, 2). A large body of evidence has established an integral role for miRNAs in cancer pathogenesis highlighting the importance of identifying miRNAs that function in the primary signaling pathways that regulate tumorigenesis. For example, the miR-17-92 cluster is directly induced by the MYC transcription factor and is able to promote cellular proliferation, survival, and tumor angiogenesis (3). miR-34 family members are directly regulated by p53 and activate cell-cycle checkpoints and apoptosis (4). The tumor suppressive miR-143/145 cluster is downregulated by oncogenic KRAS in pancreatic cancer and has been shown to abrogate tumorigenesis both in vitro and in vivo (5). Although both RAS signaling and miRNA activities can profoundly influence cancer cell behavior, the role of miRNAs in RAS-mediated phenotypes is still poorly defined.
Pancreatic cancer is a deadly and highly metastatic disease with a 5 year survival rate of less than 5%. Metastasis is the leading cause of cancer-associated death, with the majority of patients having locally advanced or distant metastatic disease at diagnosis rendering their cancers inoperable. Activating mutations in the KRAS proto-oncogene are nearly ubiquitous in pancreatic ductal adenocarcinoma (PDAC) and are found in 90–95% of cases (6). The KRAS gene mutation principally encountered in PDAC is an alteration of codon 12 from glycine to aspartic acid (G12D), which prevents GTP hydrolysis, trapping KRAS in the constitutively active configuration. KRAS activation is coupled to transcription through a number of downstream effectors including the RAF-mitogen activated protein kinase (MAPK) and phosphoinositide-3-kinase (PI3K) pathways, ultimately leading to enhanced cellular proliferation, survival, and motility all of which are commonly perturbed in cancer (7, 8). Similar activating mutations of KRAS and other RAS family members have been implicated in a broad range of human cancers including breast, colon, lymphoma malignancies (9), highlighting the widespread role for RAS signaling in tumor development and progression.
It is becoming clear that miRNAs have the potential to regulate the majority of protein coding transcripts but how miRNA-mediated regulation is integrated into RAS signaling pathways is still poorly understood. Indeed miRNAs have been found to target RAS genes themselves, including let-7 miRNA, miR-143 and miR-217 which have been experimentally demonstrated to target the KRAS 3′UTR (10, 11, 12). Recently, miR-21 has been found to be up regulated by oncogenic RAS through the MAPK pathway and the genetic deletion of miR-21 is able to suppress KRAS-driven tumors (13).
Previously, it has been demonstrated that expression of oncogenic KRAS in a transformed pancreatic ductal epithelial cell line (HPNE) leads to deregulation of four miRNAs (5). Specifically, greater than two-fold up regulation of miR-34a and miR-31 and down regulation of miR-143 and miR-145 upon expression of KRASG12D was observed. The tumor suppressive miR-143 and miR-145 were down regulated by oncogenic KRAS in pancreatic and colorectal cancers through the negative regulation of the miR-143/145 promoter by the transcription factor RREB-1 (5, 14). Restituted miR-143/145 expression in pancreatic cancer cell lines blocked anchorage independent growth in vitro and abrogated tumorigenesis in vivo and the enforced expression of either miR-143 or miR-145 blocked RAS signaling through the MAPK and PI3K pathways through the down regulation of multiple target genes including KRAS and RREB-1. These results demonstrated a feed-forward mechanism through which KRAS activation suppresses expression of an anti-tumorigenic miRNA cluster and concomitantly potentiated signaling through the RAS pathway.
Although miR-31 was identified in the aforementioned screen as being upregulated by mutant KRAS (5), its role in KRAS-mediated tumorigenesis is currently unknown. In addition, the role of miR-31 in pancreatic cancer is poorly characterized and although miR-31 has been implicated in playing a role in the regulation of invasion-migration the results are discordant among various cancer types. For example, miR-31 has been found to enhance migration-invasion of colorectal cancer cells but inhibit invasion-migration of breast cancer cells. In the present study, we have elucidated the transcriptional regulation of miR-31 expression downstream of oncogenic KRAS and the MAPK pathway. In addition, our data demonstrate miR-31 enhances invasion-migration of pancreatic cancer cells through its target gene RASA1 and subsequent activation of Rho.
Materials and Methods
Cell lines
All cancer cell lines were cultured as described previously (5, 14, 15, 16, 17, 18) and were a gift from Dr. Maitra and Dr. Eshleman at JHMI, Baltimore MD.
Chromatin immunoprecipitation
ChIP was performed in Panc-1 cells using SimpleChIP Enzymatic Chromatin IP kit with magnetic beads (Cell Signaling #9003) following the manufacturers’ protocol. For MEK and PI3K inhibitor ChIP, cells were treated with inhibitors as described below. For immunoprecipitation, ELK1 antibody (sc22804X, Cell Signaling) or 2 μg mouse IgG control antibody (Dako) were used. Real-time PCR as described below. Sequences of primers are provided in Supplemental Table 1.
Colony and growth assays
Proliferation rates were measured by plating 5000 cells/well in a 96-well plate and monitoring growth with Essen Incucyte. For colony formation assays, 5000 cells/mL were suspended in warm 0.35% agarose in DMEM (4.5 mg/ml glucose) supplemented with 10% FBS in the absence of antibiotics and layered on a 0.5% agarose/DMEM base layer. Cells were grown for 7–10 days and then photographed. Images were quantified using OpenCFU (19).
Luciferase reporter assays
Promoter and 3′UTR reporter assays were performed as described previously (14). Where indicated, control or miR-31 mimics (Dharmacon) were co-transfected at 15 nM final concentration.
Plasmid construction and design
Primer sequences used for cloning are provided in Supplemental Table 1. The miR-31 retroviral expression vector was constructed by amplifying the pre-miRNA along with ~100 bp of 5′ and 3′ flanking sequence and cloned into the XhoI site in pMSCV-Neo (Clontech). To generate the luciferase reporter vectors, digested PCR products amplified from human gDNA were cloned into NheI/BglII cut pGL3-Basic vector. For the RASA1 3′ UTR reporter construct, a segment of the 3′ UTR containing the miR-31 binding site was amplified from human gDNA and cloned into the XbaI site in the pGL3-control vector (Promega). Mutations in the miR-31 binding site were introduced using the QuikChange Site-directed mutagenesis kit (Stratagene).
Retroviral infections
Retroviruses were produced as described previously (5). Cells were infected with virus and selected by growing in 200μg/ml Neomycin for 7–14 days for creation of stable cell lines.
RhoA activation assay
2.0×105 cells were plated in a 10cm plate and grown for 24 hours followed by 24 hours in serum free media. Cells were stimulated with FBS at 10% final concentration for 1 minute to activate RhoA. RhoA-GTP pulldown was performed with RhoA activation kit (Cytoskeleton) following the manufacture’s protocol.
RNA northern blot analysis
Total RNA was isolated from cells with TRIZOL (Invitrogen) according to the manufacturer’s protocol. Northern blots were performed as described previously (17).
RNAi
2.0×105 cells were plated in a 6-well plate and then transfected with siRNA against KRAS, RASA1 (siGenome) or ELK1, GAPDH (silencer select, Invitrogen) at 5nM final concentration using RNAiMax (Invitrogen) according to the manufacturer’s protocol. Knockdown was confirmed by qPCR or western blot.
RT-PCR and qPCR
cDNAs were made using the QuantiTect kit following the manufacturer’s protocol (Qiagen). QPCR was performed using an ABI7900 system with the Fast-SYBR Green PCR core reagent (Applied Biosystems). Primer sequences are provided in Supplementary Table 1. For measuring mature miR-31, Taqman primers and probes (Applied Biosystems) were used according to manufacturer’s instructions.
Scratch wound assays
2.0×106 cells were plated in a 96-well plate with 8 replicates for each experimental condition. Scratches were made with the Essen Biosciences scratch wound maker following the manufacturer’s protocol. Cell confluency and wound closure was monitored using the Essen IncuCyte ZOOM system.
Small molecule inhibitors
2.0×105 cells were plated in a 6-well plate. Following overnight serum starvation, small molecule inhibitors were added at final concentrations of 5μM (LY294002) or 10μM (U0126) for 24 hours prior to analysis.
Transwell assays
Transwells (Corning-3422, 8μm pore size) were coated with 1:40 dilution of cold matrigel in PBS, dried overnight, rehydrated with serum free media for 2 hours, and then plated with 2.0×105 cells/mL. Complete media containing 10% FBS was added to the lower chamber. Following 24–48 hours invasion, transwells were washed with PBS, stained with 0.25% (w/v) crystal violet/70% ethanol. Matrigel was removed carefully and membranes photographed under the light microscope.
Western Blotting
Standard protocols were used for western blot. Western blots were quantified with BIO-RAD Quantity One. Antibodies used in the study are listed in the supplemental file.
Results
Oncogenic KRAS regulates miR-31 through the MAPK pathway and elevated miR-31 is a feature of pancreatic and colorectal cancer cell lines
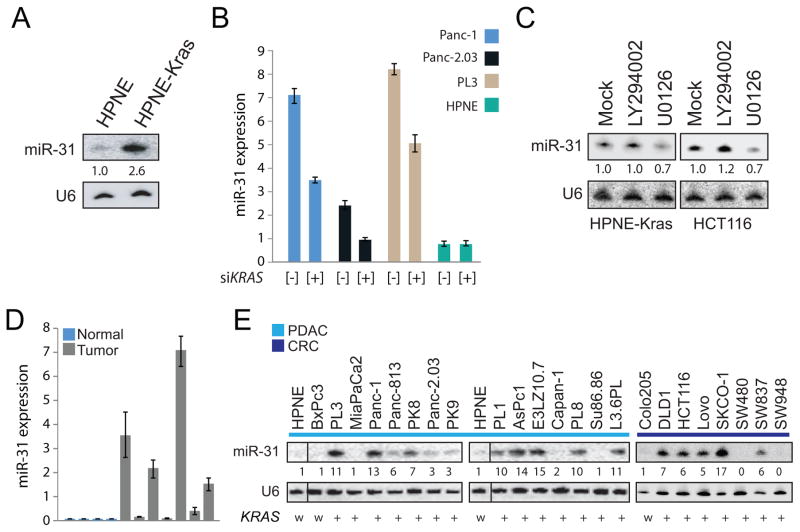
Previously, a custom microarray was used to profile miRNA expression in non-transformed HPNE cells (15) stably over expressing KRASG12D (from this point on referred to as HPNE-KRAS; ref 16) to identify miRNAs regulated by KRAS signaling (5). In the present study, the focus is on the KRAS mediated regulation of miR-31 which was upregulated by 2.6 fold in HPNE-KRAS cells (Figure 1A). To further validate the correlation between KRAS expression to miR-31 upregulation, the expression of miR-31 was examined in isogenic HCT116 cells in which either the wild-type allele of KRAS or the mutant allele was disrupted by homologous recombination (20). Using Taqman qPCR, miR-31 expression was reduced in HCT116 cells that lacked the mutant KRAS allele (Supplemental Figure 1A). In addition, acute knockdown of KRAS using siRNA partially repressed expression of miR-31 as measured by Taqman qPCR in cell lines harboring mutant KRAS (Panc-1, Panc-2.03 and PL3) but not in HPNE cells which express wild-type KRAS (Figure 1B; Supplemental Figure 1B).
Figure 1.
miR-31 is a target of oncogenic KRAS and the MAPK pathway and is highly expressed in many pancreatic and colorectal cancer cell lines. A, northern blot analysis of miR-31 expression in RNA isolated from HPNE and HPNE-KRAS(G12D) cells. U6 for this and subsequent northern blots served as a loading control. The fold change indicated. B, taqman qPCR analysis of miR-31 expression in the indicated cell lines transfected with siRNA control [−] or siRNA against KRAS [+]. Expression was normalized to U6 RNA. C, northern blot analysis of miR-31 expression in HPNE-KRAS and HCT116 cells treated with DMSO control (mock) or small molecule inhibitors against PI3K (LY294002) or MEK1/2 (U0126). D, taqman qPCR analysis of miR-31 expression normal pancreas and patient derived xenografts. Expression was normalized to U6 RNA. E, northern blot analysis of miR-31 expression in a panel of pancreatic (light blue bar, PDAC) and colorectal (dark blue bar, CRC) cancer cell lines. KRAS mutational status of each cell line is indicated as wild-type (w) or mutant (+).
RAS proteins signal through multiple effector pathways of which the RAF/MEK/ERK pathway (MAPK) and the PI3K/AKT pathways are required for transformation (7). To assess whether miR-31 expression was dependent on MAPK and/or PI3K activation, HPNE-KRAS and HCT116 cells were treated with the PI3K inhibitor LY294002 or the MEK1/2 inhibitor UO126. Levels of miR-31 decreased following MEK1/2 inhibition but no change was observed with PI3K inhibition suggesting miR-31 is activated through the MAPK pathway (Figure 1C).
Finally, miR-31 expression was analyzed from RNA isolated from a panel of patient derived xenografts, normal pancreas tissue and pancreatic and colorectal cancer cell lines. MiR-31 was moderately to highly expressed in most of the patient derived xenografts (Figure 1D). It was observed that 16/23 cell lines have moderate to high expression of miR-31 compared to HPNE which served as the “normal” control (Figure 1E). Of note, two cancer cell lines in the panel with wild-type KRAS (BxPc3 and Colo205) showed no expression of miR-31 (Figure 1E). Collectively, these results suggest that miR-31 is a downstream target gene of the KRAS-MAPK pathway and exhibits moderate to high expression in pancreatic tumors as well as pancreatic and colorectal cancer cell lines with oncogenic KRAS.
Confirmation of the miR-31 host gene expression and transcription regulation through ELK1
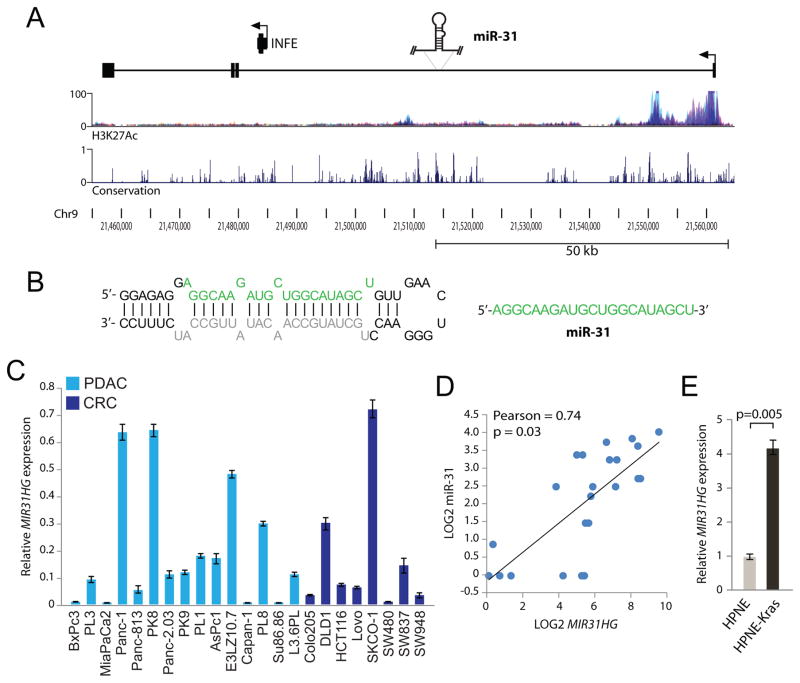
MicroRNAs are processed from the introns or exons of primary-miRNA (pri-miRNAs) which may be protein-coding or non-coding transcripts (21, 22). The mature miR-31 and pre-miRNA stem loop are contained within an NCBI RNA reference sequence transcript (MIR31HG); a long non-coding RNA that spans over 100 kb (Figures 2A and 2B). Regions within the lncRNA are highly conserved and the transcription start site (TSS) is found in a region high in histone H3-Lysine27 acetylation (H3K27Ac) a mark indicative of actively transcribed chromatin (Figure 2A). To validate MIR31HG as the miR-31 primary transcript, qPCR primers were designed within the first exon of the pri-miRNA and MIR31HG expression was measured from RNA isolated from the panel of pancreatic and colorectal cancer cell lines examined in Figure 1 (Figure 2C). The same pattern of moderate to high expression seen with mature miR-31 expression was observed with expression of MIR31HG. A dot plot analysis of MIR31HG expression versus mature miR-31 expression demonstrated a linear correlation with a Pearson correlation constant of 0.74 (p =0.03, Figure 2D). In addition, MIR31HG was found to be 4-fold upregulated in HPNE-KRAS cells relative to expression in HPNE (Figure 2E) consistent with the higher expression of mature miR-31 seen in this cell type (Figure 1A). These results strongly suggest that mature miR-31 is derived from expression and subsequent processing of the MIR31HG transcript, which like the mature miRNA, is under regulation by oncogenic KRAS.
Figure 2.
miR-31 is an intronic miRNA contained in a large non-coding primary transcript. A, structure of the human miR-31 primary transcript (MIR31HG). The plots depicted below the transcript show the layered H3K27Ac marks and the evolutionary conservation (UCSC Genome Browser 28 species conservation track, NCBI36/hg18 assembly). The INFE (interferon epsilon) gene is also contained in this region under regulation of its own promoter. The transcription start site (TSS) is indicated with an arrow. B, the proposed secondary structure of the pre-miR-31 stem loop. The mature miRNA sequence green (miR-31) and the miRNA-star sequence grey. C, quantitative PCR analysis of MIR31HG expression in the panel of pancreatic (light blue bars, PDAC) and colorectal (dark blue bars, CRC) cancer cell lines described in Figure 1E. D, dot plot analysis of LOG2 transformed mature miR-31 expression (x-axis) and MIR31HG expression (y-axis) from the panel of PDAC and CRC cell lines. The Pearson correlation and p-value (two-tailed t-test) are indicated. E, quantitative PCR analysis of MIR31HG expression in HPNE and HPNE-KRAS(G12D) cells.
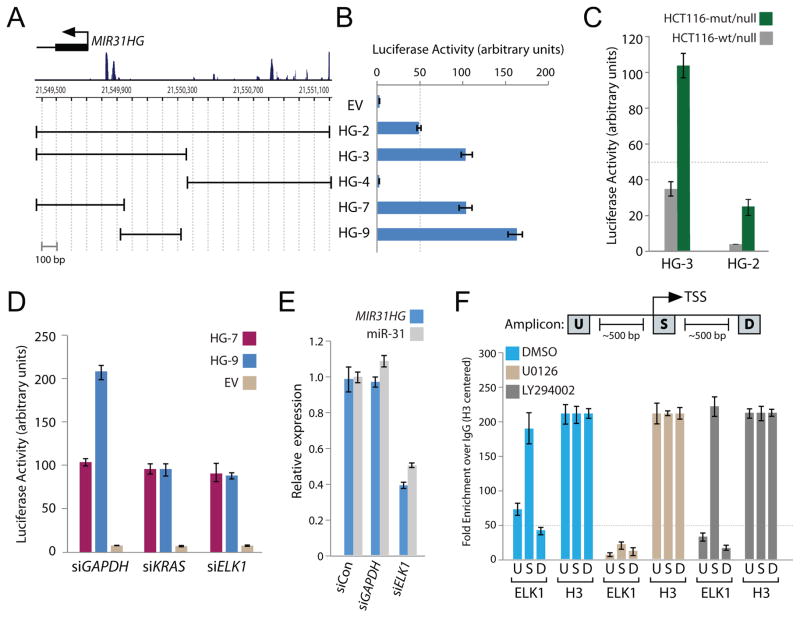
The observation that miR-31 expression was correlated with expression of oncogenic KRAS and the activation of the MAPK pathway suggested miR-31 is a transcriptional target of KRAS-MAPK signaling axis. The analysis of the miR-31 host gene and correlation of MIR31HG with miR-31 suggested that the TSS (Figure 2A) is the location of the miR-31 host gene promoter. To confirm oncogenic KRAS-mediated transcriptional regulation of miR-31, a series of genomic fragments including or excluding regions around the TSS were amplified and cloned into the pGL3 promoter-less luciferase reporter plasmid (Figure 3A). Several of the reporter constructs demonstrated robust luciferase activity in Panc-1 cells suggesting functional promoter activity (Figure 3B). Using isogenic HCT116 cells previously described, increased luciferase expressed from both HG-3 and HG-2 promoters was detected in HCT116-KRAS cells which have oncogenic KRAS relative to HCT116-null in which oncogenic KRAS has been knocked out (Figure 3C).
Figure 3.
The miR-31 proximal promoter is transactivated by oncogenic KRAS through the ELK1 transcription factor. A, the genomic region around the transcription start site (TSS) of MIR31HG. Boundaries of the MIR31HG promoter constructs cloned into pGL3-basic luciferase reporter vector are shown relative to the TSS. B, normalized luciferase activity generated from the indicated MIR31HG promoter (HG) transfected in Panc-1 cells. Luciferase activity was normalized to renilla expression and data is represented as fold change over cells expressing pGL3-promoterless empty vector (EV). Error bars in this and subsequent experiments represent standard deviations from three independent transfections each measured in triplicate. C, normalized luciferase activity from HG-2 and HG-3 promoter constructs in the indicated isogenic HCT116 cell lines. D, empty vector (EV) normalized luciferase activity from the HG-7 and HG-9 promoter constructs in Panc-1 cells pretreated with control siRNA (siGAPDH) or siRNA targeting KRAS (siKRAS) or ELK1 (siELK1). E, quantitative PCR analysis of MIR31HG and mature miR-31 expression in Panc-1 cells transfected with control siRNAs (siCon or siGAPDH) or siRNA targeting ELK1 (siELK1). F, quantitative PCR amplicons for ChIP were designed within approximately 100-bp windows at the TSS (amplicon S), 500-bp upstream of amplicon S (amplicon U), or 500-bp downstream of amplicon S (amplicon D). Quantitative PCR analysis of chromatin immunoprecipitates from Panc-1 cells treated with DMSO, U0126 or LY294002 and immunoprecipitated with antibodies recognizing ELK1 or histone H3. Fold enrichment represents the signal obtained after immunoprecipitation with a non-specific IgG antibody. Fold enrichment for each amplicon was centered to the signal obtained for histone H3. The 50-fold enrichment threshold for positive transcription factor binding is indicated as a dashed line. Data are mean ± s.d. from three independent measurements.
To identify the minimal RAS responsive region contained within the HG-3 promoter, the effect of acute KRAS knockdown on luciferase expression driven from HG-7 and HG-9 promoter fragments was queried in Panc-1 cells. HG-7 and HG-9 were designed to include or exclude respectively the two peaks of conservation near the TSS (Figure 3A). Luciferase expression driven from the HG-9 promoter was diminished when KRAS was knocked down whereas KRAS knockdown had no effect on luciferase expression produced from HG-7 (Figure 3D). This suggested that the HG-9 promoter construct contained a RAS responsive sequence required for KRAS-mediated transactivation. To find potential transcription factors (TFs) that may be regulating HG-9 through KRAS and MAPK signaling, the HG-9 sequence was examined using the ConSite algorithm (http://consite.genereg.net/). ConSite allows for the identification and visualization of conserved TF binding sites within metazoan promoters based on comparative phylogenetic footprinting (23). With an 85% discovery rate and 12-bit sequence analysis, ConSite predicted many highly conserved TF binding elements in HG-9 recognized by 15 individual TFs (Supplemental Table 2). Of particular interest, ELK1, a member of the ETS domain-containing family of transcription factors and a well-studied target of the MAPK pathway became the focus of further study. Acute knockdown of ELK1 with siRNA resulted in diminished luciferase expression from HG-9 reporter but had no effect on luciferase activity of HG-7 (Figure 3D) similar to what was observed with acute knockdown of KRAS. These results suggest that ELK1 is the dominant transcription factor downstream of KRAS driving luciferase expression from the HG-9 reporter.
To validate endogenous regulation of miR-31 by ELK1, expression of MIR31HG and the mature miR-31 was measured in Panc-1 cells following transfection with siRNA targeting ELK1 or GAPDH as a control. A significant decrease of both MIR31HG and miR-31 expression was observed in cells expressing siRNA targeting ELK1 relative to control siRNA or siRNA targeting GAPDH (Figure 3E, Supplemental Figure 2). Lastly, chromatin immunoprecipitation (ChIP) of endogenous ELK1 was performed in Panc-1 cells. Since ELK1 is activated downstream of the MAPK pathway, ChIP was also performed in lysates obtained from Panc-1 cells treated with MEK inhibitor (U0126) or as a control PI3K inhibitor (LY294002). Strong enrichment of the TSS amplicon (S) was observed in ELK1 precipitates from DMSO treated and PI3K inhibited lysates but not in MEK inhibited lysates, confirming that ELK1 interacts directly with the endogenous miR-31 promoter in a MAPK-dependent manner (Figure 3F). Collectively, these experiments demonstrate that KRAS transactivates miR-31 through the MAPK pathway and the downstream activation of ELK1 recruitment to the miR-31 proximal promoter.
Enforced expression of miR-31 increased invasion and migration in PDAC lines through activation of Rho
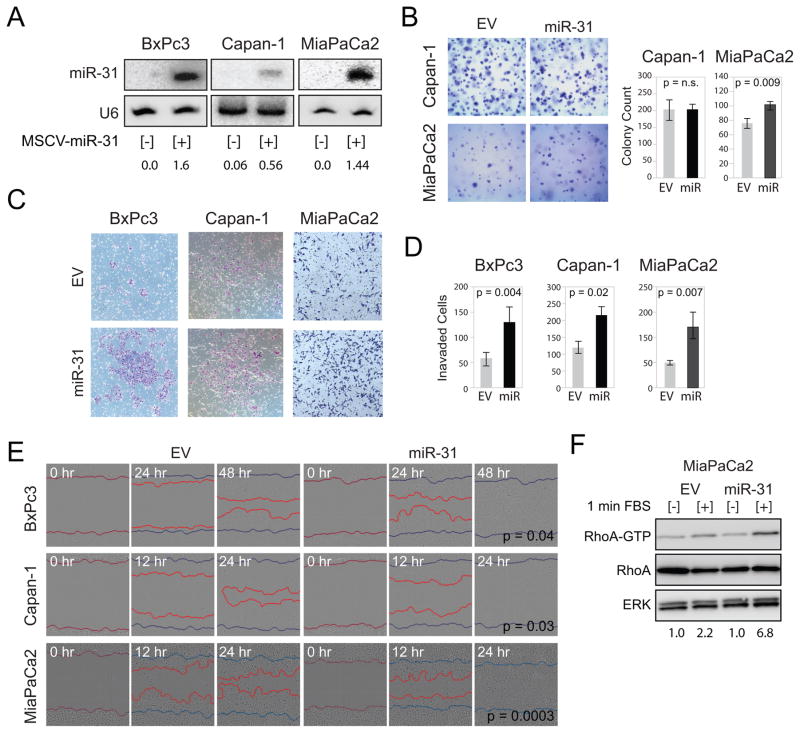
To assess if enforced expression of miR-31 enhanced KRAS-mediated phenotypes in PDAC cell lines which lacked miR-31, a retrovirus construct was used to express miR-31 in two cell lines harboring activating KRAS mutations (Capan-1 and MiaPaCa2) and in a cell line with wild-type KRAS (BxPc3). Expression of miR-31 from the stably selected retrovirally infected cell lines was validated by northern blot (Figure 4A). Importantly, enforced miR-31 expression was at physiologic levels as compared to endogenous expression of miR-31 measured in HPNE-KRAS (Supplemental Figure 3). Despite normal rates of proliferation for Capan-1 and MiaPaca2 cells with enforced miR-31 expression, BxPc3 cells with enforced expression of miR-31 grew slightly faster than BxPc3 infected with an empty vector (Supplemental Figure 4A). Enforced expression of miR-31 had no effect on the anchorage-independent growth of Capan-1 cells; however, miR-31 enhanced colony formation of MiaPaCa2 cells (Figure 4B). Enforced expression of miR-31 did not confer the ability of BxPc3 cells to grow in soft agar (data not shown). However, enforced expression of miR-31 greatly enhanced the ability of BxPc3, Capan-1 and MiaPaCa2 cells to invade through matrigel coated transwells (Figure 4C). Significant accumulation of invaded cells was observed for all three cells lines expressing miR-31 relative to empty vector (Figure 4D). Further, miR-31 expression significantly enhanced the migratory behavior of all three cell lines in scratch wound assays (Figure 4E).
Figure 4.
Enforced expression of miR-31 in pancreatic cancer cell lines enhanced invasion and migration. A, northern blot analysis of miR-31 expression in pancreatic cancer cell lines stably infected with MSCV-empty vector [−] or MSCV-miR-31 [+]. Taqman quantified expression of enforced miR-31 is shown for each cell line relative to the expression of endogenous miR-31 measured in HPNE-KRAS cells (see Supplemental Fig 3). B, representative images of Capan-1 and MiaPaCa2 cells infected with MSCV-empty vector (EV) or MSCV-miR-31 (miR-31) grown for 7 days in 0.3% agar to form colonies. Bar graphs average colony counts from 8 representative images. P-values are indicated. C, invasion of the indicated cell lines with MSCV-empty vector (EV) or MSCV-miR-31 (miR-31) through matrigel coated transwells. D, bar graphs average the number of invaded cells from 5 independent experiments. P-values are indicated. E, scratch wound analysis of the indicated cell lines with MSCV-empty vector (EV) or MSCV-miR-31 (miR-31) monitored over the indicated time course. Images from Essen IncuCyte ZOOM. Blue lines mark the starting wound boundary and the red lines mark the cell front moving into the wound. P-values calculated from the difference in wound width between EV and miR-31 expressing cells 18 hours post scratch (n=8). F, lysates derived from MiaPaCa2 cells with MSCV-empty vector (EV) or MSCV-miR-31 (miR-31) were incubated with Rhotekin-Rho binding domain (RBD) protein beads with no stimulation [−] or following 1 minute stimulation with FBS [+]. Active RhoA-GTP and total cellular RhoA (total RhoA) were detected by immunoblotting with anti-RhoA antibody. Normalization of RhoA-GTP to total cellular RhoA for each experimental condition is indicated. ERK served as a protein loading control.
Since KRAS-mediated phenotypes are dominantly determined by MAPK and PI3K signaling, we queried how miR-31 over expression would affect signaling through these pathways. Because of the high frequency of KRAS mutation in pancreatic cancer, the effect of miR-31 on signaling was assessed only in Capan-1 and MiaPaCa2 both of which have oncogenic KRAS. No differences in ERK1/2 or AKT phosphorylation was observed in either Capan-1 or MiaPaCa2 cells with enforced miR-31 expression at basal levels or following acute stimulation with serum compared to control cell lines (Supplemental Figure 4B). We conjectured that increased invasion and migration with enforced miR-31 expression was due to miR-31-mediated activation of Rho. Upon acute stimulation with serum, RhoA-GTP was significantly higher in MiaPaCa2 cells with enforced miR-31 expression compared to cells with empty vector control (Figure 4F). These data suggest that miR-31 enhances RhoA activation which likely promotes increased invasion and migration.
RASA1 is a target of miR-31
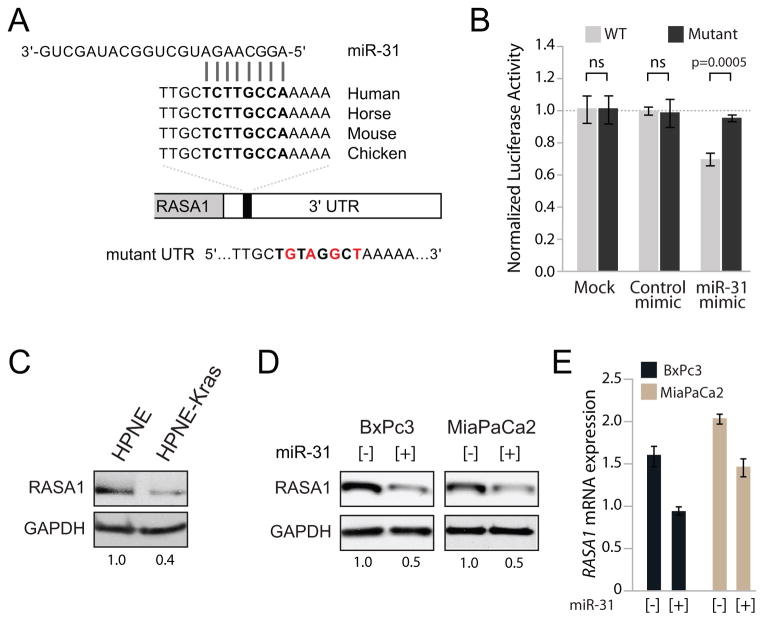
To gain insight into the mechanism through which miR-31 enhanced invasion and migration, predicted targets of miR-31 were examined. Since individual miRNA prediction programs find hundreds of potential targets, the intersection of four online searchable miRNA target prediction programs (miR-Database, miR-Target, PicTar, and Targetscan) was analyzed, revealing 18 predicted targets in common (Supplemental Table 3). Of these 18 targets, RASA1 (p120RasGAP) was of particular interest because it functions as a negative regulator of RAS pathway activation and modulates invasion-behavior through interaction with p190RhoGAP (24, 25). Moreover, RASA1 was demonstrated to be a miR-31 target in colorectal and liver cancers (26, 27). The RASA1 3′ UTR contains one predicted miR-31 binding site which is highly conserved in vertebrates (Figure 5A). We validated miR-31 regulation of RASA1 using a luciferase reporter plasmid under regulation by a fragment of the RASA1-3′UTR containing the putative miR-31 binding site. Co-transfection of this reporter with a miR-31 mimic led to significant repression of luciferase expression which was relieved by introducing mutations into the miRNA binding site (Figure 5B). RASA1 was downregulated in HPNE-KRAS cells relative to expression in HPNE (Figure 5C). We also observed decreased expression of RASA1 in isogenic HCT116-KRAS mutant versus KRAS null cells (Supplemental Figure 5). In addition, over expression of miR-31 in BxPc3 and MiaPaCa2 cells reduced endogenous RASA1 expression at both the protein and mRNA level (Figures 5D and 5E). Collectively, our results confirm that miR-31 regulates RASA1 in PDAC cells.
Figure 5.
RASA1 is a target of miR-31 in PDAC cell lines. A, sequence and evolutionary conservation of the miR-31 binding site in the 3′-UTR of RASA1 transcript. Mutations introduced into the miR-31 binding site in the mutant luciferase reporter construct are shown in red. B, luciferase activity derived from the wild-type (WT) or mutant RASA1 3′-UTR reporter constructs transfected into MiaPaCa2 cells with transfection reagent alone (mock), control or miR-31 mimics. All values normalized to renilla produced from a co-transfected control plasmid. For each transfection condition, luciferase activity produced from the wild-type construct was normalized to the activity produced by the mutant construct. Error bars represent standard deviations from 3 independent transfections, each measured in triplicate. The p-value for significant experiment is indicated. C, western blot analysis of RASA1 expression in isogenic HPNE and HPNE-KRAS cells. GAPDH served as a protein loading control. D, western blot analysis and E, quantitative PCR analysis of RASA1 expression in BxPc3 and MiaPaCa2 cells with MSCV-empty vector [−] or MSCV-miR-31 [+]. GAPDH served as a protein loading control.
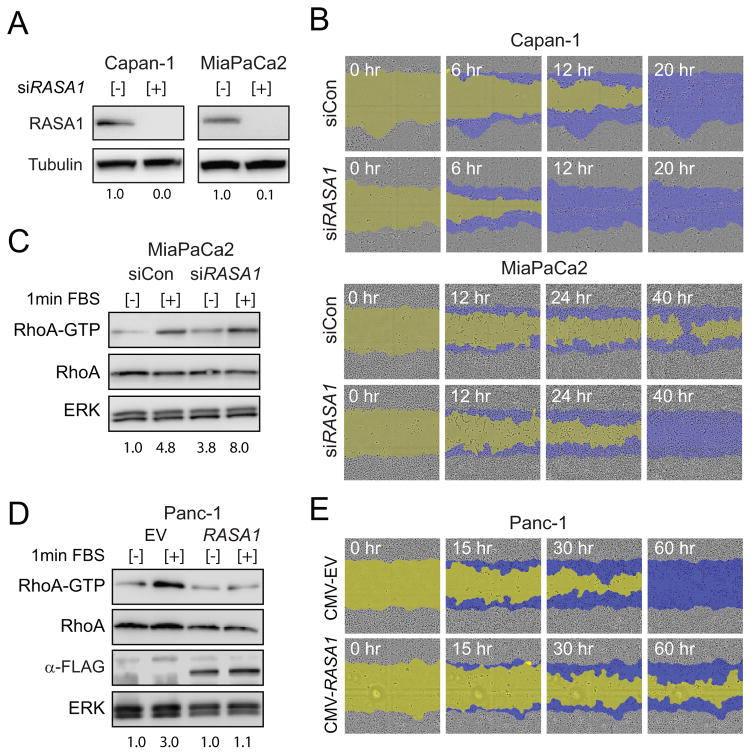
RASA1 loss phenocopies miR-31 over expression and its gain inhibits migration
To establish a genetic interaction between RASA1 and miR-31, we sought to determine whether loss of RASA1 hastened the invasion and migration behavior in PDAC cell lines seen with enforced miR-31 expression. RNAi mediated knockdown of RASA1 resulted in complete knockdown of RASA1 as confirmed by western blot in both Capan-1 and MiaPaCa2 cells (Figure 6A). Consistent with the miR-31 overexpression phenotype, RNAi knockdown of RASA1 significantly enhanced the rate of wound closure for both Capan-1 and MiaPaCa2 cell lines in scratch wound assays (Figure 6B). Serum stimulated MiaPaCa2 cells treated with siRNA targeting RASA1 had increased RhoA-GTP levels compared to siRNA control (Figure 6C). Importantly, RASA1 knockdown enhanced the serum-stimulated activation of ERK1/2 and AKT in Capan-1 and MiaPaCa2 cells, both of which have constitutively active KRAS (Supplemental Figure 6A). Since RASA1 functions to enhance the intrinsic GTPase activating proteins (GAPs) for both RHOA and RAS, suggests that RASA1 knockdown may potentiate RAS signaling by prolonging the half-life of the GTP bound state of the wild type copy of KRAS. The inability of miR-31 to alter ERK and AKT phosphorylation levels may reflect the partial effect of this miRNA on downregulation of RASA1 as compared to complete depletion of RASA1 achieved by siRNA (Supplemental Figure 4B). Future experiments will be necessary to determine the absolute levels of RASA1 required for further RAS activation in the cell lines harboring oncogenic KRAS.
Figure 6.
RASA1 negatively regulates migration in pancreatic cancer cells. A, western blot analysis of RASA1 expression in Capan-1 and MiaPaCa2 cells transfected with siRNA control [−] or siRNA targeting RASA1 [+]. Tubulin served as a protein loading control. B, scratch wound analysis of Capan-1 and MiaPaCa2 cell lines transfected with siRNA control (siCon) or siRNA targeting RASA1 (siRASA1) monitored at the indicated times. The initial scratch wound mask is colored yellow and the progression of cell migration is marked blue. C, lysates derived from indicated cells treated with siRNA control (siCon) or siRNA targeting RASA1 (siRASA1) were incubated with Rhotekin-Rho binding domain (RBD) protein beads with no stimulation [−] or following 1 minute stimulation with FBS [+]. Active RhoA-GTP and total cellular RhoA were detected by immunoblotting with anti-RhoA antibody. ERK served as a protein loading control. D, lysates derived from Panc-1 cells transfected with CMV-empty vector (EV) or CMV-flag-RASA1 (RASA1) were incubated with Rhotekin-Rho binding domain (RBD) protein beads with no stimulation [−] or following 1 minute stimulation with FBS [+] as described for panel C. Expression of flag-RASA1 confirmed with M2-anti-flag antibody. E, scratch wound analysis of Panc-1 cells transfected with CMV-empty vector (EV) or CMV-flag-RASA1 (CMV-RASA1) monitored at the indicated times. Color coding the same as panel B.
Finally, to complement our RASA1 knockdown studies, we overexpressed RASA1 in Panc-1 cells which express miR-31 and moderate levels of RASA1. Panc-1 cells were transiently transfected with a plasmid that expressed flag-tagged RASA1 lacking the endogenous 3′UTR (CMV-RASA1) and expression was confirmed by western blot (Supplemental Figure 6B). Increased expression of RASA1 blocked activation of RhoA and greatly impaired the wound closure capacity of Panc-1 cells in scratch wound assays compared to empty vector (Figure 6D and 6E). These results suggest that the stoichiometric ratio of RASA1 relative to GAPs such p190RhoGAP can create a switch controlling threshold levels of RhoA-GTP required for migration in PDAC cells. Collectively, our results suggest that RASA1, likely separate from its GAP function, can affect the rate of Rho activation and modulate migration downstream of oncogenic KRAS.
Discussion
Activating mutations of KRAS occur in approximately 20% of human cancers including 95% of pancreatic and 60% of colorectal adenocarcinomas. Dissecting how miRNAs fit into the KRAS signaling networks is critical for understanding how these pathways are regulated and may reveal novel targets for therapeutic intervention. In the present study, oncogenic KRAS has been demonstrated to upregulate miR-31 through transcriptional regulation of the MIR31HG pri-miRNA transcript via the MAPK pathway and recruitment of ELK1 transcription factor to the miR-31 promoter. Elevated levels of miR-31 negatively regulate the expression of RASA1 resulting in increased migration and invasion of pancreatic cancer cell lines. These data suggest miR-31 could be a potential therapeutic target for pancreatic cancer, especially in high risk individuals since the vast majority of patients present with metastatic disease at the time of diagnosis (28).
Multiple miRNA profiling studies have documented the upregulation of miR-31 in pancreatic cancer cell lines and tumors (17, 26, 29, 30). We have found that miR-31 is moderately to highly expressed in 16 of 23 pancreatic and colorectal cancer cell lines. Both pancreatic and colorectal cancers frequently harbor activating KRAS mutations and miR-31 has previously been shown to be overexpressed in tumor types where activating mutations of KRAS are common (31, 32, 33). A comprehensive meta-review of miRNA expression in PDAC tumors and neighboring non-cancerous pancreatic tissue found miR-31 to be upregulated from 5 independent expression studies and associated with poor survival following tumor resection (29). In addition, miR-31 was shown to be upregulated in intraductal papillary mucinous neoplasm (IPMN), a precursor cystic lesion to pancreatic cancer (34). Since acquisition of activating KRAS mutation is an early event in IPMNs and the development of pancreatic cancer (6) upregulation of miR-31 observed in these precursor lesions may account for their early metastatic behavior. Furthermore, early dissemination and invasion into the bloodstream has been shown to precede tumorigenesis in a mouse model of pancreatic cancer (28). It is plausible that oncogenic KRAS transactivates miR-31 to promote early initiation of invasion and migration programs in pancreatic cancer cells.
Our data, coupled with results from several other studies, clearly demonstrate that miR-31 is a regulator of invasion and migration in cancer. However, the positive or negative effect of miR-31 on this phenotype is discordant among different cancer types. A Pubmed search of “miR-31” and “invasion migration” results in 27 publications analyzing miR-31 function in 15 different cancer types (Supplemental Table 3 and references therein). On one hand, miR-31 is found up regulated and to enhance invasion migration in 6 cancer types (cervical, colorectal, cutaneous squamous cell, esophageal squamous cell, Kaposi’s sarcoma, lung) and on the other hand, is found down regulated and to repress invasion migration in 7 cancer types (breast, esophageal, Ewing sarcoma, glioma, melanoma, ovarian, prostate). A previous report of the role of miR-31 in pancreatic cancer is ambiguous; both inhibition and enforced expression of miR-31 reduced migration and invasion in PDAC cell lines (35). In the present study, the enforced expression of miR-31 enhanced invasion and migration in three pancreatic cancer cell lines tested irrespective of KRAS mutational status. Interestingly, an enhanced invasion-migration phenotype attributed to miR-31 is consistently seen in cancers with activating KRAS mutations suggesting a role for miR-31 as a KRAS effector in regulation of invasion-migration (36, 37). Overall, these findings suggest that miR-31 may regulate multiple targets that control invasion-migration and the repertoire of these targets that are expressed in a given cell type may dictate the impact of miR-31 expression on this phenotype.
The Rho GTPases are molecular switches that cycle between a GTP bound active state and GDP bound inactive state. Rho family members are inactivated by Rho GTPase activating proteins (GAPs) which accelerate GTP hydrolysis and activated by Rho guanine nucleotide exchange factors (GEFs) which catalyze the intrinsically slow nucleotide exchange from GDP to GTP (38). Rho GTPases regulate the actin cytoskeleton and thereby control cellular migration, morphology, motility, and other functions (39, 40). The role of Rho in migration is currently not clear. RHOA is historically thought to be involved in the moving the body and tail of the cell behind the leading edge and forming integrin-based cell contacts (40). However, other GTPases including RAS and the interplay between family members of the Rho family including Rac and Cdc42 all likely have a role in orchestrating coordinated cellular movement. Previously, TIAM1 a GEF for Rac has been identified as miR-31 target and down regulation of TIAM1 by miR-21 and miR-31 enhanced migration and invasion of colon cancer cell lines (36). RAS has long been known to make an essential contribution to cellular motility and has recently been shown to regulate an essential RhoGEF called ARHGEF2 (41) providing further evidence of Rho as RAS effector. RASA1 in addition to its RasGAP activity has been speculated to be a RAS effector mediating the cellular effects of RAS through its interaction with p190RhoGAP and the subsequent inhibition of Rho activation (38). In agreement with the work reported here, in the colorectal cancer cell line RKO, decreased RASA1 expression mediated by miR-21 enhanced RAS signaling and invasion through transwells (42).
In conclusion, we have demonstrated that KRAS signaling results in transactivation of miR-31, repression of RASA1, and subsequence activation of Rho and enhanced migration and invasion in pancreatic cancer cells lines. This provides a mechanism whereby KRAS couples the migratory machinery of the cell through coupling miR-31 to Rho. These results provide further insight into the complexity of how miRNA participate in cancer pathogenesis and highlight the importance of understanding the role of miRNAs in the RAS-transformation program. Additionally, these data suggest that miR-31 may be a biomarker for highly invasive KRAS-mutant cancers.
Supplementary Material
Implications.
Oncogenic KRAS can activate Rho through the miR-31 mediated regulation of RASA1 indicating miR-31 acts as a KRAS effector to modulate invasion and migration in pancreatic cancer.
Acknowledgments
We would like to thank Dr. Marc Halushka for helpful discussions and support with the early stages of this project and Dr. Helen Burston and Dr. Yoshi Matsumoto for proof reading the manuscript. We would like to thank Dr. Anirban Maitra and Dr. James Eshleman for kindly providing cell lines. OAK is currently a postdoctoral fellow in the laboratory of Dr. Robert Rottapel at the Princess Margaret Cancer Centre in Toronto ON. JTM is an Investigator of the Howard Hughes Medical Institute.
Financial support: This work was supported by the Canadian Institute for Health Research and a joint grant from the Terry Fox Research Institute and the Ontario Institute for Cancer Research.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Authors Contributions:
Conception and design, acquisition and analysis of data: O.A. Kent
Writing, review and revision of manuscript: O.A. Kent, J.T. Mendell, and R. Rottapel
References
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, et al. Repression of the miR-143/145 cluster by oncogenic RAS initiates a tumor-promoting feed-forward pathway. Genes & Dev. 2010;24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbacid M. RAS genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 8.Macara IG, Lounsbury KM, Richards SA, McKiernan C, Bar-Sagi D. The RAS superfamily of GTPases. FASEB J. 1996;5:625–630. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 9.Prior IA, Lewis PD, Mattos C. A comprehensive survey of RAS mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- 11.Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31:1726–1733. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
- 13.Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van Rooij E, et al. Modulation of K-RAS-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent OA, Fox-Talbot K, Halushka MK. RREB1 repressed miR-143/145 modulates KRAS signaling through downregulation of multiple targets. Oncogene. 2012;32:2576–2585. doi: 10.1038/onc.2012.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KM, Nguyen C, Ulrich AB, Pour PM, Ouellette MM. Immortalization with telomerase of the Nestin-positive cells of the human pancreas. Biochem Biophys Res Commun. 2003;301:1038–1044. doi: 10.1016/s0006-291x(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 16.Feldmann G, Habbe N, Dhara S, Bisht S, Alvarez H, Fendrich V, et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut. 2008;57:1420–1430. doi: 10.1136/gut.2007.148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent OA, Mullendore M, Wentzel EA, Lopez-Romero P, Tan AC, Alvarez H, et al. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther. 2009;8:2013–2024. doi: 10.4161/cbt.8.21.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V, Der CJ. K-RAS promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res. 2007;67:2098–2106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 19.Geissmann Q. OpenCFU, a New Free and Open-Source Software to Count Cell Colonies and Other Circular Objects. PLoS ONE. 2013;8:e54072. doi: 10.1371/journal.pone.0054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 21.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang TC, Pertea M, Lee S, Salzberg SL, Mendell JT. Genome-wide annotation of microRNA primary transcript structures reveals novel regulatory mechanisms. Genome Res. 2015;25:1401–1409. doi: 10.1101/gr.193607.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenhard B, Sandelin A, Mendoza L, Engström P, Jareborg N, Wasserman WW. Identification of conserved regulatory elements by comparative genome analysis. J Biol Chem. 2013;288:9508–9518. doi: 10.1186/1475-4924-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehler JA, Moran MF. Regulation of extracellular signal-regulated kinase activity by p120 RASGAP does not involve its pleckstrin homology or calcium-dependent lipid binding domains but does require these domains to regulate cell proliferation. Cell Growth Differ. 2001;12:551–561. [PubMed] [Google Scholar]
- 25.Pamonsinlapatham P, Hadj-Slimane R, Lepelletier Y, Allain B, Toccafondi M, Garbay C, et al. p120-RAS GTPase activating protein (RASGAP): a multi-interacting protein in downstream signaling. Biochimie. 2009;91:320–328. doi: 10.1016/j.biochi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Hu C, Huang F, Deng G, Nie W, Huang W, Zeng X. miR-31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Ther Med. 2013;6:1265–1270. doi: 10.3892/etm.2013.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J, et al. MicroRNA-31 activates the RAS pathway and functions as an oncogenic MicroRNA in human colorectal cancer by repressing RAS p21 GTPase activating protein 1 (RASA1) J Biol. 2013;288:9508–9518. doi: 10.1074/jbc.M112.367763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma MZ, Kong X, Weng MZ, Cheng K, Gong W, Quan ZW, et al. Candidate microRNA biomarkers of pancreatic ductal adenocarcinoma: meta-analysis, experimental validation and clinical significance. J Exp Clin Cancer Res. 2013;32:71–85. doi: 10.1186/1756-9966-32-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;6:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Sempere LF, Ouyang H, Memoli VA, Andrew AS, Luo Y, et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest. 2010;120:1298–1309. doi: 10.1172/JCI39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandres E, Cubedo E, Agirre X, Malumbres R, Zarate R, Ramirez N, et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29–39. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nosho K, Igarashi H, Nojima M, Ito M, Maruyama R, Yoshii S, et al. Association of microRNA-31 with BRAF mutation, colorectal cancer survival and serrated pathway. Carcinogenesis. 2014;35:776–783. doi: 10.1093/carcin/bgt374. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Paris PL, Chen J, Ngo V, Yao H, Frazier ML, et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015;356:404–409. doi: 10.1016/j.canlet.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurila EM, Sandström S, Rantanen LM, Autio R, Kallioniemi A. Both inhibition and enhanced expression of miR-31 lead to reduced migration and invasion of pancreatic cancer cells. Genes Chromosomes Cancer. 2012;51:557–568. doi: 10.1002/gcc.21941. [DOI] [PubMed] [Google Scholar]
- 36.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–35302. doi: 10.1074/jbc.M110.160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng W, Ye Z, Cui R, Perry J, Dedousi-Huebner V, Huebner A, et al. MicroRNA-31 predicts the presence of lymph node metastases and survival in patients with lung adenocarcinoma. Clin Cancer Res. 2013;19:5423–5433. doi: 10.1158/1078-0432.CCR-13-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bar-Sagi D, Hall A. RAS and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 39.Aznar S, Fernández-Valerón P, Espina C, Lacal JC. Rho GTPases: potential candidates for anticancer therapy. Cancer Lett. 2004;206:181–191. doi: 10.1016/j.canlet.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 41.Cullis J, Meiri D, Sandi MJ, Radulovich N, Kent OA, Medrano M, et al. The RhoGEF GEF-H1 is required for oncogenic RAS signaling via KSR-1. Cancer Cell. 2014;25:181–195. doi: 10.1016/j.ccr.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Gong B, Liu W, Nie W, Li D, Xie Z, Liu C, et al. MiR-21/RASA1 axis affects malignancy of colon cancer cells via RAS pathways. World J Gastroenterol. 2015;21:1488–1497. doi: 10.3748/wjg.v21.i5.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.