Abstract
Objective
We examined the emergence of chemotherapy-induced peripheral neuropathy (CIPN), a dose-limiting toxicity of oxaliplatin, over the course of oxaliplatin-based chemotherapy for colorectal cancer (CRC). Predicting which patients will likely develop CIPN is an ongoing clinical challenge.
Methods
Oxaliplatin-naïve patients with CRC underwent quantitative sensory testing (QST) before oxaliplatin-based chemotherapy and then rated CIPN-related symptoms via the MD Anderson Symptom Inventory (MDASI) weekly for 26 weeks. Mixed modeling examined the value of QST for predicting higher CIPN (MDASI numbness/tingling) during treatment. Trajectory analysis identified a patient subgroup with consistently higher CIPN symptoms.
Results
Numbness/tingling was the most-frequent, most-severe symptom, with 51% of patients clustering into a high-CIPN subgroup. Touch-sensation deficits (Bumps Detection test) significantly predicted the development of more-severe numbness/tingling (est=0.106, P=0.0003). The high-CIPN subgroup reported increased pain (est=0.472, P<0.0001) and interference with walking (est=0.840, P<0.0001). In the high-CIPN subgroup, patient-reported numbness/tingling worsened rapidly in weeks 0–5 (est=0.57, P<0.0001) and then more gradually in weeks 6–26 (est=0.07, P<0.0001).
Conclusion
Prechemotherapy screening with a simple, easily administered objective measure of touch-sensation deficits (Bumps Detection test) and monitoring of patient-reported numbness/tingling during the first 2–3 chemotherapy cycles may support improved personalized care of CRC patients with oxaliplatin-induced CIPN.
Keywords: numbness, chemotherapy-induced peripheral neuropathy (CIPN), symptom burden, oxaliplatin, quantitative sensory testing
INTRODUCTION
Colorectal cancer (CRC) is the third-most-common cancer and the third-leading cause of cancer-related death in the United States [1]. Oxaliplatin, in combination with 5-fluorouracil, leucovorin, capecitabine, and/or irinotecan, is a common chemotherapy regimen for CRC that confers significant clinical benefit in terms of progression-free survival and response rates [2–4]; however, oxaliplatin is also neurotoxic [5].
The most common toxicity associated with oxaliplatin treatment is chemotherapy-induced peripheral neuropathy (CIPN), which manifests in sensory changes and difficulty with balance and walking. The pathogenesis of CIPN is unclear, and reports of both the incidence and severity of CIPN are inconsistent in the literature. Clinically, only a proportion of chemotherapy-treated patients are expected to develop persistent CIPN, and predicting which patients undergoing a particular treatment are at higher risk for developing CIPN has been difficult [6].
The sensory changes that are hallmarks of CIPN, such as numbness and tingling, commonly start in the fingertips and toes and advance proximally in a “stocking and glove” manner with continued exposure to treatment, suggesting that primary afferent neurons are the most vulnerable to the effects of chemotherapy [7–9]. CIPN may or may not be accompanied by pain and can range from acute but transient numbness to a dose-limiting peripheral neuropathy that occurs with cumulative oxaliplatin exposure (up to 780–850 mg/m2) [10]. As a result, CIPN causes distress, can affect both daily functioning and treatment adherence [11], and often persists into survivorship for a significant proportion of patients [12]. Preventing and treating CIPN is therefore particularly important for optimizing patient outcomes. However, effective personalized management or prevention strategies for CIPN are lacking [13].
Pfeiffer et al [14] found that patients place greater importance on the toxicity profile of a treatment than on other clinical factors. Even though studies based on subjective patient-reported outcomes (PROs) provide more sensitive and reliable information about the patient’s experience during treatment than clinician-recorded data can do, current practice continues to rely largely on physician toxicity reports based on the US National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) [12;15]. PRO-based symptom profiles for CIPN have not been well characterized with quantitative, longitudinal data on the development of neuropathy in patients with CRC.
Various objective measures of sensory–functional deficits have been used to assess CIPN in patients with cancer. Quantitative sensory testing (QST) is a psychophysical battery of tests that evaluate various aspects of somatic sensory functioning, including skin temperature, sensorimotor function, sharpness detection, thermal detection, and touch detection. Although the complete battery of QST measures provides information on the mechanisms of neuropathy, it is time consuming and requires several specialized pieces of equipment and associated staff training [16–21]. Consequently, these tests are not routinely conducted in clinical practice and can result in much random missing data in longitudinal clinical studies that require repeated measurements.
The relationship between disease-driven sensory functional deficits and the development of symptom burden is unknown. We therefore conducted a study with both baseline objective and longitudinal subjective measures of CIPN-related symptom burden to portray the emergence of CIPN symptoms over the course of oxaliplatin-based chemotherapy for CRC. Using patient-reported ratings of CIPN symptoms, we characterized the prevalence and developmental trajectory of these symptoms over time. We hypothesized, on the basis of previous research by our group [22], that prechemotherapy evidence of peripheral nerve deficits would predict the emergence of CIPN symptoms during oxaliplatin-based chemotherapy and the persistence of symptoms posttreatment.
MATERIALS AND METHODS
Patients
Patients were consecutively recruited between 2008 and 2012 from the GI Medical Oncology clinic at The University of Texas MD Anderson Cancer Center in Houston, Texas, USA. Eligible patients had a confirmed pathological diagnosis of colorectal cancer, were at least 18 years old, were oxaliplatin naive, had no record of preexisting neuropathy (physician-rated NCI-CTCAE toxicity score = 0), and were scheduled for oxaliplatin-based chemotherapy. The study was approved by the MD Anderson Institutional Review Board and is registered at www.clinicaltrials.gov (trial registration ID: NCT00777192). All participants provided written informed consent.
Patient characteristics and clinical parameters (eg, age, sex, marital status, employment, and education status, cancer stage, Eastern Cooperative Oncology Group performance status, body mass index [BMI], comorbidities [including diabetes history], previous cancer therapy, total chemotherapy dose, and opioid use) were recorded during the study.
Quantitative sensory testing
Patients underwent QST prior to beginning oxaliplatin chemotherapy. To encompass areas of skin that are typically affected in chronic CIPN, we collected QST data at three sites for each participant: the tip of the index finger, the thenar eminence, and the volar surface of the forearm.
Among the QST measures used in this study were a computer-controlled Peltier device for assessing warm/cool detection thresholds and hot/cold pain thresholds in the Marstock method [23], the Grooved Pegboard for assessing sensorimotor functioning and manual dexterity [22;24;25], and von Frey filaments [26] and the new, brief Bumps Detection test [22;27] for assessing touch sensation. In the Bumps Detection test, patients are tasked with determining the location of a 5–25 µm raised cylinder that is obscured over one of five colored dots on a smooth glass panel.
To capture change in CIPN over time as the oxaliplatin dose accumulated, a qualitative assessment was performed at baseline and again after four cycles of chemotherapy. Patients answered yes-or-no questions to describe 20 specific characteristics of their neuropathy-related symptoms (for example, sharp, burning, throbbing, and so on).
Symptom assessment
Patients rated multiple symptoms via the MD Anderson Symptom Inventory (MDASI) at baseline (before beginning oxaliplatin chemotherapy), weekly during chemotherapy, and every 2 weeks posttreatment for up to 26 weeks. The MDASI assesses the severity of common cancer-related symptoms and related interference with functioning over the previous 24 hours, rated on a 0 (none) to 10 (worst possible) scale. The MDASI includes 13 core symptom items (including numbness/tingling and pain) and six interference items (general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life) [28;29]. We focused on patient ratings of numbness/tingling and pain to track the emergence of neuropathy [30], and we used the MDASI numbness/tingling item specifically to represent CIPN. MDASI interference with walking was used to represent neuropathy-related functional impairment.
Statistical analysis
Descriptive analyses (means, standard deviations, standard errors, medians, range, percentages) were used to present patient characteristics and MDASI symptom ratings over time.
To investigate the prevalence of high CIPN-related symptom burden over time, we used group-based trajectory modeling [31;32] to distinguish patients persistently having more-severe vs less-severe CIPN-related symptoms (numbness/tingling) during the first 26 weeks of therapy. Dividing patients into two subgroups with fixed membership (either high or low symptom outcomes) is based on the clinical need to identify patients with high symptom burden [32]. We used ≥4 on the MDASI’s 0–10 scale as the cut point indicating a moderate to severe rating [33;34].
Data from patients who contributed both QST and MDASI data prior to beginning oxaliplatin-based chemotherapy were analyzed to identify risk factors for high-CIPN symptom burden. Linear mixed-effect modeling, with a random effect for patient and autoregressive variance structure, was applied for each baseline QST measure, controlled for other confounders such as age, sex, Eastern Cooperative Oncology Group performance status, BMI, comorbidities, and opioid use. QST measures that were significantly predictive of increase in numbness/tingling over the 26 weeks of the study were then included in a final logistic regression model, controlled for the same covariates.
We used nonlinear mixed-effect models to describe separately the longitudinal profiles of CIPN-related symptoms (numbness/tingling) in the high-CIPN and low-CIPN subgroups. The group-based trajectory analysis showed quadratic change in CIPN symptoms over time. Thus, for each nonlinear mixed-effect model, we fitted two adjoining lines with different slopes and estimated the point (weeks from treatment start) at which the two lines would meet.
The effect of numbness/tingling on the development of pain and functional impairment (specifically, MDASI interference with walking) during chemotherapy was examined by mixed-effect models, including a random effect for patient with autoregressive variance structure, controlled for age, sex, BMI, comorbidities, and baseline opioid use.
All statistical analysis was done with SAS 9.1 for Windows (SAS Institute, Cary, NC). Statistical significance was set at P<0.05.
RESULTS
Patient characteristics
One hundred patients were enrolled in this prospective study and contributed longitudinal MDASI symptom data; of these, 60 patients contributed baseline QST data. Table 1 presents demographic and clinical characteristics of the sample. We observed no significant difference for any factor between patients who contributed QST data and those who did not. All patients received standard oxaliplatin-based treatment, with a mean dose of 85 mg/m2 administered biweekly. The mean (standard deviation) accumulated oxaliplatin dose at week 26 was 737.37 mg/m2 (313.06 mg/m2), with a median (range) of 683.50 mg/m2 (184.00–1,276.00 mg/m2).
Table 1.
Patient demographics
| Total | Patients with QST | Patients without QST | Pa | ||||
|---|---|---|---|---|---|---|---|
| (N = 100) | (n = 60) | (n = 40) | |||||
| Summary statistics | |||||||
| Age (years) | |||||||
| Mean (SD) | 56.00 (12.35) | 56.03 (12.66) | 55.97 (12.04) | 0.981 | |||
| Median (range) | 57.20 (19.55–82.67) | 57.61 (19.55–82.67) | 56.99 (27.12–81.82) | ||||
| BMI | |||||||
| Mean (SD) | 27.99 (6.03) | 28.57 (5.96) | 27.12 (6.12) | 0.243 | |||
| Median (range) | 26.94 (14.50–46.81) | 27.06 (19.91–46.81) | 26.63 (14.50–42.89) | ||||
| n | % | n | % | n | % | ||
| Age (years) | |||||||
| <60 | 63 | 63 | 38 | 63.3 | 25 | 62.5 | 0.496 |
| ≥60 | 37 | 37 | 22 | 36.7 | 15 | 37.5 | |
| Sex | |||||||
| Male | 64 | 64 | 40 | 66.7 | 24 | 60 | 0.735 |
| Female | 36 | 36 | 20 | 33.3 | 16 | 40 | |
| Race | |||||||
| Non-Hispanic white | 63 | 63 | 38 | 63.3 | 25 | 62.5 | 0.933 |
| Other | 37 | 37 | 22 | 36.7 | 15 | 37.5 | |
| Education | |||||||
| High school or less | 49 | 49 | 31 | 51.7 | 18 | 45 | 0.513 |
| College or higher | 51 | 51 | 29 | 48.3 | 22 | 55 | |
| Baseline ECOG PS | |||||||
| 0 | 70 | 70 | 42 | 70 | 28 | 70 | 0.607 |
| 1 | 25 | 25 | 14 | 23.3 | 11 | 27.5 | |
| 2 | 5 | 5 | 4 | 6.7 | 1 | 2.5 | |
| Cancer stage | |||||||
| II | 10 | 10 | 6 | 10 | 4 | 10 | 0.558 |
| III | 37 | 37 | 20 | 33.3 | 17 | 42.5 | |
| IV | 52 | 52 | 34 | 56.7 | 18 | 45 | |
| Comorbid conditions, n | |||||||
| 0 | 78 | 78 | 48 | 80 | 30 | 75 | 0.554 |
| ≥1 | 22 | 22 | 12 | 20 | 10 | 25 | |
| Previous therapy | |||||||
| Yes | 79 | 79 | 47 | 78.3 | 32 | 80 | 0.841 |
| No | 21 | 21 | 13 | 21.7 | 8 | 20 | |
| Previous radiation | |||||||
| Yes | 8 | 8 | 4 | 6.7 | 4 | 10 | 0.547 |
| No | 92 | 92 | 56 | 93.3 | 36 | 90 | |
| Previous surgery | |||||||
| Yes | 79 | 79 | 47 | 78.3 | 32 | 80 | 0.841 |
| No | 21 | 21 | 13 | 21.7 | 8 | 20 | |
| Previous non–platin-based chemotherapy | |||||||
| Yes | 10 | 10 | 4 | 6.7 | 6 | 15 | 0.174 |
| No | 90 | 90 | 56 | 93.3 | 34 | 85 | |
| Baseline opioid use | |||||||
| Yes | 28 | 28 | 16 | 26.7 | 12 | 30 | 0.716 |
| No | 72 | 72 | 44 | 73.3 | 28 | 70 | |
Abbreviations: BMI = body mass index; ECOG PS = Eastern Cooperative Oncology Group performance status; SD = standard deviation.
Comparison between patients with and without QST. We conducted t tests for continuous variables and the Fisher exact test for categorized variables.
Prevalence and characteristics of high CIPN symptom burden
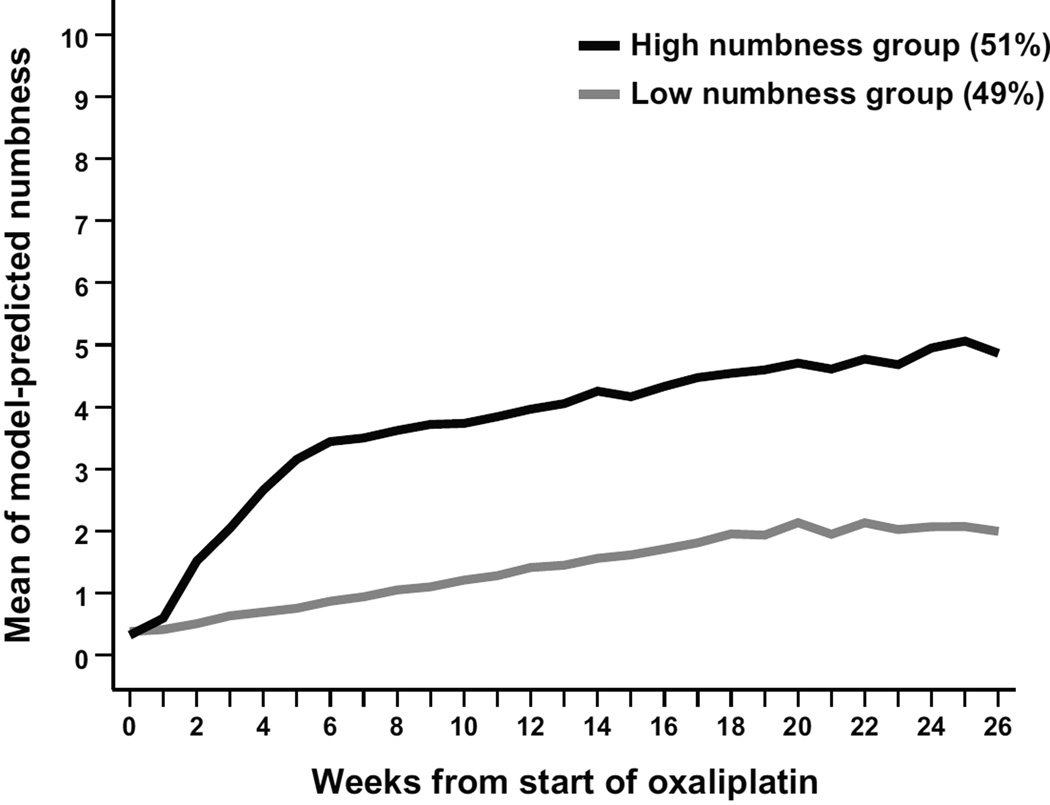
By trajectory analysis, 51% of patients (51/100) belonged to the high-CIPN subgroup, with most reporting moderate to severe (≥4 on the MDASI’s 0–10 scale) numbness/tingling after 12 weeks of oxaliplatin-based treatment. We observed no significant impact from disease (cancer stage IV vs. II or III) on baseline numbness/tingling ratings. By the end of week 26, 48% of the sample reported moderate to severe MDASI numbness/tingling. Figure 1 presents the group-based trajectory modeling analysis of the severity of numbness/tingling (N = 100).
Figure 1.
Trajectory of the MDASI item numbness/tingling in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (N=100)
Abbreviation: MDASI, MD Anderson Symptom Inventory.
For the qualitative CIPN symptom assessment, patients (n = 60) reported on 20 possible characteristics of the CIPN they were experiencing. Few patients reported any symptom at baseline; after four cycles of chemotherapy, the most frequently reported descriptors were numb, tingling, and cold. In terms of pain quality, significantly more patients in the high-CIPN subgroup reported cold pain (45% vs 19%, P=0.042).
Risk factors for development of CIPN-related symptom burden
All patients entered the study with no reported neuropathy (baseline physician-rated NCI-CTCAE score = 0). There were no significant differences in baseline QST scores between the high-CIPN vs low-CIPN subgroups (n = 60). However, adjusted for covariance, the univariate analysis indicated that several baseline QST measures were significantly predictive of increase in numbness/tingling over the 26 weeks, including the Bumps Detection test (P=0.0003), dominant-hand Grooved Pegboard test (P=0.0163), nondominant-hand Grooved Pegboard test (P=0.0005), and touch-detection thresholds at the painful area (P=0.0162), the border area (P=0.0003), and the volar area (P=0.0003). The multivariate analysis confirmed the significant predictive role of Bumps Detection test (P=0.002) and the nondominant-hand Grooved Pegboard test (P=0.002), controlling for the same covariates. Female sex, Eastern Cooperative Oncology Group performance status ≥1, BMI, and baseline opioid use showed significant association with moresevere numbness over time. See Table 2.
Table 2.
Predictive value of baseline QST on development of CIPN symptoms: multivariate analysis (n = 60, 704 observations)
| Effect | Estimate | Standard Error | Pr > |t| |
|---|---|---|---|
| Touch detection | |||
| Bumps Detection test | 0.109 | 0.035 | 0.002 |
| von Frey filament | |||
| Painful area | 0.218 | 0.333 | 0.513 |
| Border area | −0.250 | 0.476 | 0.600 |
| Volar area | 0.221 | 0.462 | 0.633 |
| Sensorimotor function | |||
| Grooved Pegboard test | |||
| Dominant hand | −0.012 | 0.008 | 0.136 |
| Nondominant hand | 0.024 | 0.008 | 0.002 |
| Other variables | |||
| Female vs. male | 0.956 | 0.241 | <0.0001 |
| ECOG PS (1–2 vs. 0) | 0.607 | 0.208 | 0.004 |
| BMI | 0.038 | 0.016 | 0.020 |
| Opioid use (yes vs. no) | 0.567 | 0.184 | 0.002 |
Abbreviations: BMI = body mass index; CIPN = chemotherapy-induced peripheral neuropathy; ECOG PS = Eastern Cooperative Oncology Group performance status; QST = quantitative sensory testing. Multivariate mixed model, with all other factors controlled.
Longitudinal profiles and the impact of CIPN-related symptom burden
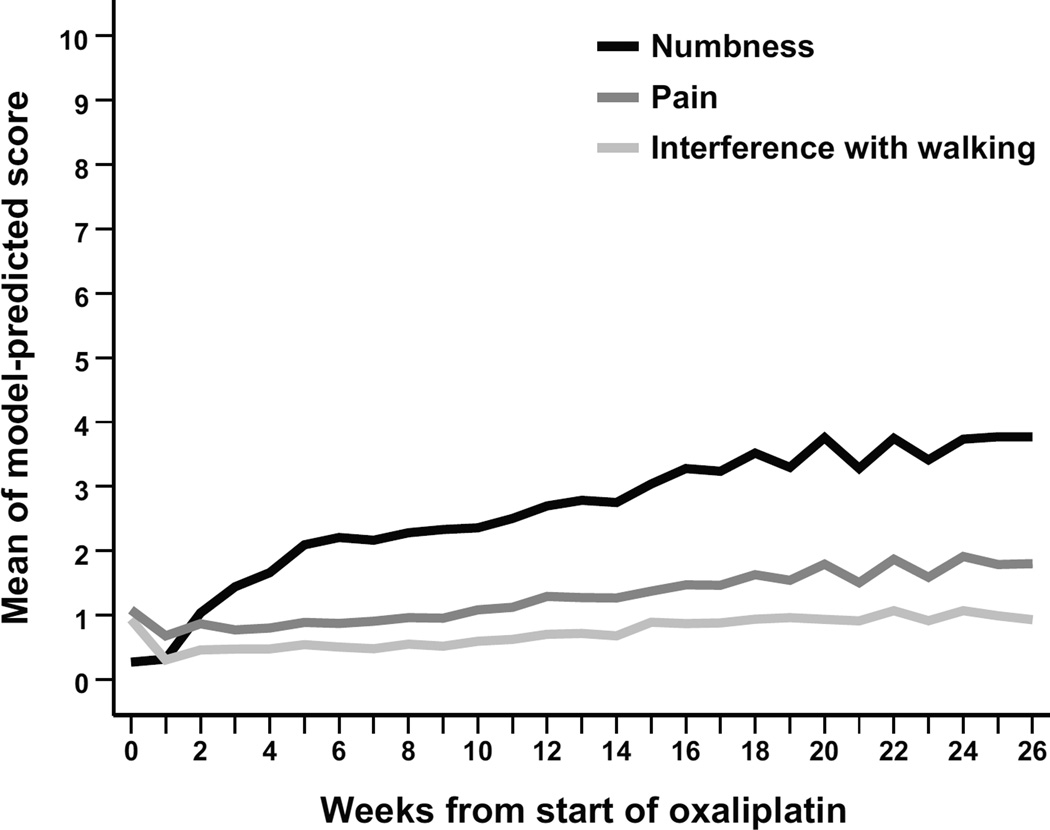
Figure 2 presents the curves of estimated means of numbness, pain, and interference with walking for all patients during the 26-week study (N = 100). As the dose of oxaliplatin-based chemotherapy accumulated over time, numbness/tingling quickly became the most severe symptom reported on the MDASI, followed by fatigue.
Figure 2.
Estimated means of MDASI symptom severity for numbness/tingling, pain, and interference with walking during 26 weeks of oxaliplatin-based chemotherapy (N=100)
Abbreviation: MDASI, MD Anderson Symptom Inventory.
Mixed modeling showed a rapid increase in numbness/tingling in the first 5 weeks of chemotherapy for the high-CIPN subgroup (est = 0.57, P<0.0001), then less rapidly but significantly in weeks 6–26 (est = 0.07, P<0.0001). In contrast, the low-CIPN subgroup had a slower increase in numbness/tingling in weeks 0–19 (est = 0.08, P<0.0001) that levelled off without further significant change during weeks 20–26 (est = 0.02, P=0.731).
Table 3 presents the impact of high CIPN on symptom burden. Mixed modeling indicated that patients in the high-CIPN subgroup were more likely to have increased pain severity during the study period (est = 0.507, P<0.0001). Other factors significantly related to higher pain score were age <60 years (P<0.0001) and baseline opioid use (P<0.0001). Nonetheless, pain was not especially prevalent, with only 5% reporting moderate to severe pain by week 26.
Table 3.
Impact of numbness/tingling on pain and interference with walking: mixed modelling (N = 100)
| Effect | Estimate | Standard Error | Pr > |t| |
|---|---|---|---|
| MDASI pain (1,540 observations) | |||
| Week | 0.013 | 0.004 | 0.002 |
| Age (<60 vs ≥60) | −0.340 | 0.071 | <0.0001 |
| Numbness (high-CIPN vs low-CIPN subgroup) | 0.507 | 0.068 | <0.0001 |
| Baseline opioid use | 0.649 | 0.074 | <0.0001 |
| MDASI interference with walking (1,538 observations) | |||
| Week | 0.0.036 | 0.006 | <0.0001 |
| Numbness (high-CIPN vs low-CIPN subgroup) | 0.795 | 0.090 | <0.0001 |
| Other variables | |||
| BMI | 0.016 | 0.007 | 0.022 |
| ECOG PS (1–2 vs. 0) | 0.239 | 0.096 | 0.022 |
| Comorbidity (yes vs. no) | 0.219 | 0.106 | 0.039 |
| Baseline opioid use | 0.538 | 0.098 | <0.0001 |
Abbreviations: BMI = body mass index; CIPN = chemotherapy-induced peripheral neuropathy; ECOG PS = Eastern Cooperative Oncology Group performance status; MDASI = MD Anderson Symptom Inventory.
Moderate to severe interference with walking was reported by 17% of patients by week 26. Membership in the high-CIPN subgroup was significantly related to the total interference score (P<0.0001), and notably to interference with walking (est = 0.795, P<0.0001) (Table 3). The magnitude of impact from the severity of numbness was larger for the physical activity interference items (general activity, walking, work; est = 1.020) than for the affective interference items (mood, enjoyment of life, relations with others; est = 0.560).
DISCUSSION
To our knowledge, this study is the first to report that baseline deficits in sensory functioning measured using the Bumps Detection test, a simple objective assessment, were predictive of increasing numbness/tingling during the first 26 weeks of oxaliplatin-based chemotherapy. We also demonstrated that more than one-half of patients with CRC (51%) reported significantly increased numbness/tingling over time; the patients in this high-CIPN subgroup experienced a rapid increase in numbness/tingling in weeks 0–5 and then a slower, but still significant, increase in the following weeks. Membership in the high-CIPN subgroup was more likely to be related to increased pain during the 26-week study, and CIPN often interfered with physical functioning, especially walking. Together, both objective measurement and corresponding subjective patient report provided evidence for identifying high risk for CIPN symptom burden, which has important implications for improving personalized management for patients who receive oxaliplatin as treatment for CRC.
A previous study reported on the use of objective QST assessment of motor-nerve excitability to establish CIPN status [35], and preexisting neuropathy was shown to be a risk indicator for possible high symptom burden for patients receiving oxaliplatin treatment [17;22]. In concert with those results, the current study demonstrates the predictive value of critical components of QST when sensory deficits exist before chemotherapy for identifying individuals with higher risk for self-reported CIPN symptom burden. Our results also extend the knowledge about the contribution of disease plus chemotherapy on CIPN-related PROs [17;22].
Comprehensive QST testing requires considerable staff and patient time (at least 45 minutes), along with specialized training and specialized equipment in the clinic. In contrast, the Bumps Detection test is relatively inexpensive and convenient to administer (requiring approximately 5 minutes) and is easy to score, making it a friendly, feasible test for inclusion in pretreatment CIPN risk assessment. The agreement among results from both conventional (von Frey monofilaments) and novel (Bumps Detection test) QST methods for assessing touch detection [27] and MDASI numbness/tingling ratings over time confirmed the potential utility of the Bumps Detection test as a standalone QST measure. The Grooved Pegboard test, a sensory-motor function measure, also was predictive of CIPN symptom development in the univariate analysis. However, results from the Grooved Pegboard should be interpreted with caution due to variations in motor functioning that could be caused by existing cognitive impairment, reported to be three to five times higher in patients with CRC than in healthy controls [36;37].
Our results also confirm clinical observations that not all patients with CRC receiving a similar dose of oxaliplatin develop high CIPN symptoms. Neurotoxicity is a well-known adverse effect of oxaliplatin, reported primarily via clinician toxicity ratings or patient yes/no responses about the presence of CIPN symptoms [38]. Limited published studies have presented longitudinal PRO-based quantitative data about the duration and dynamic developmental trend of neurotoxicity symptoms and their functional impact [39]. In the qualitative portion of our study, “numb” and “tingling” were the most-often-reported characteristics from among 20 possible CIPN symptom descriptors; this finding allowed us to use MDASI numbness/tingling as a PRO measure of CIPN in the quantitative study [40]. Interestingly, we found no significant impact on baseline (prechemotherapy) CIPN symptoms by disease stage, nor did we observe an impact on high-CIPN versus low-CIPN subgroup membership from cumulative chemotherapy dose. A possible reason for this is that, even though more patients with stage IV CRC had received prior therapy, all patients in this study were oxaliplatin naive.
The combination of frequent PRO data collection via a psychometrically validated tool (the MDASI), trajectory analysis of longitudinal data, and methods for interpreting relevant critical items (numbness/tingling, pain, and interference with walking) make the current study unique. Observation of a rapid increase in numbness/tingling in the first 2–3 cycles of oxaliplatin-based chemotherapy in the high-CIPN symptom subgroup may be early-enough information to enable personalized oncology care in terms of chemotherapy dose adjustments, symptom management, and even symptom prevention.
Previous studies described the negative impact of CIPN on quality of life and physical and emotional functioning [10–12], but few patient-reported data are available on the dynamic trajectory of oxaliplatin-induced, CIPN-related interference with usual activities. Results from the current study support the need for better patient care with more-effective strategies to prevent and control CIPN than are presently offered. In addition, our study found that even though significant neuropathic pain was prevalent in only a small proportion of patients, half of patients would ultimately develop numbness/tingling.
The study had several limitations. First, the neuropathy was not verified clinically by a neurologist and we did not have clinician-rated NCI-CTCAE neuropathy scores, especially grade 0 or 1 neuropathy. Second, we excluded patients with a history of neuropathy. Further study is needed to confirm the value of using the Bumps Detection test in patients with existing neuropathy before they begin oxaliplatin-based therapy. Third, because QST is time consuming and difficult to schedule, we collected prechemotherapy QST data from only 60% of the sample (n = 60/100). Fourth, we only enrolled patients receiving oxaliplatin-based therapeutic regimens. It would be useful to monitor the longitudinal development of CIPN and the predictive utility of the Bumps Detection test in relation to other agents known to produce peripheral neuropathy, including other platinum compounds, the taxanes, and bortezomib. This knowledge could be useful for decision making about whether or not to exclude, replace, or stop treatment with these neuropathy-inducing agents, which can sometimes be permitted in an adjuvant setting without diminishment of treatment efficacy. Finally, a study longer than 6 months for tracking symptoms and functional burden from CIPN would be helpful to describe the persistence of CIPN, recovery from CIPN, and its impact on patients with CRC.
In conclusion, we suggest that baseline screening with a simple and easily administered objective measure (the Bumps Detection test), along with early, repeated symptom monitoring of patient rated numbness/tingling in the first 2–3 chemotherapy cycles, may support improved personalized care of CRC patients with oxaliplatin-induced CIPN. The Bumps Detection test is relatively inexpensive, takes little time to complete, and requires minimal training to administer, surmounting barriers to the use of comprehensive QST in multicenter clinical trials. Overall, our results warrant the use of a symptom-focused, multidisciplinary assessment approach in patients both before they begin oxaliplatin-based therapy and throughout the course of treatment.
Acknowledgments
The authors acknowledge Jeanie F. Woodruff, BS, ELS for editorial assistance; Maggie Malekifar, Raza H. Bokhari, Donna Malveaux, and Gary M. Mobley for data gathering and management; and Anthony Perez and Tina Peters for quantitative sensory testing.
Research Support. This study (clinicaltrials.gov trial registration ID: NCT00777192) was funded by grants to Charles S. Cleeland, including grants from the National Cancer Institute of the National Institutes of Health (NCI R01 CA026582) and Genentech, and an AstraZeneca Center of Excellence grant. Additional support was provided through the MD Anderson Cancer Center Support Grant (NCI P30 CA016672; PI: Ronald A. DePinho) and a Research Scholar Grant from the American Cancer Society (125079-RSG-13-241-01-PCSM; PI: Xin Shelley Wang). None of the sponsors had any role in the study design, data collection, analysis, interpretation, or preparation of the report.
The MD Anderson Symptom Inventory (MDASI) is copyrighted and licensed by The University of Texas MD Anderson Cancer Center and Charles Cleeland.
Footnotes
Conflict of interest. The authors report no other conflicts of interest in this work.
Contributor Information
Xin Shelley Wang, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1450, Houston, TX 77030, USA, xswang@mdanderson.org, +1 713-745-3504.
Qiuling Shi, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1450, Houston, TX 77030, USA, qshi@mdanderson.org, +1 713-653-1341.
Patrick M. Dougherty, Department of Pain Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 409, Houston, TX 77030, USA, pdougherty@mdanderson.org, +1 713-745-0438.
Cathy Eng, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 426, Houston, TX 77030, USA, ceng@mdanderson.org, +1 713-792-2828.
Tito R. Mendoza, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1450, Houston, TX 77030, USA, tmendoza@mdanderson.org, +1 713-745-3486.
Loretta A. Williams, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1450, Houston, TX 77030, USA, loriwilliams@mdanderson.org, +1 713-745-0844.
David R. Fogelman, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 426, Houston, TX 77030, USA, dfogelman@mdanderson.org, +1 713-745-8516.
Charles S. Cleeland, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 1450, Houston, TX 77030, USA, ccleeland@mdanderson.org, +1 713-745-3470.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Haller DG, Poston G, Raoul JL, Spano JP, Tabernero J, Van Cutsem E. Toward optimized front-line therapeutic strategies in patients with metastatic colorectal cancer--an expert review from the International Congress on Anti-Cancer Treatment (ICACT) 2009. Ann Oncol. 2010;21:1579–1584. doi: 10.1093/annonc/mdq043. [DOI] [PubMed] [Google Scholar]
- 3.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 4.Madajewicz S, Waterhouse DM, Ritch PS, Khan MQ, Higby DJ, Leichman CG, Malik SK, Hentschel P, Gill JF, Zhao L, Nicol SJ. Multicenter, randomized phase II trial of bevacizumab plus folinic acid, fluorouracil, gemcitabine (FFG) versus bevacizumab plus folinic acid, fluorouracil, oxaliplatin (FOLFOX4) as first-line therapy for patients with advanced colorectal cancer. Invest New Drugs. 2012;30:772–778. doi: 10.1007/s10637-010-9598-9. [DOI] [PubMed] [Google Scholar]
- 5.Grothey A. Reintroduction of oxaliplatin: a viable approach to the long-term management of metastatic colorectal cancer. Oncology. 2010;79:389–399. doi: 10.1159/000323491. [DOI] [PubMed] [Google Scholar]
- 6.Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder A, Stengel M, Maag R, Wasner G, Schoch R, Moosig F, Schommer B, Baron R. Pain in oxaliplatin-induced neuropathy--sensitisation in the peripheral and central nociceptive system. Eur J Cancer. 2007;43:2658–2663. doi: 10.1016/j.ejca.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Boyette-Davis JA, Cata JP, Driver LC, Novy DM, Bruel BM, Mooring DL, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother Pharmacol. 2013;71:619–626. doi: 10.1007/s00280-012-2047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schröder S, Beckmann K, Franconi G, Meyer-Hamme G, Friedemann T, Greten HJ, Rostock M, Efferth T. Can medical herbs stimulate regeneration or neuroprotection and treat neuropathic pain in chemotherapy-induced peripheral neuropathy? Evid Based Complement Alternat Med. 2013;2013:423713. doi: 10.1155/2013/423713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PLoS One. 2011;6:e18469. doi: 10.1371/journal.pone.0018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giglio P, Gilbert MR. Neurologic complications of cancer and its treatment. Curr Oncol Rep. 2010;12:50–59. doi: 10.1007/s11912-009-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility. Oncologist. 2011;16:708–716. doi: 10.1634/theoncologist.2010-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zedan AH, Hansen TF, Fex Svenningsen A, Vilholm OJ. Oxaliplatin-induced neuropathy in colorectal cancer: many questions with few answers. Clin Colorectal Cancer. 2014;13:73–80. doi: 10.1016/j.clcc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer P, Mortensen JP, Bjerregaard B, Eckhoff L, Schonnemann K, Sandberg E, Aabo K, Jakobsen A. Patient preference for oral or intravenous chemotherapy: a randomised cross-over trial comparing capecitabine and Nordic fluorouracil/leucovorin in patients with colorectal cancer. Eur J Cancer. 2006;42:2738–2743. doi: 10.1016/j.ejca.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson TM, Li Y, Coffey CW, Sit L, Shaw M, Lavene D, Bennett AV, Fruscione M, Rogak L, Hay J, Gonen M, Schrag D, Basch E. Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;21:1159–1164. doi: 10.1007/s11136-011-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): what we need and what we know. J Peripher Nerv Syst. 2014;19:66–76. doi: 10.1111/jns5.12073. [DOI] [PubMed] [Google Scholar]
- 17.de Carvalho Barbosa M, Kosturakis AK, Eng C, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Wang XS, Cleeland CS, Dougherty PM. A quantitative sensory analysis of peripheral neuropathy in colorectal cancer and its exacerbation by oxaliplatin chemotherapy. Cancer Res. 2014;74:5955–5962. doi: 10.1158/0008-5472.CAN-14-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Griffith KA, Dorsey SG, Renn CL, Zhu S, Johantgen ME, Cornblath DR, Argyriou AA, Cavaletti G, Merkies IS, Alberti P, Postma TJ, Rossi E, Frigeni B, Bruna J, Velasco R, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galie E, Briani C, Torre CD, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey DJ, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG the CIPG. Correspondence between neurophysiological and clinical measurements of chemotherapy-induced peripheral neuropathy: secondary analysis of data from the CI-PeriNoms study. J Peripher Nerv Syst. 2014;19:127–135. doi: 10.1111/jns5.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, Cleeland C, Heijnen CJ. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One. 2014;9:e100701. doi: 10.1371/journal.pone.0100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasquez S, Guidon M, McHugh E, Lennon O, Grogan L, Breathnach OS. Chemotherapy induced peripheral neuropathy: the modified total neuropathy score in clinical practice. Ir J Med Sci. 2014;183:53–58. doi: 10.1007/s11845-013-0971-5. [DOI] [PubMed] [Google Scholar]
- 22.Boyette-Davis JA, Eng C, Wang XS, Cleeland CS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Zhang H, Dougherty PM. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012;18:3180–3187. doi: 10.1158/1078-0432.CCR-12-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruhstorfer H, Lindblom U, Schmidt WG. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiat. 1976;39:1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruff RM, Parker SB. Gender and age-specific changes in motor speed and eye-hand coordination in adults: normative values for finger tapping and grooved pegboard tests. Percept Motor Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt SL, Oliveira RM, Rocha FR, Abreu-Villaca Y. Influence of handedness and gender on the grooved pegboard test. Brain Cognit. 2000;44:445–454. doi: 10.1006/brcg.1999.1204. [DOI] [PubMed] [Google Scholar]
- 26.Dixon WJ. Efficient analysis of experimental observations. Ann Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy WR, Selim MM, Brink TS, Hodges JS, Wendelschafer-Crabb G, Foster SX, Nolano M, Provitera V, Simone DA. A new device to quantify tactile sensation in neuropathy. Neurology. 2011;76:1642–1649. doi: 10.1212/WNL.0b013e318219fadd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XS, Williams LA, Eng C, Mendoza TR, Shah NA, Kirkendoll KJ, Shah PK, Trask PC, Palos GR, Cleeland CS. Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI) Cancer. 2010;116:2053–2063. doi: 10.1002/cncr.24920. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Lewis MA, Zhao F, Jones D, Loprinzi CL, Brell J, Weiss M, Fisch MJ. Neuropathic symptoms and their risk factors in medical oncology outpatents with colorectal vs. breast, lung, or prostate cancer: results from a prospective multicenter study. J Pain Symptom Manage. 2015;49:1016–1024. doi: 10.1016/j.jpainsymman.2014.11.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 32.Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res. 2013;22:2331–2339. doi: 10.1007/s11136-013-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XS, Zhao F, Fisch MJ, O'Mara AM, Cella D, Mendoza TR, Cleeland CS. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Given B, Given CW, Sikorskii A, Jeon S, McCorkle R, Champion V, Decker D. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill A, Bergin P, Hanning F, Thompson P, Findlay M, Damianovich D, McKeage MJ. Detecting acute neurotoxicity during platinum chemotherapy by neurophysiological assessment of motor nerve hyperexcitability. BMC Cancer. 2010;10:451. doi: 10.1186/1471-2407-10-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashendorf L, Vanderslice-Barr JL, McCaffrey RJ. Motor tests and cognition in healthy older adults. Appl Neuropsychol. 2009;16:171–176. doi: 10.1080/09084280903098562. [DOI] [PubMed] [Google Scholar]
- 37.Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, Renton C, Park A, Bekele T, Ringash J, Zhang H, Burkes R, Clarke SJ, Tannock IF. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol. 2014;25:2404–2412. doi: 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, Frigeni B, Cacciavillani M, Lonardi S, Cortinovis D, Cazzaniga M, Santos C, Kalofonos HP. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014;85:392–398. doi: 10.1136/jnnp-2013-305334. [DOI] [PubMed] [Google Scholar]
- 39.Land SR, Kopec JA, Cecchini RS, Ganz PA, Wieand HS, Colangelo LH, Murphy K, Kuebler JP, Seay TE, Needles BM, Bearden JD, III, Colman LK, Lanier KS, Pajon ER, Jr, Cella D, Smith RE, O'Connell MJ, Costantino JP, Wolmark N. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 40.Tofthagen C, McAllister RD, McMillan SC. Peripheral neuropathy in patients with colorectal cancer receiving oxaliplatin. Clin J Oncol Nurs. 2011;15:182–188. doi: 10.1188/11.CJON.182-188. [DOI] [PubMed] [Google Scholar]