Abstract
Cancer cells use stress response pathways to sustain their pathogenic behavior. In breast cancer, stress response-associated phenotypes are mediated by the breast tumor kinase, Brk (PTK6), via the hypoxia-inducible factors HIF-1α and HIF-2α. Given that glucocorticoid receptor (GR) is highly expressed in triple negative breast cancer (TNBC), we investigated crosstalk between stress hormone-driven GR signaling and HIF-regulated physiologic stress. Primary TNBC tumor explants or cell lines treated with the GR ligand dexamethasone (dex) exhibited robust induction of Brk mRNA and protein that was HIF1/2-dependent. HIF and GR co-assembled on the BRK promoter in response to either hypoxia or dex, indicating that Brk is a direct GR/HIF target. Notably, HIF-2α, not HIF-1α, expression was induced by GR signaling and the important steroid receptor coactivator PELP1 was also found to be induced in a HIF-dependent manner. Mechanistic investigations showed how PELP1 interacted with GR to activate Brk expression and demonstrated that physiologic cell stress, including hypoxia, promoted phosphorylation of GR serine 134, initiating a feed-forward signaling loop that contributed significantly to Brk upregulation. Collectively, our findings linked cellular stress (HIF) and stress hormone (cortisol) signaling in TNBC, identifying the phospho-GR/HIF/PELP1 complex as a potential therapeutic target to limit Brk-driven progression and metastasis in TNBC patients.
INTRODUCTION
Breast tumor kinase (Brk), also known as protein tyrosine kinase 6 (PTK6), is a soluble tyrosine kinase, distantly related to the c-Src-family kinases (1). While Brk is absent or low in cell line models of normal mammary epithelial cells (2), recent studies show that Brk is elevated but activated (i.e. phosphorylated) and membrane-localized in cancer relative to normal tissues (3). Although Brk undergoes modest to high-level gene amplification in breast tumors (cBioPortal), Brk is most frequently upregulated at the mRNA level (4–6), with highest protein levels in advanced tumor grades (7, 8). Brk is activated downstream of multiple growth factor receptors, including MET, EGF receptor and ErbB2, and confers aggressive breast cancer phenotypes such as growth-factor induced cell migration, anchorage-independent growth, modulation of EMT markers, metastasis, and resistance to targeted therapies (2, 7, 9–13). Although precocious Brk expression clearly enhances aggressive breast cancer biology (14), a thorough understanding of the mechanisms driving persistent Brk overexpression is lacking.
We demonstrated robust Brk induction following physiologic cell stress stimuli, such as hypoxia, nutrient starvation, and reactive oxygen species (ROS) (2), mediated by the hypoxia-inducible factors (HIF), HIF-1α and HIF-2α, master regulators of responses to physiologic cell stress (15). Although triple negative breast cancers (TNBCs) lack expression of ER and PR, glucocorticoid receptor (GR), is highly expressed in 15–40% of TNBC tumors (16–18). GRs are members of the nuclear steroid receptor family and bind glucocorticoids (GCs). GCs have diverse cell-type specific effects, promoting apoptosis in cells of lymphoid origin and conversely promoting survival in cells of epithelial origin (19, 20). In solid tumors, GR/GCs are emerging as mediators of cell survival and resistance to chemotherapy induced cell death (21, 22) and GR expression is predictive of decreased survival and increased risk of metastasis in ER-negative breast tumors (18).
Herein, we report Brk induction via GR/GC and HIF signaling cross talk. Our studies demonstrate a novel mechanism of integration of physiologic cell stress (HIF-dependent) and stress hormone (cortisol) driven pathways, epigenetic signaling events that may drive persistent aggressive tumor cell behavior. Targeting the inducible mediators of tumor progression may lead to increased longevity for breast cancer survivors subjected to chronic therapy during management of metastatic disease.
MATERIALS & METHODS
Cell Culture
MDA-MB-231 cell lines were obtained in April 2012 from a collaborating lab (Dr. Roland Wenger), cultured and stable knockdown of HIF1A and HIF2A genes was generated as previously described (2). The MDA-MB-231 cell lines were authenticated December 8th, 2015 by American Type Culture Collection (ATCC) and results were compared with the ATCC short-tandem repeat (STR) database. Hs578T and BT20 cell lines were obtained in April 2012 from a collaborating lab (Dr. Doug Yee) and cultured in DMEM with 10% FBS and 1% penicillin/streptomycin. Cells were maintained in 5% CO2 at 21% O2 (normoxia, ambient air) or at 1% O2 (hypoxia).
Human breast cancer explant experiments
Fresh breast cancer tissues were obtained with informed consent from women undergoing surgery at the Hospitals of the University of Texas Southwestern Medical Center (Dallas, TX) (see Supp Table 1 for clinicopathological characteristics). The procedure for establishment of explant followed the previous description (23). The tissues were also either incubated with vehicle (ethanol) alone, dexamethasone (10 μM) and were cultured in a sterile 5% CO2 incubator at 37°C for 24h, then harvested by snap freezing for protein extraction or preserved in RNAlater (Invitrogen) for gene expression analyses.
Immunoblotting
For experiments without hormone treatment, cells were plated and treated 24hr later with 1% O2 for 6 or 24hrs or 100μM H2O2 for 1 hr. For experiments requiring hormone treatment, cells were starved for 18–24hrs in iMEM with 10% DCC, then cells were treated, if applicable (treatment conditions noted in figure legends) and whole-cell lysates were isolated as previously described (2) and probed with primary antibodies: Brk (Santa Cruz, sc-1188), GR (Santa Cruz, sc-1003), Actin, ERK1/2 (Cell Signaling, 9102L), HIF-1α (Novus Biologicals, NB100–479), HIF-2α (Novus Biologicals, NB100–122), p38 MAPK (Cell Signaling, 9212), phospho-p38 MAPK (Cell Signaling, 4511p), phospho-ser134 GR (custom made, Pierce Biotechnology), or PELP1 (Bethyl Labs, A300–180A). Representative images of triplicate experiments are shown. Densitometry was determined via ImageJ analysis and normalized to the loading control.
qRT-PCR
Quantitative real-time PCR (qRT-PCR) assays were conducted as previously described (2), with MDA-MB-231 cells cultured in normoxia or hypoxia with or without 1μM dexamethasone or ethanol vehicle for 1–24hrs. Target gene expression was normalized to the expression of internal control genes, TATA-binding protein (TBP), Actin, or 18S.
ChIP assays
Chromatin immunoprecipitation (ChIP) assays were conducted as previously described (2), with MDA-MB-231 cells treated with 1μM dex or ethanol at either normoxia or hypoxia for 1 hour. ChIP-ReChIP assays were performed; first immunoprecipitating with a GR antibody for 4hrs and subsequently immunoprecipitating was a HIF-2α antibody overnight (18hrs).
Co-immunoprecipitation assays
Co-immunoprecipitation (Co-IP) assays were performed as previously described (24). Briefly, MDA-MB-231 or HeLa cells were plated and starved (described above) before a 1hr dex (1μM) or H2O2 (100mM) treatment. Cells were lysed with ELB buffer and lysates were analyzed as previously described.
Soft agar assays
Soft agar experiments were performed as previously described (25) and results presented are representative of 3 experimental repeats. Treatment conditions included 10nm doxorubicin, 1μM dex, or both.
Gene expression analysis
Results are represented as means +/− SEM. Statistical significance for qRT-PCR and ChIP-qPCR assays was determined via unpaired Student t tests. PELP1 expression was explored among TCGA breast tumor samples stratified by clinical IHC triple-negative status (88 TNBC, 434 non-TNBC) (26). The published TCGA median-centered expression data was downloaded and quantile normalized. PELP1 expression levels were plotted along with their mean +/− 95% CI. Welch’s Two Sample t-test was performed between the groups.
RESULTS
Glucocorticoids induce Brk in TNBC
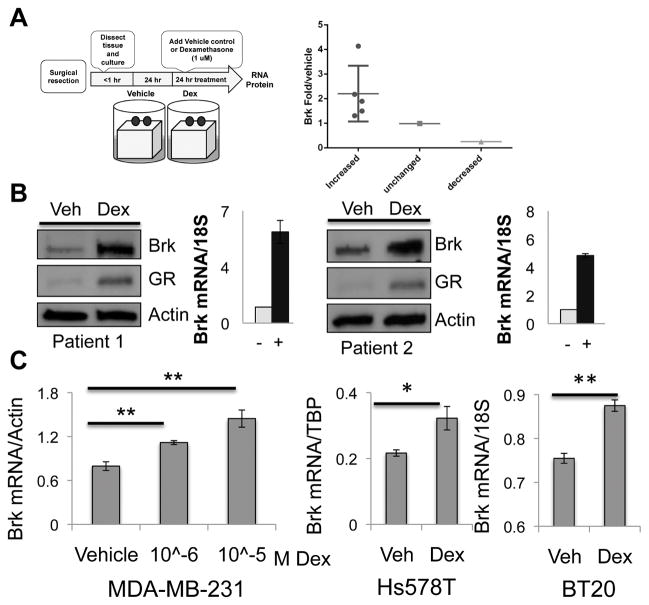
In addition to our finding of Brk upregulation via cellular stressors that input to HIF stabilization (2), we sought to investigate Brk regulation by another primary stress sensing axis, GR/GC signaling. TNBC tumor samples were obtained immediately following surgical resection (Supp. Table 1). Uniform sets of fresh tumor fragments were maintained on gelatin sponges suspended in media containing vehicle or dexamethasone (dex), a synthetic GC. Notably, 5/7 (71%) TNBC tumors showed robust induction of Brk mRNA (and protein) following dex treatment (24hrs) relative to vehicle-treated internal (i.e. same tumor) controls (Figure 1A). Brk mRNA expression remained unchanged following dex treatment in one tumor explant, while another showed decreased Brk mRNA expression (Figure 1A). Brk protein and mRNA induction relative to vehicle control are shown for two representative explants (Figure 1B). Interestingly, GR protein was also induced in 3/4 explants following dex treatment; two representative explants are shown (Figure 1B).
Figure 1.
Brk is induced following dex treatment of primary human TNBC explants and cell lines. (A) Explant experimental model and quantification of Brk mRNA levels explants. (B) Patient tumor explants were treated for 24hrs with vehicle or 10μM dex and subjected to Western blot analysis (two representative explants) Brk, GR, and Actin (loading control) antibodies. Paired (same tumor) mRNA levels were analyzed by qRT-PCR and normalized to 18S expression. (C) MDA-MB-231, Hs578T, and BT-20 cells were treated with increasing doses or 1μM dex for 24hrs and mRNA levels were analyzed by qRT-PCR after normalization to Actin, TBP, or 18S (Asterisks (**) indicate statistical significance (p<0.01; an unpaired Student t test)).
To investigate the mechanism of Brk induction by GC signaling, we utilized cell line models of TNBC. MDA-MB-231 cells, which express GR but lack ER or PR, were treated with increasing doses of dex for 24 hours. Brk mRNA was significantly increased in response to 1μM dex treatment relative to vehicle (Figure 1C). Similarly, Brk mRNA was significantly increased in response to dex treatment in Hs578T and BT-20 TNBC cell lines. Brk protein was also dose dependently induced in response to dex, and over a time course that peaked at 24–30hrs (Supp. Figure 1). Our remaining dex studies were performed using 1μM dex, a physiologically relevant dose (27, 28) that is standard in the GR field (29). Together, these data indicate that GR/GC signaling induces Brk expression in primary human TNBC tumors and cell lines.
GR requires HIFs to induce Brk
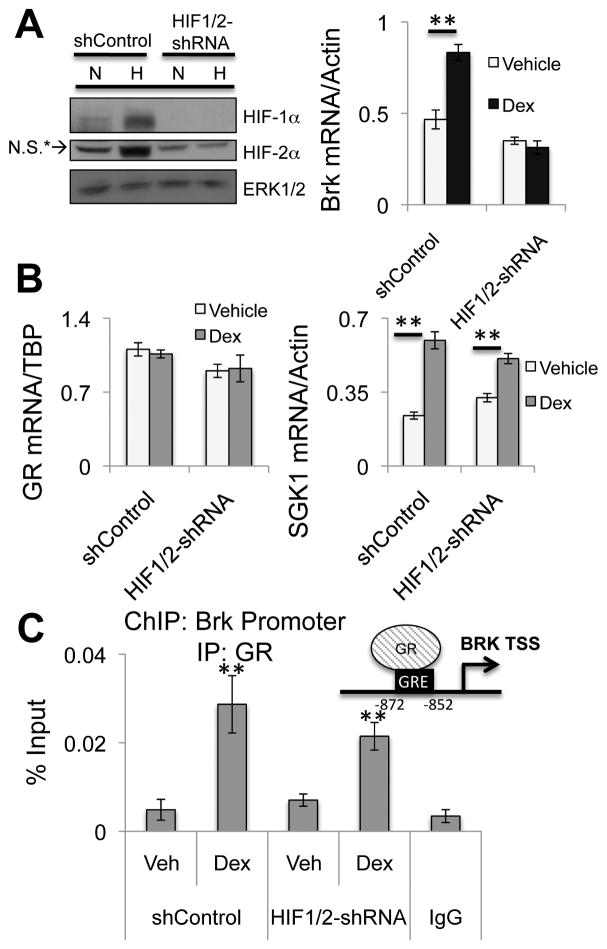
To determine if HIFs are required for GR/GC-induced Brk expression, we utilized MDA-MB-231 cells expressing a non-targeting control shRNA (shControl) or shRNAs specific to both HIF-1α and HIF-2α, resulting in a double knockdown (HIF1/2-shRNA) of HIF-1α and HIF-2α. MDA-MB-231 shControl and HIF1/2-shRNA cells were cultured in normoxia or hypoxia and treated with vehicle or dex for 24 hours and Brk mRNA expression was assessed via qRT-PCR. MDA-MB-231 shControl cells but not HIF1/2-shRNA cells significantly induced Brk expression following dex treatment compared to vehicle (Figure 2A; right). GR mRNA expression was unaltered between cell lines and the known GR target gene, SGK-1, was similarly dex-regulated in both shControl cells and HIF1/2-shRNA cells (Figure 2B). These data suggest that ligand-activated GR requires HIF-1α and/or HIF-2α to induce robust Brk mRNA expression.
Figure 2.
GR induction of Brk expression is HIF-dependent. (A) MDA-MB-231 cells expressing shControl or HIF1/2-shRNA were cultured at normoxia or hypoxia (1% O2) for 24hrs. Lysates were analyzed by Western blotting for HIF-1α, HIF-2αor ERK1/2 (loading control; N.S* indicates a non-specific band). MDA-MB-231 shControl or HIF1/2-shRNA cells were treated with vehicle or 1μM dex for 24hrs and Brk mRNA levels were assessed by qRT-PCR after normalization to Actin or (B) mRNA levels for GR and SGK-1 were assessed by qRT-PCR following normalization to TBP or Actin levels, respectively. (C) MDA-MB-231 shControl or HIF1/2-shRNA cells were treated for 1hr with vehicle or 1μM dex and ChIP assays were performed. Negative control isotype-matched IgG controls were conducted on dex treated MDA-MB-231 cells. Representative examples from triplicate experiments are shown. (**p<0.01, unpaired Student t test).
The Brk promoter contains a glucocorticoid response element (GRE) present 852 basepairs upstream of the transcriptional start site, near a known hypoxia response element (HRE) to which we previously demonstrated recruitment of both HIF-1α and HIF-2α during hypoxia (2). Chromatin immunoprecipitation (ChIP) assays showed robust recruitment of GR to the GRE in the Brk promoter following dex treatment in either cell line model relative to vehicle treatment and IgG negative controls (Figure 2C). These data suggest Brk is a direct GR target gene. Ligand-activated GR is also recruited to the Brk promoter in dex-treated HIF1/2-shRNA cells, although Brk mRNA is not induced, suggesting that HIFs are required for GR/GC co-activation of transcription or other downstream event(s) subsequent to GR recruitment to this region of chromatin.
HIF-2α is a novel GR target gene in TNBC
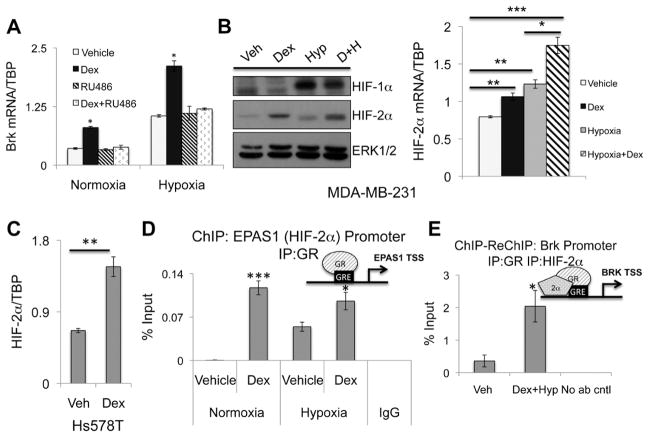
To test the dependence of HIF-induced Brk expression on GR/GC signaling, MDA-MB-231 cells were cultured in hypoxia or normoxia with or without dex or the GR antagonist, RU486. As above (Figure 1), Brk mRNA was significantly induced in response to dex treatment, but blocked by the GR antagonist, RU486, as expected (Figure 3A). Brk mRNA was significantly increased in response to hypoxia alone, relative to normoxia, and was further induced upon dex treatment during hypoxia. Interestingly, when MDA-MB-231 cells were treated with hypoxia, dex, and RU486 simultaneously, Brk mRNA expression returned to levels seen in hypoxic conditions, but were not further reduced to the basal expression levels observed during normoxia. Thus, while GR/GC-induced Brk expression requires HIFs (Figure 2A), HIFs induce Brk during hypoxia independently of ligand-activated GR (Figure 3A).
Figure 3.
GR regulates HIF-2α expression and GR/HIF-2α are co-recruited to the Brk promoter. (A) MDA-MB-231 cells cultured in normoxia or hypoxia for 24hrs with vehicle, 1μM dex, 1μM RU486 or both agents and mRNA levels were assessed by qRT-PCR after normalization to TBP levels. Asterisks indicate statistical significance from all other treatment groups in either normoxia or hypoxia. (B) MDA-MB-231 cells were treated for 24hrs with vehicle, 1μM dex, hypoxia or both agents and subjected to Western blot analysis for HIF-1α, HIF-2α or ERK1/2 (loading control); Brk mRNA levels were analyzed by qRT-PCR after normalization to TBP levels. (C) Hs578T cells treated with vehicle or 1μM dex for 24hrs and mRNA expression was analyzed via qRT-PCR following normalization to TBP expression. (D) MDA-MB-231 cells were cultured at normoxia or hypoxia and treated with vehicle or 1μM dex for 1hr and ChIP assays were performed. Negative isotype-matched IgG controls were conducted on hypoxia and dex treated MDA-MB-231. (E) MDA-MB-231 cells were treated with vehicle or 1μM dex and hypoxia for 1hr. ChIP-Re-ChIP assays were performed with initial immunoprecipitation with GR antibody and subsequently immunoprecipitation with HIF-2α antibody. No secondary antibody was included in control samples to demonstrate specificity. Representative examples from triplicate experiments are shown. (*p<0.05, **p<0.01, ***p<0.001; unpaired Student t test).
Although expression of HIF-1α/2α is canonically regulated by oxygen tension through proteasomal degradation, cancers frequently utilize alternative methods to inappropriately stabilize HIFs (30, 31). We speculated that GR may regulate HIFs in TNBC cells as a means of ‘pre-setting’ the components necessary for rapid upregulation of select target genes, including Brk. To test this, MDA-MB-231 cells were cultured with or without dex and hypoxia and as expected, HIF-1α and HIF-2α protein levels were substantially induced in hypoxia relative to normoxia. Surprisingly, HIF-2α but not HIF-1α protein levels were increased in response to dex relative to vehicle controls. Alone, dex or hypoxia significantly induced HIF2A mRNA expression relative to vehicle (Figure 3B) and combination treatment resulted in additive induction. Similar results were observed in Hs578T TNBC cells (Figure 3C). To determine if HIF-2α was a direct GR target gene, we performed ChIP assays to a GRE present in the HIF2A (EPAS1) promoter in MDA-MB-231 cells following dex and hypoxia treatment and observed robust recruitment of GR in response to dex treatment compared to vehicle (Figure 3D). Interestingly, ligand-independent recruitment of GR was observed during hypoxia relative to normoxia and may account for the modest but significant increase in HIF2A mRNA observed during hypoxia (Figure 3B). These data indicate that GR directly binds the EPAS1 promoter prior to induction of HIF2A mRNA in both ligand-dependent and -independent conditions.
HIF-1α/2α are recruited to multiple active regions of the Brk promoter in response to physiologic cell stress stimuli (2). To determine if GR and HIF-2α were present in the same transcriptional complexes at the Brk promoter in response to hypoxic cell stress and stress hormone exposure, we performed ChIP-ReChIP assays. Following first-round immunoprecipitation (IP) with an antibody specific to GR, and subsequent IP with an antibody specific for HIF-2α, we detected strong co-recruitment of these transcription factors to the Brk promoter following combined dex and hypoxia treatment and relative to vehicle controls (Figure 3E). Thus, GR and HIF-2α interact in the same transcriptional complexes at the Brk promoter following dex and during hypoxia.
PELP1 interacts with GR to induce Brk expression
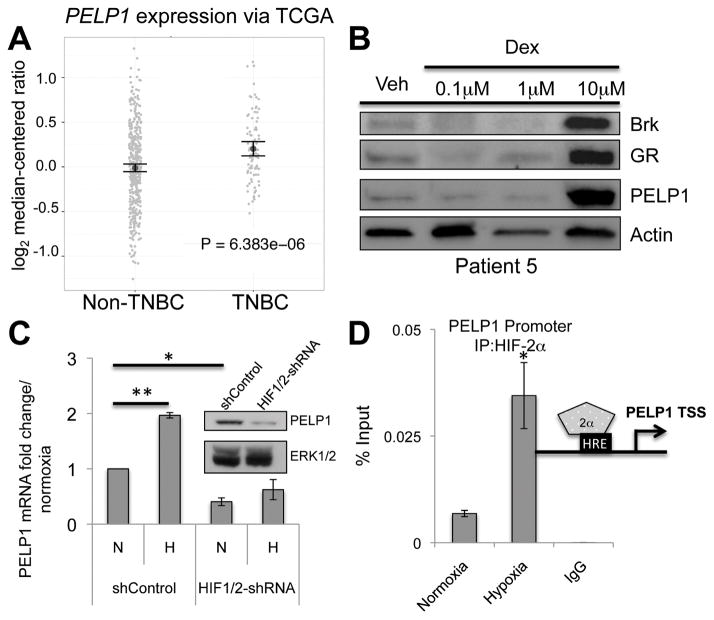
Common signaling pathways may exist across diverse hormone-driven cancers. We thus considered common SR coactivators in our models of TNBC. Proline glutamate and leucine rich protein 1 (PELP1/MNAR) is a known SR coactivator with important functions in prostate and breast tumor biology and progression (32). PELP1 mRNA expression is significantly higher in TNBC tumors compared to non-TNBC breast tumors (Figure 4A). We thus evaluated PELP1 protein expression in our ex vivo tumor explant models (as in Figure 1). Tumor samples (patient #5) were treated with increasing doses of dex and PELP1 protein was highly induced following 10μM dex treatment relative to vehicle (Figure 4B). Notably, in the same tumor, Brk and GR protein levels were also robustly induced following dex treatment. In total, PELP1 was clearly dex-induced in 4/7 tumor explants and tracked with GR induction. Conversely, we observed no increase in PELP1 mRNA or protein following dex-treatment of TNBC cell line models, perhaps due to already high basal PELP1 expression. Alternatively, tumor stromal components may be required for stable dex-induced PELP1 upregulation (Figure 4B).
Figure 4.
Hypoxic cell stress induces PELP1 expression. (A) PELP1 mRNA levels (via TCGA) comparing non-TNBC to TNBC tissues. (B) Primary human TNBC explants were treated with for 24hrs with vehicle or increasing doses of dex and subjected to Western blot analysis for Brk, GR, PELP1, and Actin (loading control). A representative patient explant is shown (n=7). (C) MDA-MB-231 shControl or HIF1/2-shRNA cells were cultured at normoxia or hypoxia for 24hrs and mRNA levels were assessed via qRT-PCR after normalization to TBP levels and shControl cell normoxia values. Inset, Western blot analysis showing PELP1 and ERK1/2 (loading control). (D) MDA-MB-231 cells were cultured at normoxia or hypoxia for 1hr and ChIP assays were performed. Negative isotype-matched controls were conducted on MDA-MB-231 cells cultured at hypoxia (1 hr). Representative examples from triplicate experiments are shown. (*p<0.05, **p<0.01; unpaired Student t test).
Patient-derived xenograft (PDX) tumors of TNBC had significant enrichment for HIF-1α and HIF-2α relative to similarly propagated luminal tumors (2). To investigate the impact of hypoxia and HIFs on the expression of PELP1 in TNBC cells, MDA-MB-231 shControl or HIF1/2-shRNA cells were cultured in normoxia or hypoxia. Interestingly, PELP1 mRNA was significantly induced in MDA-MB-231 shControl but not HIF1/2-shRNA (HIF-null) cells cultured in hypoxia relative to normoxia (Figure 4C). Additionally, basal levels of PELP1 mRNA and protein were significantly reduced in HIF1/2-shRNA cells relative to shControl cells (Figure 4C, inset). ChIP assays demonstrated robust HIF-2α recruitment to an HRE-containing region of the PELP1 promoter in MDA-MB-231 cells in response to hypoxia relative to normoxia (Figure 4D). Conversely, recruitment of HIF-1α to this region was not consistently detected in hypoxia. Taken together, these data suggest that PELP1 is a HIF-2α target gene in TNBC cells.
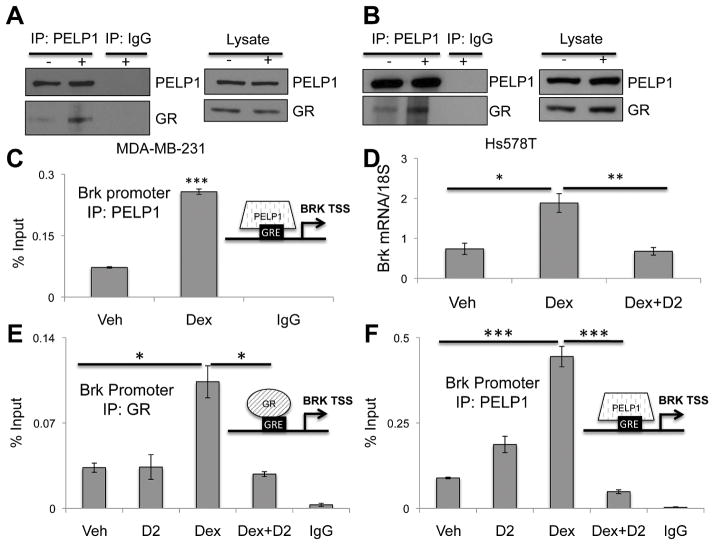
We first demonstrated ER/PR/PELP1 signaling and transcriptional complexes in luminal breast cancer models (33). However, no studies have defined a role for GR in PELP1-containing transcriptional complexes in breast cancer cells. Co-immunoprecipitation (co-IP) experiments performed in MDA-MB-231 cells treated with or without dex revealed basal GR interaction with PELP1 that increased upon dex treatment relative to vehicle controls (Figure 5A). Similar results were observed in Hs578T TNBC cells (Figure 5B). ChIP assays in MDA-MB-231 cells treated with or without dex showed robust PELP1recruitment to the GRE located in the Brk promoter following dex treatment compared to vehicle (Figure 5C). Together, these data indicate that GR and PELP1 interact in whole cell lysates and are recruited to the same location in the Brk promoter in response to dex treatment. To determine if PELP1 is required for dex induced Brk expression, MDA-MB-231 cells were treated with the PELP1 peptidomemetic inhibitor, D2, which was previously shown to inhibit PELP1/AR interaction via disruption of protein-protein interactions (23). As seen previously, dex treatment resulted in an increase in Brk mRNA expression, which was significantly diminished when cells were pre-treated with D2 prior to dex treatment (Figure 5D). Moreover, ChIP assays demonstrated inhibition of dex-induced recruitment of both GR and PELP1 to the Brk promoter in cells subjected to D2 pretreatment relative to dex treatment alone (Figure 5E, 5F). Thus, PELP1 is a key co-factor for GR/GC induction of Brk expression in TNBC cells.
Figure 5.
PELP1 interacts with GR and is required for dex-induced Brk expression. (A) MDA-MB-231 or (B) Hs578T cells were treated for 1hr with 1μM dex. Lysates were subjected to IP with PELP1 antisera or rabbit IgG (control). IP lysates and input control were assessed via Western blotting using PELP1 or GR antibodies. (C) MDA-MB-231 cells were treated for 1hr with vehicle or 1μM dex and ChIP assays were performed. Isolated DNA was analyzed by qRT-PCR. MDA-MB-231 cells pretreated with 10μM D2 or DMSO vehicle control for 1hr followed by (D) 24hr vehicle or 1μM dex treatment and mRNA expression was analyzed via qRT-PCR after normalization to 18S levels, or (E/F) 1hr dex or vehicle treatment and ChIP assays were performed with (E) GR antibody, (F) PELP1 antibody or negative isotype-matched antisera. Isolated DNA was analyzed by qRT-PCR. Representative examples from triplicate experiments are shown. (*p<0.05, ** p<0.01, ***p<0.001; unpaired Student t test).
Phosphorylation of GR Ser134 is important for Brk induction
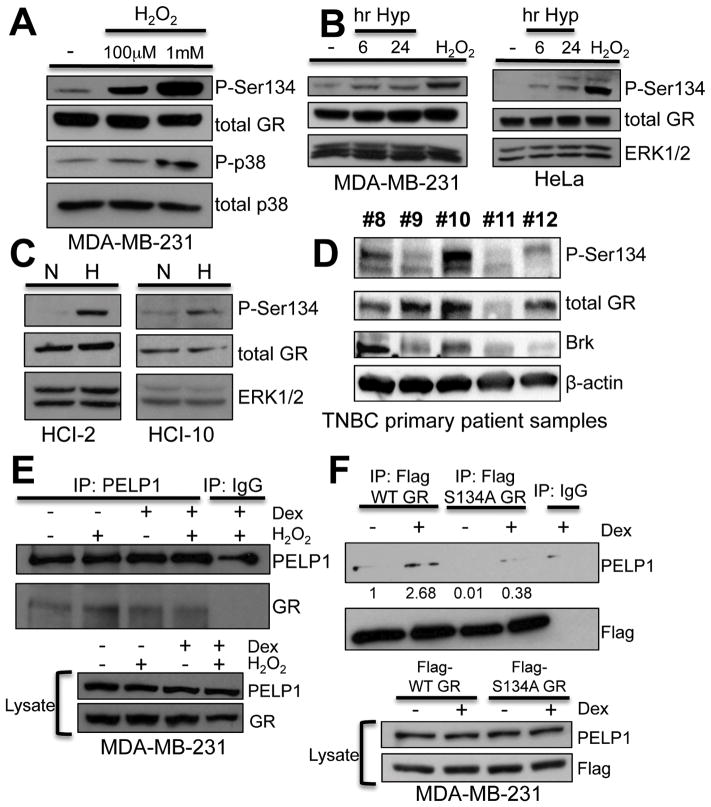
Ligand-independent phosphorylation of GR occurs at serine 134 (S134) via p38 MAPK in response to physiologic cell stress stimuli in U2OS osteosarcoma cells (34). We hypothesized that phosphorylation of GR-S134 provides a mechanistic link between HIF-and hormone-(GC) mediated cell stress-induced inputs to Brk upregulation in TNBC cells. MDA-MB-231 cells were treated with increasing doses of H2O2 and phosphorylation of GR-S134 was visualized by Western blotting using phospho-S134 antibodies. H2O2 treatment resulted in enhanced phosphorylation of GR-S134 (Figure 6A). Notably, we observed constitutive, basal phosphorylation of GR-S134, as previously observed in U2OS cells (34), as well as activation of p38 MAPK. In order to determine if GR-S134 is phosphorylated in response to hypoxic cell stress, MDA-MB-231 cells and HeLa cells were cultured in hypoxia or with H2O2 as a positive control. Phosphorylation of GR-S134 increased at 6 hours and 24 hours of hypoxia relative to normoxia in both cell lines (Figure 6B), while total GR protein levels remained unchanged. We next assessed the phosphorylation of GR-S134 in two TNBC PDX cell lines, HCI-2 and HCI-10, previously shown to have high levels of HIF-1α, HIF-2α and Brk protein expression (2). Hypoxia induced robust GR-S134 phosphorylation in both HCI-2 and HCI-10 TNBC models (Figure 6C). To determine if GR-S134 was phosphorylated in vivo, we measured Brk, phospho-S134 GR, and total GR in primary TNBC patient samples via Western blotting (Figure 6D). Patient tumors #8, #10, and #12 expressed higher levels of both total and phospho-S134 GR and also contained higher levels of Brk relative to patients #9 and #11. These data support our in vitro findings that Brk expression tracks with phospho-S134 GR in TNBC.
Figure 6.
GR Ser134 is phosphorylated in hypoxia and required for GR/PELP1 interaction. (A) MDA-MB-231 cells were treated with vehicle or increasing doses of H2O2 for 1hr and Western blot analysis was performed with antibodies to phospo-Ser134, total GR, phospho-p38 MAPK, or total p38 MAPK (loading control). (B) MDA-MB-231 or HeLa cells were cultured in normoxia or hypoxia for 6 or 24hrs or with 100μM H2O2 (positive control) for 1hr and subjected to Western blot analysis with antibodies to phospho-Ser134, total GR, or ERK1/2 (loading control). (C) Cell lines established from patient derived xenografts were cultured ex vivo for 24hrs at normoxia or hypoxia and lysates were subjected to Western blot analysis for phospho-Ser134, total GR, or ERK1/2 (loading control). (D) Whole cell lysates from five primary patient samples of TNBC were subjected to Western blot analysis with antibodies for total GR, phospho-GR-Ser134, Brk, or β-actin (loading control). (E) MDA-MB-231 cells were pretreated for 30min with 100μM H2O2 followed by 1hr vehicle or 1μM dex treatment and subjected to IP with PELP1 or rabbit IgG (control) antibodies. IP lysates or input lysates were analyzed by Western blotting for PELP1 and GR. (F) MDA-MB-231 cells stably transfected with Flag-WT GR or Flag-S134A GR constructs were treated for 1hr with 1μM dex or vehicle and subjected to IP with FLAG antisera or mouse IgG (control) antibody. IP lysates and input lysates were assessed by Western blotting using FLAG or PELP1 antisera. Relative levels of PELP1 expression, via densitometry, is shown.
To test the requirement for GR-S134 phosphorylation in GR/PELP1 interaction in TNBC cells, co-IP assays were performed with MDA-MB-231 cells. Interestingly, increased levels of GR were seen in PELP1 immunoprecipitates following H2O2 treatment (i.e. to induce phosphorylation of GR-S134) relative to vehicle (Figure 6E). To definitively assess the requirement of this phosphorylation event, co-IP assays were conducted in MDA-MB-231 cells stably expressing either flag-tagged WT GR (WT flag-GR) or a mutant GR in which S134 was mutated to a non-phosphorylated alanine residue (S134A flag-GR). We observed decreased PELP1 in S134A flag-GR immunoprecipitates relative to levels present in WT flag-GR immunoprecipitates, both basally and in response to dex treatment (Figure 6F). These data suggest that GR phosphorylation at S134 occurs during hypoxic stress and facilitates or stabilizes GR/PELP1 association.
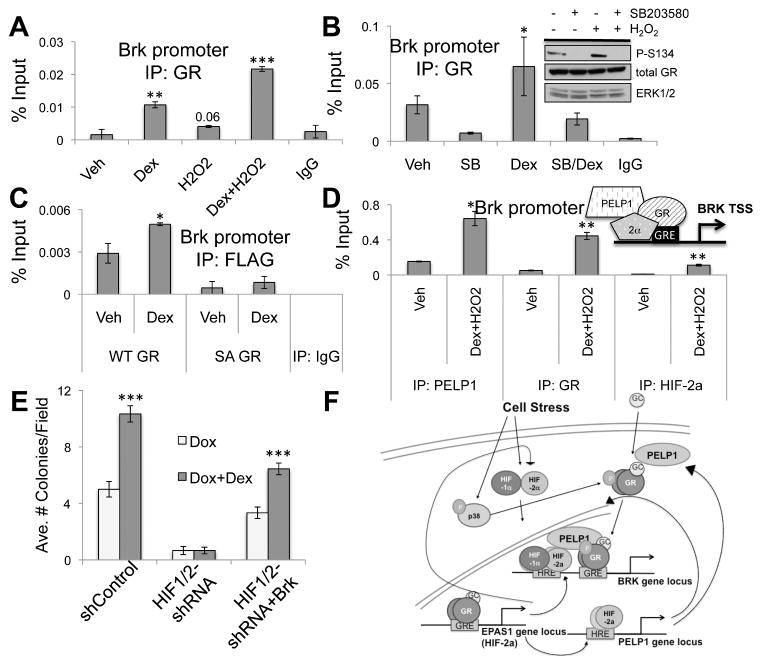
Highest levels of phosphorylation of GR-S134 were observed following exposure to H2O2 (i.e. during generation of ROS) in MDA-MB-231 cells (Figure 6). To implicate phosphorylation of GR-S134 on dex-induced GR recruitment to the Brk promoter, MDA-MB-231 cells were treated with or without dex, H2O2 or both agents and GR recruitment was assessed via ChIP assay. As previously seen, GR was recruited to the Brk promoter following dex treatment relative to vehicle. We also detected a slight increase in GR recruitment to the Brk promoter in the presence of H2O2 alone. Notably, combined dex and H2O2 treatment further enhanced recruitment of GR to the Brk promoter relative to either dex or H2O2 treatment alone (Figure 7A). We then tested the requirement for p38 MAPK activity in dex-induced recruitment of GR to the Brk promoter. MDA-MB-231 cells were pretreated with SB203580, a p38 MAPK inhibitor, which effectively blocks phosphorylation of GR-S134 (Figure 7B, inset), followed by dex or vehicle treatment. SB203580 pre-treatment resulted in diminished basal recruitment of unliganded-GR to the Brk promoter relative to vehicle control (Figure 7B). Interestingly, combined treatment with both SB203580 and dex substantially decreased GR recruitment to the Brk promoter relative to dex alone. These data suggest that inhibition of GR-S134 phosphorylation hinders the ability of GR to associate with a GRE-containing region of the Brk promoter. Regulation of two classic GR target genes, DUSP1 and GILZ, remained unaltered in similar conditions (Supp. Figure 2C–F); both were robustly dex-induced in HIF1/2-shRNA cells relative to vehicle controls (Supp. Figure 2A–B), demonstrating the specificity of phospho-GR-S134 target gene selection.
Figure 7.
The phospho-GR/HIF/PELP1 complex is recruited to the Brk promoter in response to stress. MDA-MB-231 cells were pretreated with (A)100μM H2O2 or (B) 10μM SB203580 or DMSO for 30min followed by 1hr vehicle or 1μM dex treatment or ChIP assays were performed with GR antisera or negative isotype-matched control antibodies and qRT-PCR was performed. Inset, Western blot analysis of MDA-MB-231 cells pretreated with 10μM SB203580 for 30 min followed by 100μM H2O2 treatment for 1hr and probed with antibodies for phospho-Ser134, GR or ERK1/2 (loading control). (C) MDA-MB-231 cells stably expressing Flag WT-GR or Flag SA-GR were treated with 1μM dex for 1hr and ChIP assays with Flag antibodies or negative isotype-matched controls were performed. Isolated DNA was assessed by qRT-PCR. (D) MDA-MB-231 cells were treated for 1 hr with 1μM dex and 100μM H2O2 treatment. ChIP assays were then performed with antisera for PELP1, GR, and HIF-2α or negative isotype-matched control. qRT-PCR was performed on isolated DNA. A representative experiment is shown from triplicate experiments. (E) Soft agar colony formation assays with MDA-MB-231 shControl, HIF1/2-shRNA, and HIF1/2-shRNA+Brk cells grown in 10nm doxorubicin with 1μM dex or vehicle for 18 days. (F) Model detailing feed-forward phospho-GR/HIF/PELP1 signaling loop. Stress stimuli stabilize HIFs and activate p38 MAPK; p38 phosphorylates GR on Ser134. Cortisol-bound GR induces HIF2A expression and HIFs induce PELP1. Phospho-Ser134 GR binds to PELP1, leading to formation of phospho-GR/HIF2/PELP1 complexes and induction of Brk mRNA expression.
Finally, to definitively test the requirement of GR-S134 phosphorylation for recruitment of GR to the Brk promoter, we performed ChIP assays with MDA-MB-231 cells expressing either WT flag-GR or S134A flag-GR. As expected, WT flag-GR was recruited to the Brk promoter following dex treatment relative to vehicle controls. However, the association of S134A flag-GR with the Brk promoter was basally reduced and was not significantly increased in response to dex relative to vehicle controls (Figure 7C). These data suggest that phosphorylation of GR-S134 is required for recruitment of ligand-bound GR to the Brk promoter. Our data collectively suggest a mechanism through which PELP1, GR, and HIF-2α cooperatively induce Brk expression following physiologic (HIF) and hormone (GC) stress signaling. Indeed, ChIP assays confirmed that all three molecules are simultaneously recruited to the same region of the Brk promoter in response to stress stimuli in MDA-MB-231 treated with dex and H2O2. (Figure 7D). To link GR/GC-induced TNBC cell survival to Brk expression, we performed soft agar assays with MDA-MB-231 cells expressing shControl, HIF1/2-shRNA (Brk-null) or HIF1/2-shRNA+Brk (in which Brk expression is restored) grown in the presence of doxorubicin with or without dex. MDA-MB-231 shControl cells treated with dex exhibited significantly more colonies per field relative to doxorubicin treatment alone (Figure 7E). Brk-null HIF1/2-shRNA cells exhibited decreased ability to form colonies in the presence of doxorubicin and complete loss of dex-mediated cell survival. Notably, HIF1/2-shRNA+Brk cells exhibited increased colony formation with doxorubicin treatment relative to Brk-null models, and importantly, the protective effect of dex treatment was restored. These data suggest that HIFs are required for GR/GC-induced cell survival, but that exogenous Brk expression can bypass this requirement, perhaps in part via re-establishment of feed-forward signaling (i.e. via Brk-induced activation of p38 MAPK) at additional phospho-S134 GR and PELP1 target genes (Figure 7F).
DISCUSSION
Our data demonstrate a remarkable hormone (GC) dependent signaling pathway wherein GR-S134 acts as an additional “stress sensor” of cellular stressors (ROS, hypoxia, etc) that stabilize HIFs. This phosphorylation event facilitates GR/PELP1 interactions at novel GR/PELP1/HIF target genes typified by Brk, an important mediator of advanced cancer phenotypes and tumor progression. This mechanism of integration of HIF-dependent cell stress signaling pathways with GR/GC signaling suggests that these pathways may overlap more than previously thought. Notably, many HIF responsive genes have functions relevant to cancer biology, such as glucose metabolism, angiogenesis, and cell migration (35). HIF-1α overexpression in breast cancer is predictive of relapse, higher risk of metastasis, and high levels of HIF-1α are specifically associated with TNBC (36, 37). Similarly, HIF-2α is emerging as an important mediator of cancer metastasis (38). Targeting PELP1, HIFs (including HIF-2α, and/or blocking GR-S134 phosphorylation via inhibition of upstream p38 MAPK signaling may provide a means of “redirecting” GR away from genes that promote pro-survival and tumor progression during cancer chemotherapy while preserving the desired protective (i.e. to inflammation) effects of therapeutic corticosteroids.
Remarkably, PELP1 expression is HIF-dependent (Figure 4C). PELP1 is primarily associated with ER or AR transactivation but can both transrepress and transactivate GR in a cell-type specific manner (39). Our data support a role for PELP1 as an important GR co-activator. Like GR, PELP1 mRNA expression is significantly increased in TNBC compared to non-TNBC (Figure 4A). PELP1 expression is dysregulated in multiple cancer types, including 60–80% of breast tumors (40, 41). Notably, high PELP1 expression in breast tumors is associated with increased tumor grade, cell proliferation, metastasis and decreased disease free survival as well as the appearance of basal cytokeratin markers (40–43). Patients with PELP1/Ki-67 double high tumors experienced shorter disease free survival and overall survival (44). Expression of PELP1 is inversely associated with expression of ER, PR, or AR (41). Ours is the first study to link PELP1 expression and function to GR. Consistent with the concept of GR is a key mediator of increased TNBC cell survival ((18, 29, 45) and Figure 7E), PELP1 knockdown in mutant p53 TNBC cells enhanced chemotherapy-induced cell death, in part via modulation of gain-of-function mutant p53 activity (46). PELP1 inhibitors are currently in development, primarily for prostate cancer and ER/PR positive luminal breast tumors (23). Our studies suggest that PELP1 inhibitors may have utility in treating aggressive TNBC, especially in patients whose tumors exhibit PELP1/Ki-67 double high expression (44).
Like Brk (47), GR is a mediator of pro-survival in breast cancer. TNBC cells treated with combination chemotherapy and dex, in vitro and in vivo, undergo significantly decreased cell death relative to chemotherapy alone (29). Conzen and colleagues identified multiple GR target genes that mediate this pro-survival effect, such as serum and glucocorticoid regulated kinase 1 (SGK-1) and dual-specificity serine phosphatase 1 (DUSP1) (29, 45). Relevant to these findings, increased phosphorylation of GR at S134 resulted in association of GR with the adaptor protein 14-3-3zeta and ultimately globally changed GR target genes in response to dex-treatment of osteosarcoma cell models (34). Notably, as with Brk, GR, PELP1, and HIFs, expression of 14-3-3zeta is also very high in TNBC (i.e. as determined via TCGA analysis), suggesting that these molecules cooperate to alter gene expression and cell fate in TNBC. Our present study identifies phospho-S134 GR as a critical regulator of novel GR/PELP1/HIF complexes. We suspect that phospho-S134-GR/PELP1/HIF signaling complexes are markers of aggressive tumors and drive a new gene program to promote tumor cell pro-survival and progression to metastasis. As Brk is known to promote these aggressive phenotypes, it is likely a key downstream effector of GR/HIF-dependent stress signaling in TNBC.
Brk may be representative of a larger gene program that is jointly regulated by HIFs, phospho-GR, 14-3-3zeta, and PELP1. Going forward, it will be important to identify risks associated with GC-based therapies. Breast cancer patients typically receive high-dose GC treatment prior to chemotherapy to alleviate adverse side effects. Furthermore, organ transplant patients chronically treated with GCs to achieve immunosuppression (48) experience greatly increased rates of metastatic melanoma (49), an aggressive cancer that is typically Brk+ (50). Thus, a detailed understanding GR/GC signaling in cancer biology and its impact on aggressive tumor phenotypes is urgently needed. Our studies demonstrate a novel feed-forward loop, in which components of the stress pathways (p38 MAPK, Brk, HIFs, GR and PELP1) regulate expression and activity of other members of the pathway (i.e. Brk activates p38 MAPK and GR induces HIF-2α, while HIFs induce PELP1, which binds to phospho-GR), to ultimately potentiate stress signaling, persistently augment pathway activity, and drive gene expression (i.e. epigenetic events typified by Brk induction) required for aggressive tumor biology (Figure 7F).
Supplementary Material
Acknowledgments
Financial support: This work was supported by the Tickle Family Land Grant Endowed Chair in Breast Cancer Research (held by C.A. Lange) and the Mary Kay Ash Breast Cancer Foundation (to C.A. Lange) and by an NIH/NCI F31 predoctoral fellowship (CA195877-01 to T.M. Regan Anderson), an NIH/NCI T32 training grant fellowship (CA009138 to T.M. Regan Anderson) and NIH/NCI 1R01 (CA138488 to T.N. Seagroves).
We thank Dr. Julie Ostrander (U of MN Dept of Medicine) for helpful advice on PELP1 signaling and reagents.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–19. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- 2.Regan Anderson TM, Peacock DL, Daniel AR, Hubbard GK, Lofgren KA, Girard BJ, et al. Breast tumor kinase (Brk/PTK6) is a mediator of hypoxia-associated breast cancer progression. Cancer Res. 2013;73:5810–20. doi: 10.1158/0008-5472.CAN-13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng M, Emmadi R, Wang Z, Wiley EL, Gann PH, Khan SA, et al. PTK6/BRK is expressed in the normal mammary gland and activated at the plasma membrane in breast tumors. Oncotarget. 2014;5:6038–48. doi: 10.18632/oncotarget.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, et al. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–90. [PubMed] [Google Scholar]
- 6.Aubele M, Walch AK, Ludyga N, Braselmann H, Atkinson MJ, Luber B, et al. Prognostic value of protein tyrosine kinase 6 (PTK6) for long-term survival of breast cancer patients. Br J Cancer. 2008;99:1089–95. doi: 10.1038/sj.bjc.6604660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 8.Born M, Quintanilla-Fend L, Braselmann H, Reich U, Richter M, Hutzler P, et al. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J Pathol. 2005;205:592–6. doi: 10.1002/path.1720. [DOI] [PubMed] [Google Scholar]
- 9.Castro NE, Lange CA. Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res. 2010;12:R60. doi: 10.1186/bcr2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One. 2010;5:e11729. doi: 10.1371/journal.pone.0011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ai M, Liang K, Lu Y, Qiu S, Fan Z. Brk/PTK6 cooperates with HER2 and Src in regulating breast cancer cell survival and epithelial-to-mesenchymal transition. Cancer Biol Ther. 2013;14 doi: 10.4161/cbt.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J, et al. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc Natl Acad Sci U S A. 2008;105:12463–8. doi: 10.1073/pnas.0805009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Lu Y, Liang K, Hsu JM, Albarracin C, Mills GB, et al. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene. 2012;31:4372–83. doi: 10.1038/onc.2011.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrander JH, Daniel AR, Lange CA. Brk/PTK6 signaling in normal and cancer cell models. Curr Opin Pharmacol. 2010;10:662–9. doi: 10.1016/j.coph.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buxant F, Engohan-Aloghe C, Noel JC. Estrogen receptor, progesterone receptor, and glucocorticoid receptor expression in normal breast tissue, breast in situ carcinoma, and invasive breast cancer. Appl Immunohistochem Mol Morphol. 2010;18:254–7. doi: 10.1097/PAI.0b013e3181c10180. [DOI] [PubMed] [Google Scholar]
- 17.Belova L, Delgado B, Kocherginsky M, Melhem A, Olopade OI, Conzen SD. Glucocorticoid receptor expression in breast cancer associates with older patient age. Breast Cancer Res Treat. 2009;116:441–7. doi: 10.1007/s10549-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan D, Kocherginsky M, Conzen SD. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71:6360–70. doi: 10.1158/0008-5472.CAN-11-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frei E, 3rd, Karon M, Levin RH, Freireich EJ, Taylor RJ, Hananian J, et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood. 1965;26:642–56. [PubMed] [Google Scholar]
- 20.Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11(Suppl 1):S45–55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Wenger T, Mattern J, Ilea S, Frey C, Gutwein P, et al. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol Ther. 2007;6:278–87. doi: 10.4161/cbt.6.2.3652. [DOI] [PubMed] [Google Scholar]
- 22.Rutz HP. Effects of corticosteroid use on treatment of solid tumours. Lancet. 2002;360:1969–70. doi: 10.1016/S0140-6736(02)11922-2. [DOI] [PubMed] [Google Scholar]
- 23.Ravindranathan P, Lee TK, Yang L, Centenera MM, Butler L, Tilley WD, et al. Peptidomimetic targeting of critical androgen receptor-coregulator interactions in prostate cancer. Nat Commun. 2013;4:1923. doi: 10.1038/ncomms2912. [DOI] [PubMed] [Google Scholar]
- 24.Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, et al. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–80. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels MH. Effects of variations in physiological cortisol levels on thyrotropin secretion in subjects with adrenal insufficiency: a clinical research center study. J Clin Endocrinol Metab. 2000;85:1388–93. doi: 10.1210/jcem.85.4.6540. [DOI] [PubMed] [Google Scholar]
- 28.Raubenheimer PJ, Young EA, Andrew R, Seckl JR. The role of corticosterone in human hypothalamic-pituitary-adrenal axis feedback. Clin Endocrinol (Oxf) 2006;65:22–6. doi: 10.1111/j.1365-2265.2006.02540.x. [DOI] [PubMed] [Google Scholar]
- 29.Pang D, Kocherginsky M, Krausz T, Kim SY, Conzen SD. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther. 2006;5:933–40. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 30.Cowey CL, Rathmell WK. VHL gene mutations in renal cell carcinoma: role as a biomarker of disease outcome and drug efficacy. Curr Oncol Rep. 2009;11:94–101. doi: 10.1007/s11912-009-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinengo C, Poggio T, Menotti M, Scalzo MS, Mastini C, Ambrogio C, et al. ALK-dependent control of hypoxia-inducible factors mediates tumor growth and metastasis. Cancer Res. 2014;74:6094–106. doi: 10.1158/0008-5472.CAN-14-0268. [DOI] [PubMed] [Google Scholar]
- 32.Ravindranathan P, Lange C, Raj GV. Invited Minireview: Deciphering the cellular functions of PELP1. Mol Endocrinol. 2015:me20151049. doi: 10.1210/ME.2015-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniel AR, Gaviglio AL, Knutson TP, Ostrander JH, D’Assoro AB, Ravindranathan P, et al. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1-and estrogen receptor-containing transcription complexes. Oncogene. 2015;34:506–15. doi: 10.1038/onc.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011;31:4663–75. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dales JP, Garcia S, Meunier-Carpentier S, Andrac-Meyer L, Haddad O, Lavaut MN, et al. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer. 2005;116:734–9. doi: 10.1002/ijc.20984. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Ibusuki M, Okumura Y, Kawasoe T, Kai K, Iyama K, et al. Hypoxia-inducible factor 1alpha is closely linked to an aggressive phenotype in breast cancer. Breast Cancer Res Treat. 2008;110:465–75. doi: 10.1007/s10549-007-9742-1. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–43. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayahara M, Ohanian J, Ohanian V, Berry A, Vadlamudi R, Ray DW. MNAR functionally interacts with both NH2-and COOH-terminal GR domains to modulate transactivation. Am J Physiol Endocrinol Metab. 2008;295:E1047–55. doi: 10.1152/ajpendo.90429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–32. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2010;120:603–12. doi: 10.1007/s10549-009-0419-9. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009;15:4123–30. doi: 10.1158/1078-0432.CCR-08-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res. 2007;67:5505–12. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Dai J, McNamara KM, Bai B, Shi M, Chan MS, et al. Prognostic significance of proline, glutamic acid, leucine rich protein 1 (PELP1) in triple-negative breast cancer: a retrospective study on 129 cases. BMC Cancer. 2015;15:699. doi: 10.1186/s12885-015-1694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–64. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan SR, Nair BC, Sareddy GR, Roy SS, Natarajan M, Suzuki T, et al. Novel role of PELP1 in regulating chemotherapy response in mutant p53-expressing triple negative breast cancer cells. Breast Cancer Res Treat. 2015;150:487–99. doi: 10.1007/s10549-015-3339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lofgren KA, Ostrander JH, Housa D, Hubbard GK, Locatelli A, Bliss RL, et al. Mammary gland specific expression of Brk/PTK6 promotes delayed involution and tumor formation associated with activation of p38 MAPK. Breast Cancer Res. 2011;13:R89. doi: 10.1186/bcr2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16:1611–28. doi: 10.1016/j.bbmt.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Green AC, Olsen CM. Increased Risk of Melanoma in Organ Transplant Recipients: Systematic Review and Meta-analysis of Cohort Studies. Acta Derm Venereol. 2015 doi: 10.2340/00015555-2148. [DOI] [PubMed] [Google Scholar]
- 50.Lee ST, Strunk KM, Spritz RA. A survey of protein tyrosine kinase mRNAs expressed in normal human melanocytes. Oncogene. 1993;8:3403–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.