Abstract
Recombinant immunotoxins (RITs) have been highly successful in cancer therapy due in part to the high cancer-specific expression of cell-surface antigens such as mesothelin which is overexpressed in mesothelioma, ovarian, lung, breast, and pancreatic cancers, but is limited in normal cells. RG7787 is a clinically optimized RIT consisting of a humanized anti-mesothelin Fab fused to domain III of Pseudomonas exotoxin A in which immunogenic B cell epitopes are silenced. To enhance the therapeutic efficacy of RITs, we conducted a kinome RNAi sensitization screen which identified discoidin domain receptor 1 (DDR1), a collagen-activated tyrosine kinase, as a potential target. The collagen/DDR1 axis is implicated in tumor-stromal interactions and potentially affects tumor response to therapy. Therefore, we investigated the effects of DDR1 on RIT. Knockdown of DDR1 by siRNA or treatment with inhibitor, 7rh, greatly enhanced the cytotoxic activity of RG7787 in several cancer cell lines. Investigation into the mechanism of action showed DDR1 silencing was associated with decreased expression of several ribosomal proteins and enhanced inhibition of protein synthesis. Conversely, induction of DDR1 expression or collagen-stimulated DDR1 activity protected cancer cells from RG7787 killing. Moreover, the combination of RG7787 and DDR1 inhibitor caused greater shrinkage of tumor xenografts than either agent alone. These data demonstrate that DDR1 is a key modulator of RIT activity and represents a novel therapeutic strategy to improve targeting of mesothelin-expressing cancers.
Keywords: Immunotoxins, Mesothelin, Cancer therapy, Discoidin Domain Receptor 1, Collagen
Introduction
Recombinant immunotoxins (RITs) are novel cytotoxic agents composed of an antibody fragment (Fv or Fab) targeted to a cell-surface antigen and a protein toxin. Our laboratory has developed RITs by attaching the catalytic domain of a potent bacterial toxin Pseudomonas exotoxin A (PE) to fragments of antibodies targeting cell surface antigens that exhibit relatively high cancer-specific expression such as CD22 or mesothelin (MSLN) (1). MSLN is a cell-surface glycoprotein whose expression is restricted to mesothelial cells. It is an excellent tumor target because it is highly expressed in mesothelioma, lung, gastric, pancreatic, ovarian, and triple-negative breast cancers (TNBC) (2–8).
SS1P is the first-generation PE38 based RIT targeted to MSLN. While it showed a favorable safety profile when tested as a single agent in phase I clinical trials (9), its activity was limited due to formation of neutralizing antibodies against the toxin in 90% of patients. Combination with the T- and B-cell depleting drugs, pentostatin and cyclophosphamide, allowed prolonged dosing of SS1P and resulted in striking regression of some advanced refractory mesotheliomas (10). To minimize PE immunogenicity and improve clinical efficacy, a re-engineered version of SS1P called RG7787 was developed in collaboration with Roche Innovation Center Penzberg, Germany (11). RG7787, currently in phase I of clinical trials, consists of a humanized anti-MSLN Fab linked to a PE24 moiety generated by silencing B-cell epitopes and truncating protease sensitive regions. In mice, RG7787 has a longer half-life than SS1P and can be administered at a higher dose (11). Preclinical testing of RG7787 showed tumor growth inhibition when used as a single agent and significant tumor regression in combination with taxol in TNBC, gastric, and pancreatic cancer xenograft models (12, 13). RITs are internalized by receptor-mediated endocytosis after binding of their antibody portion to the cell-surface antigen. Cleavage by a cellular protease, furin, separates the toxin moiety, which then traffics to the endoplasmic reticulum via retrograde transport. Once in the cytosol, the toxin ADP-ribosylates elongation factor 2 preventing the elongation step of protein translation resulting in inhibition of protein synthesis and eventually cell death. Understanding which proteins may inhibit toxin-mediated cell killing is critical in designing combination therapies for improved efficacy of RITs. Several receptor tyrosine kinases (RTKs) are known to play a major role in cell survival and can be activated by cells under stress. Previous work in our lab has shown that the activity of SS1P can be enhanced by lowering levels of the insulin receptor (14) or HCK or PDGFR2 or SRC (15). To expand on this knowledge we conducted a comprehensive kinome RNAi screen to identify kinases that may regulate the activity of RITs. Among the top hits identified from this screen was the RTK discoidin domain receptor 1 (DDR1). In this study, we examined the role of DDR1 in modulating activity of RG7787 and SS1P.
Collagen mediated activation of DDR1 facilitates cell adhesion, migration, proliferation and matrix remodeling (16, 17). Under physiological conditions, DDR1 controls cell polarity and tissue morphogenesis by acting as a collagen sensor. ECM-mediated aberrant DDR1 activation contributes to the migratory and pro-invasive phenotype of cancer cells. In several cancer types, overexpression of DDR1 is correlated with the severity of disease (18). The collagen/DDR1 axis also modulates tumor-stromal interaction and potentially can affect tumor response to therapy (19).
The aim of this study was to understand whether DDR1 regulates the cellular response to immunotoxins and whether RG7787 activity can be enhanced by combination with a small molecule inhibitor of DDR1.
Materials and Methods
Cell culture and reagents
A431 is an epidermoid cancer cell line that was obtained from Dr. George Todaro (NCI, Bethesda, MD) in 1983. A431/H9 was transfected to stably express MSLN (20). KB31 was provided by Dr. Michael Gottesman (NCI, Bethesda, MD) in 1985. HAY (mesothelioma) cell line was provided by Stehlin Foundation for Cancer Research (Houston, TX) in 2004. A431/H9 cells were verified by STR-PCR in 2014, KB31 in 2009 and HAY in 2012. SUM149 (Triple negative breast cancer) were a gift of Dr. Stanley Lipkowitz (NCI, Bethesda, MD), and authenticated in 2013. KLM1 (pancreatic cancer) cells were provided by Dr. U. Rudloff (NCI, Bethesda, MD) and their identity was verified in 2012. HT-1080 is a human fibrosarcoma-derived cell line that was originally obtained from ATCC in 2013. It was transfected to express the tetracycline (Tet)-regulated transactivator Tet-Off (pLVX-Tet-Off advanced; Clontech) by Marco Prunotto in 2013 and verified by STR-PCR in 2013. Inducible expression of human DDR1b was achieved by transducing this cell line with a Lentivirus containing human DDR1b under the control of a tetracycline-responsive promoter (pLVX-Tight-Puro; Clontech). Expression is induced by the withdrawal of doxycycline (Dox) from the culture medium. Identity of tet-inducible cell lines was confirmed by STR testing in 2013. All cells were grown in RPMI-1640 medium (Lonza) supplemented with 10% FBS (HyClone; Thermo Scientific), 1mM sodium pyruvate and 1% penicillin-streptomycin (Gibco Life Technologies) in humidified atmosphere of 5% CO2 at 37°C. Native PE was produced as previously described (21). SS1P was manufactured by Advanced Bioscience Laboratories, Inc and RG7787 was supplied by Roche Holding AG. 3H-Leucine was purchased from Perkin Elmer and leucine-free medium from Invitrogen. DDR1 inhibitor '7rh' was synthesized as described (22).
Cell survival after DDR1 RNAi and DDR1 inhibitor
A431/H9 cells (3000/well; 10nM siRNA) or KB cells (2800/well; 25nM siRNA) were reverse transfected using lipofectamine RNAi Max with scramble control siRNA (Ambion) or DDR1 siRNA #9 or #10 in a 96 well plate for 48 hours followed by treatment with different concentrations of RIT (SS1P, RG7787, or native PE) for 72 hours. Sequences for siRNAs are listed in Supplementary Table S1. To evaluate the effect of DDR1 inhibitor 7rh, cells were plated overnight and treated with 7rh for 3 hours before treatment with RIT for 72 hours. ATP levels were measured to assess cell viability using the Cell Titer-Glo Luminescent assay (Promega). Data were normalized and shown as percent of control.
Measurement of protein synthesis
Incorporation of radio-labeled leucine was used to measure cellular protein synthesis. Briefly, cells were reverse transfected for 48 hours or treated with 7rh for 3 hours, as described above, followed by treatment with RIT for 18 hours. Media was replaced with leucine free medium containing 3H-Leucine (2uCi/well) for 3 hours at 37°C. After a freeze thaw cycle, plates were counted in a microplate beta counter. Counts from wells containing only medium with 3H-Leucine were used as background.
Collagen treatment
A431/H9 and KLM1 cells were plated on collagen I-coated plates (Corning) overnight followed by treatment with soluble collagen I (10 or 25 μg/ml; Invitrogen) for 3–6 hours before treatment with various concentrations of RG7787. Images were taken 48 hours after exposure to RG7787 using a Zeiss microscope with a 10X EC Plan-NeoFluar objective using the AxioCam MRc camera and the AxioVision 4.7.2 acquisition software.
DDR1 overexpression
The HT1080 (fibrosarcoma) cell line was generated by stable transfection with a DDR1b inducible vector. DDR1 expression was induced by culturing cells without doxycycline for 48 hours and expression compared with cells grown with doxycycline. Cells were treated with different concentrations of native PE for 48 hours and cell viability was assessed as described above.
Animal studies
A431/H9 cells (2 × 106) or KLM1 cells (4 × 106) supplemented with Matrigel (4 mg/ml; BD Bioscience) were injected subcutaneously into the flank of athymic nude mice. 7rh was dissolved in DMSO: EtOH: Cremaphor EL: H2O = 4:4:4:88. RG7787 was dissolved in 0.1%HSA/Dulbecco’s phosphate buffered saline (D-PBS). When tumors reached 120–150mm3, DDR1 inhibitor 10 or 20 mg/kg 7rh was given daily by oral gavage while 75 μg (3.75 mg/kg) RG7787 was given by intravenous tail injection every other day. The group of mice with combination treatment received RG7787 3 hours after receiving 7rh. Tumor volume was measured every other day using a digital caliper and calculated using the formula: 0.4 x width2 x length. Liquid diet was provided if at least one mouse in an experiment lost more than 2g. Mice were euthanized when tumors reached >500mm3 in size or became necrotic. All animal experiments were approved by the NCI Animal Care and Use Committee and conducted according to the NIH guidelines.
Statistical analysis
Data were analyzed and statistical analyses were performed using GraphPad Prism 6 software and Excel. Each experiment was performed at least twice with 3–6 replicates per assay. Data are presented as mean ± SEM. Unpaired student t test and one way analysis of variance (ANOVA) with Bonferroni test for multiple comparisons was performed. A p-value of <0.05 was considered statistically significant.
Results
DDR1 knockdown enhanced activity of immunotoxins and native PE
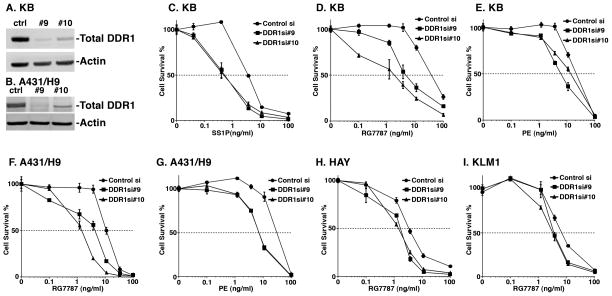
To identify regulators of the cytotoxicity of immunotoxins, we performed a RNAi kinome screen in the KB cervical cancer cell line (details will be reported elsewhere) and identified DDR1 as a top candidate whose inhibition significantly enhanced cytotoxicity of SS1P (RSA(23) p-value <0.001). In this study, we began by treating a panel of cancer cells with two siRNAs targeting DDR1 in combination with SS1P, RG7787 or native PE. Treatment with the siRNA reduced DDR1 protein levels in KB and A431/H9 cells (Fig. 1A–B) and enhanced the cytotoxicity of the immunotoxins in all cell lines tested (Fig. 1C–I). The magnitude of enhancement varied among cell lines from 2–50 fold. Table 1 summarizes the change in IC50 for all cell lines treated with DDR1 siRNAs and RG7787. These studies show that DDR1 can regulate immunotoxin activity in multiple cancer types.
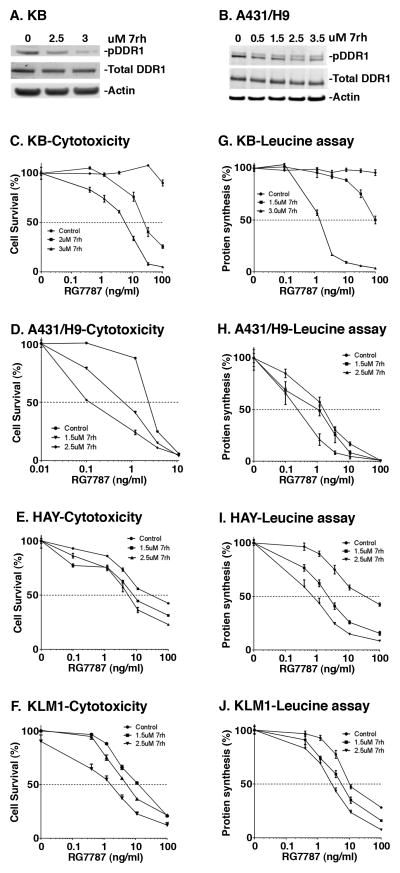
Figure 1.
Effect of DDR1 knockdown on protein expression and cytotoxicity of RITs. A, B) Reverse transfection with scramble control siRNA (ctrl) or siRNA targeting DDR1 (#9 and #10) was performed for 48 hours in KB and A431/H9 cells, respectively. Cells were harvested and lysates were subjected to western blotting. Blots were probed using anti-DDR1 or anti-actin antibodies. After transfection for 48 hours, KB cells were treated with varying concentrations of C) SS1P, D) RG7787 or E) native PE for 72 hours. Effect on DDR1 knockdown on cytotoxicity of F) RG7787 and G) PE in A431/H9 cells are shown. Cell survival was measured using cell titer glow ATP assay. Activity of RG7787 after DDR1 knockdown in H) HAY (mesothelioma) and I) KLM1 (pancreatic cancer) cells are shown. Data are represented as percentage of control by normalizing to transfected cells treated with PBS alone. Each data point represents a mean of four replicates.
Table 1.
Summary of enhancement of RG7787 activity by combination with DDR1 siRNA or with DDR1 Inhibitor 7rh in MSLN-expressing cell lines
| Cell line | Control si + RG7787 IC50 (ng/ml) | DDR1 siRNA + RG7787 IC50 (ng/ml) | RG7787 alone IC50 (ng/ml) | 7rh + RG7787 IC50 (ng/ml) |
|---|---|---|---|---|
| KB | 40 | 2 | >100 | 6 |
| A431/H9 | 11 | 1.5 | 2 | 0.1 |
| HAY | 11 | 1.5 | 35 | 5 |
| KLM1 | 8 | 3.7 | 11 | 2 |
| ASPC1 | 40 | 0.7 | -- | -- |
| L55S | -- | -- | 10 | 3 |
| SUM149 | -- | -- | 11 | 1 |
| HCC70 | -- | -- | 4 | 0.4 |
Immunotoxin internalization after DDR1 knockdown
Internalization of immunotoxins by receptor-mediated endocytosis is required for immunotoxin action. We tested whether enhanced activity of RG7787 after DDR1 knockdown was associated with increased RIT internalization. A panel of cell lines was transfected with control or DDR1-specific siRNAs for 48 hours and treated with Alexa-647 labeled RG7787 for varying times. Uptake as measured by FACs demonstrated that Alexa-647-RG7787 internalization did not change significantly after DDR1 silencing in 3 out of 4 cell lines indicating that enhanced activity of RG7787 after DDR1 knockdown is mediated by a later step in the intoxication pathway (Supplementary Fig. S1A–C).
However, A431/H9 cells have increased internalization of Alexa-647 labeled RG7787 after DDR1 silencing (Supplementary Fig. S1D). Consistent with this observation, higher surface expression of MSLN was observed after DDR1 knockdown in A431/H9 cells (Supplementary Fig. S2A). To understand whether this was due to increased MSLN expression, mRNA levels were measured by RT-PCR. A 2-fold increase in mRNA levels was observed following DDR1 silencing (Fig. S2B). It has been reported that miRNA198 regulates MSLN expression in transfected cells (24). DDR1 knockdown decreased miRNA198 levels by 2-fold (Supplementary Fig. S2C). These data indicate that in A431/H9 cells, DDR1 silencing can also regulate surface MSLN expression and RG7787 uptake via miRNA198.
Effect of DDR1 knockdown on protein synthesis
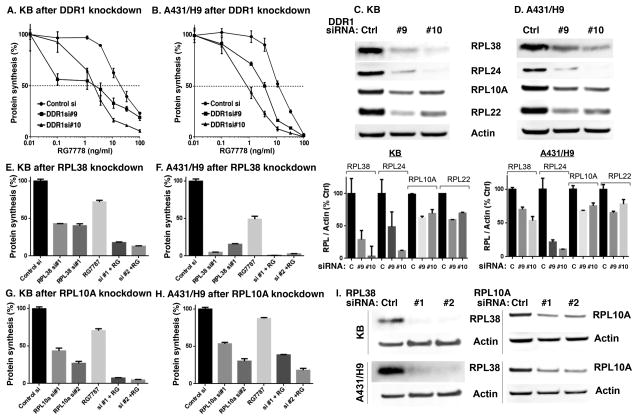
PE-based RITs kill cells by inhibiting protein synthesis (25). Therefore, we examined whether depleting DDR1 enhances toxin-mediated inhibition of protein synthesis, A431/H9 or KB cells were transfected with DDR1 siRNA for 48 hours, treated with RG7787 for 18 hours, and then labeled with 3H-Leucine for 3 hours. In both KB and A431/H9 cells, DDR1 knockdown significantly enhanced protein synthesis inhibition (Fig. 2A–B).
Figure 2.
DDR1 knockdown enhances inhibition of protein synthesis by RG7787 and reduces levels of ribosomal proteins. Forty-eight hours after transfection, A) KB or B) A431/H9 cells were treated with RG7787 for 18 hours and assayed for incorporation of 3H-Leucine to measure the level of protein synthesis. C, D) After DDR1 knockdown, cell lysates were analyzed for the expression of ribosomal proteins by western blot analysis using anti-RPL38, anti-RPL24, anti-RPL10A, and anti-RPL22 antibodies. The relative band intensities were quantified (Multigauge software), normalized to actin levels and represented as percentage of control. E, F) KB or A431/H9 cells were transfected with control siRNA or RPL38 siRNAs or G, H) RPL10A siRNAs for 48 hours then treated with 11ng/ml RG7787 for 18 hours before assaying for protein synthesis by measuring incorporation of 3H-Leucine. I) Knockdown of RPL38 or RPL10A by siRNAs was verified by WB in KB and A431/H9 cells.
We recently reported that knockdown of several ribosomal proteins enhanced the cytotoxicity of SS1P (26). Loss of one or more ribosomal proteins inhibits proper assembly of ribosomes and protein synthesis (27, 28). RPL38, RPL24, and RPL10A were identified amongst the top 5 sensitizers to RIT treatment in a genome knockdown screen (26). To determine if DDR1 knockdown affects these ribosomal proteins, we examined mRNA and protein expression of RPLs after transfection with control or DDR1 siRNA in KB and A431/H9 cells. DDR1 knockdown lowered RPL22 mRNA levels but not the others (Supplementary Fig. S3). However, DDR1 knockdown significantly reduced protein levels of RPL38, RPL24, RPL10A, and RPL22 by 40–90% in KB and A431/H9 cells (Fig. 2C–D). Importantly, silencing of RPL38 or RPL10A alone inhibits protein synthesis and this effect is further enhanced by RG7787 (Fig. 2E–I). RPL24 or RPL22 did not enhance RG7787 activity (data not shown). These results indicate that RPL10A and RPL38 play a specific role in protein synthesis inhibition and toxin activity.
DDR1 overexpression decreased sensitivity of native toxin
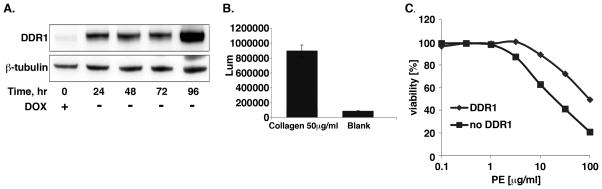
To evaluate the opposite effect (i.e. DDR1 protection from toxin induced cell death) TET-off inducible DDR1b overexpressing fibrosarcoma (HT1080) cells were generated. Cells cultured without doxycycline showed induction of DDR1 protein expression for 24–96 hours relative to cells cultured with doxycycline (Fig. 3A). Increased expression results in a fully functional DDR1b receptor as shown by the collagen stimulated DDR1 phosphorylation (Fig. 3B).
Figure 3.
Induction of DDR1 expression protects cells from killing by the native PE. A) HT1080 cells transfected with inducible DDR1b vector were cultured in medium without doxycycline for indicated times while changing media daily. Cell lysates were analyzed by WB for DDR1 expression. B) Cells were cultured in serum free medium for 24 hours with or without doxycycline for 48 hours before plating into dishes. Plated cells were stimulated with collagen I for 18 hours then DDR1 phosphorylation was measured in the cell lysate by ELISA kit (R&D) according to the manufacturer’s protocol. C) Cells cultured in the presence or absence of doxycycline for 48 hours were treated with varying concentrations of native PE for 48 hours and cell viability assessed by ATP assay. Results are presented as a percentage of the PBS-treated control.
Because HT1080 cells do not express MSLN, we used native PE to examine the effect of enhanced DDR1 expression on toxin-mediated cell death. Cells cultured with and without doxycycline were treated with varying concentrations of native PE and cell viability was assessed. Figure 3C demonstrates that cells with high expression of DDR1 (IC50 100ng/ml) showed nearly 5-fold resistance to killing by PE relative to cells with no DDR1 (IC50 20ng/ml). These data indicate that DDR1 negatively regulates the activity of PE-based toxins and confers protection to cells from PE mediated killing.
Collagen protects cells from immunotoxin mediated cell killing
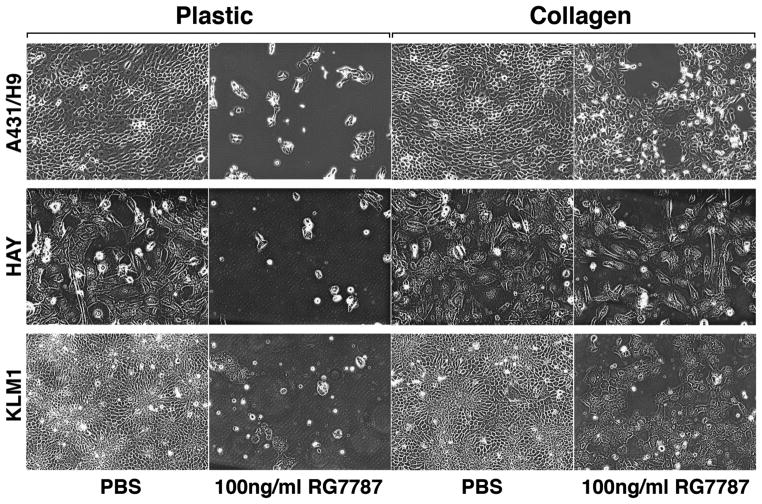
Deposition of collagen, abundant in tumor stroma, has been shown to modulate therapeutic efficacy of drugs (19, 29). DDR1 tyrosine kinase activity is activated by collagen I (16). We hypothesized that collagen will protect cells from killing by immunotoxins. To examine this, A431/H9 cells were plated in culture dishes coated with or without collagen I and allowed to attach overnight. Cells on collagen I-coated dishes were incubated with 25 μg/ml soluble collagen I for 3 hours, followed by treatment with PBS or 100ng/ml RG7787 for 48 hours. The photomicrographs shown in Figure 4 demonstrate that collagen I protects A431/H9, HAY, and KLM1 cells from killing by RG7787. Treatment with RG7787 killed almost all cells on plastic dishes, however, more than 50% cells survived in the presence of collagen I for all cell types (Fig. 4). Because collagen is a ligand for DDR1, we measured DDR1 activation in response to collagen I treatment. Growing A431/H9 and KLM1 cells in the presence of collagen I induced DDR1 phosphorylation (Supplementary Fig. S4A–B). These data suggest that collagen protects cells from RG7787 mediated killing via activation of DDR1 signaling.
Figure 4.
Collagen protects cells from immunotoxin mediated cell killing. A431/H9, HAY, or KLM1 cells were seeded on plastic or collagen I-coated dishes at the same density, allowed to attach for 24 hours, treated with 25 μg/ml soluble collagen I for 3 hours followed by treatment with 100ng/ml RG7787 for 48 hours then images were taken. Images shown are representative of two independent experiments and of replicate fields per treatment.
Combination of DDR1 inhibitor enhanced toxin activity
Multi-kinase inhibitors such as Nilotinib, Dasatinib, and Imatinib have been shown to inhibit DDR1 but are unselective (30). We found that Nilotinib enhanced the cytotoxic activity of RG7787 in A431/H9 and KLM1 cells in a dose-dependent manner (Supplementary Fig. S5A–B). Recently, a more selective inhibitor of DDR1, called 7rh, was reported (22). We found that treatment with 7rh for 3 hours reduced phosphorylation of DDR1 in both KB and A431/H9 cells (Fig. 5A–B). Combining 7rh with RG7787 increased cytotoxicity. KB cells are highly resistant to RG7787 when treated as a single-agent, however, pretreatment with 3μM 7rh resulted in up to a 100-fold enhancement in cell killing (IC50 of >100 vs. 6 ng/ml (Fig. 5C)). Similarly, combination of 7rh and RG7787 resulted in 7 to 20-fold enhancement relative to RG7787 treatment alone in several cell lines (Fig. 5D–F, Table 1). Consistent with these observations protein synthesis was significantly decreased when cells were pretreated with the combination relative to cells treated with either drug alone (Fig. 5G–J). In addition, combination of 7rh with either native PE or HB21-PE40, an immunotoxin targeted to transferrin receptor, also reduced survival of multiple cancer cell lines (Supplementary Fig. S6A–F). These data indicate that the DDR1 inhibitor 7rh significantly enhances cancer cell sensitivity to PE-based immunotoxins.
Figure 5.
DDR1 inhibitor 7rh inhibits DDR1 phosphorylation and enhances cytotoxicity and protein synthesis inhibition by RG7787. A) KB or B) A431/H9 cells were treated with different concentrations of 7rh inhibitor for 3 hours and harvested for western blotting. Blots were probed using anti-phospho DDR1, anti-DDR1 or anti-Actin antibody. C–F) KB, A431/H9, HAY, and KLM1 cells, respectively, were treated with DDR1 inhibitor for 3 hours, followed by varying concentrations of RG7787. After 72 hours, cell viability was assessed by cell titer glow ATP assay. G–J) Protein synthesis was measured by radioactive leucine incorporation after 18 hours of RG7787 treatment. Each point represents a mean of four to six treated wells.
7rh enhanced antitumor activity of RG7787 in A431/H9 and KLM1 xenografts
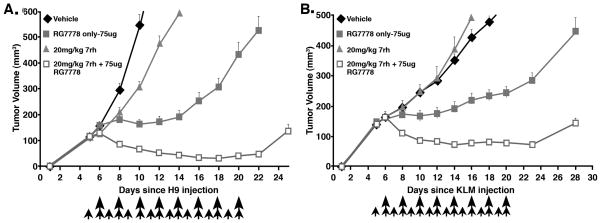
We recently reported that RG7787 inhibits growth of pancreatic, breast, and stomach tumors in mice (12,13), but does not produce complete responses as a single agent. To investigate whether 7rh can enhance the anti-tumor activity of RG7787, A431/H9 cells were implanted subcutaneously in athymic nude mice. After tumors reached an average volume 100–120mm3, mice received a single dose of 10mg/kg 7rh with RG7787 at 7.5mg/kg. Mice treated with this combination showed nearly 30% tumor shrinkage but tumors started to regrow after treatment was stopped. While single agent RG7787 only halted tumor growth briefly (data not shown). To verify target-inhibition by 7rh, we harvested treated A431/H9 tumors and measured DDR1 phosphorylation by SDS-PAGE. The level of DDR1 phosphorylation significantly decreased (p<0.05) in tumors after 7rh kinase inhibitor treatment in a dose-dependent manner (Supplementary Fig. S7). Sustained treatment with 7rh in combination with RG7787 resulted in improved anti-tumor effect. Tumors treated with 7rh alone grew slower than vehicle treated tumors. RG7787 halted tumor growth, but tumors started to regrow after day 12 despite continued treatment (Fig. 6A–B). In contrast, A431/H9 tumors treated with the 7rh/RG7787 combination shrank 34% by day 8 (p<0.0001) and showed continued shrinkage up to 61% by day 22 (Fig. 6A). Tumor volume in vehicle-treated mice reached a threshold of 400mm3 at 10 days compared to an average of 20 days (range 18–22 days) for RG7787. Combination-treated mice took an average of 33 days to reach this threshold (p<0.0001). Similar results were seen with KLM1 pancreatic tumors (Fig. 6B); combination treatment resulted in 89% reduction in tumor volume but RG7787 treatment alone, reduced tumor volume by only 58% (p<0.0001). Combination-treated KLM1 tumors did not reach the 400mm3 threshold volume over the 30-day course of the experiment, while vehicle- and RG7787-treated tumors grew to this size by day 17 and 27, respectively (p<0.0001). Overall, antitumor activity of RG7787 was enhanced by combination treatment relative to either drug alone in both A431/H9 and KLM1 tumor models.
Figure 6.
DDR1 inhibitor 7rh enhances antitumor activity of RG7787 in A431/H9 and pancreatic cancer KLM1 xenograft tumor models. Female athymic nude mice were subcutaneously injected with A) A431/H9 or B) KLM1 tumor cells. When tumors reached 100–120mm3, mice were treated with vehicle, 7rh alone, RG7787 alone, or a combination of 7rh and RG7787. 7rh (20mg/kg) was given daily by oral gavage starting on day 5 (indicated by small arrows) for a total of 16 doses. RG7787 (75 μg) was administered by intravenous tail injection every other day starting on day 6 for a total of eight doses, as indicated by long arrows. Each data point represents average of mean tumor volume of 4–7 mice per group that are followed over time from the day of inoculation. Statistically significant difference in tumor volume was observed between mice treated with RG7787 alone compared to the mice treated with the combination starting from day 8 and this difference was maintained during the course of experiment. Results shown are representative of more than two similar experiments.
These data suggest DDR1 inhibition as an excellent therapeutic strategy to enhance in vivo efficacy of RG7787.
Discussion
DDR1 is a tyrosine kinase that is activated by collagen and is emerging as a regulator of tumor microenvironments in several malignancies (18). RITs are novel anti-cancer drugs designed to kill MSLN-expressing tumors such as lung, pancreatic, TNBC and mesothelioma (1, 12). Our present results show that DDR1 regulates activity of RITs; silencing of DDR1 enhanced killing by RITs whereas induction of DDR1 expression decreased sensitivity of cancer cells to toxin. To our knowledge, this is the first report implicating a matrix-regulating tyrosine kinase like DDR1 in regulation of immunotoxin-based cancer therapy. Moreover, we showed that collagen protects cancer cells from killing by RG7787 and this effect may be mediated by DDR1. Preventing this activation with a novel DDR1 kinase inhibitor, 7rh, significantly enhanced antitumor activity of RG7787 in epithelial and pancreatic tumor models.
The discoidin domain receptor sub-family consists of DDR1 and DDR2. Both are activated by fibrillary collagens, but they differ in their cellular expression pattern and preference for collagen type (17). DDR1 is implicated in cancer progression due to its role in cancer cell survival, migration, and adhesion, however, little is known about the role of DDR2 in cancer, if any. In a kinome RNAi screen, we saw enhancement of RIT activity by DDR1 silencing, and saw no changes in the activity of RITs after knockdown of DDR2. While there is limited knowledge about signaling downstream of DDR1, various studies have shown that overexpression and activation of DDR1 results in activation of the mitogen-activated protein kinase, phosphoinositide 3-kinase, Janus kinase, and NF-κB pathways (31). It has also been reported that DDR1 is a transcriptional target of p53 under genotoxic stress, and that inhibition of DDR1 sensitizes WT p53 expressing cells to apoptosis under these conditions (32). We found that DDR1 inhibition enhanced anti-tumor activity of RIT in both WT (A431/H9) and mutant (KLM1) p53 tumors, indicating inhibition of DDR1 may be an attractive strategy to enhance therapeutic efficacy of RITs.
Consistent with the established pro-survival role of DDR1 in cancers, here we have shown that overexpression of DDR1 protects cells from RITs, while DDR1 silencing or small molecule inhibition enhances killing by RITs. Knockdown or inhibition of DDR1 enhanced inhibition of protein synthesis by RG7787. Together, these data provide strong evidence that DDR1 regulates the toxin intoxication pathway through regulating protein synthesis either directly or indirectly. Consistent with this observation, reduction of DDR1 was associated with decrease in ribosomal proteins, RPL10A, RPL24, and RPL38 that play a pivotal role in ribosome assembly and protein synthesis (25). Silencing of RPL10A and RPL38 but not RPL22 and RPL24 inhibited protein synthesis. This selective role of RPL38 is consistent with previous reports illustrating translation of specific mRNAs of pro-survival genes by RPL38 (33). The mechanism of how DDR1 regulates expression of ribosomal proteins is beyond the scope of this manuscript and is currently under investigation. Nonetheless, these data strongly suggest a novel role of DDR1 in modulating protein synthesis via ribosomal proteins.
Collagen is the most abundant component of the ECM and is thought to play a role in cancer progression and resistance to therapy via aberrant signaling. In particular, pancreatic cancer is known to have a collagen-dense stroma (19) and is resistant to most standard therapeutics. Recently, Aguilera et al. have shown that resistance to VEGF therapy in an animal model of pancreatic cancer is due to collagen mediated DDR1 activation (19). Cader et al. also reported collagen/DDR1-mediated protection of Hodgkin lymphoma cells from chemotherapy (29). It has been reported that DDR1 causes chemo-resistance by activating NF-κB pathway and its target Cox2 (31). This is the first report that collagen protects cancer cells from killing by a RIT and that this protection can be overcome by combining RIT with the DDR1 inhibitor, 7rh. We found a significant anti-tumor response with the combination of 7rh and RG7787 in xenograft mouse models of epithelial and pancreatic cancer. The maximum tolerated single dose for RG7787 in mice is 150 μg. We were able to achieve a therapeutic effect at half the maximal tolerated dose. Repeated dosing is needed for maximal effect in mice. Because humans metabolize the drugs more slowly than mice, it usually requires about 10% as much drug to be effective in humans. For example, for HA22 the effective dose in humans is 50 μg/kilo whereas in mice it is 400 μg/kilo. So 75 μg of RG7787/20 grams would translate into 3.75 mg/kilo in mice or 375 kg/kilo in humans. RG7787 is now in clinical testing but a safe dose has not yet been established. DDR1 inhibitor 7rh is under preclinical testing and newer inhibitors are being developed. In addition, Nilotinib inhibits DDR1 and is approved for the treatment of several malignancies (30). We found that combining Nilotinib with RG7787 significantly enhanced cytotoxic activity in epithelial and pancreatic cancer cells. Nilotinib also enhanced activity of SS1P (David Fitzgerald; personal communication). Therefore, it has the potential to be used in combination with immunotoxins in clinical trials.
We propose that resistance to killing of cancer cells by RG7787 in the presence of collagen is mediated by DDR1. In addition to DDRs, integrins are also activated by collagen and thought to play a role in ECM remodeling. While it has been reported that collagen mediated DDR1 activation does not require integrins (34), DDR1 induction was shown to cooperatively activate β1 integrins to mediate cell adhesion (35). Knockdown of integrins did not alter RIT activity (26) therefore; it is likely that protection of cells by collagen is primarily mediated by DDR1. Our findings indicate that DDR1 inhibition may be a good strategy to inhibit collagen-mediated resistance to therapeutics. Our in vivo data provide strong support for this.
In summary, this study presents a clinically relevant finding that collagen protects cancer cells from killing by RITs by activating DDR1, and inhibition of DDR1 enhances killing of many cancer cell types by RITs. Our data support the use of DDR1 inhibitors in combination with RITs to enhance their clinical efficacy.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, in part by the Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Cancer Institute, Center for Cancer Research, and in part with a Cooperative Research and Development Agreement (#2791) with Roche Pharmaceuticals. We thank Drs. Xui-Fen Liu and Christine Alewine for helpful discussion and critically reviewing the manuscript.
Footnotes
Disclosure of Potential Conflict of Interest
I. Pastan and D. FitzGerald are inventors on immunotoxin patents that have all been assigned to NIH. The authors declare no conflict of interest.
References
- 1.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 2.Alewine C, Xiang L, Yamori T, Niederfellner G, Bosslet K, Pastan I. Efficacy of RG7787, a next generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13:2653–61. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argani P, Iacobuzioi-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 4.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–5. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RJ, Pastan I, Cowan ML, Mongtomgery E, Hassan R, Alewine CC, et al. Mesothelin expression is a novel target in gastric cancer. J Clin Oncol. 2014;32(3s) suppl:abstr 61. [Google Scholar]
- 7.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–7. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 8.Tchou J, Wang LC, Selven B, Zhang H, Conejo-Garcia J, Borghaei H, et al. Mesothelin a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat. 2012;133:799–804. doi: 10.1007/s10549-012-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Williamham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 10.Hassan R, Sharon E, Thomas A, Zhang J, Ling A, Miettinen M, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer. 2014;120:3311–9. doi: 10.1002/cncr.28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederfellner G, Bauss F, Imhof-Jung S, Hesse F, Kronenberg S, Staak R, et al. RG7787-a novel de-immunized PE based fusion protein for therapy of mesothelin-positive solid tumors. Cancer Res. 2014;74(suppl):19. abstr 4510. [Google Scholar]
- 12.Alewine C, Xiang L, Yamori T, Niederfellner G, Bosslet K, Pastan I. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13:2653–61. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollevoet K, Mason-Osann E, Liu XF, Imhof-Jung S, Niederfellner G, Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin rg7787 in pancreatic cancer. Mol Cancer Ther. 2014;13:2040–9. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XF, FitzGerald DJ, Pastan I. The insulin receptor negatively regulates the action of Pseudomonas toxin-based immunotoxins and native Pseudomonas toxin. Cancer Res. 2013;73:2281–8. doi: 10.1158/0008-5472.CAN-12-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XF, Xiang L, FitzGerald DJ, Pastan I. Antitumor effects of immunotoxins are enhanced by lowering HCK or treatment with SRC kinase inhibitors. Mol Cancer Ther. 2014;13:82–9. doi: 10.1158/1535-7163.MCT-13-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 17.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilera KY, Rivera LB, Hur H, Carbon JG, Toombs JE, Goldstein CD, et al. Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1032–44. doi: 10.1158/0008-5472.CAN-13-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–20. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhary VK, Jinno Y, Gallo MG, FitzGerald D, Pastan I. Mutagenesis of Pseudomonas exotoxin in identification of sequences responsible for the animal toxicity. J Biol Chem. 1990;265:16306–10. [PubMed] [Google Scholar]
- 22.Gao M, Duan L, Luo J, Zhang L, Lu X, Zhang Y, et al. Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem. 2013;56:3281–95. doi: 10.1021/jm301824k. [DOI] [PubMed] [Google Scholar]
- 23.König R, Chiang CY, Tu BP, Yam SF, DeJesus PD, Romero A, et al. A probability-based approach for the analysis of large-scale RNAi screens. Nat Methods. 2007;4:847–9. doi: 10.1038/nmeth1089. [DOI] [PubMed] [Google Scholar]
- 24.Marin-Muller C, Li D, Bharadqaj U, Li M, Chen C, Hodges SE, et al. A tumorigenic factor interactome connected through tumor suppressor microRNA-198 in human pancreatic cancer. Clin Cancer Res. 2013;19:5901–13. doi: 10.1158/1078-0432.CCR-12-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weldon JE, Pastan I. A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasetto M, Antignani A, Ormanoglu P, Buehler E, Guha R, Pastan I, et al. Whole-genome RNAi screen highlights components of the endoplasmic reticulum/Golgi as a source of resistance to immunotoxin-mediated cytotoxicity. Proc Natl Acad Sci USA. 2015;112:E1135–42. doi: 10.1073/pnas.1501958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenmochi N, Kawaguchi T, Rozen S, Davis E, Goodman N, Hudson TJ, et al. A map of 75 human ribosomal protein genes. Genome Res. 1998;8:509–23. doi: 10.1101/gr.8.5.509. [DOI] [PubMed] [Google Scholar]
- 28.Buszczak M, Signer RA, Morrison SJ. Cellular differences in protein synthesis regulate tissue homeostasis. Cell. 2014;159:242–51. doi: 10.1016/j.cell.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cader FZ, Vockerodt M, Bose S, Nagy E, Brundler MA, Kearns P, et al. The EBV oncogene LMP1 protects lymphoma cells from cell death through the collagen-mediated activation of DDR1. Blood. 2013;122:4237–45. doi: 10.1182/blood-2013-04-499004. [DOI] [PubMed] [Google Scholar]
- 30.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–63. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 31.Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-κB pathway activation. Cancer Res. 2006;66:8123–8130. doi: 10.1158/0008-5472.CAN-06-1215. [DOI] [PubMed] [Google Scholar]
- 32.Han JA, Kim JI, Ongusaha PP, Hwang DH, Ballou LR, Mahale A, et al. p53-mediated induction of Cox-2 counteracts p53- or genotoxic stress-induced apoptosis. EMBO J. 2002;21:5635–44. doi: 10.1093/emboj/cdf591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsiech AC, Xue S, et al. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145:383–97. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel W, Brakebusch C, Fässler R, Alves F, Ruggiero F, Pawson T. Discoidin domain receptor 1 is activated independently of β1 integrin. J Biol Chem. 2000;275:5779–84. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Bihan D, Chang F, Huang PH, Farmdale RW, Leitinger B. Discoidin domain receptors promote α1β1- and α2β1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS One. 2012;7:e52209. doi: 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.